In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement
Abstract
:1. Introduction
2. Methods
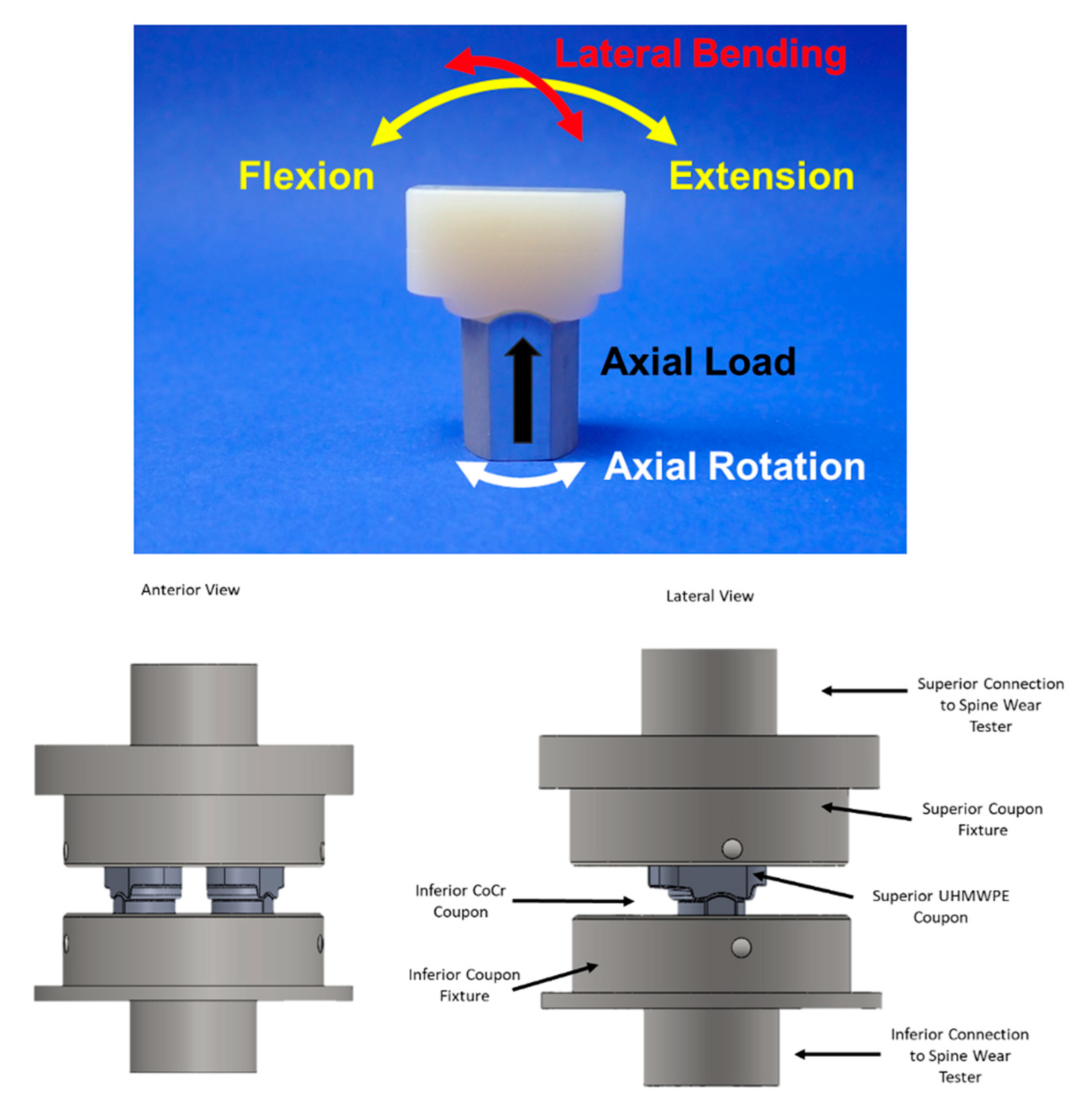
2.1. Standard Wear Testing
2.2. Adverse Abrasive Testing
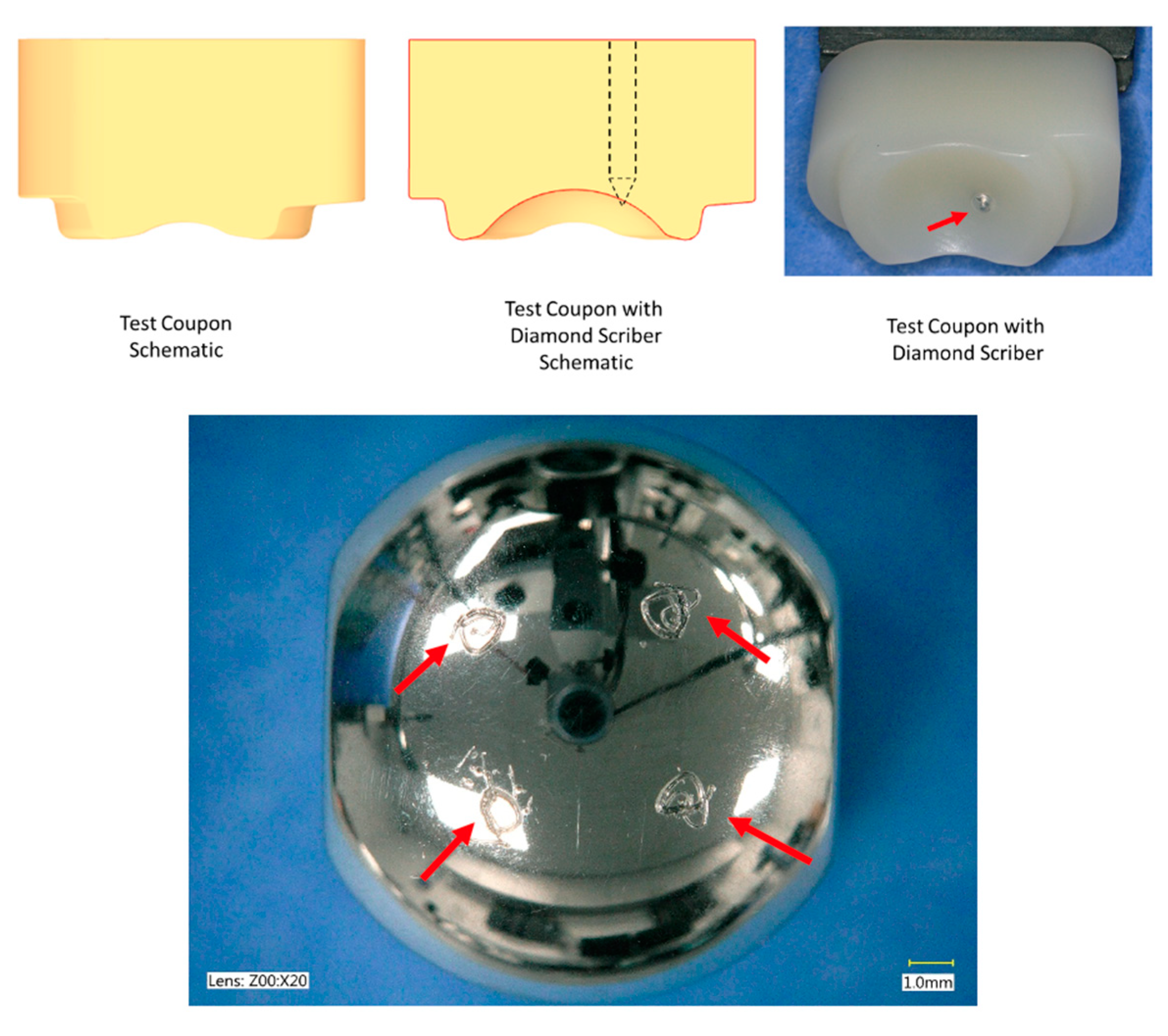
2.3. Impingement Testing
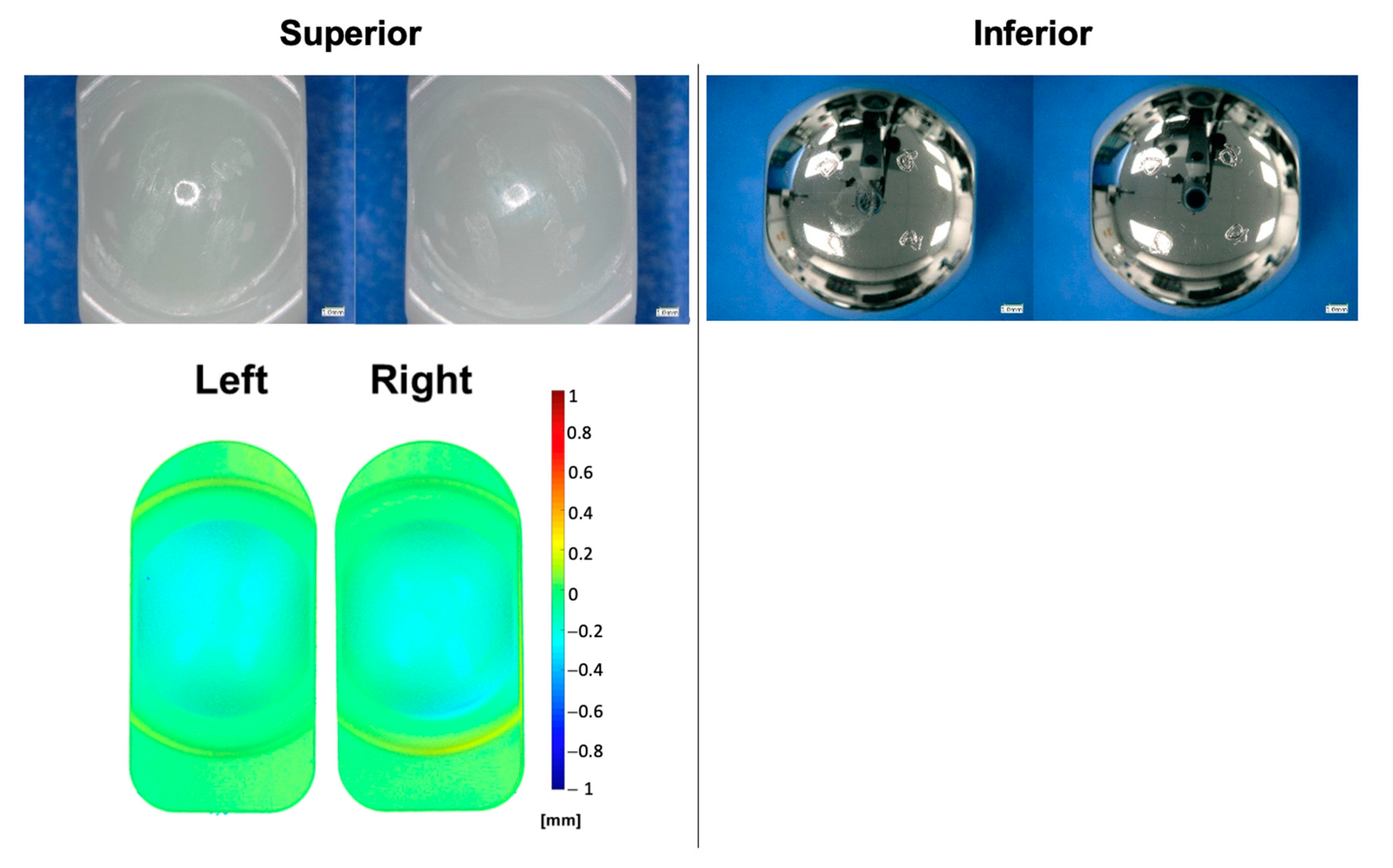
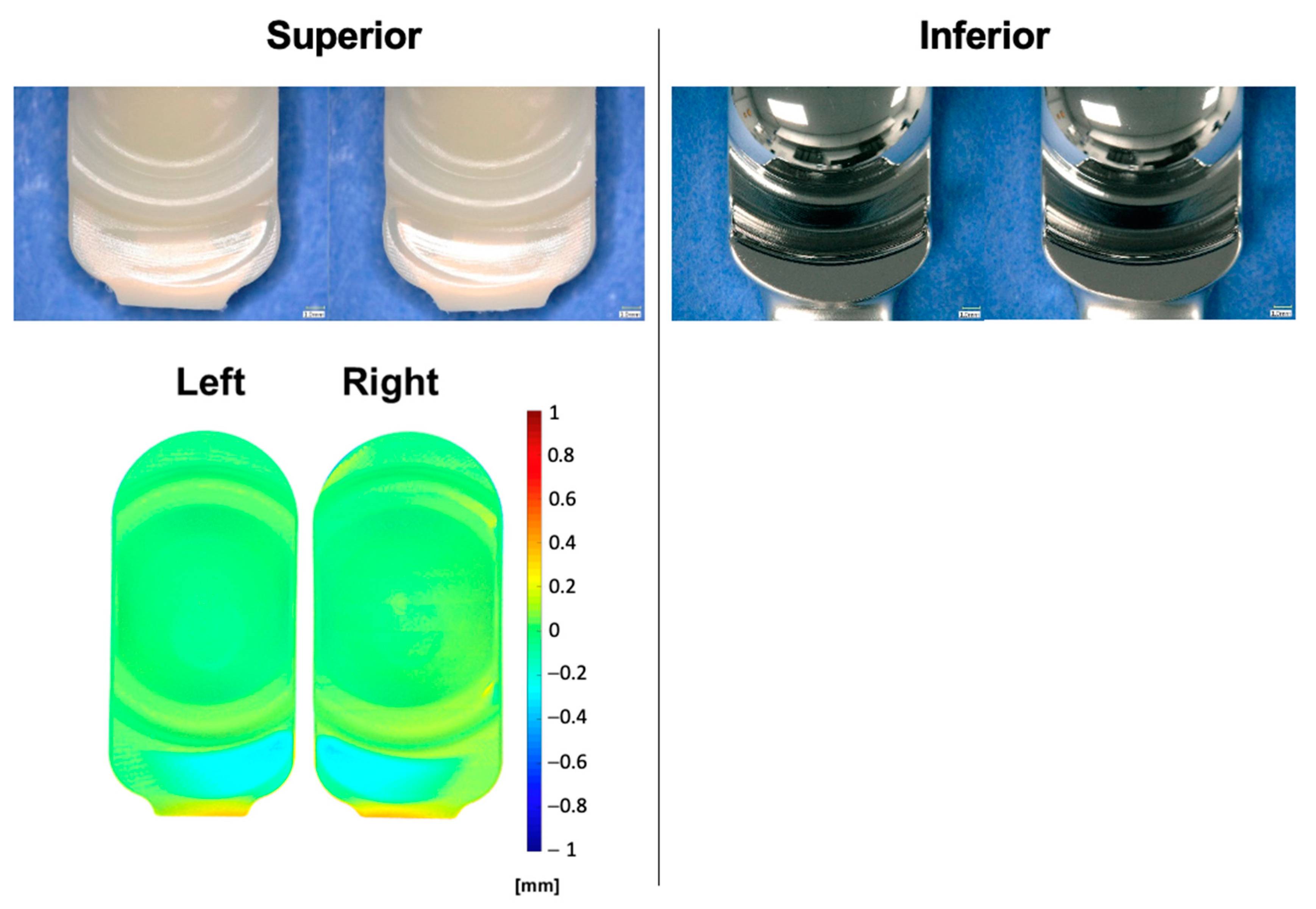
2.4. Interval Analyses
3. Results
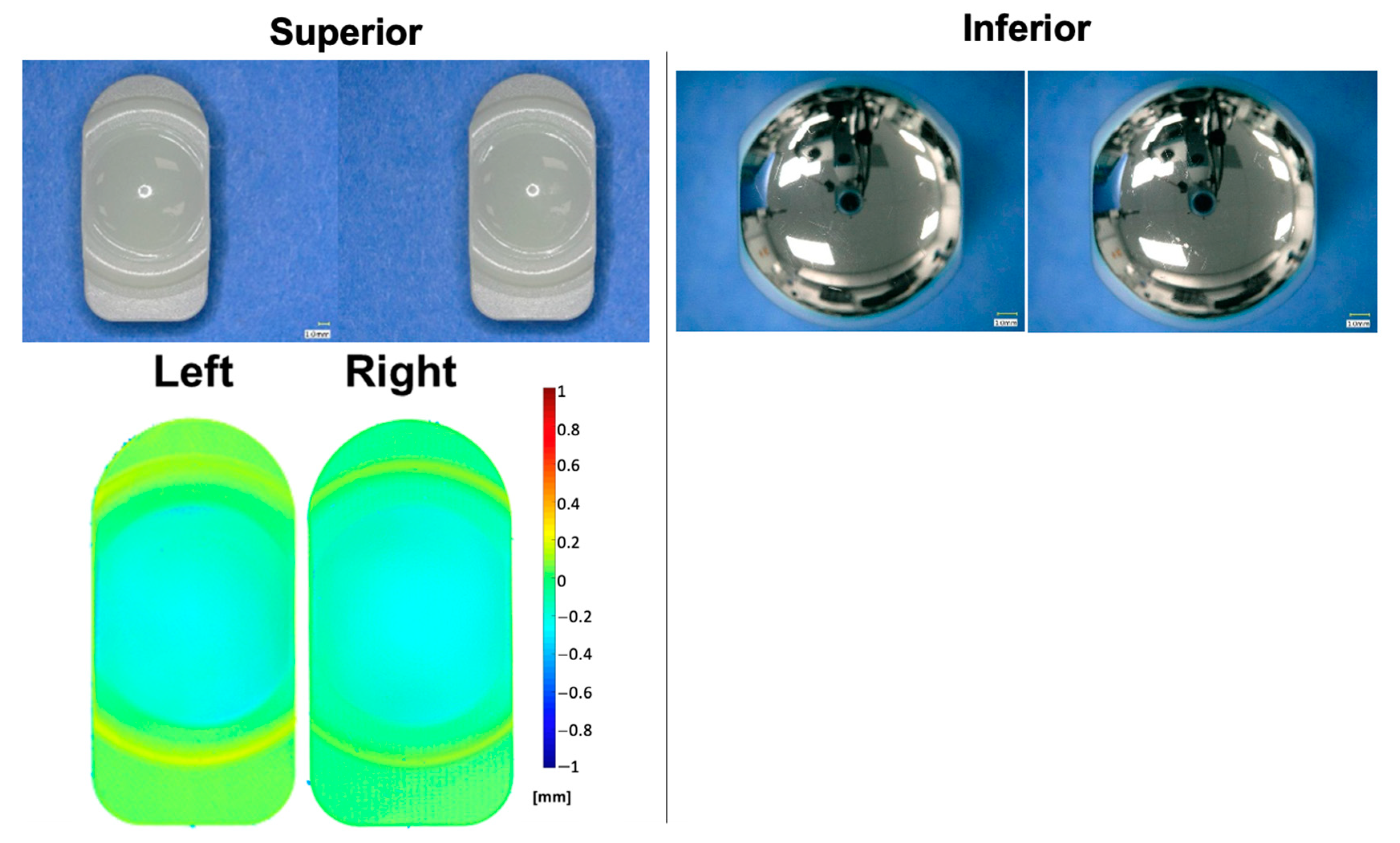
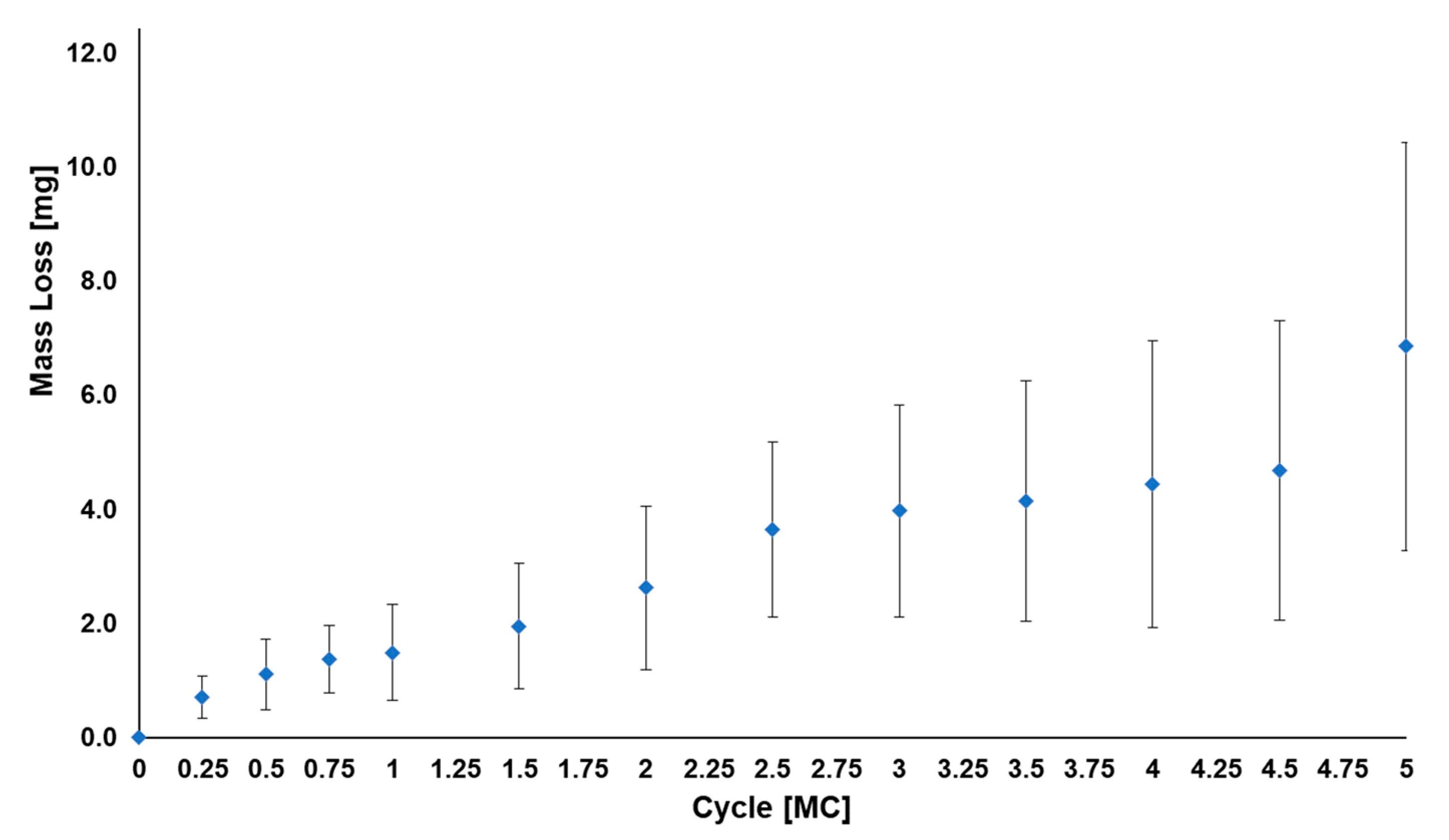
3.1. Standard Wear Testing
3.2. Adverse Abrasive Testing
3.3. Impingement Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Franco, D.; Largoza, G.; Montenegro, T.S.; Gonzalez, G.A.; Hines, K.; Harrop, J. Lumbar Total Disc Replacement: Current Usage. Neurosurg. Clin. N. Am. 2021, 32, 511. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.J.; Garcia, R.; Blumenthal, S.; Coric, D.; Patel, V.V.; Dinh, D.H.; Buttermann, G.R.; Deutsch, H.; Miller, L.E.; Persaud, E.J. Five-year results of a randomized controlled trial for lumbar artificial discs in single-level degenerative disc disease. Spine J. 2019, 44, 1685–1696. [Google Scholar] [CrossRef]
- Schroeder, G.D.; Vaccaro, A.R.; Divi, S.N.; Reyes, A.A.; Goyal, D.K.; Phillips, F.M.; Zigler, J. 2021 Position Statement From the International Society for the Advancement of Spine Surgery on Cervical and Lumbar Disc Replacement. Int. J. Spine Surg. 2021, 15, 37–46. [Google Scholar] [CrossRef]
- Zigler, J.; Gornet, M.F.; Ferko, N.; Cameron, C.; Schranck, F.W.; Patel, L. Comparison of Lumbar Total Disc Replacement With Surgical Spinal Fusion for the Treatment of Single-Level Degenerative Disc Disease: A Meta-Analysis of 5-Year Outcomes From Randomized Controlled Trials. Glob. Spine J. 2018, 8, 413–423. [Google Scholar] [CrossRef]
- Bai, D.-Y.; Liang, L.; Zhang, B.-B.; Zhu, T.; Zhang, H.-J.; Yuan, Z.-G.; Chen, Y.-F. Total disc replacement versus fusion for lumbar degenerative diseases - a meta-analysis of randomized controlled trials. Medicine 2019, 98, e16460. [Google Scholar] [CrossRef]
- Mills, E.S.; Shelby, T.; Bouz, G.J.; Hah, R.J.; Wang, J.C.; Alluri, R.K. A Decreasing National Trend in Lumbar Disc Arthroplasty. Glob. Spine J. 2022, 13, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, R.; Bourghli, A.; Obeid, I. Revision surgery of spinal dynamic implants: A literature review and algorithm proposal. Eur. Spine J. 2020, 29, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V. Effect of type of contact, counterface surface roughness, and contact area on the wear and friction of extensively cross-linked, vitamin E stabilized UHMWPE. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1985. [Google Scholar] [CrossRef]
- Chakravarty, R.; Elmallah, R.D.K.; Cherian, J.J.; Kurtz, S.M.; Mont, M.A. Polyethylene Wear in Knee Arthroplasty. J. Knee Surg. 2015, 28, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Liza, S.; Shahemi, N.H.; Yee, T.M.; Syed, S.K.; Puad, M. Biomedical Tribology: Wear of Polyethylene in Total Joint Replacement. In Tribology and Sustainability; CRC Press: Boca Raton, FL, USA, 2021; p. 353. [Google Scholar]
- Cheung, A.; Yan, C.H.; Fu, H.; Cheung, M.H.; Chan, P.K.; Chiu, K.Y. Ten-to sixteen-year follow-up of highly cross-linked polyethylene in total hip arthroplasty: What factors affect wear? J. Arthroplast. 2019, 34, 2016. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Gawel, H.A.; Patel, J.D. History and Systematic Review of Wear and Osteolysis Outcomes for First-generation Highly Crosslinked Polyethylene. Clin. Orthop. Relat. Res. 2011, 469, 2262–2277. [Google Scholar] [CrossRef]
- Bistolfi, A.; Giustra, F.; Bosco, F.; Sabatini, L.; Aprato, A.; Bracco, P.; Bellare, A. Ultra-high molecular weight polyethylene (UHMWPE) for hip and knee arthroplasty: The present and the future. J. Orthop. 2021, 25, 98–106. [Google Scholar] [CrossRef]
- Sielatycki, J.A.; Devin, C.J.; Pennings, J.; Koscielski, M.; Metcalf, T.; Archer, K.R.; Dunn, R.; Humphreys, S.C.; Hodges, S. A novel lumbar total joint replacement may be an improvement over fusion for degenerative lumbar conditions: A comparative analysis of patient-reported outcomes at one year. Spine J. 2021, 21, 829–840. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Sielatycki, J.A.; Havey, R.M.; Humphreys, S.C.; Hodges, S.D.; Blank, K.R.; Muriuki, M.G. Loading of the lumbar spine during transition from standing to sitting: Effect of fusion versus motion preservation at L4–L5 and L5–S1. Spine J. 2021, 21, 708–719. [Google Scholar] [CrossRef]
- Veruva, S.Y.; Steinbeck, M.J.; Toth, J.; Alexander, D.D.; Kurtz, S.M. Which Design and Biomaterial Factors Affect Clinical Wear Performance of Total Disc Replacements? A Systematic Review. Clin. Orthop. Relat. Res. 2014, 472, 3759–3769. [Google Scholar] [CrossRef]
- Spece, H.; Yarbrough, R.V.; Kurtz, S.M. In Vivo Performance of Vitamin E Stabilized Polyethylene Implants for Total Hip Arthroplasty: A Review. J. Arthroplast. 2022, 38, 970–979. [Google Scholar] [CrossRef]
- Massier, J.R.; Van Erp, J.H.; Snijders, T.E.; Gast, A.D. A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients. Acta Orthop. 2020, 91, 705. [Google Scholar] [CrossRef] [PubMed]
- Spece, H.; Yarbrough, R.V.; Kurtz, S.M. A Review of Early In Vivo Performance of Antioxidant Stabilized Polyethylene for Total Knee Arthroplasty. J. Arthroplast. 2023, 38, 970. [Google Scholar] [CrossRef] [PubMed]
- Reeks, J.; Liang, H. Materials and Their Failure Mechanisms in Total Disc Replacement. Lubricants 2015, 3, 346–364. [Google Scholar] [CrossRef]
- ASTM F3295-18; Standard Guide for Impingement Testing of Total Disc Prostheses. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 18192-1:2011; Implants for Surgery—Wear of Total Intervertebral Spinal Disc Prostheses—Part 1: Loading and Displacement Parameters for Wear Testing and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2011.
- Shkolnikov, Y.P.; Bowden, A.; MacDonald, D.; Kurtz, S.M. Wear pattern observations from TDR retrievals using autoregistration of voxel data. J. Biomed. Mater. Res. 2010, 94, 312. [Google Scholar] [CrossRef] [PubMed]
- Scott-Young, M.; Rathbone, E.; Grierson, L. Midterm osteolysis-induced aseptic failure of the M6-C cervical total disc replacement secondary to polyethylene wear debris. Eur. Spine J. 2022, 31, 1273–1282. [Google Scholar] [CrossRef]
- Wahbeh, J.M.; Park, S.-H.; Campbell, P.; Ebramzadeh, E.; Sangiorgio, S.N. The lexicon for periprosthetic bone loss versus osteolysis after cervical disc arthroplasty: A systematic review. Eur. Spine J. 2022, 31, 830–842. [Google Scholar] [CrossRef]
- Kurtz, S.; Siskey, R.; Ciccarelli, L.; van Ooij, A.; Peloza, J.; Villarrage, M.; Dean, S. Retrieval Analysis of Total Disc Replacements: Implications for Standardized Wear Testing. J. ASTM Int. 2006, 3, 1. [Google Scholar] [CrossRef]
- Siskey, R.; Peck, J.; Mehta, H.; Kosydar, A.; Kurtz, S.; Hill, G. Development of a clinically relevant impingement test method for a mobile bearing lumbar total disc replacement. Spine J. 2016, 16, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Hozack, W.J.; Purtill, J.J.; Marcolongo, M.; Kraay, M.J.; Goldberg, V.M.; Sharkey, P.F.; Parvizi, J.; Rimnac, C.M.; Edidin, A.A. Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin. Orthop. Relat. Res. 2006, 453, 47. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, M.P.; Bennett, A.P.; Rimnac, C.M.; Wright, T.M. The natural history of ultra high molecular weight polyethylene. Clin. Orthop. Relat. Res. 1994, 309, 20–28. [Google Scholar]
- Kurtz, S.M.; Patel, J.D. The clinical performance of highly crosslinked UHMWPE in hip replacements. In The UHMWPE Biomaterials Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, 3rd ed.; Kurtz, S.M., Ed.; Elsevier Inc.: Oxford, UK, 2016; p. 57. [Google Scholar]
- U.S. Food and Drug Administration. PMA P050010/S020: FDA Summary of Safety and Effectiveness Data; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Affatato, S.; De Mattia, J.S.; Bracco, P.; Pavoni, E.; Taddei, P. Wear performance of neat and vitamin E blended highly cross-linked PE under severe conditions: The combined effect of accelerated ageing and third body particles during wear test. J. Mech. Behav. Biomed. Mater. 2016, 64, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Tokuhashi, Y.; Sawada, H.; Saito, S.; Suzuki, S.; Ozaki, R.; Nakanishi, K. Fatigue wear test comparing vitamin-E-blended crosslinked polyethylene and conventional polyethylene in a Posterior Dynamic Stabilization System of the spine in the laboratory. J. Orthop. Sci. 2022, 27, 558–562. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. PMA P120024: FDA Summary of Safety and Effectiveness Data; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2015.
- FDA Summary of Safety and Effectiveness Data Template (SSED). Available online: https://www.fda.gov/media/113810/download (accessed on 13 October 2023).
- Hyde, P.J.; Fisher, J.; Hall, R.M. Wear characteristics of an unconstrained lumbar total disc replacement under a range of in vitro test conditions. J. Biomed. Mater. Res. 2017, 105, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vicars, R.; Prokopovich, P.; Brown, T.D.; Tipper, J.L.; Ingham, E.; Fisher, J.; Hall, R.M. The Effect of Anterior-Posterior Shear on the Wear of CHARITÉ Total Disc Replacement. Spine 2012, 37, E528–E534. [Google Scholar] [CrossRef]
- Kettler, A.; Bushelow, M.; Wilke, H.-J. Influence of the loading frequency on the wear rate of a polyethylene-on-metal lumbar intervertebral disc replacement. Eur. Spine J. 2012, 21, S709. [Google Scholar] [CrossRef]
- Grupp, T.M.; Yue, J.J.; Garcia, R., Jr.; Basson, J.; Schwiesau, J.; Fritz, B.; Blömer, W. Biotribological evaluation of artificial disc arthroplasty devices: Influence of loading and kinematic patterns during in vitro wear simulation. Eur. Spine J. 2009, 18, 98–108. [Google Scholar] [CrossRef]
- Serhan, H.A.; Dooris, A.P.; Parsons, M.L.; Ares, P.J.; Gabriel, S.M.P. In Vitro Wear Assessment of the Charite Artificial Disc According to ASTM Recommendations. Spine 2006, 31, 1900–1910. [Google Scholar] [CrossRef]
- Grupp, T.M.; Yue, J.J.; Garcia, R., Jr.; Kaddick, C.; Fritz, B.; Schilling, C.; Schwiesau, J.; Blömer, W. Evaluation of impingement behaviour in lumbar spinal disc arthroplasty. Eur. Spine J. 2015, 24, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
- Wang, A. A unified theory of wear for ultra-high molecular weight polyethylene in multi-directional sliding. Wear 2001, 248, 38–47. [Google Scholar] [CrossRef]
- McKellop, H.A.; Campbell, P.; Park, S.H.; Schmalzried, T.P.; Grigoris, P.; Amstutz, H.C.; Sarmiento, A. The origin of submicron polyethylene wear debris in total hip arthroplasty. Clin. Orthop. 1995, 311, 3. [Google Scholar]
- Bragdon, C.R.; O’Connor, D.O.; Lowenstein, J.D.; Jasty, M.; Syniuta, W.D. The importance of multidirectional motion on the wear of polyethylene. Proc. Instn. Mech. Eng. 1996, 210, 157–165. [Google Scholar] [CrossRef]
- Prock-Gibbs, H.; Pumilia, C.A.; Meckmongkol, T.; Lovejoy, J.; Mumith, A.; Coathup, M. Incidence of Osteolysis and Aseptic Loosening Following Metal-on-Highly Cross-Linked Polyethylene Hip Arthroplasty: A Systematic Review of Studies with Up to 15-Year Follow-up. J. Bone Jt. Surg. 2021, 103, 728. [Google Scholar] [CrossRef]
- Bladen, C.; Teramura, S.; Russell, S.; Fujiwara, K.; Fisher, J.; Ingham, E.; Tomita, N.; Tipper, J. Analysis of wear, wear particles, and reduced inflammatory potential of vitamin E ultrahigh-molecular-weight polyethylene for use in total joint replacement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 458. [Google Scholar] [CrossRef]
- Galliera, E.; Ragone, V.; Marazzi, M.G.; Selmin, F.; Banci, L.; Romanelli, M.M.C. Vitamin E-stabilized UHMWPE: Biological response on human osteoblasts to wear debris. Clin. Chim. Acta 2018, 486, 18–25. [Google Scholar] [CrossRef]
- MOTUS Total Joint Replacement Investigational Device Exemption Study, NCT05438719. In ClinicalTrials.gov; 2023. Available online: https://www.clinicaltrials.gov/study/NCT05438719 (accessed on 13 October 2023).




| Test | VE-HXLPE TJR | activL SSED [34] | ProDisc SSED [31] |
|---|---|---|---|
| Standard Wear Testing | The average mass wear rate up to 10 MC was 1.2 ± 0.5 mg/MC for the superior UHMWPE components and 0.4 ± 0.1 mg/MC for the inferior (CoCr) components. | Average cumulative wear at 10 million cycles was 25.3 mg and the mean wear rate was 2.7 mg/MC. The test setup was unable to create any backside wear of the polyethylene inlay. | The average mass wear rate of the polyethylene insert up to 5.0 MC was 5.4 ± 1.3 mg/MC and 4.8 ± 1.1 mg/MC for the large- and medium-sized devices, respectively. |
| Adverse Abrasive Testing | The average mass wear rate up to 5 MC was 1.1 ± 0.6 mg/MC for the superior UHMWPE components and 0.2 ± 0.1 mg/MC for the inferior (CoCr) components. | Not included in SSED. | Not included in SSED. |
| Impingement Testing | The average mass wear rate up to 1.0 MC was 1.7 ± 1.1 mg/MC (short) and 3.9 ± 1.1 mg/MC (long) for the superior UHMWPE components and 1.2 ± 0.4 mg/MC (short) and 1.2 ± 0.1 mg/MC (long) for the inferior (CoCr) components. | Impingement behavior of the activL® included contact between the cobalt chromium endplates. Based on gravimetric measurements, the mean total material loss from both endplates was 1.5 ± 0.4 mm3. The UHMWPE inlays gained mass during testing.Volume to mass loss using 8.4 mg/mm3 for density: 12.6 ± 3.4 mg/MC. | Under impingement conditions, the rate of mass loss of the polyethylene insert was less than in the Mode I testing condition. The polyethylene demonstrated impingement on the posterior surface. Characteristic of the contact observed on published retrievals, metal-on-metal contact was observed in the 2 mm offset test group. The maximum mass loss experienced by the metal components was converted to maximum volume losses of 0.6 mm3 and 0.5 mm3 for the inferior and superior components, respectively.Volumes to mass loss using 8.4 mg/mm3 for density: 5.4 mg/MC. |
| Authors | Lumbar ADR Design Evaluated | Clean Standard Wear Test Methods | Wear Rate | Notes |
|---|---|---|---|---|
| Hyde et al. (2017) [36] | CHARITÉ | ISO 18192-1 for 5 MC (“Baseline”), followed by testing a lower cross shear, lower loads, and changes in center of rotation | 14.4 ± 2.1 mm3/MC | Lower cross shear reduced baseline wear by 49% |
| Siskey et al. (2016) [27] | CHARITÉ | ISO 18192-1 | 13.8 ± 3.8 mg/MC | Conventional polyethylene cores were reverse engineered and tested with retrieved endplates |
| Vicars et al. (2012) [37] | CHARITÉ | ISO 18192-1 for 5 MC (“4DOF”), then 5 MC with additional shear load profile (“5DOF”) | 12.2 ± 1.0 mg/MC for 5 MC standard test (“4DOF”); 22.3 ± 2.0 mg/MC for 5 MC standard test (“5DOF”); | Height loss of polyethylene cores was not sensitive to changes in the standard test method |
| Kettler et al. (2012) [38] | ProDisc L | ISO 18192-1 for 6 MC at 1 and 2 Hz | 5.6 ± 2.3 mg/MC at 1 Hz 7.7 ± 1.6 mg/MC at 2 Hz | Authors recommended testing at 1 Hz |
| Grupp et al. (2009) [39] | Activ-L | ISO/FDIS18192-1 (2006) for 10 MC followed by ASTM F2423-05 | 2.7 ± 0.3 mg/MC (ISO test method) 0.14 ± 0.06 mg/MC (ASTM 2005 test method) | ASTM 2005 lower wear rate explained by linear wear track |
| Serhan et al. (2006) [40] | CHARITÉ | Adaptation of ASTM Draft 2 Protocol | 0.13 mg/MC | Linear wear track explains low wear rate |
| Authors | Lumbar TDR Design Evaluated | Test Methods | Wear Rate | Notes |
|---|---|---|---|---|
| Siskey et al. (2016) [27] | CHARITÉ | Impingement protocols (1 MC) with and without facet engagement developed | −1.0 ± 1.2 mg/MC (wear not detectable due to UHMWPE deformation and mass gain due to fluid adsorption) | Test protocols and impingement scars validated with clinical retrievals |
| Grupp et al. (2015) [41] | Activ-L | Four different protocols: flexion, extension, lateral bending, flexion and bending | Flexion: 0.67 mm3/MC Extension: 0.21 mm3/MC Lateral bending: 0.06 mm3/MC Flexion and bending: 1.44 mm3/MC | CoCr-CoCr endplate impingement simulated, validated with clinical retrievals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siskey, R.L.; Yarbrough, R.V.; Spece, H.; Hodges, S.D.; Humphreys, S.C.; Kurtz, S.M. In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement. Bioengineering 2023, 10, 1198. https://doi.org/10.3390/bioengineering10101198
Siskey RL, Yarbrough RV, Spece H, Hodges SD, Humphreys SC, Kurtz SM. In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement. Bioengineering. 2023; 10(10):1198. https://doi.org/10.3390/bioengineering10101198
Chicago/Turabian StyleSiskey, Ryan L., Ronald V. Yarbrough, Hannah Spece, Scott D. Hodges, Steven C. Humphreys, and Steven M. Kurtz. 2023. "In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement" Bioengineering 10, no. 10: 1198. https://doi.org/10.3390/bioengineering10101198
APA StyleSiskey, R. L., Yarbrough, R. V., Spece, H., Hodges, S. D., Humphreys, S. C., & Kurtz, S. M. (2023). In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement. Bioengineering, 10(10), 1198. https://doi.org/10.3390/bioengineering10101198






