Application of Escherichia coli-Derived Recombinant Human Bone Morphogenic Protein-2 to Unstable Spinal Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Undergoing Spinal Surgery
2.2. Surgical Techniques
2.3. Radiologic and Clinical Parameters
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Radiographic Outcomes
3.3. Clinical Outcomes
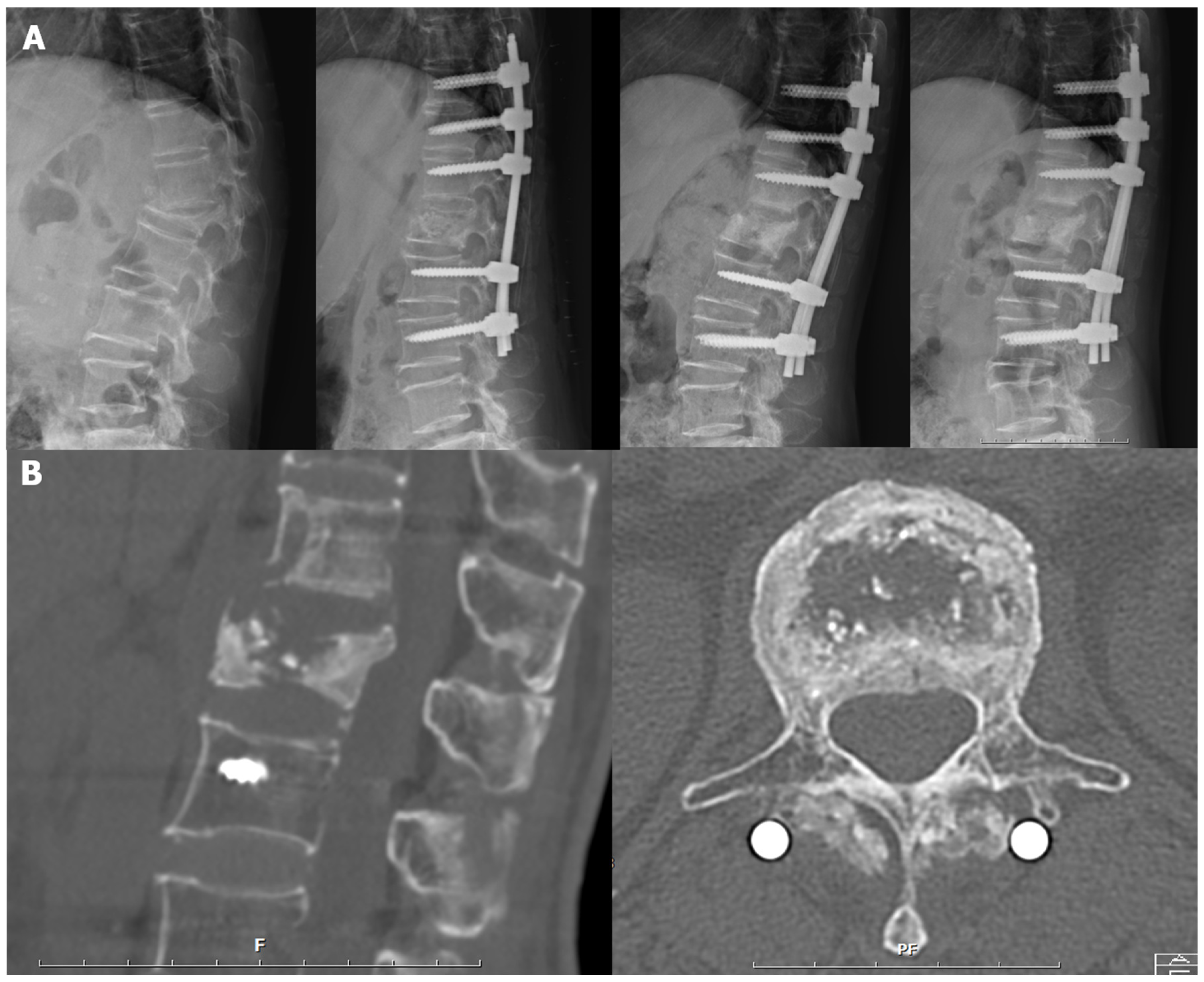
3.4. Representative Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, H.D.; Kim, E.H.; Lee, J.C.; Choi, S.W.; Kim, H.S.; Cha, J.S.; Shin, B.J. Management of Osteoporotic Vertebral Fracture: Review Update 2022. Asian Spine J. 2022, 16, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Ha, K.Y.; Kim, Y.S.; Kim, K.W.; Rhyu, K.W.; Park, J.B.; Shin, J.H.; Kim, Y.Y.; Lee, J.S.; Park, H.Y.; et al. Lumbar Interbody Fusion and Osteobiologics for Lumbar Fusion. Asian Spine J. 2022, 16, 1022–1033. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.; Fischgrund, J.S. Bone morphogenetic proteins for spinal fusion. Spine J. 2005, 5, 240s–249s. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Z.; Zheng, G.B.; Lee, J.H. Escherichia coli BMP-2 showed comparable osteoinductivity with Chinese hamster ovary derived BMP-2 with demineralized bone matrix as carrier. Growth Factors 2019, 37, 85–94. [Google Scholar] [CrossRef]
- Israel, D.I.; Nove, J.; Kerns, K.M.; Moutsatsos, I.K.; Kaufman, R.J. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors 1992, 7, 139–150. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Brokelmann, M.; Marten, S.; Trappe, S.; Cabrera-Crespo, J.; Hoffmann, A.; Gross, G.; Weich, H.A.; Rinas, U. Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J. Biotechnol. 2002, 94, 185–194. [Google Scholar] [CrossRef]
- Kimura, M.; Zhao, M.; Zellin, G.; Linde, A. Bone-inductive efficacy of recombinant human bone morphogenetic protein-2 expressed in Escherichia coli: An experimental study in rat mandibular defects. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2000, 34, 289–299. [Google Scholar]
- Yano, K.; Hoshino, M.; Ohta, Y.; Manaka, T.; Naka, Y.; Imai, Y.; Sebald, W.; Takaoka, K. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J. Bone Miner. Metab. 2009, 27, 355–363. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Yeom, J.S.; Chang, B.S.; Yang, J.J.; Koo, K.H.; Hwang, C.J.; Lee, K.B.; Kim, H.J.; Lee, C.K.; et al. Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: An open, active-controlled, randomized, multicenter trial. Spine J. 2017, 17, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Chang, B.S.; Chang, S.Y.; Park, H.S.; Kim, H. Anterior Cervical Discectomy and Fusion Using Escherichia coli-Derived Recombinant Human Bone Morphogenetic Protein-2: A Pilot Study. Clin. Orthop. Surg. 2022, 14, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Im, S.K.; Lee, J.H.; Lee, K.Y.; Yoo, S.J. Effectiveness and Feasibility of Injectable Escherichia coli-Derived Recombinant Human Bone Morphogenetic Protein-2 for Anterior Lumbar Interbody Fusion at the Lumbosacral Junction in Adult Spinal Deformity Surgery: A Clinical Pilot Study. Orthop. Surg. 2022, 14, 1350–1358. [Google Scholar] [CrossRef]
- Dai, L.Y. Principles of management of thoracolumbar fractures. Orthop. Surg. 2012, 4, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, Y.H.; Ha, K.Y.; Chang, D.G.; Kim, S.I.; Park, S.B. Are the Choice of Frame and Intraoperative Patient Positioning Associated with Radiologic and Clinical Outcomes in Long-instrumented Lumbar Fusion for Adult Spinal Deformity? Clin. Orthop. Relat. Res. 2022, 480, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Kanayama, M.; Oha, F.; Hashimoto, T.; Iwasaki, N. Effect of teriparatide (rh-PTH 1-34) versus bisphosphonate on the healing of osteoporotic vertebral compression fracture: A retrospective comparative study. BMC Musculoskelet. Disord. 2017, 18, 148. [Google Scholar] [CrossRef]
- Kim, G.W.; Jang, J.W.; Hur, H.; Lee, J.K.; Kim, J.H.; Kim, S.H. Predictive factors for a kyphosis recurrence following short-segment pedicle screw fixation including fractured vertebral body in unstable thoracolumbar burst fractures. J. Korean Neurosurg. Soc. 2014, 56, 230–236. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Liang, Z.; Zhou, M.; Chen, C. Comparative Clinical Effectiveness and Safety of Bone Morphogenetic Protein Versus Autologous Iliac Crest Bone Graft in Lumbar Fusion: A Meta-analysis and Systematic Review. Spine 2020, 45, E729–E741. [Google Scholar] [CrossRef]
- Sandler, A.B.; Scanaliato, J.P.; Raiciulescu, S.; Nesti, L.; Dunn, J.C. Bone Morphogenic Protein for Upper Extremity Fractures: A Systematic Review. Hand 2023, 18, 80–88. [Google Scholar] [CrossRef]
- Fuchs, T.; Stolberg-Stolberg, J.; Michel, P.A.; Garcia, P.; Amler, S.; Wähnert, D.; Raschke, M.J. Effect of Bone Morphogenetic Protein-2 in the Treatment of Long Bone Non-Unions. J. Clin. Med. 2021, 10, 4597. [Google Scholar] [CrossRef]
- Henssler, L.; Kerschbaum, M.; Mukashevich, M.Z.; Rupp, M.; Alt, V. Molecular enhancement of fracture healing—Is there a role for Bone Morphogenetic Protein-2, parathyroid hormone, statins, or sclerostin-antibodies? Injury 2021, 52 (Suppl. S2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Jeong, S.; Lee, K.B. Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid. Bioengineering 2023, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.J.; Longobardi, L.; Willcockson, H.; Temple, J.D.; Tagliafierro, L.; Ye, P.; Li, T.; Esposito, A.; Moats-Staats, B.M.; Spagnoli, A. BMP2 Regulation of CXCL12 Cellular, Temporal, and Spatial Expression is Essential During Fracture Repair. J. Bone Miner. Res. 2015, 30, 2014–2027. [Google Scholar] [CrossRef]
- Morimoto, T.; Kaito, T.; Kashii, M.; Matsuo, Y.; Sugiura, T.; Iwasaki, M.; Yoshikawa, H. Effect of Intermittent Administration of Teriparatide (Parathyroid Hormone 1-34) on Bone Morphogenetic Protein-Induced Bone Formation in a Rat Model of Spinal Fusion. J. Bone Jt. Surg. Am. 2014, 96, e107. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Kim, K.C. Does Teriparatide Improve Fracture Union?: A Systematic Review. J. Bone Metab. 2020, 27, 167–174. [Google Scholar] [CrossRef]
- Ebata, S.; Takahashi, J.; Hasegawa, T.; Mukaiyama, K.; Isogai, Y.; Ohba, T.; Shibata, Y.; Ojima, T.; Yamagata, Z.; Matsuyama, Y.; et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months after Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J. Bone Jt. Surg. Am. 2017, 99, 365–372. [Google Scholar] [CrossRef]
- Ueno, M.; Toriumi, E.; Yoshii, A.; Tabata, Y.; Furudate, T.; Tajima, Y. Use of Parathyroid Hormone and Rehabilitation Reduces Subsequent Vertebral Body Fractures after Balloon Kyphoplasty. Asian Spine J. 2022, 16, 432–439. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, K.W.; Ryu, J.H.; Yoon, S.T.; Baek, I.H.; Jang, T.Y.; Lee, J.S. Long-Segmental Posterior Fusion Combined with Vertebroplasty and Wiring: Alternative Surgical Technique for Kummell’s Disease with Neurologic Deficits—A Retrospective Case Series. Geriatr. Orthop. Surg. Rehabil. 2021, 12, 21514593211027055. [Google Scholar] [CrossRef]
- Kim, W.J.; Ma, S.B.; Shin, H.M.; Song, D.G.; Lee, J.W.; Chang, S.H.; Park, K.Y.; Choy, W.S.; Oh, T.H. Correlation of Sagittal Imbalance and Recollapse after Percutaneous Vertebroplasty for Thoracolumbar Osteoporotic Vertebral Compression Fracture: A Multivariate Study of Risk Factors. Asian Spine J. 2022, 16, 231–240. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef]
- Lim, J.; Choi, S.W.; Youm, J.Y.; Kwon, H.J.; Kim, S.H.; Koh, H.S. Posttraumatic Delayed Vertebral Collapse: Kummell’s Disease. J. Korean Neurosurg. Soc. 2018, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alhashash, M.; Shousha, M. Minimally Invasive Short-Segment Anteroposterior Surgery for Thoracolumbar Osteoporotic Fractures with Canal Compromise: A Prospective Study with a Minimum 2-Year Follow-up. Asian Spine J. 2022, 16, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Suh, S.P.; Yeom, J.; Kim, J.Y.; Lee, S.G.; Han, J.W. Minimally Invasive Spine Surgery versus Open Posterior Instrumentation Surgery for Unstable Thoracolumbar Burst Fracture. Asian Spine J. 2021, 15, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Jover-Mendiola, A.D.; Lopez-Prats, F.A.; Lizaur-Utrilla, A.; Vizcaya-Moreno, M.F. Patient-Reported Outcomes of Minimally Invasive versus Open Transforaminal Lumbar Interbody Fusion for Degenerative Lumbar Disc Disease: A Prospective Comparative Cohort Study. Clin. Orthop. Surg. 2023, 15, 257–264. [Google Scholar] [CrossRef]


| Variables | Number (Percent) |
|---|---|
| Number of patients | 10 |
| Sex (M:F) | 5:5 |
| Age (years) | 71.7 ± 7.9 |
| Smoking | 2 (20%) |
| Bone mineral density (T-score) | −1.6 ± 1.7 |
| Fractured level | D11: 1 D12: 3 L1: 4 L3: 1 L5: 1 |
| Level of fusion | 4.1 ± 1.0 |
| Cement augmentation | 4 (40%) |
| Operation time (minutes) | 184.6 ± 70.0 |
| Bleeding (mL) | 315.0 ± 152.8 |
| ASA (1:2:3:4) | 0:9:1:0 |
| Patients | Age/Sex | Diagnosis | Fused Level | Anabolic Agent | Radiographic Healing (Days) | Follow-Up Period (Months) |
|---|---|---|---|---|---|---|
| 1 | 71/M | L5 unstable burst fracture | L3-S1 with S2AI screws | Teriparatide (3 months) | 96 | 17 |
| 2 | 75/M | D12 chalky stick fracture Underlying AS | D10-L2 (D10, L2 cement augmentation) | Teriparatide (6 months) | 173 | 13 |
| 3 | 81/F | D12 chalky stick fracture Underlying AS | D9-L2 (L2 cement augmentation) | Teriparatide (8 months) | 100 | 23 |
| 4 | 75/F | L1 unstable burst fracture | D11-L2 (D11,12,L2 cement augmentation) | Teriparatide (1 month) | 68 | 18 |
| 5 | 68/M | L3 flexion-distraction injury | L1–4 | Teriparatide (1 month) | 83 | 18 |
| 6 | 70/M | L1 unstable burst fracture | D11–L2 | Teriparatide (5 months) | 67 | 17 |
| 7 | 78/F | L1 unstable burst fracture Combined prevertebral abscess | D10–L3 | Teriparatide (1 month) → Romosozumab (7 months) | 84 | 13 |
| 8 | 74/F | D12 unstable burst fracture | D10–L2 | Romosozumab (12 months) | 192 | 12 |
| 9 | 52/M | L1 unstable burst fracture | D11–L3 | None | 74 | 12 |
| 10 | 73/F | D11 unstable burst fracture | D8–L2 (L2 cement augmentation) | Teriparatide (2 months) → Romosozumab (6 months) | 62 | 12 |
| Total | - | - | 4.1 ± 1.0 levels | 5.2 ± 3.9 months | 99.9 ± 45.4 | 15.5 ± 3.7 |
| LKA | AVH | VWA | |
|---|---|---|---|
| Pre-operative | 9.9 ± 17.9 | 19.1 ± 8.3 | 12.3 ± 8.3 |
| Post-operative | −0.8 ± 16.5 | 28.7 ± 3.2 | 2.5 ± 4.6 |
| p-value * | 0.002 | 0.001 | 0.001 |
| Last follow-up | 3.8 ± 18.6 | 26.4 ± 4.9 | 4.7 ± 5.6 |
| p-value ** | 0.072 | 0.006 | 0.006 |
| Pre-Operative | Last Follow-Up | p-Value | |
|---|---|---|---|
| NRS (back pain) | 8.6 ± 1.8 | 3.7 ± 1.8 | <0.001 |
| NRS (leg pain) | 3.4 ± 3.7 | 2.4 ± 2.3 | 0.393 |
| ODI | 67.8 ± 17.5 | 36.0 ± 13.3 | 0.002 |
| EQ-5D index | 0.21 ± 0.28 | 0.68 ± 0.18 | 0.001 |
| ASIA impairment scale | |||
| A | 0 | 0 | - |
| B | 0 | 0 | |
| C | 1 | 0 | |
| D | 3 | 1 | |
| E | 6 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Lee, J.-S.; Ha, K.-Y.; Kim, S.-I.; Jung, H.-Y.; Kim, G.-U.; Joh, Y.; Park, H.-Y. Application of Escherichia coli-Derived Recombinant Human Bone Morphogenic Protein-2 to Unstable Spinal Fractures. Bioengineering 2023, 10, 1114. https://doi.org/10.3390/bioengineering10101114
Kim Y-H, Lee J-S, Ha K-Y, Kim S-I, Jung H-Y, Kim G-U, Joh Y, Park H-Y. Application of Escherichia coli-Derived Recombinant Human Bone Morphogenic Protein-2 to Unstable Spinal Fractures. Bioengineering. 2023; 10(10):1114. https://doi.org/10.3390/bioengineering10101114
Chicago/Turabian StyleKim, Young-Hoon, Jun-Seok Lee, Kee-Yong Ha, Sang-Il Kim, Ho-Young Jung, Geon-U Kim, Yongwon Joh, and Hyung-Youl Park. 2023. "Application of Escherichia coli-Derived Recombinant Human Bone Morphogenic Protein-2 to Unstable Spinal Fractures" Bioengineering 10, no. 10: 1114. https://doi.org/10.3390/bioengineering10101114
APA StyleKim, Y.-H., Lee, J.-S., Ha, K.-Y., Kim, S.-I., Jung, H.-Y., Kim, G.-U., Joh, Y., & Park, H.-Y. (2023). Application of Escherichia coli-Derived Recombinant Human Bone Morphogenic Protein-2 to Unstable Spinal Fractures. Bioengineering, 10(10), 1114. https://doi.org/10.3390/bioengineering10101114









