Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedures
2.3. Radiographic Evaluation
2.4. Micro-CT Analysis
2.5. Manual Palpation and Manipulation
2.6. Histology Analysis
2.7. Statistical Analysis
3. Results
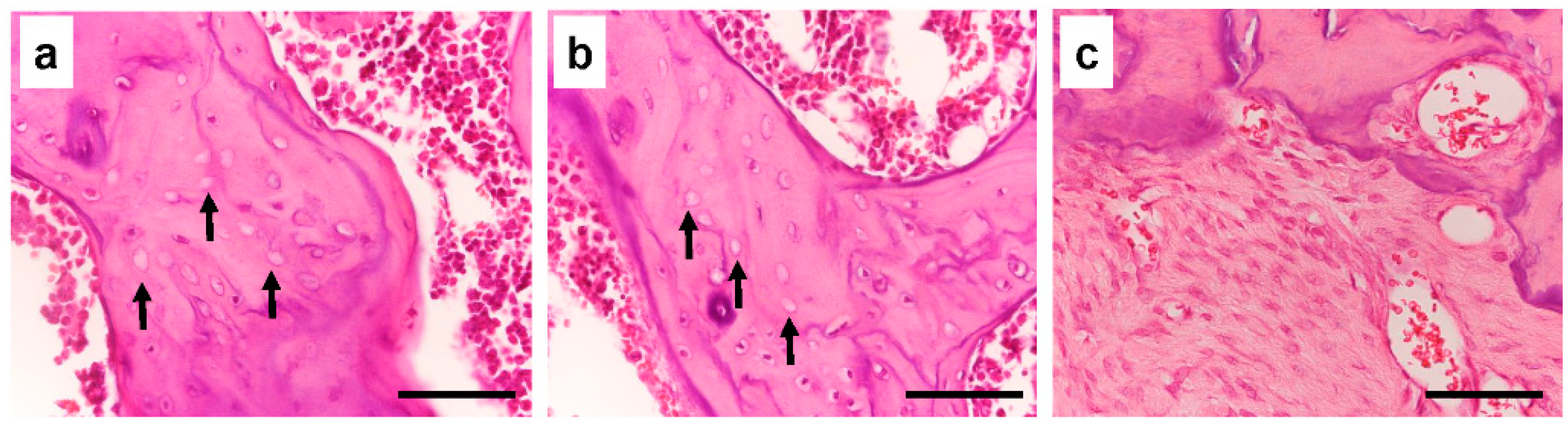
3.1. The Effect of Long-Term Treatment with Bisphosphonate
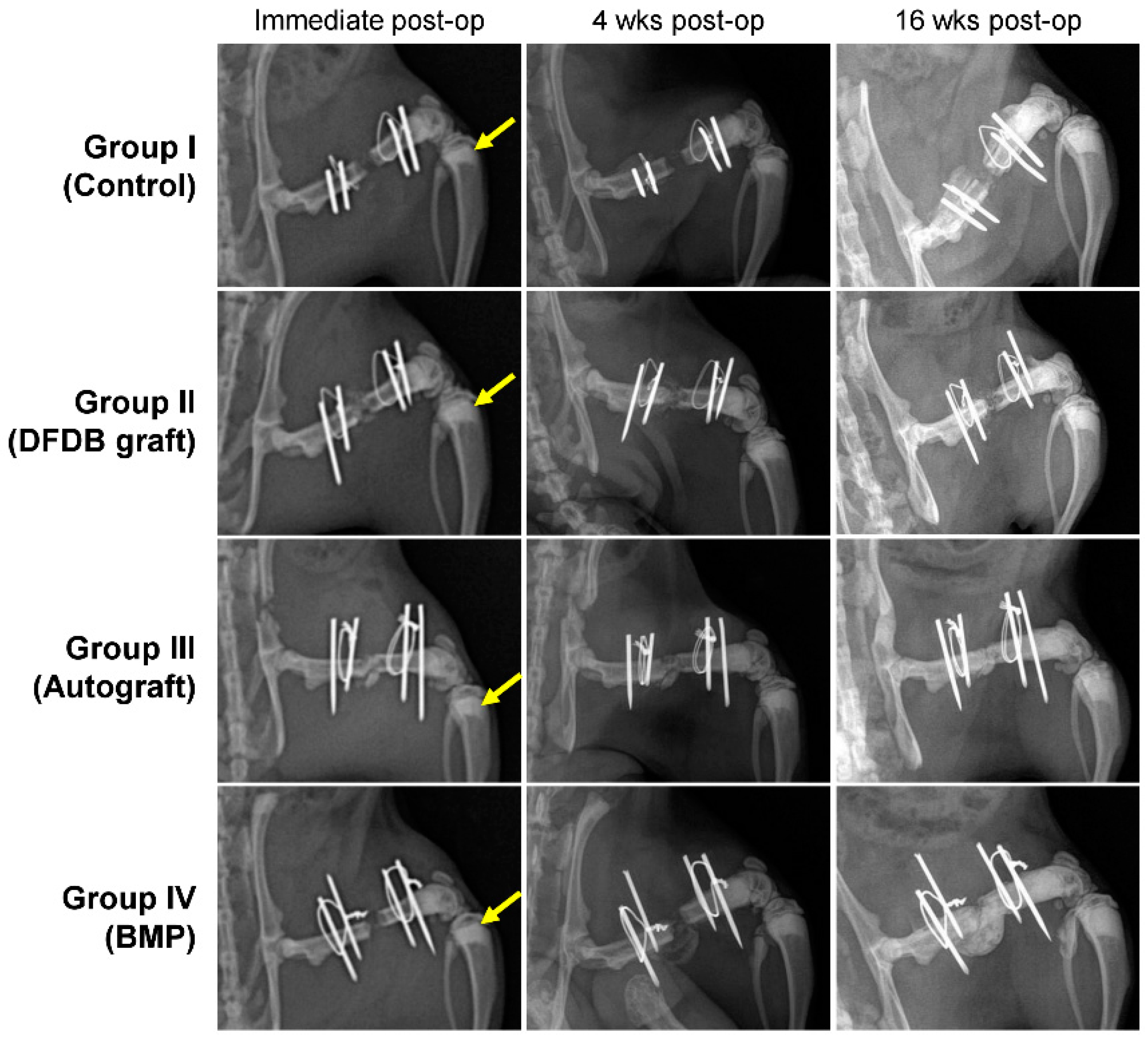
3.2. Radiographic Evaluation
3.3. Manual Palpation and Manipulation
3.4. Micro-CT Analysis—Bone Volume
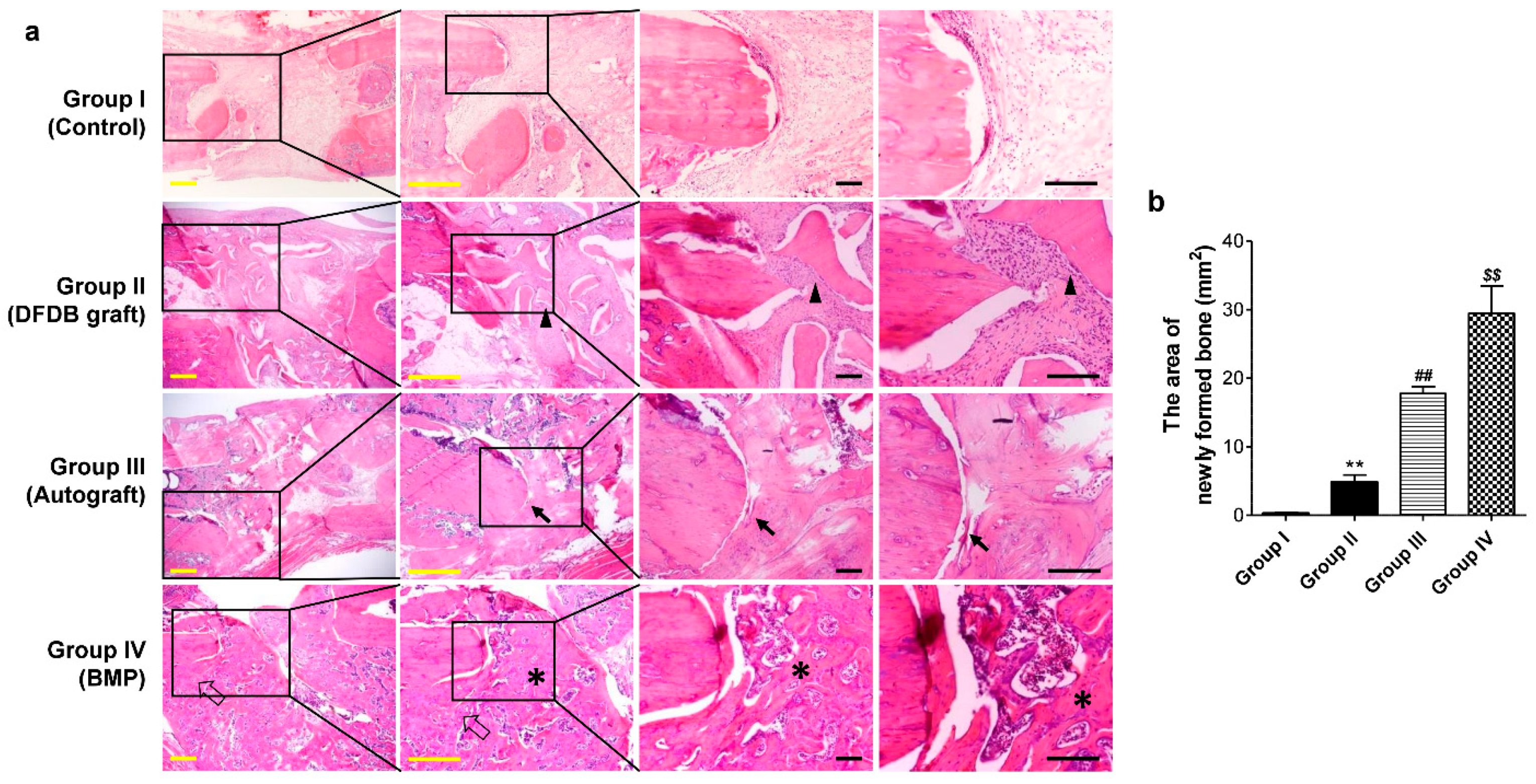
3.5. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M.J. Nitrogen-Containing Bisphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. J. Bone Miner. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.; Lyles, K.; Brown, J.P.; Fraser, W.D.; Miller, P.; Curiel, M.D.; Devogelaer, J.P.; Hooper, M.; Su, G.; Zelenakas, K.; et al. Long-term control of bone turnover in Paget’s disease with zoledronic acid and risedronate. J. Bone Miner. Res. 2007, 22, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.R.; Saunders, Y.; Edmonds, P.M.; Patel, S.; Broadley, K.E.; Johnston, S.R.D. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 2003, 327, 469. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Siegrist, M.; Denzer, A.; Saulacic, N.; Grosjean, J.; Bohner, M.; Hofstetter, W. Bisphosphonates reduce biomaterial turnover in healing of critical-size rat femoral defects. J. Orthop. Surg. 2018, 26, 2309499018802487. [Google Scholar] [CrossRef] [PubMed]
- Kates, S.L.; Ackert-Bicknell, C.L. How do bisphosphonates affect fracture healing? Injury 2016, 47 (Suppl. 1), 65–68. [Google Scholar] [CrossRef]
- Pozzi, S.; Vallet, S.; Mukherjee, S.; Cirstea, D.; Vaghela, N.; Santo, L.; Rosen, E.; Ikeda, H.; Okawa, Y.; Kiziltepe, T.; et al. High-Dose Zoledronic Acid Impacts Bone Remodeling with Effects on Osteoblastic Lineage and Bone Mechanical Properties. Clin. Cancer Res. 2009, 15, 5829–5839. [Google Scholar] [CrossRef]
- Puhaindran, M.E.; Farooki, A.; Steensma, M.R.; Hameed, M.; Healey, J.H.; Boland, P.J. Atypical Subtrochanteric Femoral Fractures in Patients with Skeletal Malignant Involvement Treated with Intravenous Bisphosphonates. J. Bone Jt. Surg. 2011, 93, 1235–1242. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical Subtrochanteric and Diaphyseal Femoral Fractures: Second Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef]
- Vasanwala, R.F.; Sanghrajka, A.; Bishop, N.J.; Högler, W. Recurrent Proximal Femur Fractures in a Teenager With Osteogenesis Imperfecta on Continuous Bisphosphonate Therapy: Are We Overtreating? J. Bone Miner. Res. 2016, 31, 1449–1454. [Google Scholar] [CrossRef]
- Grady, M.K.; Watson, J.T.; Cannada, L.K. Treatment of Femoral Fracture Nonunion After Long-term Bisphosphonate Use. Orthopedics 2012, 35, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Hyodo, K.; Matsumoto, Y.; Yanagisawa, Y.; Yoshizawa, T.; Yamazaki, M. Surgical results of atypical femoral fractures in long-term bisphosphonate and glucocorticoid users—Relationship between fracture reduction and bone union. J. Orthop. 2019, 19, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-C.; Tzeng, S.-T.; Keorochana, G.; Lee, K.-B.; Johnson, J.S.; Morishita, Y.; Murray, S.S.; Wang, J.C. Enhancement of recombinant human BMP-7 bone formation with bmp binding peptide in a rodent femoral defect model. J. Orthop. Res. 2011, 29, 753–759. [Google Scholar] [CrossRef]
- Lissenberg-Thunnissen, S.N.; de Gorter, D.J.J.; Sier, C.F.M.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- van Baardewijk, L.J.; van Der Ende, J.; Lissenberg-Thunnissen, S.; Romijn, L.M.; Hawinkels, L.J.A.C.; Sier, C.F.M.; Schipper, I.B. Circulating bone morphogenetic protein levels and delayed fracture healing. Int. Orthop. 2013, 37, 523–527. [Google Scholar] [CrossRef]
- Ghodadra, N.S.; Singh, K. Recombinant human bone morphogenetic protein-2 in the treatment of bone fractures. Biol. Targets Ther. 2008, 2, 345–354. [Google Scholar] [CrossRef]
- Jones, A.L.; Bucholz, R.W.; Bosse, M.J.; Mirza, S.K.; Lyon, T.R.; Webb, L.X.; Pollak, A.N.; Golden, J.D.; Valentin-Opran, A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J. Bone Jt. Surg. 2006, 88, 1431–1441. [Google Scholar] [CrossRef]
- Axelrad, T.W.; Steen, B.; Lowenberg, D.W.; Creevy, W.R.; Einhorn, T.A. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J. Bone Jt. Surg. 2008, 90, 1617–1622. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef]
- Vantucci, C.E.; Krishan, L.; Cheng, A.; Prather, A.; Roy, K.; Guldberg, R.E. BMP-2 delivery strategy modulates local bone regeneration and systemic immune responses to complex extremity trauma. Biomater. Sci. 2021, 9, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and Radiographic Outcomes of Anterior Lumbar Interbody Fusion Using Recombinant Human Bone Morphogenetic Protein-2. Spine 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Angle, S.R.; Sena, K.; Sumner, D.R.; Virkus, W.W.; Virdi, A.S. Healing of rat femoral segmental defect with bone morphogenetic protein-2: A dose response study. J. Musculoskelet. Neuronal Interact. 2012, 12, 28–37. [Google Scholar] [PubMed]
- Morishita, Y.; Naito, M.; Miyazaki, M.; He, W.; Wu, G.; Wei, F.; Sintuu, C.; Hymanson, H.; Brochmann, E.J.; Murray, S.S.; et al. Enhanced effects of BMP-binding peptide combined with recombinant human BMP-2 on the healing of a rodent segmental femoral defect. J. Orthop. Res. 2010, 28, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Miyazaki, M.; Yoshiiwa, T.; Hara, K.; Kataoka, M.; Tsumura, H. Manipulation of the anabolic and catabolic responses with BMP-2 and zoledronic acid in a rat femoral fracture model. Bone 2011, 49, 777–782. [Google Scholar] [CrossRef]
- Kanezaki, S.; Miyazaki, M.; Ishihara, T.; Notani, N.; Abe, T.; Tsubouchi, Y.; Kataoka, M.; Tsumura, H. Enhancement of the effects of intermittent parathyroid hormone (1–34) by bone morphogenetic protein in a rat femoral open fracture model. J. Orthop. Surg. Res. 2019, 14, 403. [Google Scholar] [CrossRef]
- Miyazaki, M.; Toyoda, M.; Yoshiiwa, T.; Kawano, M.; Kaku, N.; Tsumura, H. Enhancement of the Effects of Exfoliated Carbon Nanofibers by Bone Morphogenetic Protein in a Rat Femoral Fracture Model. J. Orthop. Res. 2015, 33, 185–192. [Google Scholar] [CrossRef]
- Sharma, D.; Ivanovski, S.; Slevin, M.; Hamlet, S.; Pop, T.S.; Brinzaniuc, K.; Petcu, E.B.; Miroiu, R.I. Bisphosphonate-related osteonecrosis of jaw (BRONJ): Diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc. Cell 2013, 5, 1. [Google Scholar] [CrossRef]
- Al Muderis, M.; Azzopardi, T.; Cundy, P. Zebra lines of pamidronate therapy in children. J. Bone Jt. Surg. 2007, 89, 1511–1516. [Google Scholar] [CrossRef]
- Bosemark, P.; Isaksson, H.; Tägil, M. Influence of systemic bisphosphonate treatment on mechanical properties of BMP-induced calluses in a rat fracture model: Comparison of three-point bending and twisting test. J. Orthop. Res. 2014, 32, 721–726. [Google Scholar] [CrossRef]
- Mathavan, N.; Bosemark, P.; Isaksson, H.; Tägil, M. Investigating the synergistic efficacy of BMP-7 and zoledronate on bone allografts using an open rat osteotomy model. Bone 2013, 56, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed Bone Turnover by Bisphosphonates Increases Microdamage Accumulation and Reduces Some Biomechanical Properties in Dog Rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef]
- Park, J.-B.; Cho, S.-H.; Kim, I.; Lee, W.; Kang, S.-H.; Kim, H. Evaluation of the bisphosphonate effect on stem cells derived from jaw bone and long bone rabbit models: A pilot study. Arch. Oral Biol. 2018, 85, 178–182. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.B.; Zhang, J.; Waits, C.; Skikne, B.; Garimella, R.; Anderson, H.C. Localization of bone morphogenetic proteins (BMPs)-2, -4, and -6 within megakaryocytes and platelets. Bone 2004, 35, 1316–1322. [Google Scholar] [CrossRef]
- Kugimiya, F.; Kawaguchi, H.; Kamekura, S.; Chikuda, H.; Ohba, S.; Yano, F.; Ogata, N.; Katagiri, T.; Harada, Y.; Azuma, Y.; et al. Involvement of Endogenous Bone Morphogenetic Protein (BMP) 2 and BMP6 in Bone Formation. J. Biol. Chem. 2005, 280, 35704–35712. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.L.; Howie, R.N.; Hall, S.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; LaRue, A.C.; Muise-Helmericks, R.; et al. Optimizing bone wound healing using BMP2 with absorbable collagen sponge and Talymed nanofiber scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef]
- Edgar, C.M.; Chakravarthy, V.; Barnes, G.; Kakar, S.; Gerstenfeld, L.C.; Einhorn, T.A. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone 2007, 40, 1389–1398. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Hochberg, M.C.; Mogun, H.; Schneeweiss, S. The relation between bisphosphonate use and non-union of fractures of the humerus in older adults. Osteoporos. Int. 2009, 20, 895–901. [Google Scholar] [CrossRef]
- den Boer, F.C.; Bramer, J.A.M.; Blokhuis, T.J.; Van Soest, E.J.; Jenner, J.M.G.T.; Patka, P.; Bakker, F.C.; Burger, E.H.; Haarman, H.J.T.M. Effect of recombinant human osteogenic protein-1 on the healing of a freshly closed diaphyseal fracture. Bone 2002, 31, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Baffes, G.C.; Wolfe, M.W.; Sampath, T.K.; Rueger, D.C. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin. Orthop. Relat. Res. 1994, 301, 302–312. [Google Scholar] [CrossRef]
- Li, F.; Yu, F.; Liao, X.; Wu, C.; Wang, Y.; Li, C.; Lou, F.; Li, B.; Yin, B.; Wang, C.; et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci. Rep. 2019, 9, 8073. [Google Scholar] [CrossRef]
- Barba-Recreo, P.; Del Castillo Pardo de Vera, J.L.; García-Arranz, M.; Yébenes, L.; Burgueño, M. Zoledronic acid—Related osteonecrosis of the jaws. Experimental model with dental extractions in rats. J. Craniomaxillofac. Surg. 2014, 42, 744–750. [Google Scholar] [CrossRef]


| Complete/Partial Union | 4 Weeks | 8 Weeks | 12 Weeks | 16 Weeks |
|---|---|---|---|---|
| Group I (Control, n = 10) | 0/0 | 0/1 | 0/1 | 0/1 |
| Group II (DFDB graft, n = 10) | 0/2 | 0/2 | 1/3 | 2/2 |
| Group III (Autograft, n = 10) | 0/3 | 1/3 | 4/3 | 6/3 |
| Group IV (BMP, n = 10) | 0/3 | 1/4 | 3/4 | 6/2 |
| Complete Union | 16 Weeks | Union Rate |
|---|---|---|
| Group I (Control, n = 10) | 0 | 0 |
| Group II (DFDB graft, n = 10) | 2 | 20% |
| Group III (Autograft, n = 10) | 6 | 60% |
| Group IV (BMP, n = 10) | 6 | 60% |
| BV (mm³) | BV/TV (%) | |
|---|---|---|
| Group I (Control, n = 10) | 42.8 ± 19.8 | 15.79 ± 5.6 |
| Group II (DFDB graft, n = 10) | 72.93 ± 15.6 | 36.44 ± 2.5 |
| Group III (Autograft, n = 10) | 168.78 ± 20.1 | 64.3 ± 1.4 |
| Group IV (BMP, n = 10) | 193.94 ± 61.5 | 56.59 ± 9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.J.; Jeong, S.; Lee, K.-B. Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid. Bioengineering 2023, 10, 86. https://doi.org/10.3390/bioengineering10010086
Moon YJ, Jeong S, Lee K-B. Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid. Bioengineering. 2023; 10(1):86. https://doi.org/10.3390/bioengineering10010086
Chicago/Turabian StyleMoon, Young Jae, Seongyup Jeong, and Kwang-Bok Lee. 2023. "Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid" Bioengineering 10, no. 1: 86. https://doi.org/10.3390/bioengineering10010086
APA StyleMoon, Y. J., Jeong, S., & Lee, K.-B. (2023). Bone Morphogenetic Protein 2 Promotes Bone Formation in Bone Defects in Which Bone Remodeling Is Suppressed by Long-Term and High-Dose Zoledronic Acid. Bioengineering, 10(1), 86. https://doi.org/10.3390/bioengineering10010086





