The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs
Abstract
1. Introduction
2. Materials and Methods
- Etanercept 2.5 μg/mL (E)
- Infliximab 50 μg/mL (I)
- Metotrexat 2.5 μg/mL (M)
- Etanercept 2.5 μg/mL + Metotrexat 2.5 μg/mL (E + M)
- Infliximab 50 μg/mL + Metotrexat 2.5 μg/mL (I + M)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapila, Y.L. Oral health’s inextricable connection to systemic health: Special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontology 2000 2021, 87, 11–16. [Google Scholar] [CrossRef] [PubMed]
- González-Febles, J.; Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontology 2000 2021, 87, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, J.; Zhao, L. The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: A systematic review and meta-analysis. Lasers Med. Sci. 2021, 36, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Bedoya-García, J.A.; Arrubla-Escobar, D.E. Antibiotic resistance in periodontitis patients: A systematic scoping review of randomized clinical trials. Oral Dis. 2022, 23–29, 1–11. [Google Scholar] [CrossRef]
- Akram, Z.; Hyder, T.; Al-Hamoudi, N.; Binshabaib, M.S.; Alharthi, S.S.; Hanif, A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 19, 86–92. [Google Scholar] [CrossRef]
- Pummer, A.; Knüttel, H.; Hiller, K.A.; Buchalla, W.; Cieplik, F.; Maisch, T. Antimicrobial efficacy of irradiation with visible light on oral bacteria in vitro: A systematic review. Future Med. Chem. 2017, 9, 1557–1574. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic Profiles of Patients with Dental Prosthetic Treatment and Periodontitis before and after Photoactivation Therapy—Randomized Clinical Trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- Sculean, A.; Deppe, H.; Miron, R.; Schwarz, F.; Romanos, G.; Cosgarea, R. Effectiveness of photodynamic therapy in the treatment of periodontal and peri-implant diseases. Oral Biofilms 2021, 29, 133–143. [Google Scholar]
- Nicolae, V.; Chiscop, I.; Cioranu, V.S.I.; Martu, M.A.; Luchian, A.I.; Martu, S.; Solomon, S.M. The use of photoactivated blue-o toluidine for periimplantitis treatment in patients with periodontal disease. Rev. Chim. 2015, 66, 2121–2123. [Google Scholar]
- Rola, P.; Włodarczak, S.; Lesiak, M.; Doroszko, A.; Włodarczak, A. Changes in Cell Biology under the Influence of Low-Level Laser Therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Colaco, A.S. An update on the effect of low-level laser therapy on growth factors involved in oral healing. J. Dent. Lasers 2018, 12, 46. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef]
- Valle, L.A.; Lopes, M.M.; Zangrando, M.S.; Sant’Ana, A.C.; Greghi, S.L.; de Rezende, M.L.; Damante, C.A. Blue photosensitizers for aPDT eliminate Aggregatibacter actinomycetemcomitans in the absence of light: An in vitro study. J. Photochem. Photobiol. B Biol. 2019, 194, 56–60. [Google Scholar] [CrossRef]
- Wang, D.; Pan, H.; Yan, Y.; Zhang, F. Rose bengal-mediated photodynamic inactivation against periodontopathogens in vitro. Photodiagnosis Photodyn. Ther. 2021, 34, 102250. [Google Scholar] [CrossRef]
- Tantivitayakul, P.; Rassameemasmaung, S.; Thapanabhiboonsuk, S. In vitro effect of diode laser against biofilm of Aggregatibacter actinomycetemcomitans. Eur. J. Dentistry. 2018, 12, 485–490. [Google Scholar] [CrossRef]
- Etemadi, A.; Azizi, A.; Pourhajibagher, M.; Chiniforush, N. In Vitro Efficacy of Antimicrobial Photodynamic Therapy with Phycocyanin and Diode Laser for the Reduction of Porphyromonas gingivalis. J. Lasers Med. Sci. 2022, 13, e55. [Google Scholar]
- Picchianti-Diamanti, A.; Rosado, M.M.; D’Amelio, R. Infectious agents and inflammation: The role of microbiota in autoimmune arthritis. Front. Microbiol. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Wysocki, T.; Paradowska-Gorycka, A. Pharmacogenomics of Anti-TNF Treatment Response Marks a New Era of Tailored Rheumatoid Arthritis Therapy. Int. J. Mol. Sci. 2022, 23, 2366. [Google Scholar] [CrossRef]
- Zamri, F.; De Vries, T.J. Use of TNF inhibitors in rheumatoid arthritis and implications for the periodontal status: For the benefit of both? Front. Immunol. 2020, 11, 591365. [Google Scholar] [CrossRef]
- Cheng, W.C.; van Asten, S.D.; Burns, L.A.; Evans, H.G.; Walter, G.J.; Hashim, A.; Hughes, F.J.; Taams, L.S. Periodontitis-associated pathogens P. gingivalis and A. actinomycetemcomitans activate human CD14+ monocytes leading to enhanced Th17/IL-17 responses. Eur. J. Immunol. 2016, 46, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Ibbotson, S.H. A perspective on the use of NB-UVB phototherapy versus PUVA photochemotherapy. Front. Med. 2018, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Matsushita, K.; Horioka, S.; Furuichi, Y.; Sumi, Y. Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria. BMC Oral Health 2017, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Elnazar, A.; Salma, Y.; Ghazy, A.A.; Ghoneim, H.E.; Taha, A.R.; Abouelella, A.M. Effect of ultra violet irradiation on the interplay between Th1 and Th2 lymphocytes. Front. Pharmacol. 2015, 6, 56. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Dawson, D.; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K.; et al. The periodontal war: Microbes and immunity. Periodontology 2000 2017, 75, 52–115. [Google Scholar] [CrossRef]
- Cieplik, F.; Späth, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.A.; Maisch, T.; Schmalz, G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef]
- Oruba, Z.; Łabuz, P.; Macyk, W.; Chomyszyn-Gajewska, M. Periopathogens differ in terms of the susceptibility to toluidine blue O-mediated photodynamic inactivation. Photodiagnosis Photodyn. Ther. 2017, 20, 28–34. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Monzavi, A.; Chiniforush, N.; Monzavi, M.M.; Sobhani, S.; Shahabi, S.; Bahador, A. Real-time quantitative reverse transcription-PCR analysis of expression stability of Aggregatibacter actinomycetemcomitans fimbria-associated gene in response to photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 18, 78–82. [Google Scholar] [CrossRef]
- Fekrazad, R.; Khoei, F.; Bahador, A.; Hakimiha, N. Photo-activated elimination of Aggregatibacter actinomycetemcomitans in planktonic culture: Comparison of photodynamic therapy versus photothermal therapy method. Photodiagnosis Photodyn. Ther. 2017, 19, 28–32. [Google Scholar] [CrossRef]
- Alvarenga, L.H.; Prates, R.A.; Yoshimura, T.M.; Kato, I.T.; Suzuki, L.C.; Ribeiro, M.S.; Ferreira, L.R.; dos Santos Pereira, S.A.; Martinez, E.F.; Saba-Chujfi, E. Aggregatibacter actinomycetemcomitans biofilm can be inactivated by methylene blue-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 131–135. [Google Scholar] [CrossRef]
- Saitawee, D.; Teerakapong, A.; Morales, N.P.; Jitprasertwong, P.; Hormdee, D. Photodynamic therapy of Curcuma longa extract stimulated with blue light against Aggregatibacter actinomycetemcomitans. Photodiagnosis Photodyn. Ther. 2018, 22, 101–105. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Chiniforush, N.; Monzavi, A.; Barikani, H.; Monzavi, M.M.; Sobhani, S.; Shahabi, S.; Bahador, A. Inhibitory Effects of Antimicrobial Photodynamic Therapy with Curcumin on Biofilm-Associated Gene Expression Profile of Aggregatibacter actinomycetemcomitans. J. Dent. 2018, 15, 169. [Google Scholar]
- Azizi, B.; Budimir, A.; Bago, I.; Mehmeti, B.; Jakovljević, S.; Kelmendi, J.; Stanko, A.P.; Gabrić, D. Antimicrobial efficacy of photodynamic therapy and light-activated disinfection on contaminated zirconia implants: An in vitro study. Photodiagnosis Photodyn. Ther. 2018, 21, 328–333. [Google Scholar] [CrossRef]
- de Sousa, G.R.; Soares, L.O.; Soares, B.M.; de Carvalho Cruz, R.; Uliana Junior, P.; Santiago, T.; Farias, L.M.; Magalhães, P.P.; Silveira, L.B.; Almeida Lopes, L.; et al. In vitro evaluation of physical and chemical parameters involved in aPDT of Aggregatibacter actinomycetemcomitans. Lasers Med. Sci. 2022, 37, 391–401. [Google Scholar] [CrossRef]
- Sales, L.S.; Miranda, M.L.; de Oliveira, A.B.; Ferrisse, T.M.; Fontana, C.R.; Milward, M.; Brighenti, F.L. Effect of the technique of photodynamic therapy against the main microorganisms responsible for periodontitis: A systematic review of in-vitro studies. Arch. Oral Biol. 2022, 138, 105425. [Google Scholar] [CrossRef]
- Aabed, K.; Moubayed, N.; BinShabaib, M.S.; ALHarthi, S.S. Is a single session of antimicrobial photodynamic therapy as an adjuvant to non-surgical scaling and root planing effective in reducing periodontal inflammation and subgingival presence of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans in patients with periodontitis? Photodiagnosis Photodyn. Ther. 2022, 38, 102847. [Google Scholar]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 176–198. [Google Scholar] [CrossRef]
- Choe, R.; Balhaddad, A.A.; Fisher, J.P.; Melo, M.A.; Huang, H.C. Photodynamic Therapy for Biomodulation and Disinfection in Implant Dentistry: Is It Feasible and Effective? Photochem. Photobiol. 2021, 97, 916–929. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Martu, M.-A.; Luchian, I.; Stoleriu, S.; Diaconu-Popa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. The Effects of 810 nm Diode Laser and Indocyanine Green on Periodontal Parameters and HbA1c in Patients with Periodontitis and Type II Diabetes Mellitus: A Randomized Controlled Study. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef] [PubMed]
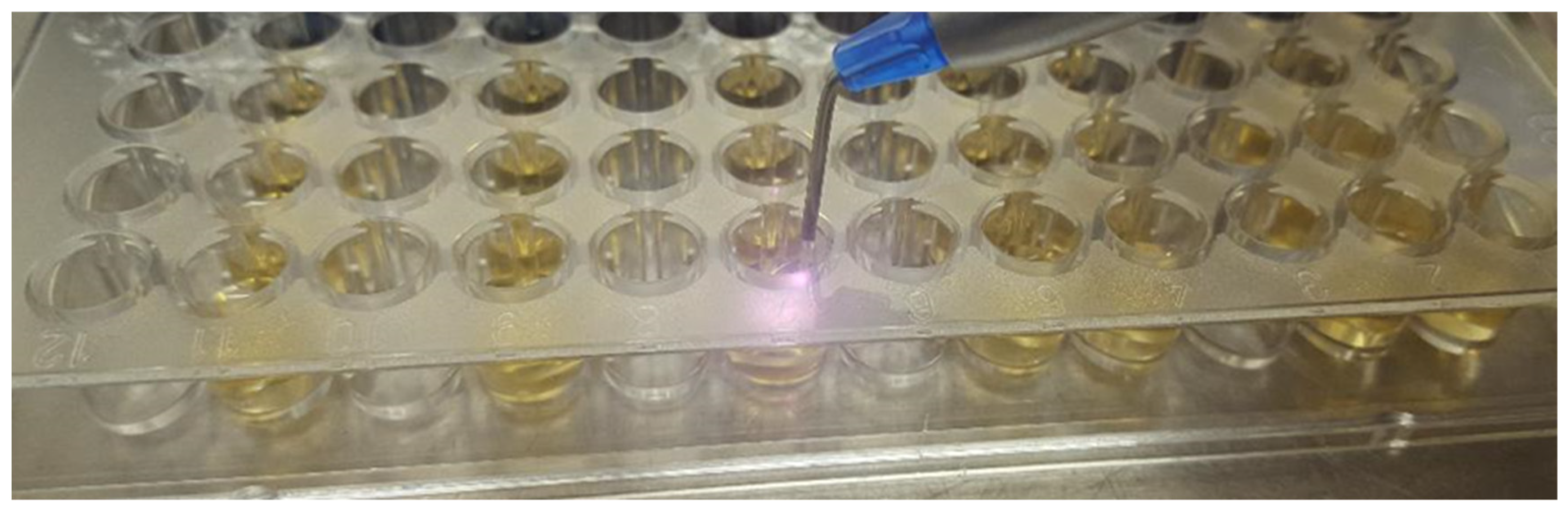
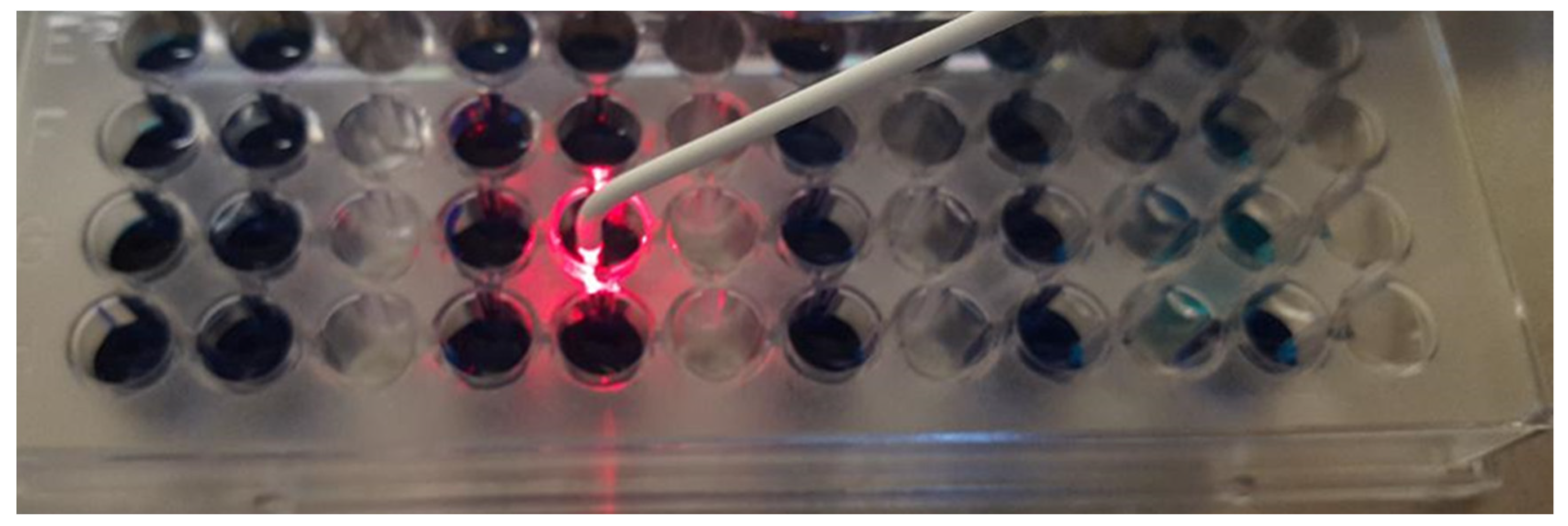
| Technique | Bacteria | Effect | Author |
|---|---|---|---|
| PDT + toluidine or methylene blue dye | A. actinomycetemcomitans | 100% eradication at 10 mg/mL | Valle et al., 2019 [14] |
| PDT + rose bengal | P. gingivalis, A. actinomycetemcomitans, F. nucleatum | Maximal reduction at 160 μg/mL rose bengal | Wang et al., 2021 [15] |
| Diode Laser 810-nm | A. actinomycetemcomitans | 93% reduction at 2.5 W; 30 s | Tantivitayakul et al., 2018 [16] |
| Diode laser 635 nm + phycocyanin | P. gingivalis | Mean reduction 44.24% | Etemadi et al., 2022 [17] |
| Active Substance | E | E + M | I | I + M | M | CP | CN |
|---|---|---|---|---|---|---|---|
| Absorbance value (mean and standard deviation) | 2.054 ± 0.197 | 1.922 ± 0.189 | 2.031 ± 0.214 | 1.968 ± 0.191 | 2.075 ± 0.174 | 2.163 ± 0.209 | 0.154 ± 0.028 |
| Active Substance | E | E + M | I | I + M | M | CP | CN |
|---|---|---|---|---|---|---|---|
| Absorbance value (mean and standard deviation) | 0.402 ± 0.0 | 0.384 ± 0.051 | 0.358 ± 0.067 | 0.353 ± 0.046 | 0.398 ± 0.051 | 2.163 ± 0.209 | 0.154 ± 0.028 |
| Degree of reduction (%) | 87.7 | 88.6 | 90.1 | 90.1 | 87.9 | 0.00 | - |
| Absorbance value (mean and standard deviation) 5W | 0.176 ± 0.052 | 0.172 ± 0.048 | 0.168 ± 0.051 | 0.161 ± 0.039 | 0.189 ± 0.044 | 2.163 ± 0.209 | 0.154 ± 0.028 |
| Degree of reduction %) | 98.9 | 99.1 | 99.3 | 99.7 | 98.3 | 0.00 | - |
| CFU/mL resulting from cultivation (mean) | <102 | <102 | <102 | <102 | <102 | 2.6 ± 105 | - |
| Degree of reduction % (log10) | >99.9 (3log) | >99.9 (3log) | >99.9 (3log) | >99.9 (3log) | >99.9 (3log) | 0.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martu, M.-A.; Luchian, I.; Mares, M.; Solomon, S.; Ciurcanu, O.; Danila, V.; Rezus, E.; Foia, L. The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs. Bioengineering 2023, 10, 61. https://doi.org/10.3390/bioengineering10010061
Martu M-A, Luchian I, Mares M, Solomon S, Ciurcanu O, Danila V, Rezus E, Foia L. The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs. Bioengineering. 2023; 10(1):61. https://doi.org/10.3390/bioengineering10010061
Chicago/Turabian StyleMartu, Maria-Alexandra, Ionut Luchian, Mihai Mares, Sorina Solomon, Oana Ciurcanu, Vlad Danila, Elena Rezus, and Liliana Foia. 2023. "The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs" Bioengineering 10, no. 1: 61. https://doi.org/10.3390/bioengineering10010061
APA StyleMartu, M.-A., Luchian, I., Mares, M., Solomon, S., Ciurcanu, O., Danila, V., Rezus, E., & Foia, L. (2023). The Effectiveness of Laser Applications and Photodynamic Therapy on Relevant Periodontal Pathogens (Aggregatibacter actinomycetemcomitans) Associated with Immunomodulating Anti-rheumatic Drugs. Bioengineering, 10(1), 61. https://doi.org/10.3390/bioengineering10010061











