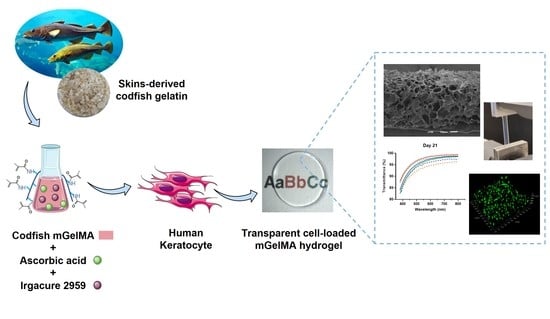
Cell-Laden Marine Gelatin Methacryloyl Hydrogels Enriched with Ascorbic Acid for Corneal Stroma Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Material
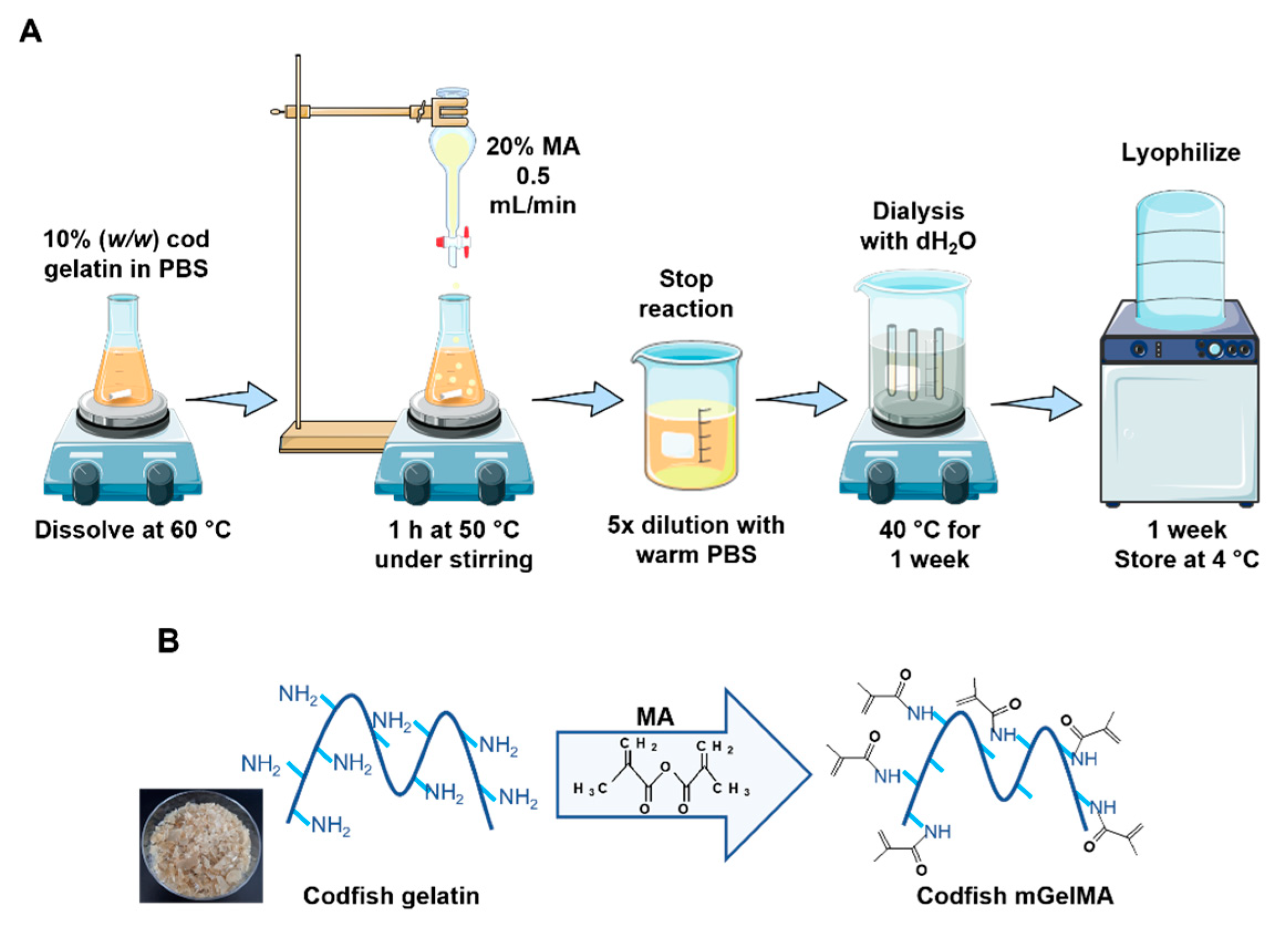
2.2. Synthesis of Marine Gelatin Methacryloyl (mGelMA)
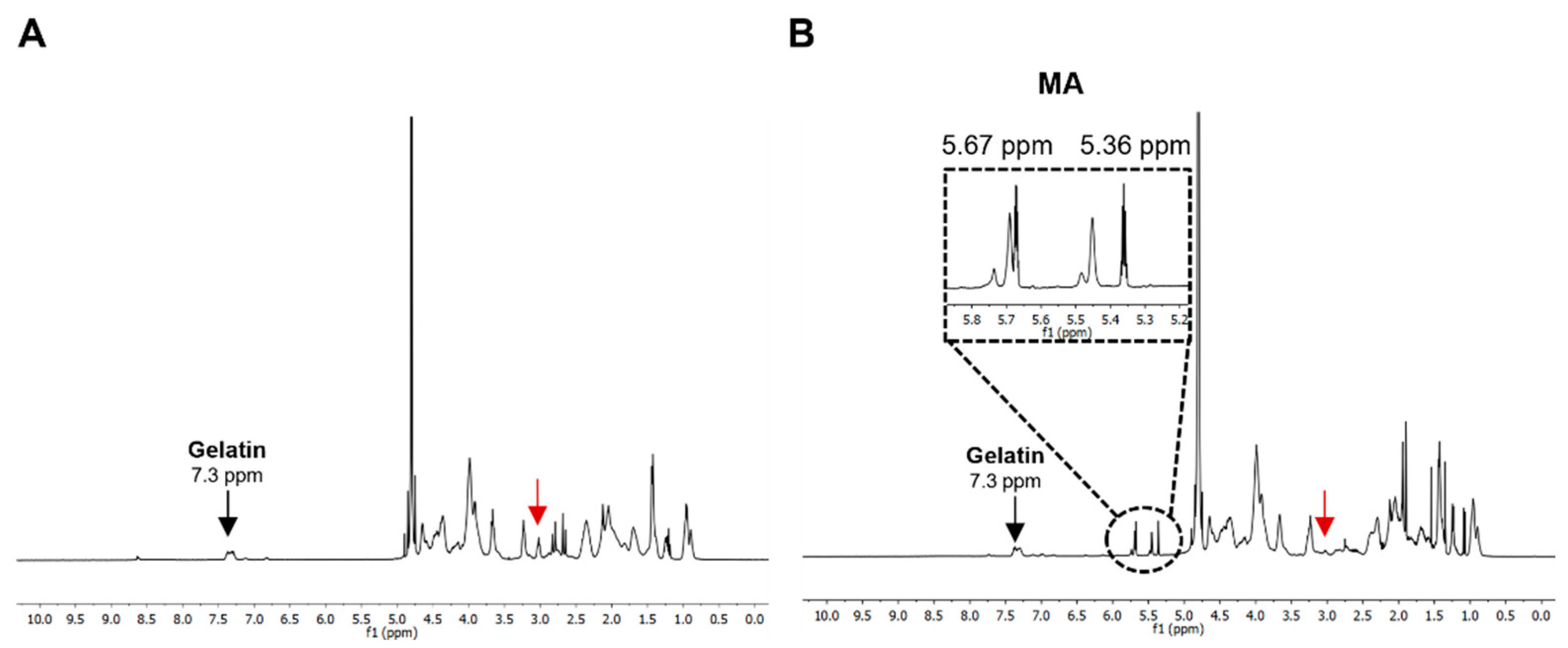
2.3. 1H-Nucleic Magnetic Resonance (1H-NMR) of mGelMA
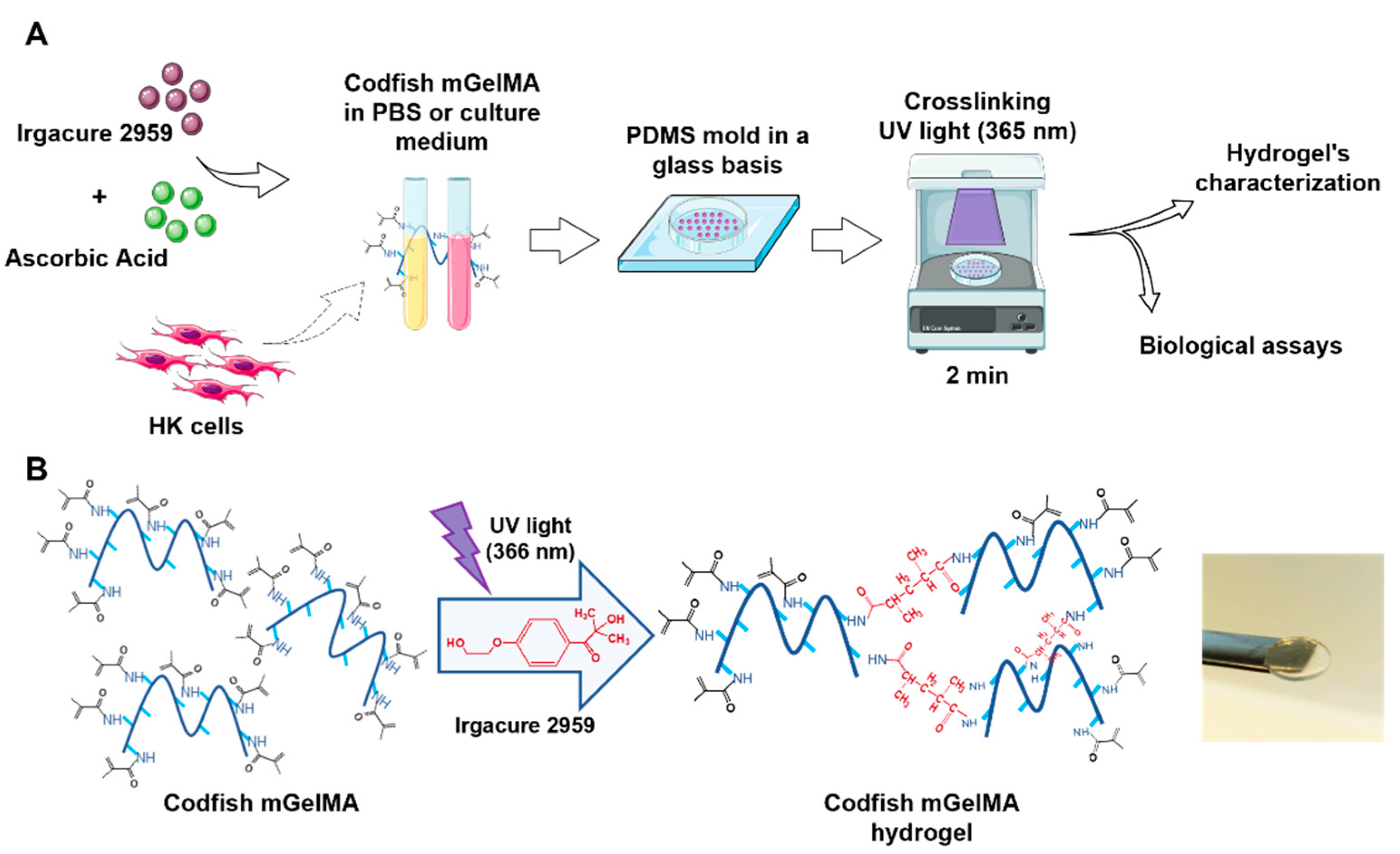
2.4. Production of mGelMA Hydrogels
2.5. Characterization of mGelMA Hydrogels
2.5.1. Chemical Analysis
2.5.2. Equilibrium Water Content (EWC)
2.5.3. Degradation in PBS
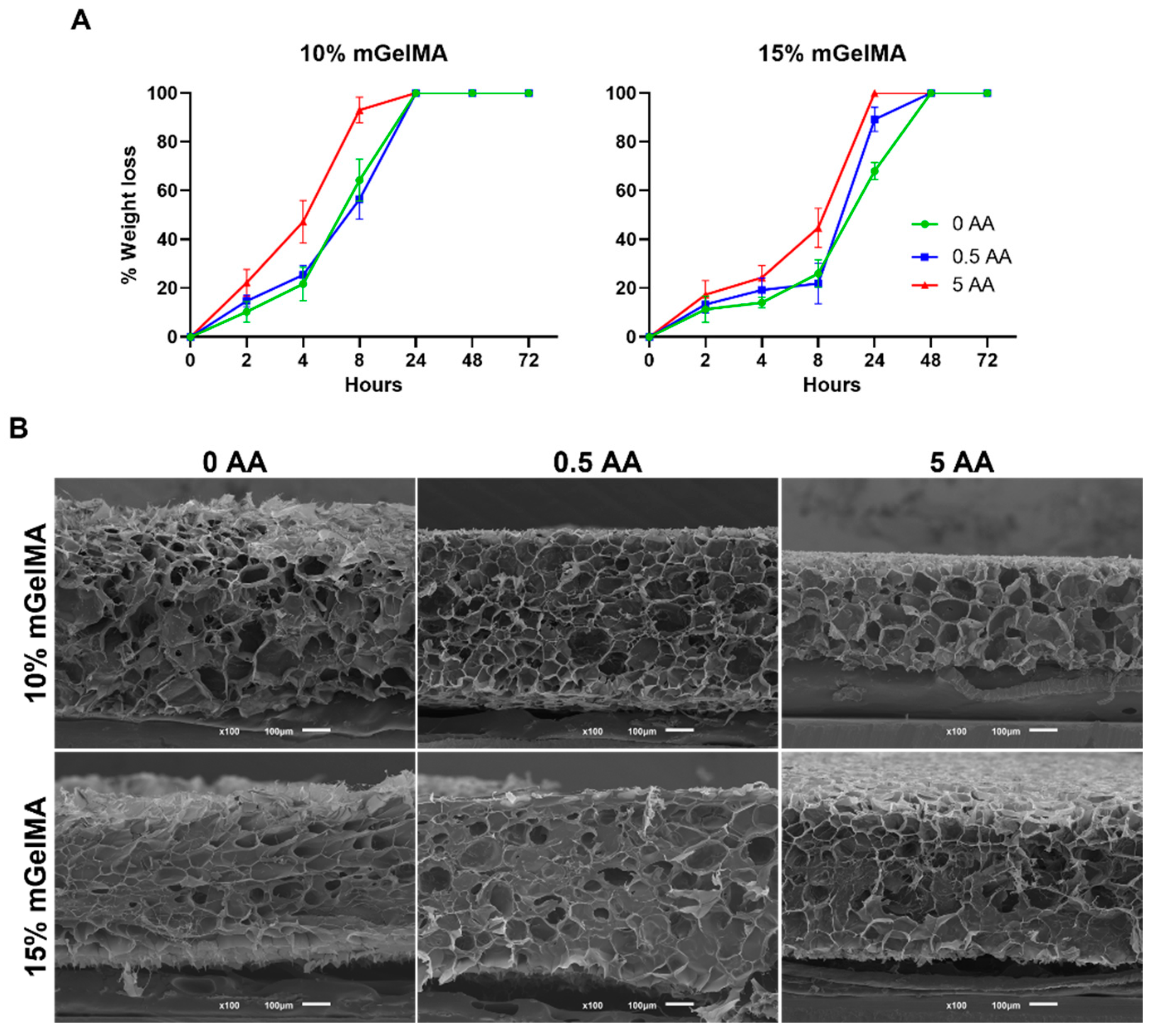
2.5.4. Enzymatic Degradation
2.5.5. Mechanical Tests
2.5.6. Transparency of mGelMA Hydrogels
2.5.7. Release Profile of Ascorbic Acid
2.6. Production of Cell-Loaded mGelMA Hydrogels
2.6.1. Cell Culture
2.6.2. Cell Encapsulation and Hydrogel Preparation
2.7. Performance of the Encapsulated Cells
2.7.1. Cell Viability
Live–Dead Staining
MTS Assay
Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
3.1. Gelatin Methacrylation
3.2. Production of mGelMA Hydrogels and Characterization
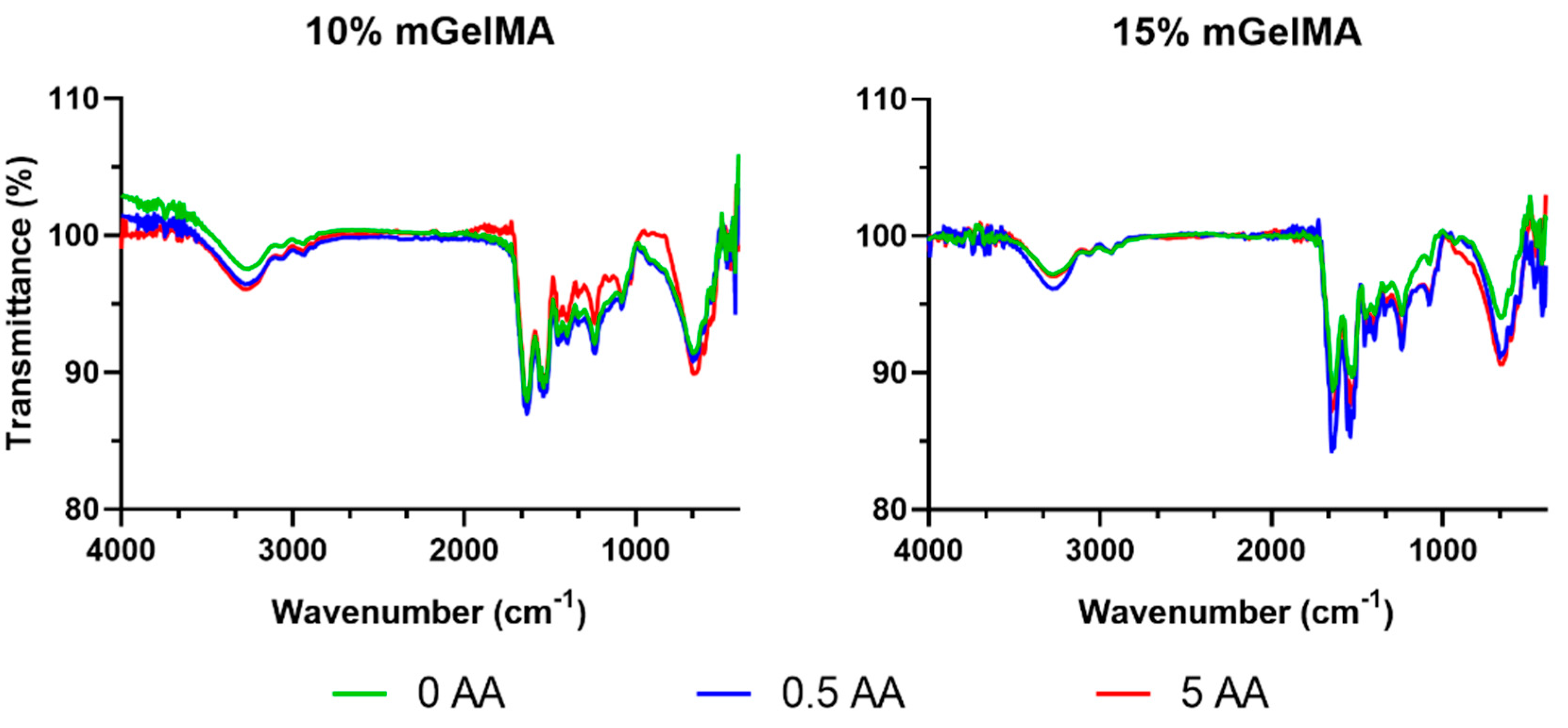
3.2.1. ATR-FTIR Analysis
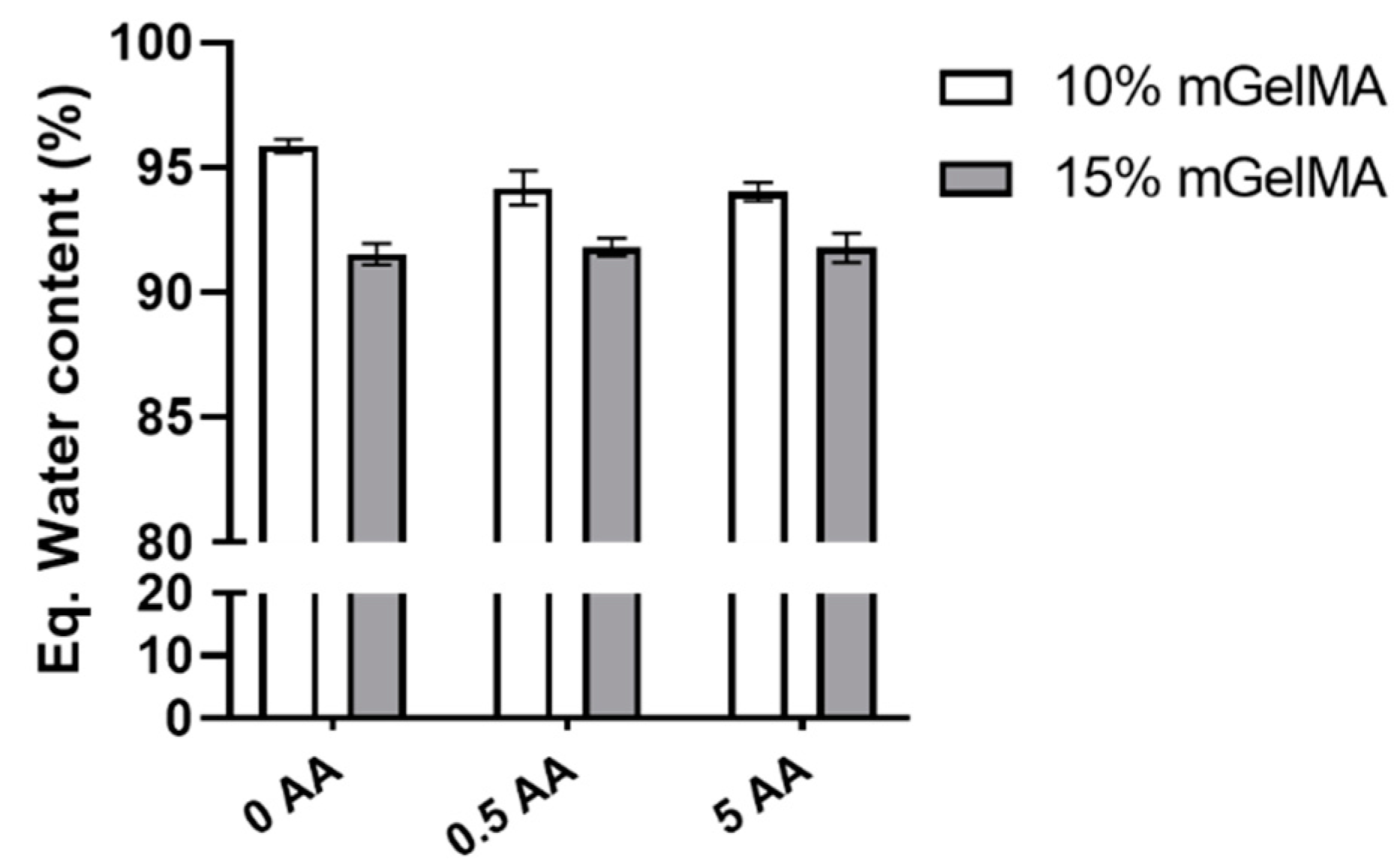
3.2.2. Equilibrium Water Content of mGelMA Hydrogels
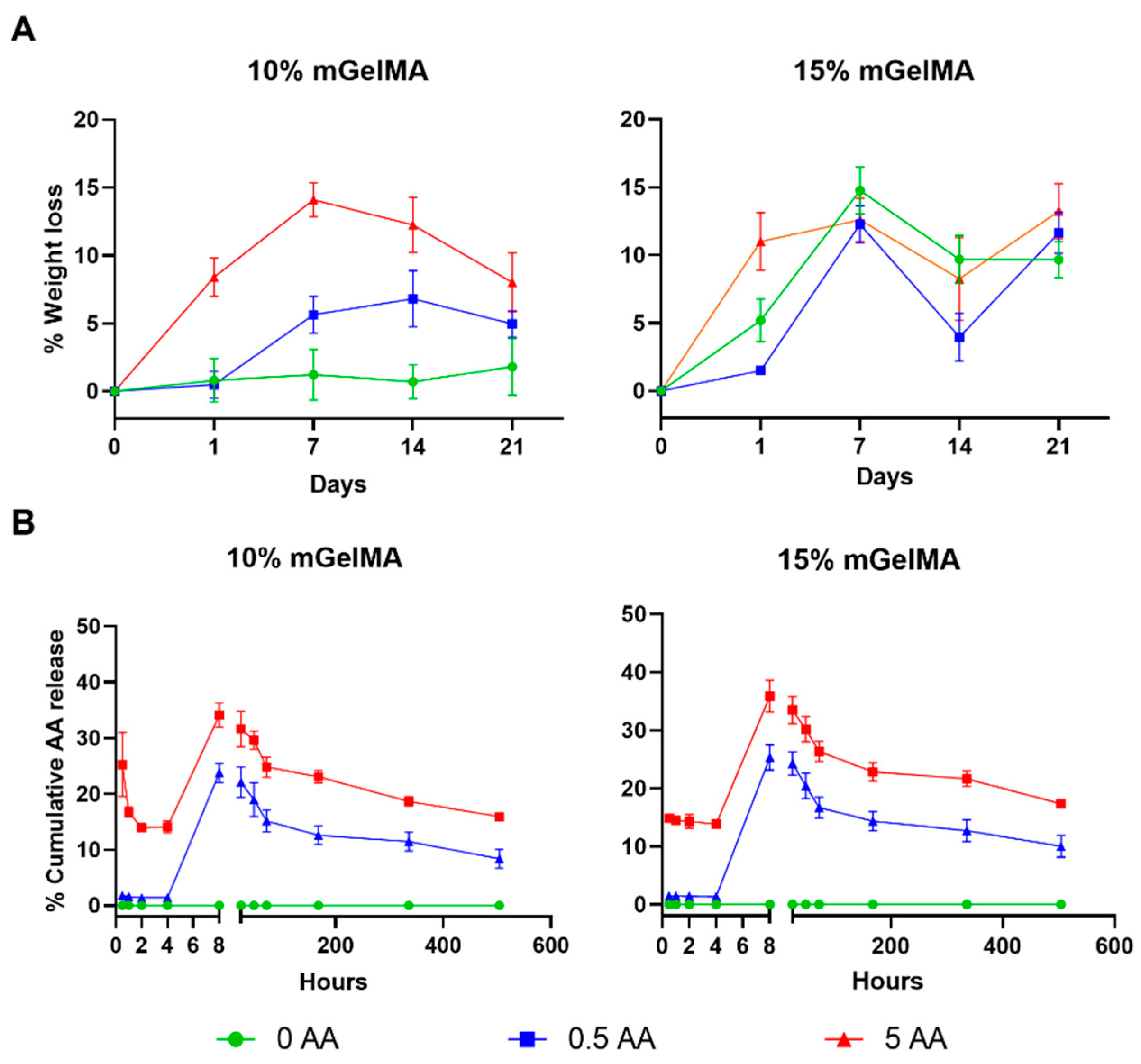
3.2.3. Stability of mGelMA Hydrogels and Release of AA
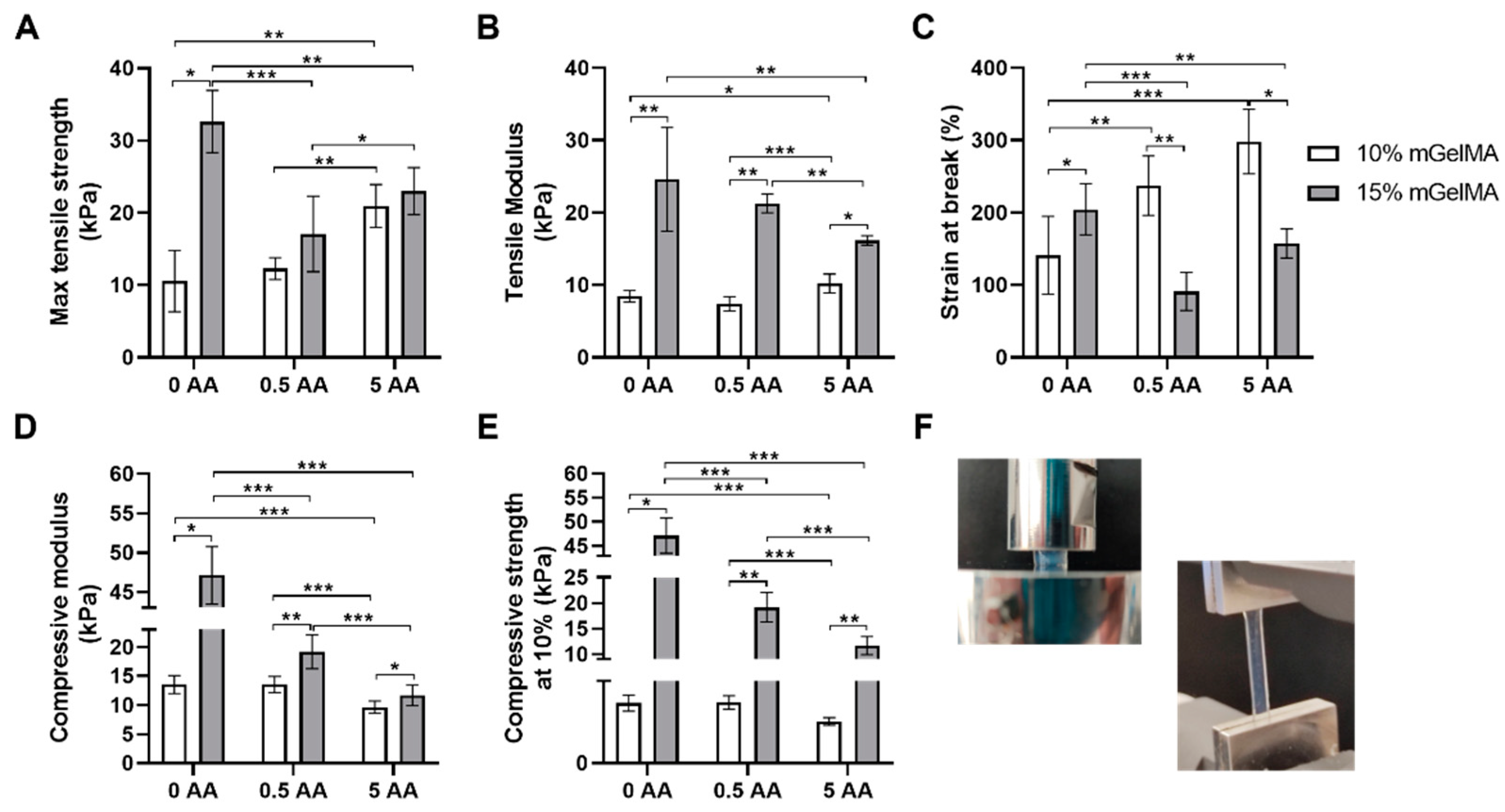
3.2.4. Mechanical Properties of mGelMA Hydrogels
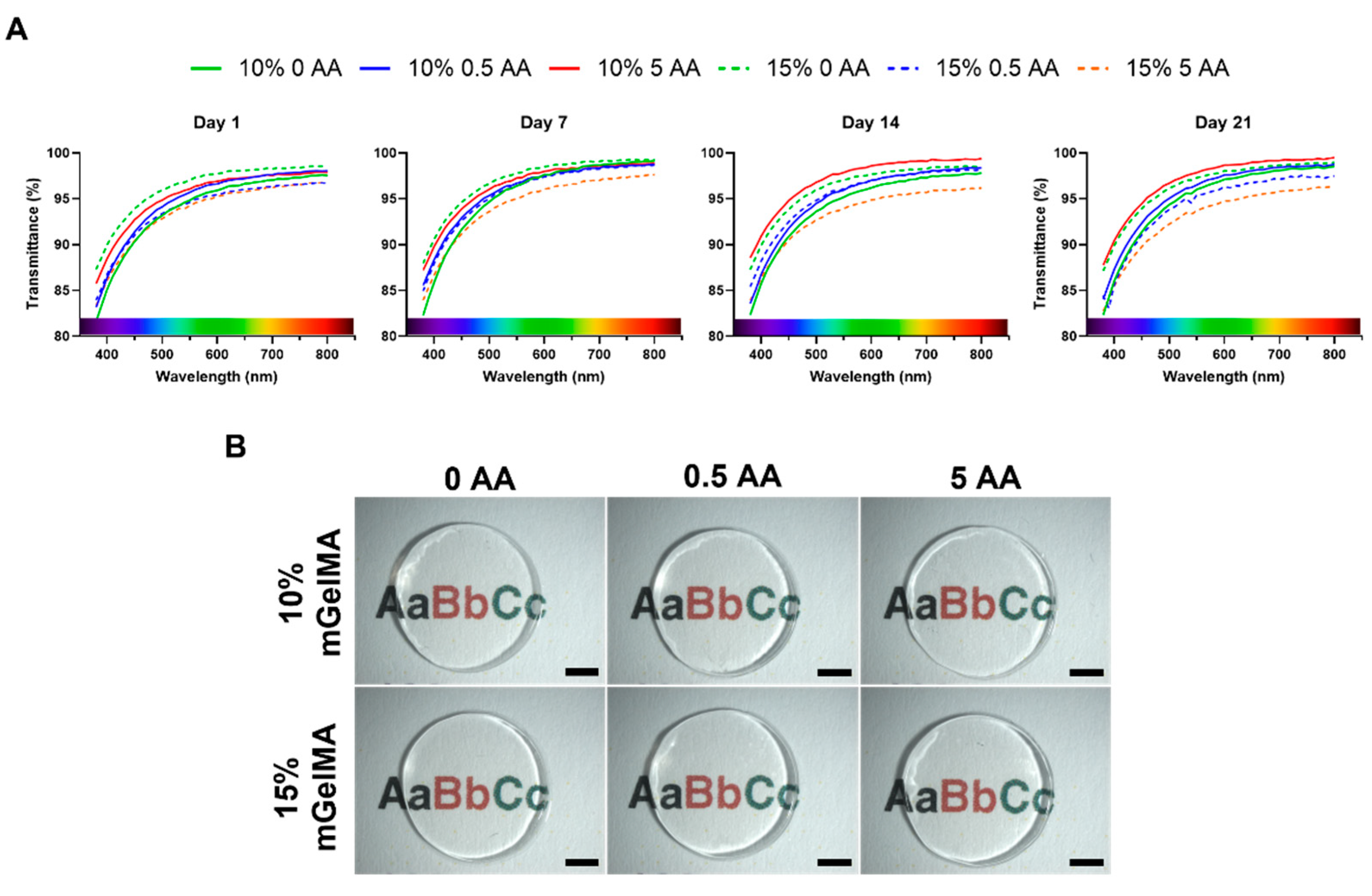
3.2.5. Optical Properties of mGelMA Hydrogels
3.3. Encapsulation of Human Keratocytes
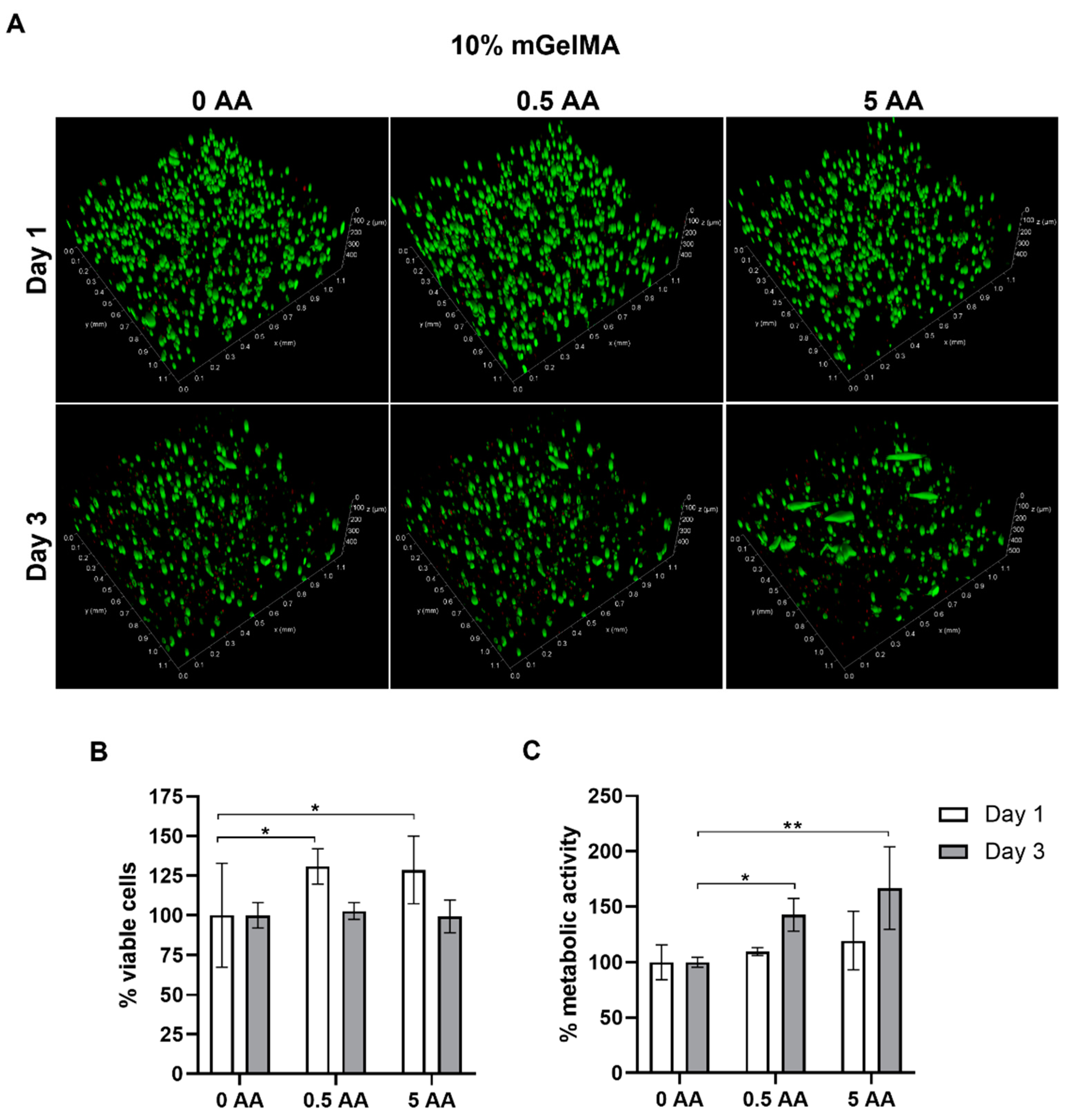
3.3.1. Cell Viability
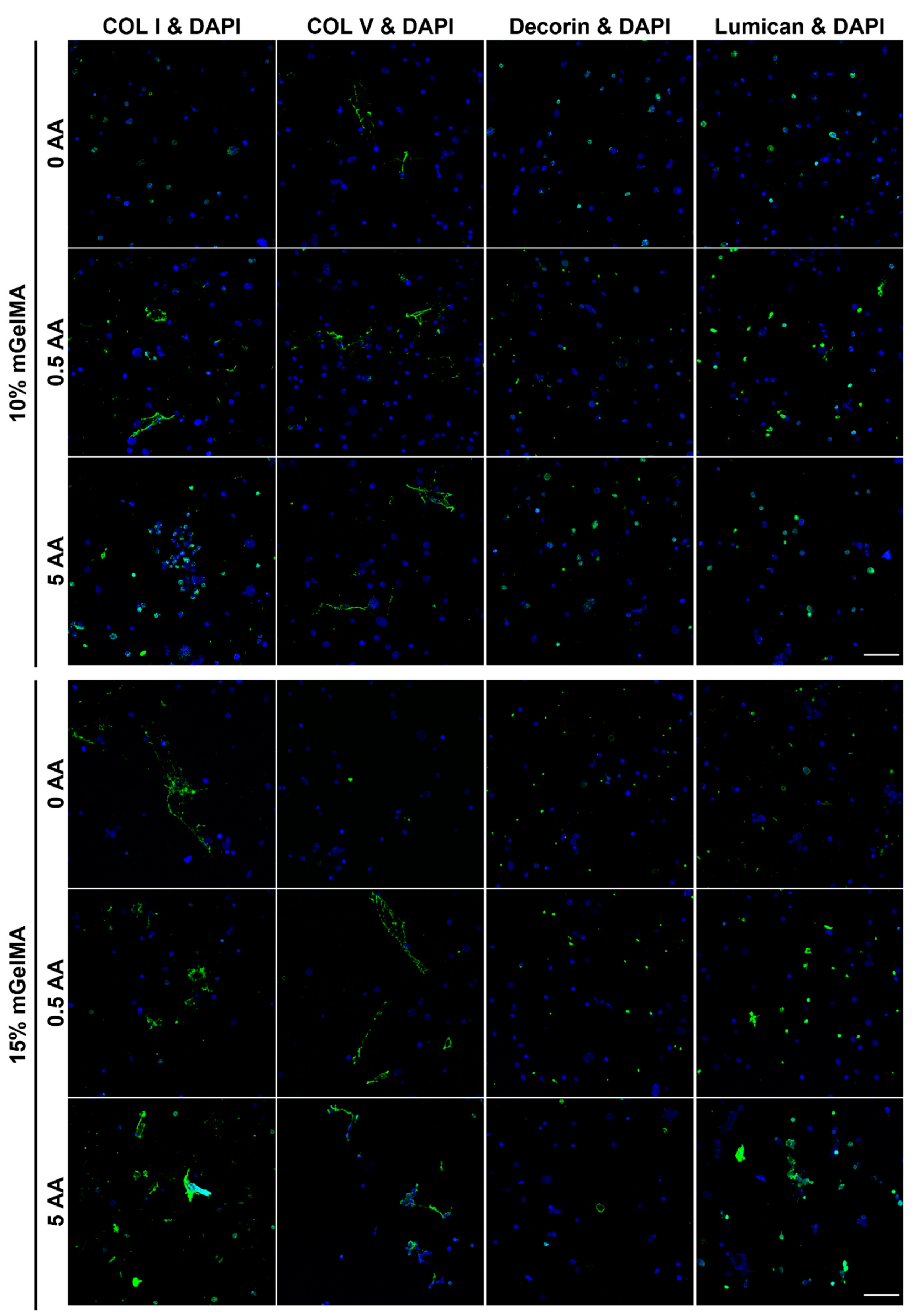
3.3.2. Immunofluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Land, M.F.; Fernald, R.D. The evolution of eyes. Annu. Rev. Neurosci. 1992, 15, 1–29. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the cornea: Structure, function, and development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar]
- Chen, Z.; You, J.; Liu, X.; Cooper, S.; Hodge, C.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomaterials for corneal bioengineering. Biomed. Mater. 2018, 13, 032002. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing scaffolds for corneal regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef]
- Nonpassopon, M.; Niparugs, M.; Cortina, M.S. Boston type 1 keratoprosthesis: Updated perspectives. Clin. Ophthalmol. 2020, 14, 1189–1200. [Google Scholar] [CrossRef]
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal repair and regeneration: Current concepts and future directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Brunette, I.; Roberts, C.J.; Vidal, F.; Harissi-Dagher, M.; Lachaine, J.; Sheardown, H.; Durr, G.M.; Proulx, S.; Griffith, M. Alternatives to eye bank native tissue for corneal stromal replacement. Prog. Retin. Eye Res. 2017, 59, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio, J.L.A.; Arnalich-Montiel, F.; De Miguel, M.P.; El Zarif, M.; Alió, J.L. Corneal stroma regeneration: Preclinical studies. Exp. Eye Res. 2021, 202, 108314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gan, L.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A.; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A simple, cross-linked collagen tissue substitute for corneal implantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Builles, N.; Janin-Manificat, H.; Malbouyres, M.; Justin, V.; Rovère, M.-R.; Pellegrini, G.; Torbet, J.; Hulmes, D.J.; Burillon, C.; Damour, O. Use of magnetically oriented orthogonal collagen scaffolds for hemi-corneal reconstruction and regeneration. Biomaterials 2010, 31, 8313–8322. [Google Scholar] [CrossRef]
- Calderón-Colón, X.; Xia, Z.; Breidenich, J.L.; Mulreany, D.G.; Guo, Q.; Uy, O.M.; Tiffany, J.E.; Freund, D.E.; McCally, R.L.; Schein, O.D. Structure and properties of collagen vitrigel membranes for ocular repair and regeneration applications. Biomaterials 2012, 33, 8286–8295. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Ma, D.H.-K.; Lai, M.-H.; Li, Y.-T.; Chang, R.-J.; Chen, L.-M. Characterization of cross-linked porous gelatin carriers and their interaction with corneal endothelium: Biopolymer concentration effect. PLoS ONE 2013, 8, e54058. [Google Scholar] [CrossRef]
- Zhang, J.; Sisley, A.M.; Anderson, A.J.; Taberner, A.J.; McGhee, C.N.; Patel, D.V. Characterization of a novel collagen scaffold for corneal tissue engineering. Tissue Eng. Part C Methods 2016, 22, 165–172. [Google Scholar] [CrossRef]
- Rafat, M.; Jabbarvand, M.; Sharma, N.; Xeroudaki, M.; Tabe, S.; Omrani, R.; Thangavelu, M.; Mukwaya, A.; Fagerholm, P.; Lennikov, A.; et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Fernández-Figueras, M.T.; Puig, L. Inflammatory, Immune-Mediated Adverse Reactions Related to Soft Tissue Dermal Fillers; Seminars in arthritis and rheumatism; Elsevier: Amsterdam, The Netherlands, 2013; pp. 241–258. [Google Scholar]
- Zhang, L.; Niu, X.; Sun, L.; She, Z.; Tan, R.; Wang, W. Immune response of bovine sourced cross-linked collagen sponge for hemostasis. J. Biomater. Appl. 2018, 32, 920–931. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, I.H.; Dutille, E.K.; Bailey, R.; Shaker, M.S. Preventing iatrogenic gelatin anaphylaxis. Ann. Allergy Asthma Immunol. 2019, 123, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Capella, G.L. Foot and mouth disease in human beings. Lancet 2001, 358, 1374. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Burcharth, J.; Rosenberg, J. Animal derived products may conflict with religious patients’ beliefs. BMC Med. Ethics 2013, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Moreira-Silva, J.; Diogo, G.S.; Marques, A.L.; Silva, T.H.; Reis, R.L. Marine collagen isolation and processing envisaging biomedical applications. In Biomaterials from Nature for Advanced Devices and Therapies; John Wiley & Sons: New York, NY, USA, 2016; pp. 16–36. [Google Scholar]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Alves, A.L.; Costa-Gouveia, J.; Vieira de Castro, J.; Sotelo, C.G.; Vázquez, J.A.; Pérez-Martín, R.I.; Torrado, E.; Neves, N.; Reis, R.L.; Castro, A.G.; et al. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomater. 2022, 141, 123–131. [Google Scholar] [CrossRef]
- Coelho, R.C.; Marques, A.L.; Oliveira, S.M.; Diogo, G.S.; Pirraco, R.P.; Moreira-Silva, J.; Xavier, J.C.; Reis, R.L.; Silva, T.H.; Mano, J.F. Extraction and characterization of collagen from antarctic and sub-antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng.: C 2017, 78, 787–795. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Diogo, G.S.; López-Senra, E.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Pérez-Martín, R.I.; Sotelo, C.G.; Marques, A.P.; González, P. Marine collagen/apatite composite scaffolds envisaging hard tissue applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from atlantic cod (Gadus morhua) swim bladders envisaging health-related applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Alves, A.L.; Fraguas, F.J.; Carvalho, A.C.; Valcárcel, J.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A.; Silva, T.H. Characterization of codfish gelatin: A comparative study of fresh and salted skins and different extraction methods. Food Hydrocoll. 2022, 124, 107238. [Google Scholar] [CrossRef]
- Lin, C.C.; Ritch, R.; Lin, S.M.; Ni, M.H.; Chang, Y.C.; Lu, Y.L.; Lai, H.J.; Lin, F.H. A new fish scale-derived scaffold for corneal regeneration. Eur. Cells Mater. 2010, 19, 50–57. [Google Scholar] [CrossRef]
- Feng, H.; Li, X.; Deng, X.; Li, X.; Guo, J.; Ma, K.; Jiang, B. The lamellar structure and biomimetic properties of a fish scale matrix. RSC Adv. 2020, 10, 875–885. [Google Scholar] [CrossRef]
- van Essen, T.H.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.; Salvatori, D.C.; Luyten, G.P.; Jager, M.J. A fish scale-derived collagen matrix as artificial cornea in rats: Properties and potential. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3224–3233. [Google Scholar] [CrossRef]
- van Essen, T.H.; van Zijl, L.; Possemiers, T.; Mulder, A.A.; Zwart, S.J.; Chou, C.H.; Lin, C.C.; Lai, H.J.; Luyten, G.P.M.; Tassignon, M.J.; et al. Biocompatibility of a fish scale-derived artificial cornea: Cytotoxicity, cellular adhesion and phenotype, and in vivo immunogenicity. Biomaterials 2016, 81, 36–45. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, L.; Lin, C.-C.; Chou, C.-H.; Li, L. A cornea substitute derived from fish scale: 6-month followup on rabbit model. J. Ophthalmol. 2014, 2014, 914542. [Google Scholar] [CrossRef]
- Chen, S.-C.; Telinius, N.; Lin, H.-T.; Huang, M.-C.; Lin, C.-C.; Chou, C.-H.; Hjortdal, J. Use of fish scale-derived biocornea to seal full-thickness corneal perforations in pig models. PLoS ONE 2015, 10, e0143511. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N. Corneal stromal regeneration: Current status and future therapeutic potential. Curr. Eye Res. 2020, 45, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin c. Adv. Free. Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553. [Google Scholar]
- Saika, S.; Uenoyama, K.; Hiroi, K.; Ooshima, A. L-ascorbic acid 2-phosphate enhances the production of type i and type iii collagen peptides in cultured rabbit keratocytes. Ophthalmic Res. 1992, 24, 68–72. [Google Scholar] [CrossRef]
- Luo, L.-J.; Lai, J.-Y.; Chou, S.-F.; Hsueh, Y.-J.; Ma, D.H.-K. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018, 65, 123–136. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Science. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Kanďár, R.; Drábková, P.; Hampl, R. The determination of ascorbic acid and uric acid in human seminal plasma using an hplc with uv detection. J. Chromatogr. B 2011, 879, 2834–2839. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J. Tissue Eng. Regen. Med. 2018, 12, e1899–e1910. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Burcu, A.; Gedikoglu, G.; Telek, H.H.; Ornek, F.; Hasirci, V. Methacrylated gelatin hydrogels as corneal stroma substitutes: In vivo study. J. Biomater. Sci. Polym. Ed. 2019, 30, 1803–1821. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced gelma hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.; Melotti, S.A.; Zieske, J.D.; Trinkaus-Randall, V.; Ruberti, J.W. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid–stimulated human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4050–4060. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3d culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.F.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Vilela, C.; Silvestre, A.J.; Freire, C.S. Swellable gelatin methacryloyl microneedles for extraction of interstitial skin fluid toward minimally invasive monitoring of urea. Macromol. Biosci. 2020, 20, 2000195. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Bock, N.; Dargaville, B.L.; Hutmacher, D.W. Deciphering the molecular mechanism of water interaction with gelatin methacryloyl hydrogels: Role of ionic strength, ph, drug loading and hydrogel network characteristics. Biomedicines 2021, 9, 574. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ecm production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 59, 63–72. [Google Scholar] [CrossRef]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.; Hubschman, J.P.; Deng, S.; et al. Thz and mm-wave sensing of corneal tissue water content: Electromagnetic modeling and analysis. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 170–183. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of scaffold degradation in tissue engineering: A review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stöckle, U.; Stegemann, J.P.; Aicher, W.K. The geometrical shape of mesenchymal stromal cells measured by quantitative shape descriptors is determined by the stiffness of the biomaterial and by cyclic tensile forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Lee, J.-J.; Ho, C.-C.; Yen, S.-Y.; Ng, H.Y.; Chen, Y.-W. Effects of gelatin methacrylate bio-ink concentration on mechano-physical properties and human dermal fibroblast behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Petsche, S.J.; Chernyak, D.; Martiz, J.; Levenston, M.E.; Pinsky, P.M. Depth-dependent transverse shear properties of the human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, J.; Huang, K.; Lee, Z.; Lee, X. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 2001, 34, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.E.; Pye, D.C. Young’s modulus in normal corneas and the effect on applanation tonometry. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2008, 85, 445–450. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for polymers used in ocular drug delivery. Front. Med. 2021, 8, 787644. [Google Scholar] [CrossRef]
- Doutch, J.; Quantock, A.J.; Smith, V.A.; Meek, K.M. Light transmission in the human cornea as a function of position across the ocular surface: Theoretical and experimental aspects. Biophys. J. 2008, 95, 5092–5099. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Backman, L.J.; Malm, A.D.; Danielson, P. Surface topography and mechanical strain promote keratocyte phenotype and extracellular matrix formation in a biomimetic 3d corneal model. Adv. Healthc. Mater. 2017, 6, 1601238. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.L.; Carvalho, A.C.; Machado, I.; Diogo, G.S.; Fernandes, E.M.; Castro, V.I.B.; Pires, R.A.; Vázquez, J.A.; Pérez-Martín, R.I.; Alaminos, M.; et al. Cell-Laden Marine Gelatin Methacryloyl Hydrogels Enriched with Ascorbic Acid for Corneal Stroma Regeneration. Bioengineering 2023, 10, 62. https://doi.org/10.3390/bioengineering10010062
Alves AL, Carvalho AC, Machado I, Diogo GS, Fernandes EM, Castro VIB, Pires RA, Vázquez JA, Pérez-Martín RI, Alaminos M, et al. Cell-Laden Marine Gelatin Methacryloyl Hydrogels Enriched with Ascorbic Acid for Corneal Stroma Regeneration. Bioengineering. 2023; 10(1):62. https://doi.org/10.3390/bioengineering10010062
Chicago/Turabian StyleAlves, Ana L., Ana C. Carvalho, Inês Machado, Gabriela S. Diogo, Emanuel M. Fernandes, Vânia I. B. Castro, Ricardo A. Pires, José A. Vázquez, Ricardo I. Pérez-Martín, Miguel Alaminos, and et al. 2023. "Cell-Laden Marine Gelatin Methacryloyl Hydrogels Enriched with Ascorbic Acid for Corneal Stroma Regeneration" Bioengineering 10, no. 1: 62. https://doi.org/10.3390/bioengineering10010062
APA StyleAlves, A. L., Carvalho, A. C., Machado, I., Diogo, G. S., Fernandes, E. M., Castro, V. I. B., Pires, R. A., Vázquez, J. A., Pérez-Martín, R. I., Alaminos, M., Reis, R. L., & Silva, T. H. (2023). Cell-Laden Marine Gelatin Methacryloyl Hydrogels Enriched with Ascorbic Acid for Corneal Stroma Regeneration. Bioengineering, 10(1), 62. https://doi.org/10.3390/bioengineering10010062