Evaluation of Different Cannulation Strategies for Aortic Arch Surgery Using a Cardiovascular Numerical Simulator
Abstract
1. Introduction
2. Materials and Methods
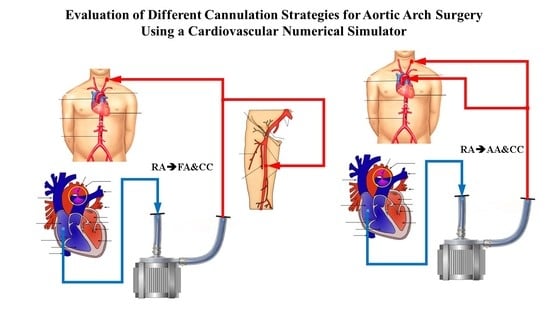
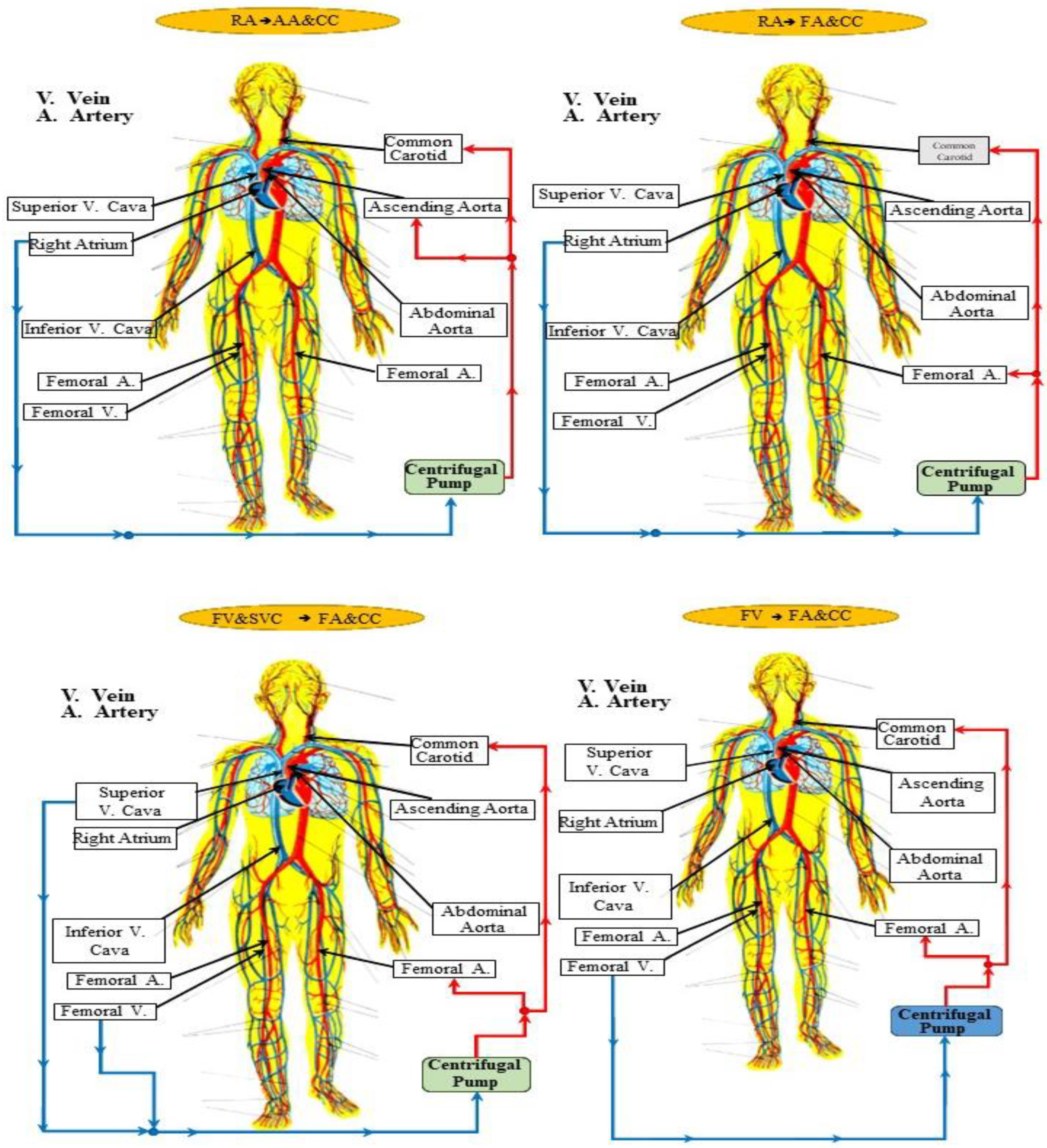
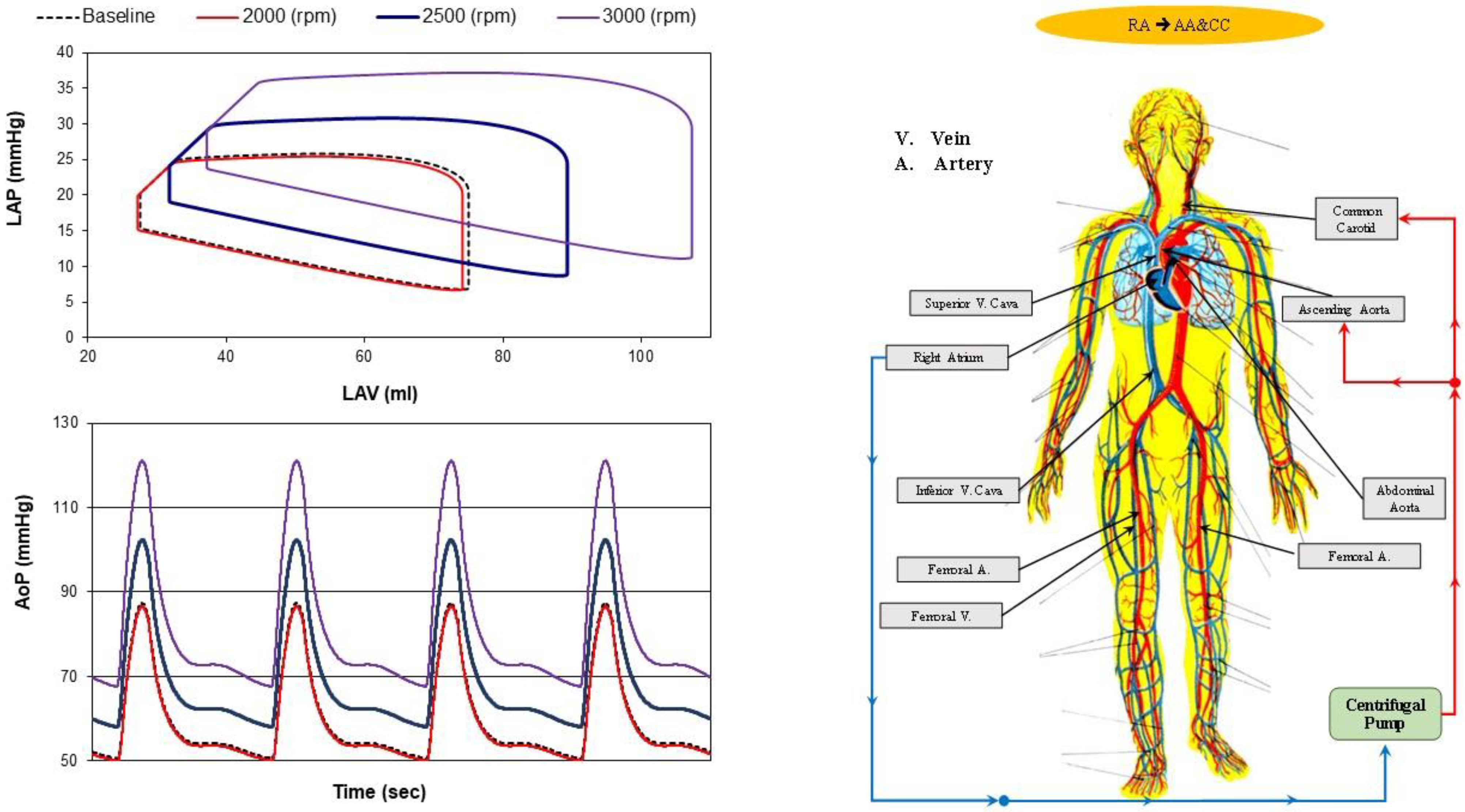
2.1. Cannulation Strategies for Aortic Arch Surgery
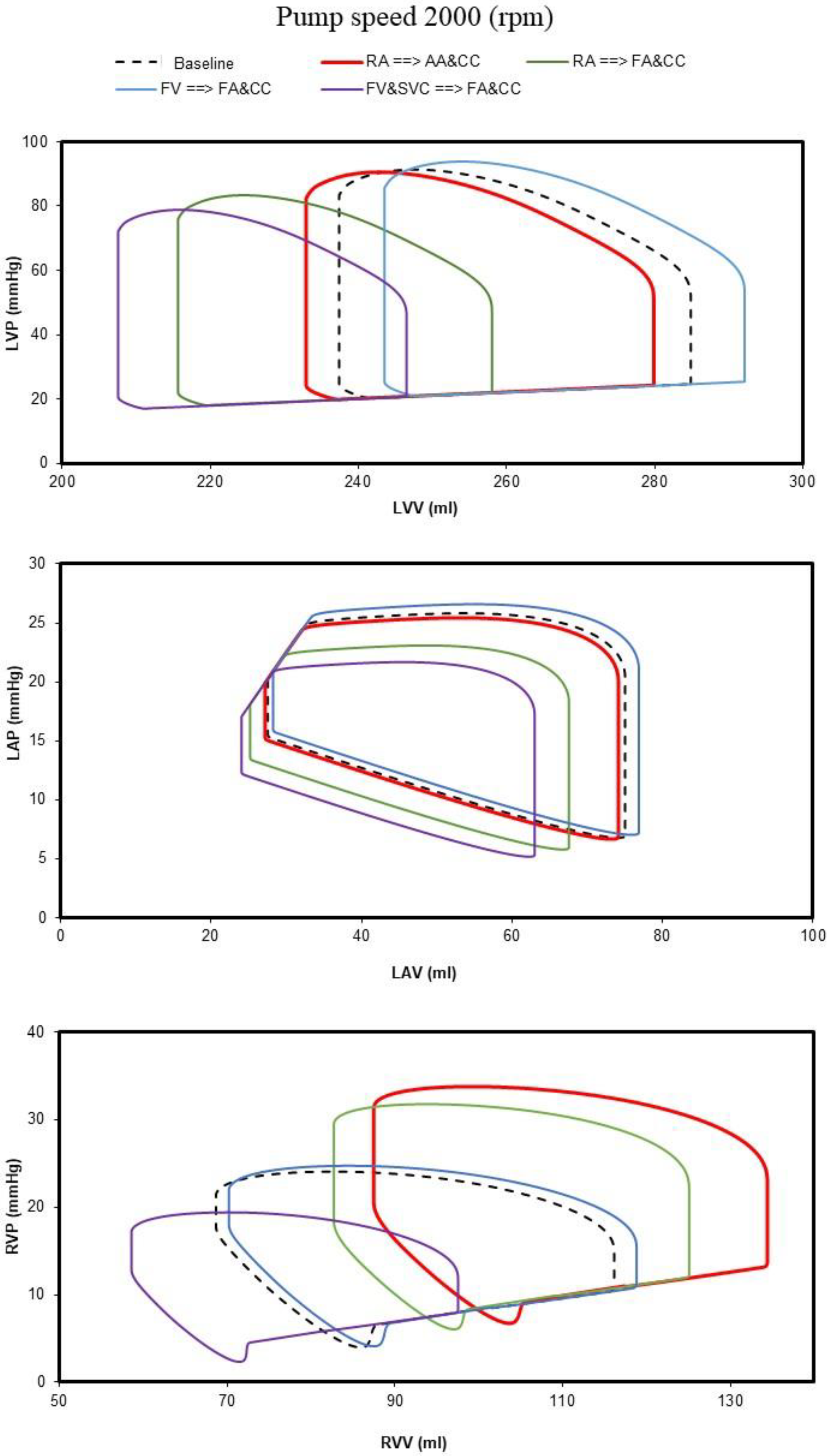
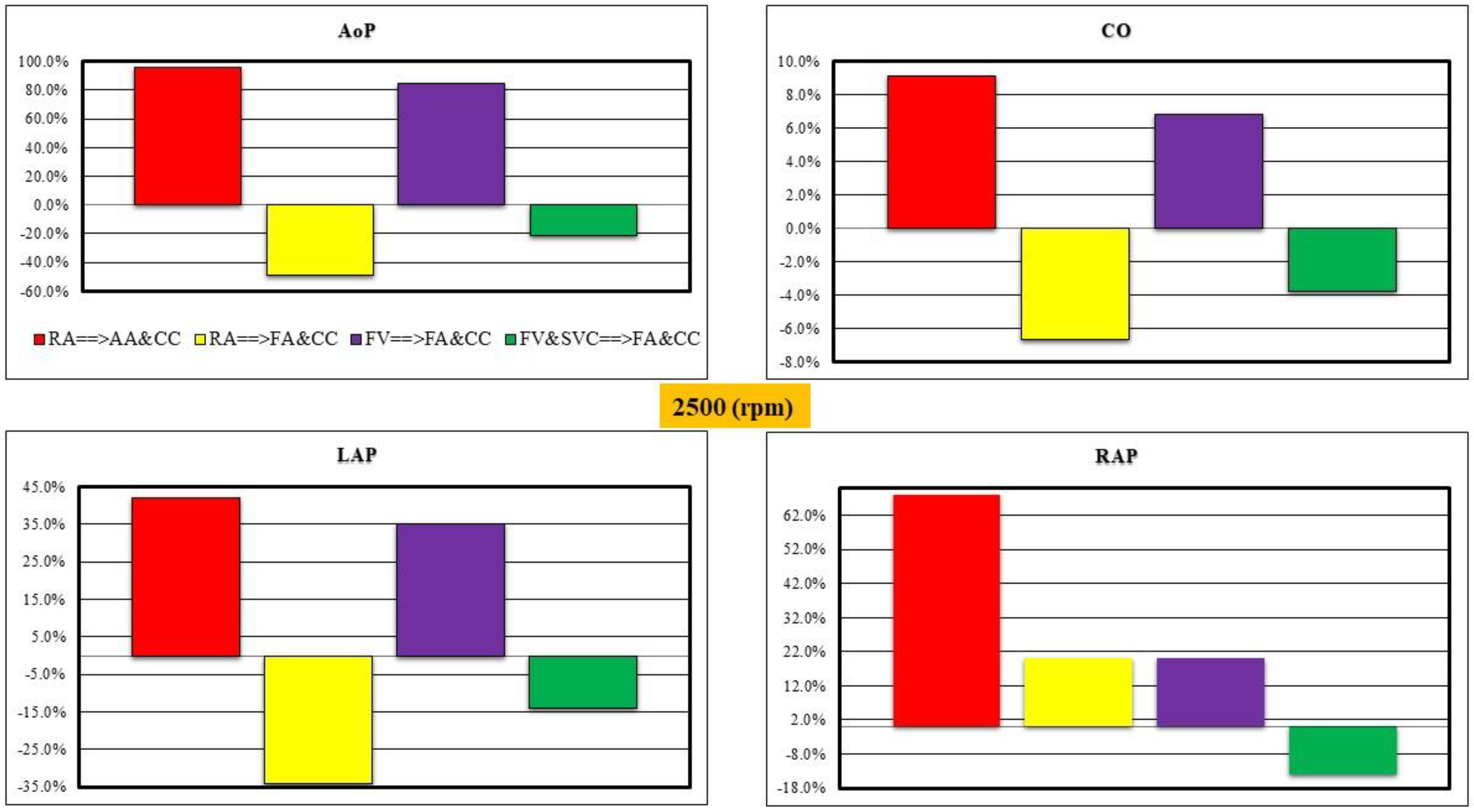
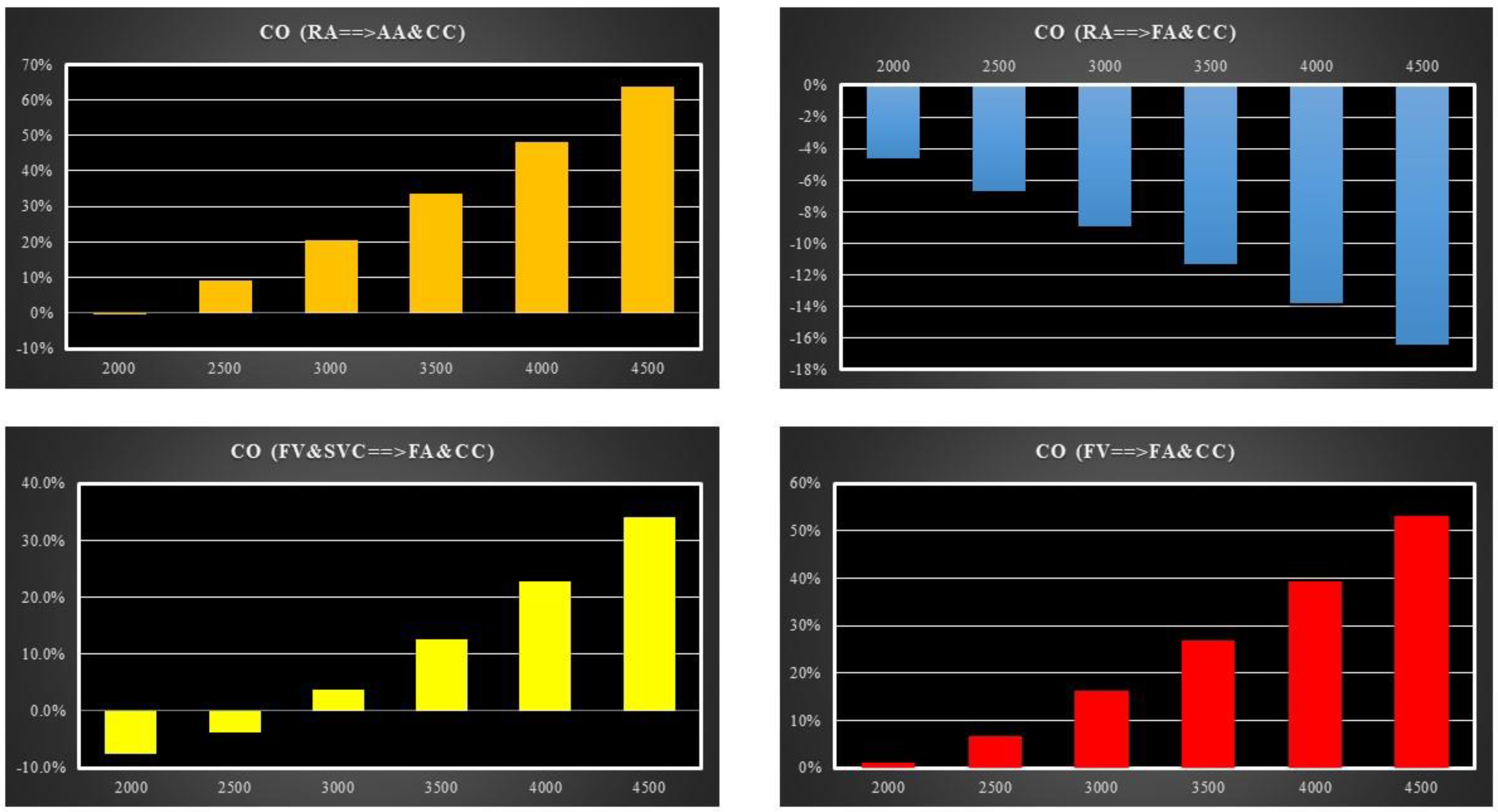
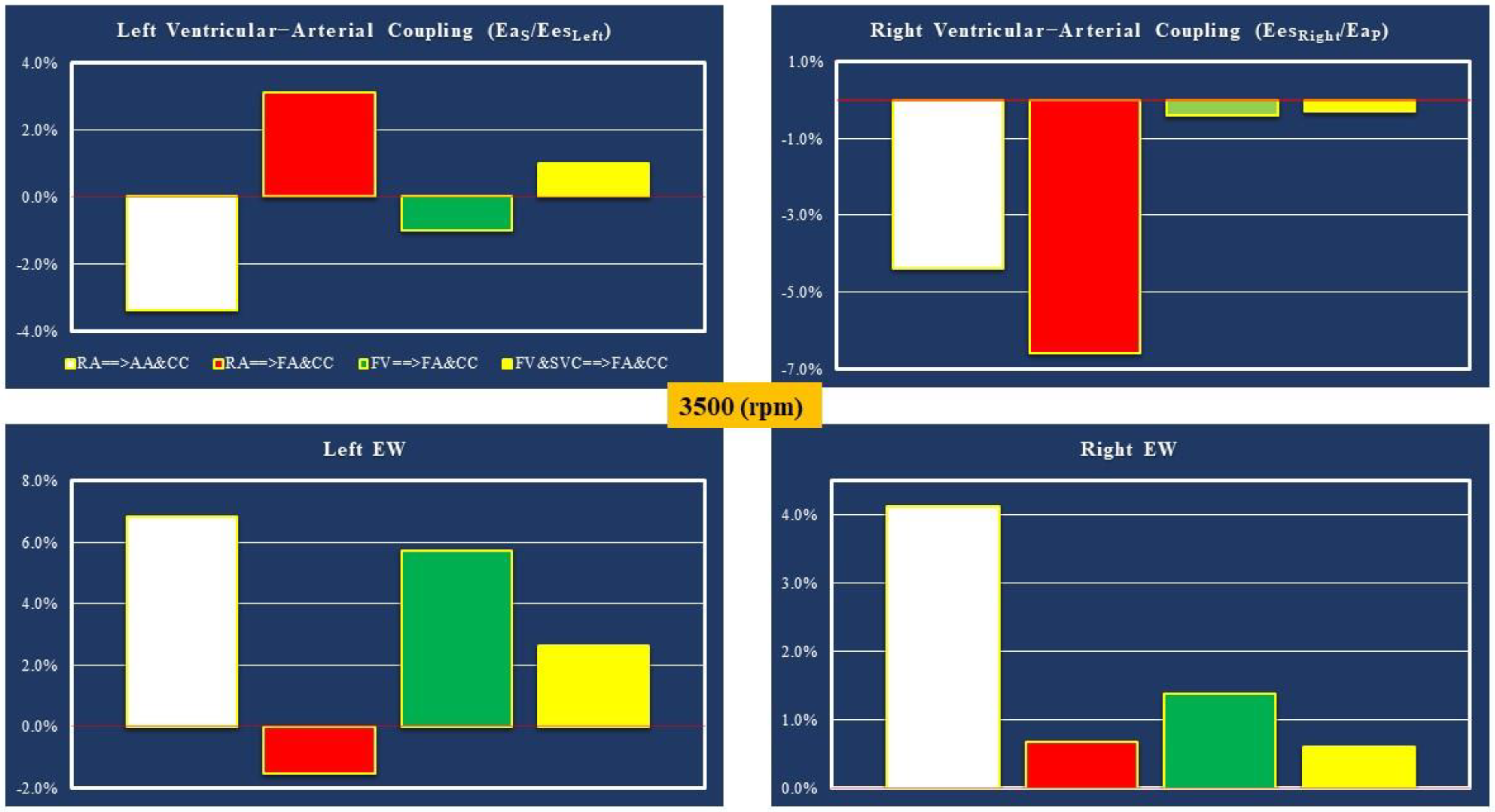
- Right atrial (RA) cannulation to ascending aorta (AA) and bilateral common carotid artery (CC) return (top-left panel), RA→AA&CC.
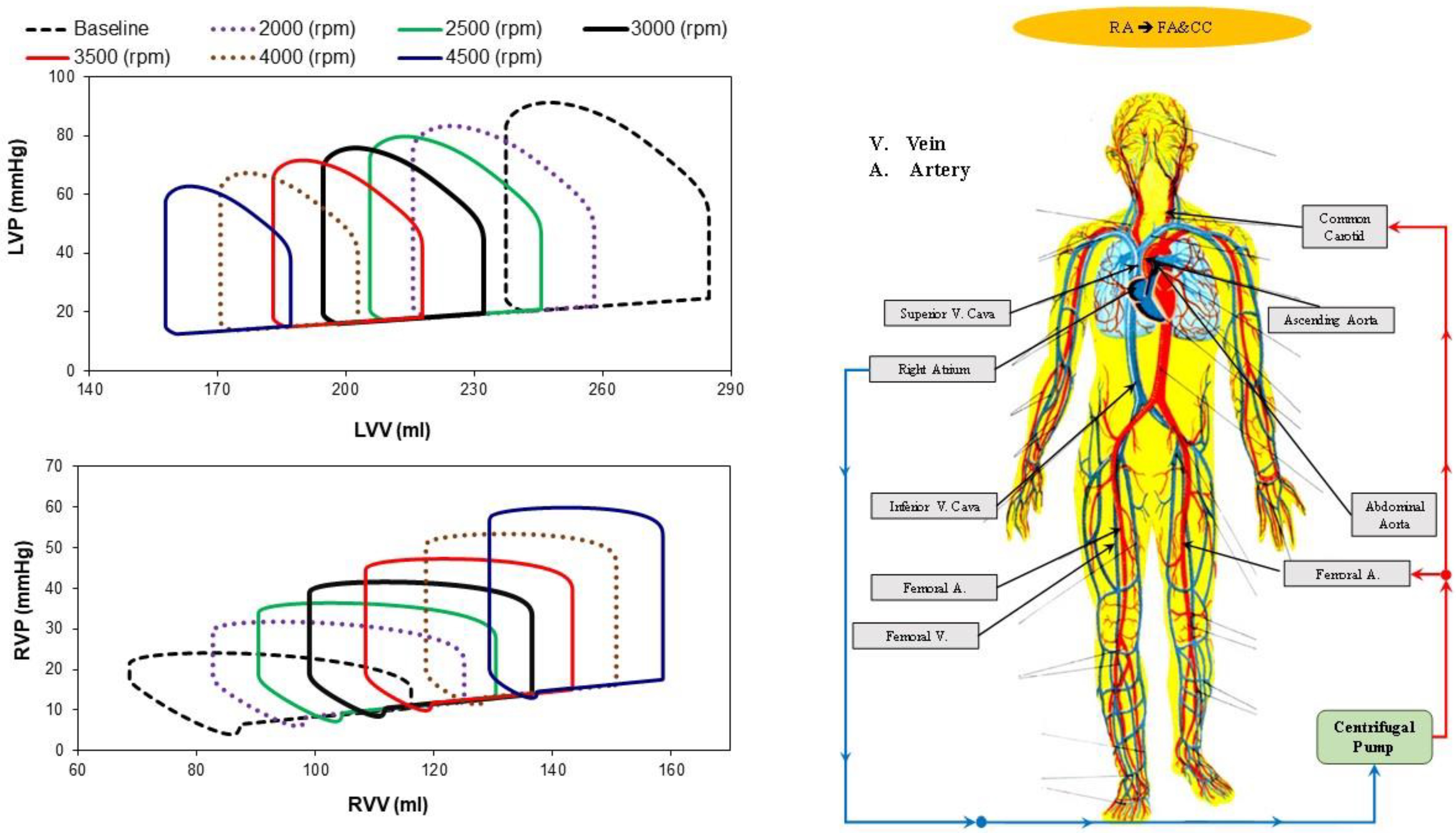
- Right atrial cannulation to femoral artery (FA) and bilateral common carotid artery return (top-right panel), RA→FA&CC.
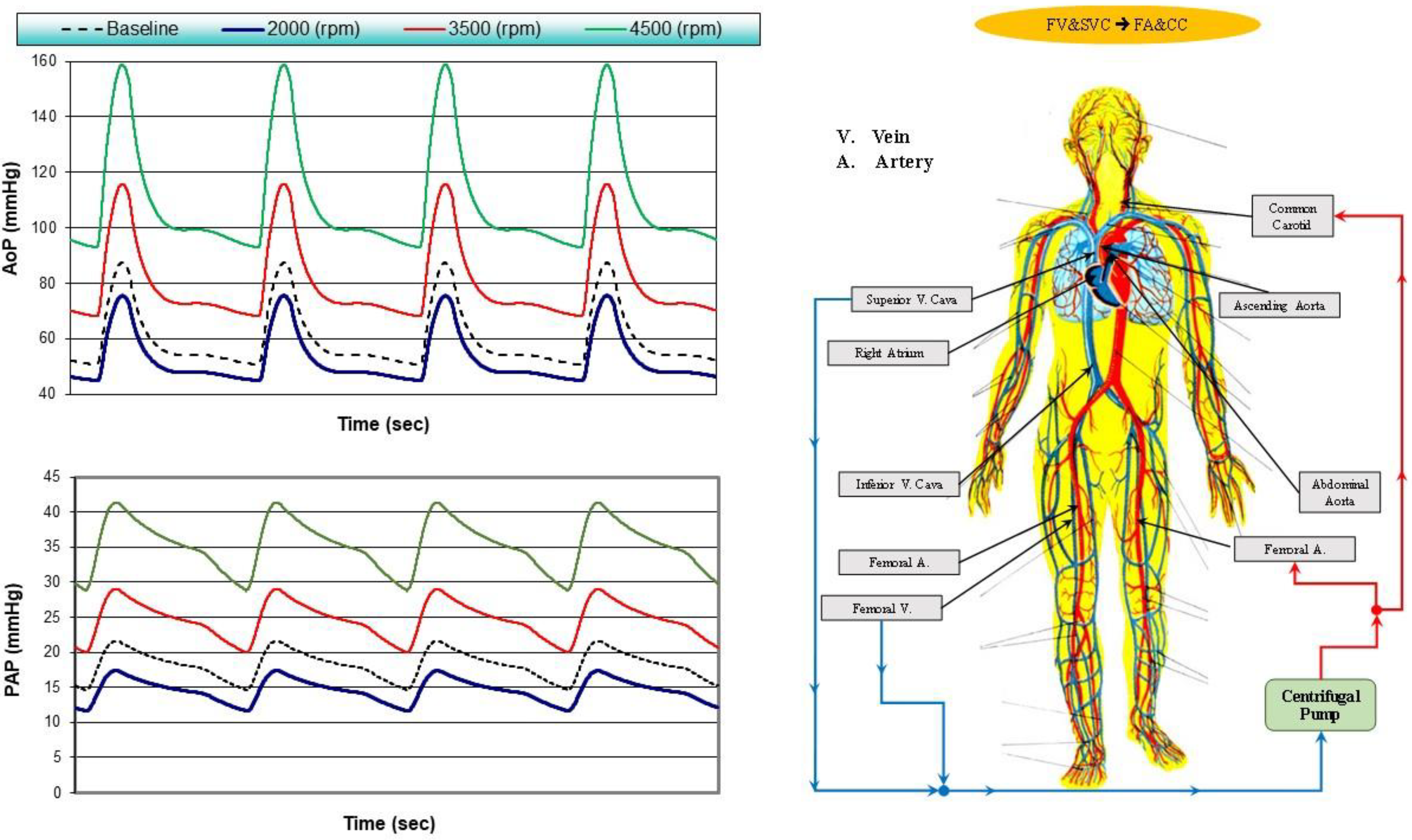
- Femoral vein (FV) and superior vena cava (SVC) cannulation to femoral artery and bilateral common carotid artery return (bottom-left panel), FV&SVC→FA&CC.
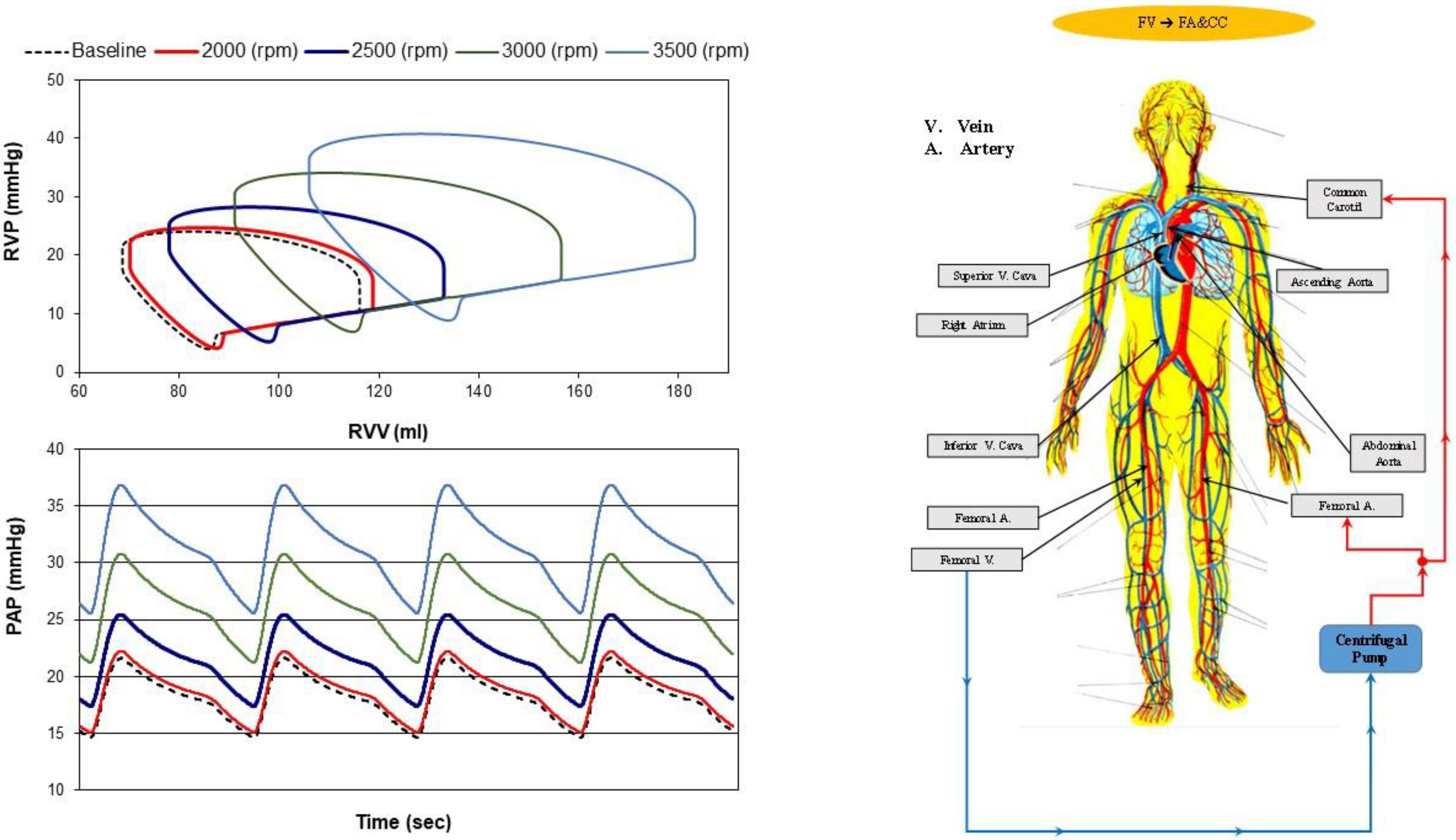
- Femoral vein cannulation to femoral artery and bilateral common carotid artery return (bottom-right panel), FV→FA&CC.
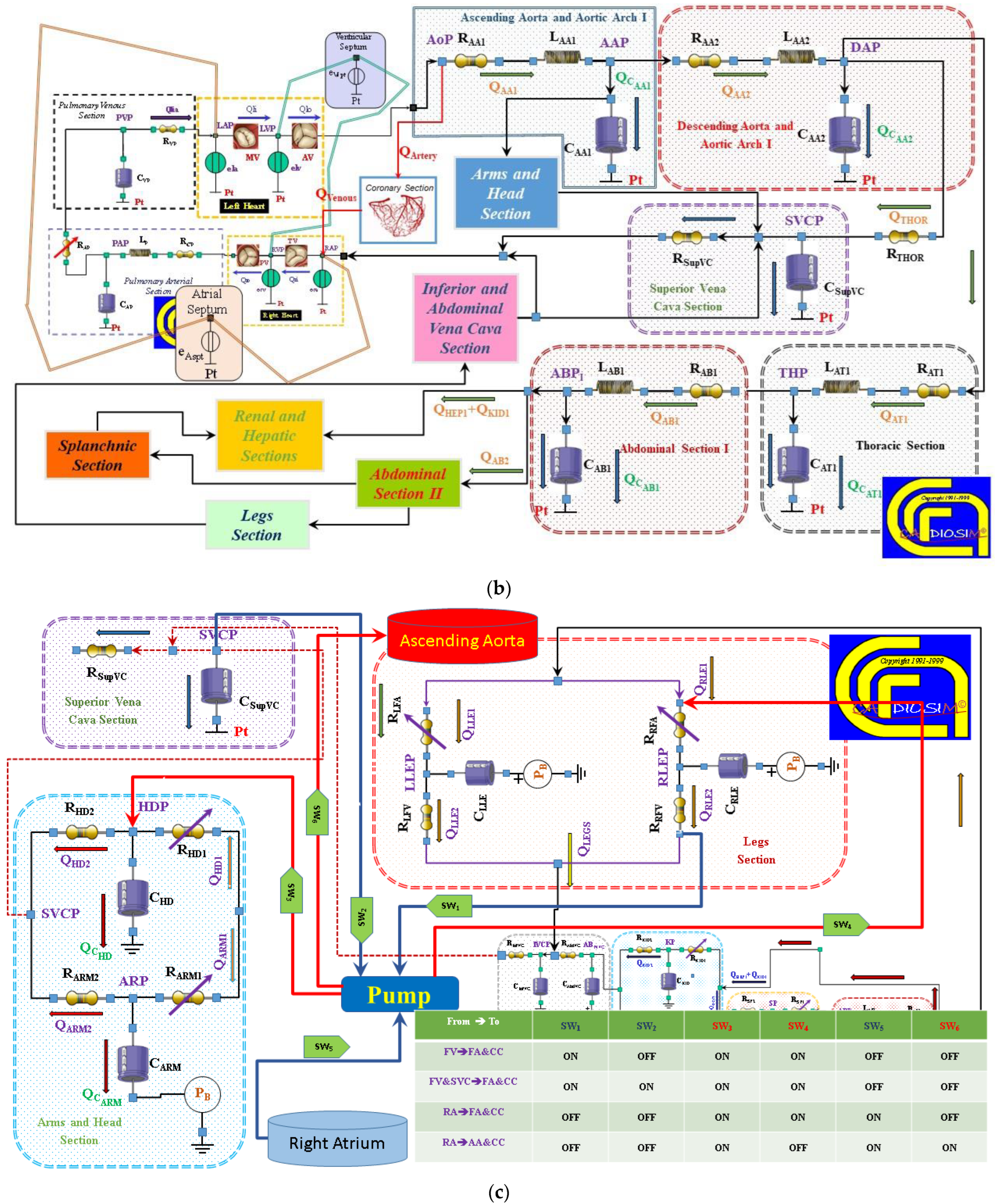
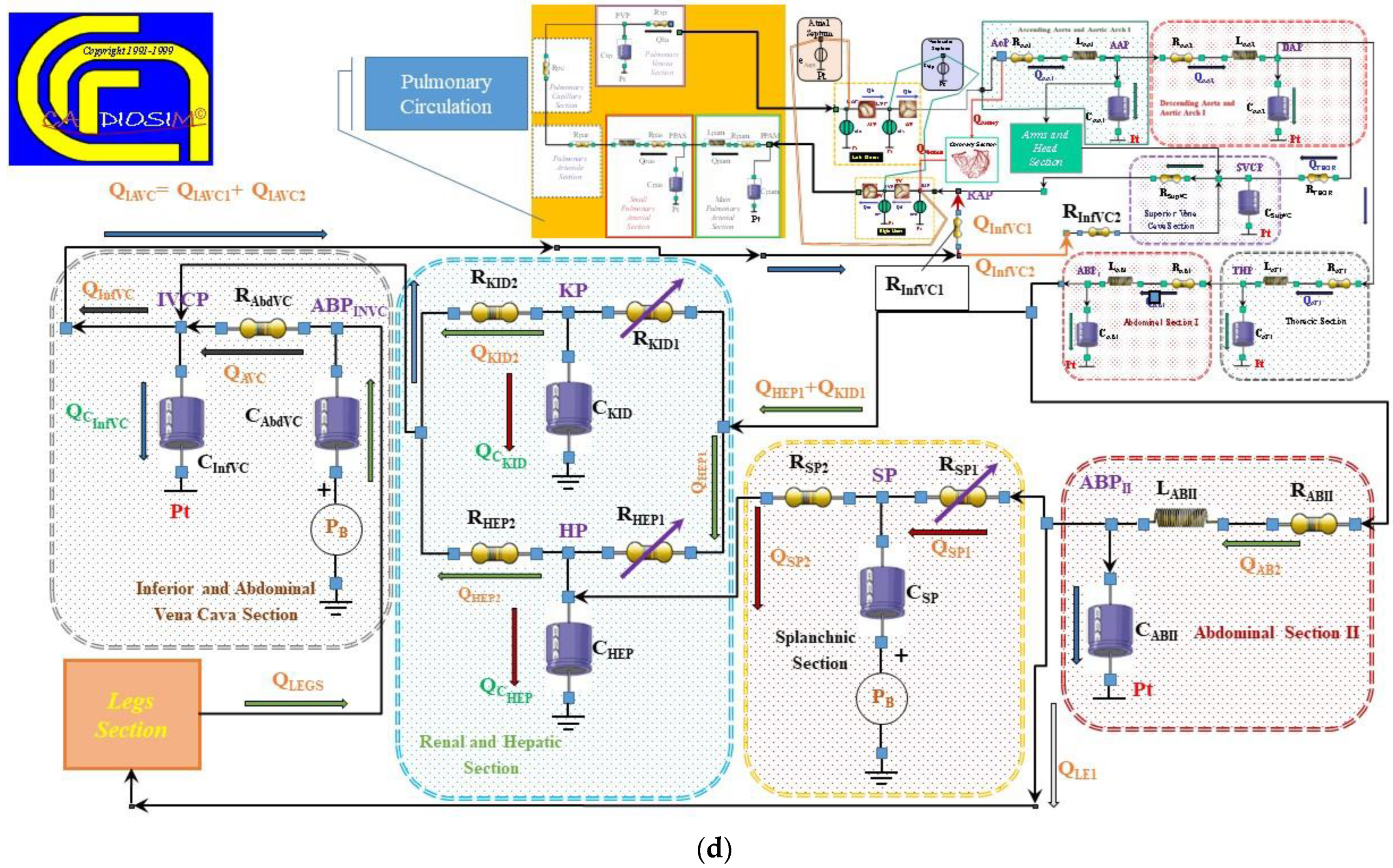
2.2. The Heart and Circulatory Numerical Network
2.3. Numerical Models of the Cannulae and Centrifugal Pump
2.4. Simulation Protocol
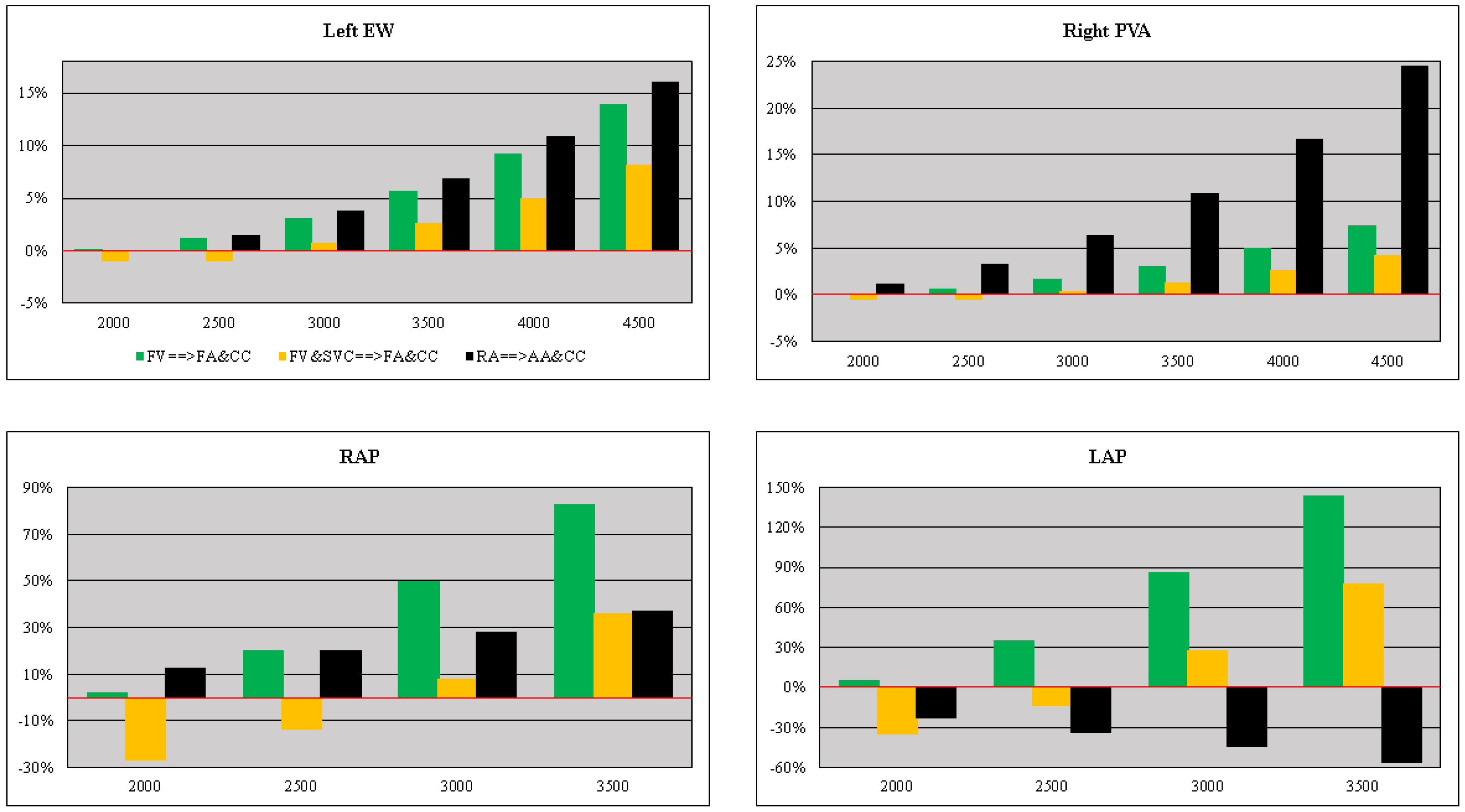
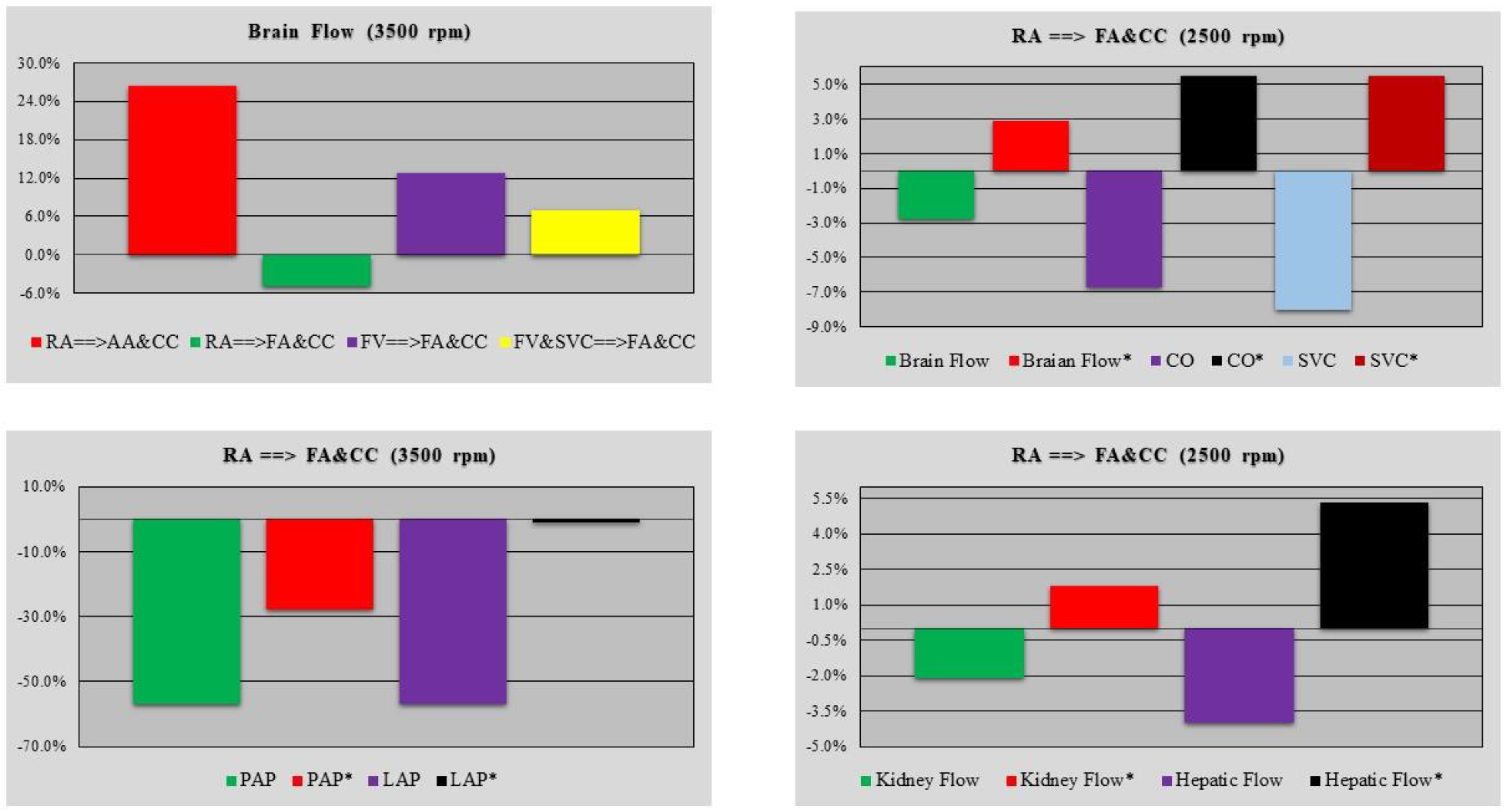
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachet, J. Open repair techniques in the aortic arch are still superior. Ann. Cardiothorac. Surg. 2018, 7, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Chakos, A.; Jbara, D.; Yan, T.D.; Tian, D.H. Long-term survival and related outcomes for hybrid versus traditional arch repair—A meta-analysis. Ann. Cardiothorac. Surg. 2018, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Schepens, M.A.; Dossche, K.M.; Morshuis, W.J.; van den Barselaar, P.J.; Heijmen, R.H.; Vermeulen, F.E. The elephant trunk technique: Operative results in 100 consecutive patients. Eur. J. Cardiothorac. Surg. 2002, 21, 276–281. [Google Scholar] [CrossRef]
- Shrestha, M.; Martens, A.; Krüger, H.; Krüger, H.; Maeding, I.; Ius, F.; Fleissner, F.; Haverich, A. Total aortic arch replacement with the elephant trunk technique: Single-centre 30-year results. Eur. J. Cardiothorac. Surg. 2014, 45, 289–296. [Google Scholar] [CrossRef]
- Karck, M.; Chavan, A.; Hagl, C.; Friedrich, H.; Galanski, M.; Haverich, A. The frozen elephant trunk technique: A new treatment for thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2003, 125, 1550–1553. [Google Scholar] [CrossRef]
- Shrestha, M.; Bachet, J.; Bavaria, J.; Carrel, T.P.; De Paulis, R.; di Bartolomeo, R.; Etz, C.D.; Grabenwöger, M.; Grimm, M.; Haverich, A.; et al. Current status and recommendations for use of the frozen elephant trunk technique: A position paper by the Vascular Domain of EACTS. Eur. J. Cardiothorac. Surg. 2015, 47, 759–769. [Google Scholar] [CrossRef]
- De Bakey, M.E.; Crawford, E.S.; Cooley, D.A.; Morris, G.C., Jr. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg. Gynecol. Obstet. 1957, 105, 657–664. [Google Scholar]
- De Bakey, M.E.; Beall, A.C., Jr.; Cooley, D.A.; Crawford, E.S.; Morris, G.C., Jr.; Garrett, H.E. Resection and graft replacement of aneurysms involving the transverse arch of the aorta. Surg. Clin. N. Am. 1966, 46, 1057–1071. [Google Scholar] [CrossRef]
- Gega, A.; Rizzo, J.A.; Johnson, M.H.; Tranquilli, M.; Farkas, E.A.; Elefteriades, J.A. Straight Deep Hypothermic Arrest: Experience in 394 Patients Supports Its Effectiveness as a Sole Means of Brain Preservation. Ann. Thorac. Surg. 2007, 84, 759–767. [Google Scholar] [CrossRef]
- Ehrlich, M.P.; McCullough, J.N.; Zhang, N.; Weisz, D.J.; Juvonen, T.; A Bodian, C.; Griepp, R.B. Effect of Hypothermia on Cerebral Blood Flow and Metabolism in the Pig. Ann. Thorac. Surg. 2002, 73, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miki, S.; Kusuhara, K.; Okita, Y.; Tahata, T.; Yamanaka, K. Deep Hypothermic Systemic Circulatory Arrest and Continuous Retrograde Cerebral Perfusion for Surgery of Aortic Arch Aneurysm. Eur. J. Cardiothorac. Surg. 1992, 6, 36–41; Discussion 42. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, C.J.; Flameng, W.J. Retrograde Cerebral Perfusion Does Not Perfuse the Brain in Nonhuman Primates. Ann. Thorac. Surg. 1995, 60, 319–328. [Google Scholar] [CrossRef] [PubMed]
- de Brux, J.L.; Subayi, J.B.; Pegis, J.D.; Pillet, J. Retrograde Cerebral Perfusion: Anatomic Study of the Distribution of Blood to the Brain. Ann. Thorac. Surg. 1995, 60, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.L.; Uysal, S.; Ergin, M.A.; Griepp, R.B. Retrograde Cerebral Perfusion as a Method of Neuroprotection during Thoracic Aortic Surgery. Ann Thorac. Surg. 2001, 72, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.P.; Hagl, C.; McCullough, J.N.; Zhang, N.; Shiang, H.; Bodian, C.; Griepp, R.B. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J. Thorac. Cardiovasc. Surg. 2001, 122, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Künzli, A.; Zingg, P.O.; Zünd, G.; Leskosek, B.; von Segesser, L.K. Does retrograde cerebral perfusion via superior vena cava cannulation protect the brain? Eur. J. Cardiothorac. Surg. 2006, 30, 906–909. [Google Scholar] [CrossRef][Green Version]
- Estrera, A.L.; Miller, I.I.I.C.C.; Lee, T.Y.; Shah, P.; Safi, H.J. Ascending and transverse aortic arch repair: The impact of retrograde cerebral perfusion. Circulation 2008, 118, S160–S166. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Gaudino, M.; Iannacone, E.M.; Gambardella, I.; Munjal, M.; Ohmes, L.B.; Degner, B.C.; Girardi, L.N. Retrograde cerebral Perfusion is Effective for Prolonged Circulatory Arrest in Arch Aneurysm Repair. Ann. Thorac. Surg. 2018, 105, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kazui, T.; Inoue, N.; Yamada, O.; Komatsu, S. Selective Cerebral Perfusion During Operation for Aneurysms of the Aortic Arch: A Reassessment. Ann. Thorac. Surg. 1992, 53, 109–114. [Google Scholar] [CrossRef]
- Kazui, T.; Yamashita, K.; Washiyama, N.; Terada, H.; Bashar, A.H.M.; Suzuki, T.; Ohkura, K. Usefulness of Antegrade Selective Cerebral Perfusion during Aortic Arch Operations. Ann. Thorac. Surg. 2002, 74, S1806–S1809. [Google Scholar] [CrossRef] [PubMed]
- Kazui, T. Total arch replacement with separated graft technique and selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2013, 2, 353–357. [Google Scholar] [PubMed]
- Bachet, J.; Guilmet, D.; Goudot, B.; Termignon, J.L.; Teodori, G.; Dreyfus, G.; Brodaty, D.; Dubois, C.; Delentdecker, P.; Cabrol, C. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J. Thorac Cardiovasc. Surg. 1991, 102, 85–94. [Google Scholar] [CrossRef]
- Bachet, J.; Guilmet, D.; Goudot, B.; Dreyfus, G.D.; Delentdecker, P.; Brodaty, D.; Dubois, C. Antegrade Cerebral Perfusion with Cold Blood: A 13-Year Experience. Ann. Thorac. Surg. 1999, 67, 1874–1878. [Google Scholar] [CrossRef]
- Pacini, D.; Leone, A.; Di Marco, L.; Marsilli, D.; Sobaih, F.; Turci, S.; Masieri, V.; Di Bartolomeo, R. Antegrade selective cerebral perfusion in thoracic aorta surgery: Safety of moderate hypothermia. Eur. J. Cardiothorac. Surg. 2007, 31, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Coleman, R.; Griffith, S.; McNeil, J.; Young, H.; Calhoon, J.; Serrano, F.; DiGeronimo, R. Brain preservation with selective cerebral perfusion for operations requiring circulatory arrest: Protection at 25 °C is similar to 18 °C with shorter operating times. Eur. J. Cardiothorac. Surg. 2009, 36, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Küçüker, S.A.; Özatik, M.A.; Saritaş, A.; Taşdemir, O. Arch repair with unilateral antegrade cerebral perfusion. Eur. J. Cardiothorac. Surg. 2005, 27, 638–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malvindi, P.G.; Scrascia, G.; Vitale, N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact. Cardiovasc Thorac. Surg. 2008, 7, 891–897. [Google Scholar] [CrossRef]
- Krähenbühl, E.S.; Reineke, D.; Aymard, T.; Clément, M.; Czerny, M.; Stalder, M.; Schmidli, J.; Carrel, T. Antegrade cerebral protection in thoracic aortic surgery: Lessons from the past decade. Eur. J. Cardiothorac. Surg. 2010, 38, 46–51. [Google Scholar] [CrossRef]
- Urbanski, P.P.; Lenos, A.; Blume, J.C.; Ziegler, V.; Griewing, B.; Schmitt, R.; Diegeler, A.; Dinkel, M. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur. J. Cardiothorac. Surg. 2008, 33, 402–408. [Google Scholar] [CrossRef]
- Matalanis, G.; Koirala, R.S.; Shi, W.Y.; Hayward, P.A.; McCall, P.R. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J. Thorac. Cardiovasc. Surg. 2011, 142, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Matalanis, G.; Ip, S. Total aortic repair for acute type an aortic dissection: A new paradigm. J. Vis. Surg. 2018, 4, 79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sabik, J.F.; Lytle, B.W.; McCarthy, P.M.; Cosgrove, D.M. Axillary artery: An alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J. Thorac. Cardiovasc. Surg. 1995, 109, 885–890; Discussion 890–891. [Google Scholar] [CrossRef]
- Schachner, T.; Nagiller, J.; Zimmer, A.; Laufer, G.; Bonatti, J. Technical problems and complications of axillary artery cannulation. Eur. J. Cardiothorac. Surg. 2005, 27, 634–637. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Ciano, M.; Labriola, G.; Lionetti, G.; Di Eusanio, G. Cannulation of the innominate artery during surgery of the thoracic aorta: Our experience in 55 patients. Eur. J. Cardiothorac. Surg. 2007, 32, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, P.P.; Lenos, A.; Lindemann, Y.; Weigang, E.; Zacher, M.; Diegeler, A. Carotid artery cannulation in aortic surgery. J. Thorac. Cardiovasc. Surg. 2006, 132, 1398–1403. [Google Scholar] [CrossRef]
- Capoccia, M.; Nienaber, C.A.; Mireskandari, M.; Sabetai, M.; Young, C.; Cheshire, N.J.; Rosendahl, U.P. Alternative Approach for Cerebral Protection during Complex Aortic Arch and Redo Surgery. J. Cardiovasc. Dev. Dis. 2021, 8, 86. [Google Scholar] [CrossRef]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Original Website Platform Regarding the Implementation of the Cardiovascular Software Simulator CARDIOSIM©. Available online: https://cardiosim.dsb.cnr.it/ (accessed on 6 April 2022).
- De Lazzari, C.; Darowski, M.; Ferrari, G.; Clemente, F.; Guaragno, M. Ventricular energetics during mechanical ventilation and intraaortic balloon pumping? Computer simulation. J. Med. Eng. Technol. 2001, 25, 103–111. [Google Scholar] [CrossRef]
- De Lazzari, C.; Darowski, M.; Ferrari, G.; Pisanelli, D.M.; Tosti, G. The Impact of Rotary Blood Pump in Conjunction with Mechanical Ventilation on Ventricular Energetic Parameters. Methods Inf. Med. 2006, 45, 574–583. [Google Scholar]
- Shi, Y.; Korakianitis, T. Numerical Simulation of Cardiovascular Dynamics With Left Heart Failure and In-series Pulsatile Ventricular Assist Device. Artif. Organs 2006, 30, 929–948. [Google Scholar] [CrossRef]
- Shi, Y.; Korakianitis, T.; Bowles, C. Numerical simulation of cardiovascular dynamics with different types of VAD assistance. J. Biomech. 2007, 40, 2919–2933. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Brown, A.G.; Lawford, P.V.; Arndt, A.; Nuesser, P.; Hose, D.R. Computational modelling and evaluation of cardiovascular response under pulsatile impeller pump support. Interface Focus 2011, 1, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Korakianitis, T. Impeller-pump model derived from conservation laws applied to the simulation of the cardiovascular system coupled to heart-assist pumps. Comput. Biol. Med. 2018, 93, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, Z.; Yin, M.; Fan, X.; Yang, X.; Luo, X. A patient-specific lumped-parameter model of coronary circulation. Sci. Rep. 2018, 8, 874. [Google Scholar] [CrossRef]
- Doshi, D.; Burkhoff, D. Cardiovascular Simulation of Heart Failure Pathophysiology and Therapeutics. J. Cardiac. Fail. 2016, 22, 303–311. [Google Scholar] [CrossRef]
- Morris, P.D.; Narracott, A.; von Tengg-Kobligk, H.; Silva Soto, D.A.; Hsiao, S.; Lungu, A.; Evans, P.; Bressloff, N.W.; Lawford, P.V.; Hose, D.R.; et al. Computational fluid dynamics modelling in cardiovascular medicine. Heart. 2016, 102, 18–28. [Google Scholar] [CrossRef]
- Morris, P.D.; Ryan, D.; Morton, A.C.; Lycett, R.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual Fractional Reserve From Coronary Angiography: Modelling the Significance of Coronary Lesions. Results From the VIRTU-1 (VIRTUal Fractional Flow Reserve From Coronary Angiography) Study. J. Am. Coll. Cardiol. Intv. 2013, 6, 149–157. [Google Scholar] [CrossRef]
- Kim, H.J.; Vignon-Clementel, I.E.; Figueroa, C.A.; LaDisa, J.F.; Jansen, K.E.; Feinstein, J.A.; Taylor, C.A. On Coupling a Lumped Parameter Heart Model and a Three-Dimensional Finite Element Aorta Model. Ann. Biomed. Eng. 2009, 37, 2153–2169. [Google Scholar] [CrossRef]
- Vignon-Clementel, I.E.; Figueroa, C.A.; Jansen, K.E.; Taylor, C.A. Outflow boundary conditions for three-dimensional finite element modelling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 2006, 195, 3776–3796. [Google Scholar] [CrossRef]
- Niederer, S.A.; Lumens, J.; Trayanova, N.A. Computational models in cardiology. Nat. Rev. Cardiol. 2019, 16, 100–111. [Google Scholar] [CrossRef]
- Bozkurt, S. Mathematical modelling of cardiac function to evaluate clinical cases in adults and children. PLoS ONE 2019, 14, e0224663. [Google Scholar] [CrossRef] [PubMed]
- Hose, D.R.; Lawford, P.V.; Huberts, W.; Hellevik, L.R.; Omholt, S.W.; van de Vosse, F.N. Cardiovascular models for personalised medicine: Where now and where next? Med. Eng. Phys. 2019, 72, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ruijsink, B.; Zugaj, K.; Wong, J.; Pushparajah, K.; Hussain, T.; Moireau, P.; Razavi, R.; Chapelle, D.; Chabiniok, R. Dobutamine stress testing in patients with Fontan circulation augmented by biomechanical modelling. PLoS ONE 2020, 15, e0229015. [Google Scholar] [CrossRef] [PubMed]
- Garber, L.; Khodaei, S.; Keshavarz-Motamed, Z. The Critical Role of Lumped Parameter Models in Patient-Specific Cardiovascular Simulations. Arch. Comput. Methods Eng. 2022, 29, 2977–3000. [Google Scholar] [CrossRef]
- Chakos, A.; Yan, T.D. Mini-access branch-first total arch replacement and frozen elephant trunk procedure. Ann. Cardiothorac. Surg. 2020, 9, 236–243. [Google Scholar] [CrossRef]
- Zanotti, G.; Reece, T.B.; Aftab, M. Aortic Arch Pathology. Surgical Options for the Aortic Arch Replacement. Cardiol. Clin. 2017, 35, 367–385. [Google Scholar] [CrossRef]
- Qu, J.Z.; Kao, L.W.; Smith, J.E.; Kuo, A.; Xue, A.; Iyer, M.H.; Essandoh, M.K.; Dalia, A.A. Brain Protection in Aortic Arch Surgery: An Evolving Field. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1176–1188. [Google Scholar] [CrossRef]
- De Lazzari, C.; Genuini, I.; Pisanelli, D.M.; D’Ambrosi, A.; Fedele, F. Interactive simulator for e-Learning environments: A teaching software for health care professionals. BioMed. Eng. Online 2014, 13, 172. [Google Scholar] [CrossRef]
- De Lazzari, C. Interaction between the septum and the left (right) ventricular free wall in order to evaluate the effects on coronary blood flow: Numerical simulation. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 1359–1368. [Google Scholar] [CrossRef]
- De Lazzari, B.; Iacovoni, A.; Capoccia, M.; Papa, S.; Badagliacca, R.; Filomena, D.; De Lazzari, C. Ventricular and Atrial Pressure—Volume Loops: Analysis of the Effects Induced by Right Centrifugal Pump Assistance. Bioengineering 2022, 9, 181. [Google Scholar] [CrossRef]
- Capoccia, M.; Marconi, S.; Singh, S.A.; Pisanelli, D.M.; De Lazzari, C. Simulation as a preoperative planning approach in advanced heart failure patients. A retrospective clinical analysis. BioMed. Eng. Online 2018, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, B.; Iacovoni, A.; Mottaghy, K.; Capoccia, M.; Badagliacca, R.; Vizza, C.D.; De Lazzari, C. ECMO Assistance During Mechanical Ventilation: Effects Induced on Energetic and Haemodynamic Variables. Comput. Methods. Programs. Biomed. 2021, 202, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, C.; De Lazzari, B.; Iacovoni, A.; Marconi, S.; Papa, S.; Capoccia, M.; Badagliacca, R.; Vizza, C.D. Intra-Aortic Balloon Counterpulsation Timing: A New Numerical Model for Programming and Training in the Clinical Environment. Comput. Methods. Programs. Biomed. 2020, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/CirculationModels/ncm2 (accessed on 6 April 2022).
- Capoccia, M.; Marconi, S.; De Lazzari, C. Decision-making in advanced heart failure patients requiring LVAD insertion: Can preoperative simulation become the way forward? A case study. J. Biomed. Eng. Inform. 2018, 4, 8–20. [Google Scholar] [CrossRef]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/CirculationModels/pci4 (accessed on 6 April 2022).
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it/AssistDevices/ecmo (accessed on 6 April 2022).
- Safi, H.J.; Miller, I.I.I.C.C.; Estrera, A.L.; Villa, M.A.; Goodrick, J.S.; Porat, E.; Azizzadeh, A. Optimization of Aortic Arch Replacement: Two-Stage Approach. Ann. Thorac. Surg. 2007, 83, S815–S818. [Google Scholar] [CrossRef]
- Leone, A.; Di Marco, L.; Murana, G.; Coppola, G.; Fiorentino, M.F.; Amodio, C.; Di Bartolomeo, R.; Pacini, D. The Decision-Making Process in Acute Type an Aortic Dissection: When to Replace the Aortic Arch. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 691–696. [Google Scholar] [CrossRef]
- Czerny, M.; Schmidli, J.; Adler, S.; Van Den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Editor’s Choice—Current Options and Recommendations for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2019, 57, 165–198. [Google Scholar]
- Sakurada, T.; Kazui, T.; Tanaka, H.; Komatsu, S. Comparative Experimental Study of Cerebral Protection During Aortic Arch Reconstruction. Ann. Thorac. Surg. 1996, 61, 1348–1354. [Google Scholar] [CrossRef]
- Henriksen, L.; Hjelms, E. Cerebral blood flow during cardiopulmonary bypass in man: Effect of arterial filtration. Thorax 1986, 41, 386–395. [Google Scholar] [CrossRef]
- Alaoja, H.; Niemelä, E.; Anttila, V.; Dahlbacka, S.; Mäkelä, J.; Kiviluoma, K.; Laurila, P.; Kaakinen, T.; Juvonem, T. Leukocyte filtration to decrease the number of adherent leukocytes in the cerebral microcirculation after a period of deep hypothermic circulatory arrest. J. Thorac. Cardiovasc. Surg. 2006, 132, 1339–1347. [Google Scholar] [CrossRef]
- Dahlbacka, S.; Alaoja, H.; Mäkelä, J.; Niemelä, E.; Laurila, P.; Kiviluoma, K.; Honkanen, A.; Ohtonen, P.; Anttila, V.; Juvonen, T. Effects of pH Management During Selective Antegrade Cerebral Perfusion on Cerebral Microcirculation and Metabolism: Alpha-Stat Versus pH-Stat. Ann. Thorac. Surg. 2007, 84, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Hugenroth, K.; Borchardt, R.; Ritter, P.; Groβ-Hardt, S.; Meyns, B.; Verbelen, T.; Steinseifer, U.; Kaufmann, T.A.S.; Engelmann, U.M. Optimising cerebral perfusion and hemodynamics during cardiopulmonary bypass through cannula design combining in silico, in vitro and in vivo input. Sci. Rep. 2021, 11, 16800. [Google Scholar] [CrossRef] [PubMed]
- Coogan, J.S.; Humphrey, J.D.; Figueroa, C.A. Computational simulations of hemodynamic changes within thoracic, coronary and cerebral arteries following early wall remodelling in response to distal aortic coarctation. Biomech. Model Mechanobiol. 2013, 12, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Imasaka, K.; Tayama, E.; Tomita, Y. The impact of carotid or intracranial atherosclerosis on perioperative stroke in patients undergoing open aortic arch surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Brady, K.; Lee, J.; Easley, B.; Panigrahi, R.; Smielewski, P.; Czosnyka, M.; Hogue, C.W., Jr. Impaired Autoregulation of Cerebral Blood Flow During Rewarming from Hypothermic Cardiopulmonary Bypass and Its Potential Association with Stroke. Anesth. Analg. 2010, 110, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Brady, K.; Easley, R.B.; Brown, C.; Kraut, M.; Gottesman, R.F.; Hogue, C.W., Jr. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J. Thorac. Cardiovasc. Surg. 2014, 147, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Akiyoshi, K.; Nakano, M.; Brady, K.; Bush, B.; Nadkarni, R.; Venkataraman, A.; Koehler, R.C.; Lee, J.K.; Hogue, C.W.; et al. Determining Thresholds for Three Indices of Autoregulation to Identify the Lower Limit of Autoregulation During Cardiac Surgery. Crit. Care Med. 2020, 49, 650–660. [Google Scholar] [CrossRef]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W., Jr. Real-Time Continuous Monitoring of Cerebral Blood Flow Autoregulation Using Near-Infrared Spectroscopy in Patients Undergoing Cardiopulmonary Bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef]
- Ono, M.; Joshi, B.; Brady, K.; Easley, R.B.; Zheng, Y.; Brown, C.; Baumgartner, W.; Hogue, C.W. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br. J. Anaesth. 2012, 109, 391–398. [Google Scholar] [CrossRef]
- Liu, X.; Pu, F.; Fan, Y.; Deng, X.; Li, D.; Li, S. A numerical study on the flow of blood and the transport of LDL in the human aorta: The physiological significance of the helical flow in the aortic arch. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H163–H170. [Google Scholar] [CrossRef]
- Liu, H.; Lan, L.; Abrigo, J.; Ip, H.L.; Soo, Y.; Zheng, D.; Wong, K.S.; Wang, D.; Shi, L.; Leung, T.W.; et al. Comparison of Newtonian and Non-newtonian Fluid Models in Blood Flow Simulation in Patients With Intracranial Arterial Stenosis. Front. Physiol. 2021, 12, 718540. [Google Scholar] [CrossRef]
- Thondapu, V.; Shishikura, D.; Dijkstra, J.; Zhu, S.J.; Revalor, E.; Serruys, P.W.; van Gaal, W.J.; Poon, E.K.W.; Ooi, A.; Barlis, P. Non-Newtonian Endothelial Shear Stress Simulation: Does It Matter? Front. Cardiovasc. Med. 2022, 9, 835270. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lawford, P.; Hose, R. Review of Zero-D and 1-D Models of Blood Flow in the Cardiovascular System. Biomed. Eng. Online 2011, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Frolov, S.V.; Sindeev, S.V.; Lischouk, V.A.; Gazizova, D.S.H.; Liepsch, D.; Balasso, A. A Lumped Parameter Model of Cardiovascular System with Pulsating Heart for Diagnostic Studies. J. Mech. Med. Biol. 2017, 17, 1750056. [Google Scholar] [CrossRef]
- Civilla, L.; Sbrollini, A.; Burattini, L.; Morettini, M. An integrated lumped-parameter model of the cardiovascular system for the simulation of acute ischemic stroke: Description of instantaneous changes in hemodynamics. Math. Biosci. Eng. 2021, 18, 3993–4010. [Google Scholar] [CrossRef]
- Chaudhuri, K.; Pletzer, A.; Smith, N.P. A predictive patient-specific computational model of coronary artery bypass grafts for potential use by cardiac surgeons to guide selection of graft configurations. Front. Cardiovasc. Med. 2022, 9, 953109. [Google Scholar] [CrossRef]
- Hose, D.R.; Lawford, P.V.; Halliday, I.; Rafiroiu, D.; Lungu, A. Challenges and progress in the application of physiological models for clinical decision support in cardiovascular medicine. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1254, 012005. [Google Scholar] [CrossRef]
- Casas, B.; Lantz, J.; Viola, F.; Cedersund, G.; Bolger, A.F.; Carlhäll, C.-J.; Karlsson, M.; Ebbers, T. Bridging the gap between measurements and modelling: A cardiovascular avatar. Sci. Rep. 2017, 7, 6214. [Google Scholar] [CrossRef]
- Laubscher, R.; Van Der Merwe, J.; Herbst, P.; Liebenberg, J. Estimation of Simulated Left Ventricle Elastance Using Lumped Parameter Modelling and Gradient-Based Optimisation With Forward-Mode Automatic Differentiation Based on Syhthetically Generated Noninvasive Data. J. Biomech Eng. 2023, 145, 021008-1–021008-14. [Google Scholar] [CrossRef]
- Manganotti, J.; Caforio, F.; Kimmig, F.; Moireau, P.; Imperiale, S. Coupling reduced-order blood flow and cardiac models through energy-consistent strategies: Modelling and discretisation. Adv. Model. Simul. Eng. Sci. 2021, 8, 21. [Google Scholar] [CrossRef]
- Regazzoni, F.; Salvador, M.; Africa, P.C.; Fedele, M.; Dedè, L.; Quarteroni, A. A cardiac electromechanical model coupled with a lumped-parameter model for closed-loop blood circulation. J. Comput. Phys. 2022, 457, 111083. [Google Scholar] [CrossRef]
- Saltarocchi, S. Simulation in Advanced Heart Failure with a View to Selection and Optimisation of Device Therapy. Ph.D. Thesis, Sapienza University, Rome, Italy, 2018. [Google Scholar]


| Symbol | Description | Unit |
|---|---|---|
| AoP (AAP) | Aortic (ascending and aortic) pressure | mmHg |
| LAP (RAP) | Left (right) atrial pressure | mmHg |
| LVP (RVP) | Left (right) ventricular pressure | mmHg |
| DAP (SVCP) | Descending aortic (superior vena cava) pressure | mmHg |
| THP (ABPI) | Thoracic (abdominal) pressure | mmHg |
| HDP (ARP) | Brain (Arm) pressure | mmHg |
| LLEP (RLEP) | Left (right) leg pressure | mmHg |
| Pt | Intrathoracic pressure | mmHg |
| PB | Breathing pressure | mmHg |
| SP (ABPII) | Splanchnic (abdominal II) pressure | mmHg |
| HP (KP) | Hepatic (renal) pressure | mmHg |
| IVCP | Inferior vena cava pressure | mmHg |
| RAA1 (RAA2) | Ascending (descending) and aortic arch resistance | mmHg·cm−3·sec |
| LAA1 (LAA2) | Ascending (descending) and aortic arch inertance | mmHg·cm−3·sec2 |
| CAA1 (CAA2) | Ascending (descending) and aortic arch compliance | mmHg−1·cm−3 |
| RTHOR (RSupVC) | Thoracic (superior vena cava) resistance | mmHg·cm−3·sec |
| CSupVC | Superior vena cava compliance | mmHg−1·cm−3 |
| RAT1 (RAB1) | Thoracic (abdominal) resistance | mmHg·cm−3·sec |
| LAT1 (LAB1) | Thoracic (abdominal) inertance | mmHg·cm−3·sec2 |
| CAT1 (CAB1) | Thoracic (abdominal) compliance | mmHg−1·cm−3 |
| RLFA (RLFV) | Left femoral arterial (venous) resistance | mmHg·cm−3·sec |
| RRFA (RRFV) | Right femoral arterial (venous) resistance | mmHg·cm−3·sec |
| CLLE (CRLE) | Left (right) femoral compliance | mmHg−1·cm−3 |
| RARM1 and RARM2 (CARM) | Upper limb resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RHD1 and RHD2 (CHD) | Brain resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RinfVC1 and RinfVC2 (CInfVC) | Inferior vena cava resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RHEP1 and RHEP2 (CHEP) | Hepatic resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RKID1 and RKID2 (CKID) | Renal resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RSP1 and RSP2 (CSP) | Splanchnic resistances (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| RABII (CABII) | Abdominal (II) resistance (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| LABII | Abdominal (II) inertance | mmHg·cm−3·sec2 |
| RAbdVC (CAbdVC) | Abdominal vena cava resistance (compliance) | mmHg·cm−3·sec (mmHg−1·cm−3) |
| EesLeft (EesRight) | Left (right) ventricular end-systolic elastance | mmHg/ml |
| EaS (EaP) | Systemic (pulmonary) arterial elastance | mmHg/ml |
| EaS/EesLeft (EesRight/EaP) | Left (right) ventricular–arterial coupling | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lazzari, B.; Capoccia, M.; Cheshire, N.J.; Rosendahl, U.P.; Badagliacca, R.; De Lazzari, C. Evaluation of Different Cannulation Strategies for Aortic Arch Surgery Using a Cardiovascular Numerical Simulator. Bioengineering 2023, 10, 60. https://doi.org/10.3390/bioengineering10010060
De Lazzari B, Capoccia M, Cheshire NJ, Rosendahl UP, Badagliacca R, De Lazzari C. Evaluation of Different Cannulation Strategies for Aortic Arch Surgery Using a Cardiovascular Numerical Simulator. Bioengineering. 2023; 10(1):60. https://doi.org/10.3390/bioengineering10010060
Chicago/Turabian StyleDe Lazzari, Beatrice, Massimo Capoccia, Nicholas J. Cheshire, Ulrich P. Rosendahl, Roberto Badagliacca, and Claudio De Lazzari. 2023. "Evaluation of Different Cannulation Strategies for Aortic Arch Surgery Using a Cardiovascular Numerical Simulator" Bioengineering 10, no. 1: 60. https://doi.org/10.3390/bioengineering10010060
APA StyleDe Lazzari, B., Capoccia, M., Cheshire, N. J., Rosendahl, U. P., Badagliacca, R., & De Lazzari, C. (2023). Evaluation of Different Cannulation Strategies for Aortic Arch Surgery Using a Cardiovascular Numerical Simulator. Bioengineering, 10(1), 60. https://doi.org/10.3390/bioengineering10010060