Biological Scaffolds for Congenital Heart Disease
Abstract
1. Introduction
2. Tissue Engineering Approaches
3. Production of Biological Scaffolds
3.1. Chemical Fixation of Biological Scaffolds
3.2. Decellularisation of Biological Scaffolds
4. Biological Scaffolds for CHD
4.1. Acellular Scaffolds for CHD
4.1.1. Homografts for CHD
4.1.2. Xenografts for CHD
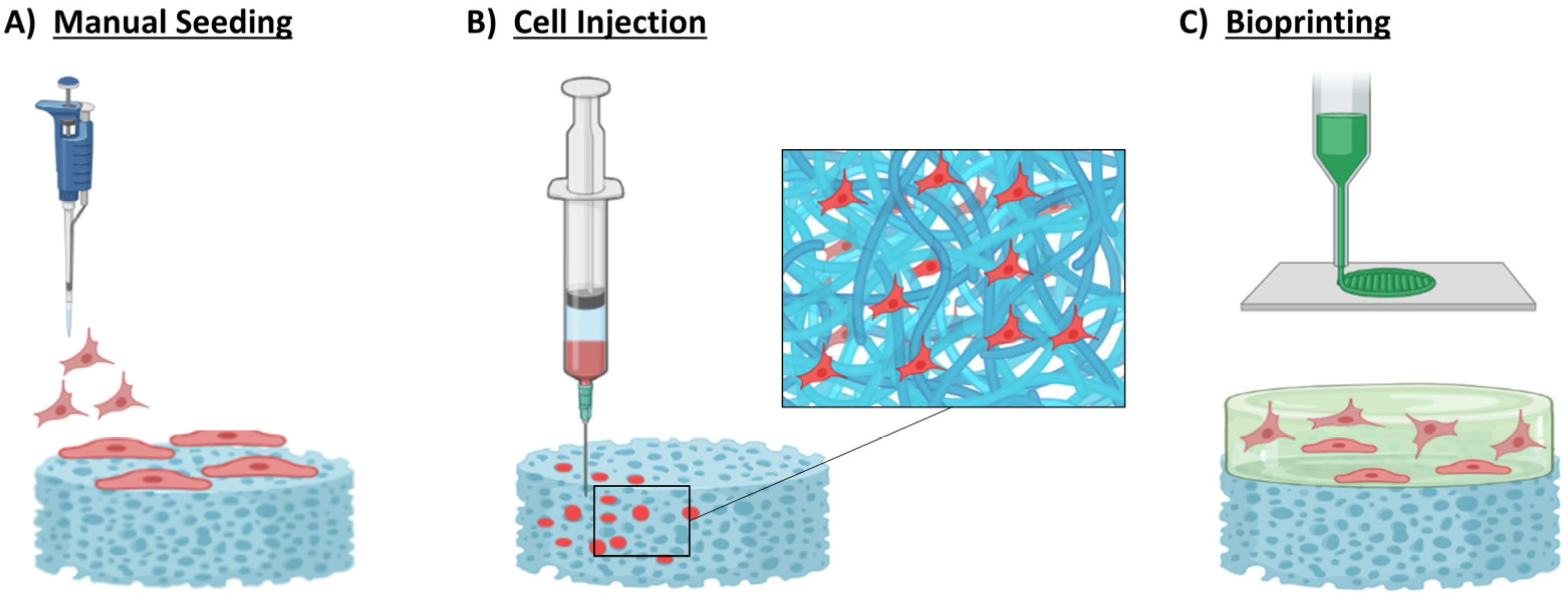
4.2. Cellular Scaffolds for CHD
4.2.1. Manual Seeding
4.2.2. Cell Injection
4.2.3. 3D Bioprinting
| Cell Type | Application | Seeding Method | Biological Scaffold |
|---|---|---|---|
| Peripheral mononuclear cells | Human use: paediatric patient | Manual | Decellularised pulmonary heart valve allograft [140] |
| hiPSC-cardiomyocytes and cardiac fibroblast | In vitro | Manual | dECM [141] |
| hiPSCs-cardiomyocytes | In vitro | Bioprinting | 3D bioprinted cardiac tissue grafts from cell spheroids [161] |
| hiPSC-cardiomyocytes, HUVECs, and fibroblast | In vivo | Bioprinting | Cardiac Organoids forming EHTS [162] |
| hUCB-MSCs | In vivo | Manual | AM-based scaffold [133] |
| MSCs | In vivo | Manual | SIS [148] |
| MSCs | In vivo | Manual | Ultra-foam collagen sponge [149] |
| MSCs | In vitro | Bioprinting | GelMa/poly (eythelene glycol) diacrylate hydrogels supported by PCL [159] |
| U-SCs | In vivo | Manual | Polyurethane/SIS patch [144] |
| RAECs and RMCs | In vitro | Manual | Hybrid HPM [143] |
| PCs | In vitro | Manual | Decellularised porcine pulmonary artery [145] |
| PCs | In vitro | Manual | Decellularised cardiac ECM [146] |
| EPCs | In vitro | Manual | Decellularised aortic heart valve [147] |
| hCPCs | In vivo | Bioprinting | Patch consisting of a hybrid bioink made of cardiac dECM hydrogel and GelMA loaded with paediatric hCPCs [155] |
| Pericytes | In vivo | Manual | Proxicor® [2] |
| SMCs | In vitro | Manual | Reinforced porcine blood-derived fibrinogen [142] |
| SMCs | In vivo | Manual | Decellularised swine SIS graft [80] |
| SMCs | In vivo | Manual | Scaffold-free. Ten-layered cell sheets of SMCs [150] |
| ARS-SMCs and AV-VICs | In vitro | Bioprinting | Aortic valve conduits composed of alginate/gelatin hybrid hydrogel [158] |
| AV-VICs | In vitro | Bioprinting | Heart trileaflet valve conduits are composed of Me-HA and GelMa hydrogels [160] |
| Neonatal cardiac Fibroblast | In vitro | Injection | Direct injection encapsulating with a 3D fibrin gel artificial heart muscle patch [151] |
| Neonatal cardiac Fibroblast | In vitro | Injection | Direct injecting into the novel BECV [152] |
5. Future Work and Translation to Clinic
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernier, P.L.; Stefanescu, A.; Samoukovic, G.; Tchervenkov, C.I. The Challenge of Congenital Heart Disease Worldwide: Epidemiologic and Demographic Facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010, 13, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Alvino, V.V.; Kilcooley, M.; Thomas, A.C.; Carrabba, M.; Fagnano, M.; Cathery, W.; Avolio, E.; Iacobazzi, D.; Ghorbel, M.; Caputo, M.; et al. In Vitro and In Vivo Preclinical Testing of Pericyte-Engineered Grafts for the Correction of Congenital Heart Defects. J. Am. Heart Assoc. 2020, 9, e014214. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.A.; et al. Global, Regional, and National Burden of Congenital Heart Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Strouse, R.; Hor, K.; Pepper, V.; Tipton, A.; Kelly, J.; Shinoka, T.; Breuer, C. Toward a Patient-Specific Tissue Engineered Vascular Graft. J. Tissue Eng. 2018, 9, 2041731418764709. [Google Scholar] [CrossRef] [PubMed]
- Mirani, B.; Parvin Nejad, S.; Simmons, C.A. Recent Progress Toward Clinical Translation of Tissue-Engineered Heart Valves. Can. J. Cardiol. 2021, 37, 1064–1077. [Google Scholar] [CrossRef]
- Marelli, A.J.; Mackie, A.S.; Ionescu-Ittu, R.; Rahme, E.; Pilote, L. Congenital Heart Disease in the General Population: Changing Prevalence and Age Distribution. Circulation 2007, 115, 163–172. [Google Scholar] [CrossRef]
- Moons, P.; Engelfriet, P.; Kaemmerer, H.; Meijboom, F.J.; Oechslin, E.; Mulder, B.J.M. Delivery of Care for Adult Patients with Congenital Heart Disease in Europe: Results from the Euro Heart Survey. Eur. Heart J. 2006, 27, 1324–1330. [Google Scholar] [CrossRef]
- Rohit, M.; Shrivastava, S. Acyanotic and Cyanotic Congenital Heart Diseases. Indian J. Pediatr. 2018, 85, 454–460. [Google Scholar] [CrossRef]
- Stout, K.K.; Broberg, C.S.; Book, W.M.; Cecchin, F.; Chen, J.M.; Dimopoulos, K.; Everitt, M.D.; Gatzoulis, M.; Harris, L.; Hsu, D.T.; et al. Chronic Heart Failure in Congenital Heart Disease: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 770–801. [Google Scholar] [CrossRef]
- Yuan, S.M.; Jing, H. Palliative Procedures for Congenital Heart Defects. Arch. Cardiovasc. Dis. 2009, 102, 549–557. [Google Scholar] [CrossRef]
- Karsenty, C.; Zhao, A.; Marijon, E.; Ladouceur, M. Risk of Thromboembolic Complications in Adult Congenital Heart Disease: A Literature Review. Arch. Cardiovasc. Dis. 2018, 111, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Yang, T.; Huang, P.; Wang, L.; Zhao, L.; Zhang, S.; Ye, Z.; Chen, L.; Zheng, Z.; et al. Congenital Heart Disease and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2019, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lueders, C.; Jastram, B.; Hetzer, R.; Schwandt, H. Rapid Manufacturing Techniques for the Tissue Engineering of Human Heart Valves. Eur. J. Cardio-Thorac. Surg. 2014, 46, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Drenthen, W.; Boersma, E.; Balci, A.; Moons, P.; Roos-Hesselink, J.W.; Mulder, B.J.M.; Vliegen, H.W.; van Dijk, A.P.J.; Voors, A.A.; Yap, S.C.; et al. Predictors of Pregnancy Complications in Women with Congenital Heart Disease. Eur. Heart J. 2010, 31, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Freling, H.G.; van Slooten, Y.J.; van Melle, J.P.; Mulder, B.J.M.; van Dijk, A.P.J.; Hillege, H.L.; Post, M.C.; Sieswerda, G.T.; Jongbloed, M.R.M.; Willems, T.P.; et al. Prosthetic Valves in Adult Patients with Congenital Heart Disease: Rationale and Design of the Dutch PROSTAVA Study. Neth. Heart J. 2012, 20, 419–424. [Google Scholar] [CrossRef]
- Kiraly, L.; Vijayavenkataraman, S. Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines 2021, 12, 332. [Google Scholar] [CrossRef]
- Taksande, A.; Jameel, Z.P. Critical Congenital Heart Disease in Neonates: A Review Article. Curr. Pediatr. Rev. 2021, 17, 120–126. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. Choice of Prosthetic Heart Valve in Adults. An Update. J. Am. Coll. Cardiol. 2010, 55, 2413–2426. [Google Scholar] [CrossRef]
- Tasca, G.; Mhagna, Z.; Perotti, S.; Centurini, P.B.; Sabatini, T.; Amaducci, A.; Brunelli, F.; Cirillo, M.; Tomba, M.D.; Quiani, E.; et al. Impact of Prosthesis-Patient Mismatch on Cardiac Events and Midterm Mortality after Aortic Valve Replacement in Patients with Pure Aortic Stenosis. Circulation 2006, 113, 570–576. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Durko, A.P.; Yacoub, M.H.; Kluin, J. Tissue Engineered Materials in Cardiovascular Surgery: The Surgeon’s Perspective. Front. Cardiovasc. Med. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.S.; Go, J.L.; Hennessy, R.R.; Tefft, B.J.; Jana, S.; Stoyles, N.J.; Al-Hijji, M.A.; Thaden, J.J.; Pislaru, S.V.; Simari, R.D.; et al. Recellularization of a Novel Off-the-Shelf Valve Following Xenogenic Implantation into the Right Ventricular Outflow Tract. PLoS ONE 2017, 12, e0181614. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, M.; Madeddu, P. Current Strategies for the Manufacture of Small Size Tissue Engineering Vascular Grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Grande-Allen, K.J. Which Biological Properties of Heart Valves Are Relevant to Tissue Engineering? Front. Cardiovasc. Med. 2020, 7, 63. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Prat-Vidal, C.; Bayes-Genis, A. In Vivo Experience with Natural Scaffolds for Myocardial Infarction: The Times They Are a-Changin’. Stem. Cell Res. Ther. 2015, 6, 248. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. [Google Scholar] [CrossRef]
- Brown, B.; Lindberg, K.; Reing, J.; Stolz, D.B.; Badylak, S.F. The Basement Membrane Component of Biologic Scaffolds Derived from Extracellular Matrix. Tissue Eng. 2006, 12, 519–526. [Google Scholar] [CrossRef]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L.; Kraine, M.R.; Simmons, C. The Use of Xenogeneic Small Intestinal Submucosa as a Biomaterial for Achille’s Tendon Repair in a Dog Model. J. Biomed. Mater. Res. 1995, 29, 977–985. [Google Scholar] [CrossRef]
- Hodde, J.; Record, R.; Tullius, R.; Badylak, S. Fibronectin Peptides Mediate HMEC Adhesion to Porcine-Derived Extracellular Matrix. Biomaterials 2002, 23, 1841–1848. [Google Scholar] [CrossRef]
- Hodde, J.P.; Badylak, S.F.; Brightman, A.O.; Voytik-Harbin, S.L. Glycosaminoglycan Content of Small Intestinal Submucosa: A Bioscaffold for Tissue Replacement. Tissue Eng. 1996, 2, 209–217. [Google Scholar] [CrossRef] [PubMed]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Voytik-Harbin, S.L.; Brightman, A.O.; Kraine, M.R.; Waisner, B.; Badylak, S.F. Identification of Extractable Growth Factors from Small Intestinal Submucosa. J. Cell. Biochem. 1997, 67, 478–491. [Google Scholar] [CrossRef]
- Hodde, J.P.; Record, R.D.; Liang, H.A.; Badylak, S.F. Vascular Endothelial Growth Factor in Porcine-Derived Extracellular Matrix. Endothelium 2001, 8, 11–24. [Google Scholar] [CrossRef]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering Artificial Blood Vessels from Natural Materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; de Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Yang, J.; Dang, H.; Xu, Y. Recent Advancement of Decellularization Extracellular Matrix for Tissue Engineering and Biomedical Application. Artif. Organs 2022, 46, 549–567. [Google Scholar] [CrossRef]
- Blum, K.M.; Mirhaidari, G.J.M.; Breuer, C.K. Tissue Engineering: Relevance to Neonatal Congenital Heart Disease. Semin. Fetal Neonatal Med. 2022, 27, 101225. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1801217. [Google Scholar] [CrossRef]
- Santschi, M.; Vernengo, A.; Eglin, D.; D’Este, M.; Wuertz-Kozak, K. Decellularized Matrix as a Building Block in Bioprinting and Electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Xing, Q.; Qian, Z.; Jia, W.; Ghosh, A.; Tahtinen, M.; Zhao, F. Natural Extracellular Matrix for Cellular and Tissue Biomanufacturing. ACS Biomater. Sci. Eng. 2017, 3, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Fedak, P.W.M. Using Acellular Bioactive Extracellular Matrix Scaffolds to Enhance Endogenous Cardiac Repair. Front. Cardiovasc. Med. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A Review of Decellurization Methods Caused by an Urgent Need for Quality Control of Cell-Free Extracellular Matrix’ Scaffolds and Their Role in Regenerative Medicine. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Senage, T.; Paul, A.; le Tourneau, T.; Fellah-Hebia, I.; Vadori, M.; Bashir, S.; Galiñanes, M.; Bottio, T.; Gerosa, G.; Evangelista, A.; et al. The Role of Antibody Responses against Glycans in Bioprosthetic Heart Valve Calcification and Deterioration. Nat. Med. 2022, 28, 283–294. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised Scaffolds: Just a Framework? Current Knowledge and Future Directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lian, Y.; Zhou, Z.; Lu, Y.; Li, S.; Pei, F.; Cheng, J. Distribution of the Alpha-Gal Epitope on Adult Porcine Bone Tissue. Transplant. Proc 2006, 38, 2247–2251. [Google Scholar] [CrossRef]
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of α-Gal Epitope, Anti-Gal Antibody, A1,3 Galactosyltransferase and Its Clinical Exploitation (Review). Int. J. Mol. Med. 2016, 37, 11–20. [Google Scholar] [CrossRef]
- Abay, A.; Simionato, G.; Chachanidze, R.; Bogdanova, A.; Hertz, L.; Bianchi, P.; van den Akker, E.; von Lindern, M.; Leonetti, M.; Minetti, G.; et al. Glutaraldehyde—A Subtle Tool in the Investigation of Healthy and Pathologic Red Blood Cells. Front. Physiol. 2019, 10, 514. [Google Scholar] [CrossRef]
- Maizato, M.J.S.; Higa, O.Z.; Mathor, M.B.; Camillo, M.A.P.; Spencer, P.J.; Pitombo, R.N.; Zavaglia, C.A.C.; Leirner, A.A. Glutaraldehyde-Treated Bovine Pericardium: Effects of Lyophilization on Cytotoxicity and Residual Aldehydes. Artif. Organs 2003, 27, 692–694. [Google Scholar] [CrossRef]
- Choe, J.A.; Jana, S.; Tefft, B.J.; Hennessy, R.S.; Go, J.; Morse, D.; Lerman, A.; Young, M.D. Biomaterial Characterization of Off-the-Shelf Decellularized Porcine Pericardial Tissue for Use in Prosthetic Valvular Applications. J. Tissue Eng. Regen. Med. 2018, 12, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Consolo, F.; Spiccia, M.; Piola, M.; Kassem, S.; Prandi, F.; Vinci, M.C.; Forti, E.; Polvani, G.; Fiore, G.B.; et al. Feasibility of Pig and Human-Derived Aortic Valve Interstitial Cells Seeding on Fixative-Free Decellularized Animal Pericardium. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 345–356. [Google Scholar] [CrossRef]
- Aldridge, A.; Desai, A.; Owston, H.; Jennings, L.M.; Fisher, J.; Rooney, P.; Kearney, J.N.; Ingham, E.; Wilshaw, S.P. Development and Characterisation of a Large Diameter Decellularised Vascular Allograft. Cell Tissue Bank. 2018, 19, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, N.; Gangwar, A.; Saxena, A. Using Acellular Aortic Matrix to Repair Umbilical Hernias of Calves. Aust. Vet. J. 2013, 91, 251–253. [Google Scholar] [CrossRef]
- Tsai, T.N.; Kirton, J.P.; Campagnolo, P.; Zhang, L.; Xiao, Q.; Zhang, Z.; Wang, W.; Hu, Y.; Xu, Q. Contribution of Stem Cells to Neointimal Formation of Decellularized Vessel Grafts in a Novel Mouse Model. Am. J. Pathol. 2012, 181, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Cahill, P.A.; Lally, C. Investigation of a Small-Diameter Decellularised Artery as a Potential Scaffold for Vascular Tissue Engineering; Biomechanical Evaluation and Preliminary Cell Seeding. J. Mech. Behav. Biomed. Mater. 2012, 14, 130–142. [Google Scholar] [CrossRef]
- Halfwerk, F.R.; Rouwkema, J.; Gossen, J.A.; Grandjean, J.G. Supercritical Carbon Dioxide Decellularised Pericardium: Mechanical and Structural Characterisation for Applications in Cardio-Thoracic Surgery. J. Mech. Behav. Biomed. Mater. 2018, 77, 400–407. [Google Scholar] [CrossRef]
- Prat-Vidal, C.; Gálvez-Montón, C.; Puig-Sanvicens, V.; Sanchez, B.; Díaz-Güemes, I.; Bogónez-Franco, P.; Perea-Gil, I.; Casas-Solà, A.; Roura, S.; Llucià-Valldeperas, A.; et al. Online Monitoring of Myocardial Bioprosthesis for Cardiac Repair. Int. J. Cardiol. 2014, 174, 654–661. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Sabetkish, S.; Akbarzadeh, A.; Muhammadnejad, A.; Heidari, R.; Kajbafzadeh, A.M. Effect of Three Decellularisation Protocols on the Mechanical Behaviour and Structural Properties of Sheep Aortic Valve Conduits. Adv. Med. Sci. 2014, 59, 299–307. [Google Scholar] [CrossRef]
- Yu, B.T.; Li, W.T.; Song, B.Q.; Wu, Y.L. Comparative Study of the Triton X-100-Sodium Deoxycholate Method and Detergent-Enzymatic Digestion Method for Decellularization of Porcine Aortic Valves. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2179–2184. [Google Scholar]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.T.; Ross, D.B. Comparison of Aortic Valve Allograft Decellularization Techniques in the Rat. J. Biomed. Mater. Res. A 2006, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Somers, P.; de Somer, F.; Cornelissen, M.; Thierens, H.; van Nooten, G. Decellularization of Heart Valve Matrices: Search for the Ideal Balance. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Joyce, E.M.; Sacks, M.S. Effects of Decellularization on the Mechanical and Structural Properties of the Porcine Aortic Valve Leaflet. Biomaterials 2008, 29, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Guhathakurta, S.; Varghese, S.; Balasubramanian, V.; Agarwal, R.; Murthy, B.S.; Veerappan, S.; Padmaja, P.; Cherian, K.M. Technique to Process Xenogenic Tissues for Cardiovascular Implantation—A Preliminary Report. Curr. Sci. 2006, 91, 1068–1073. [Google Scholar]
- Luo, J.; Korossis, S.A.; Wilshaw, S.P.; Jennings, L.M.; Fisher, J.; Ingham, E. Development and Characterization of Acellular Porcine Pulmonary Valve Scaffolds for Tissue Engineering. Tissue Eng. Part A 2014, 20, 2963–2974. [Google Scholar] [CrossRef]
- Vafaee, T.; Thomas, D.; Desai, A.; Jennings, L.M.; Berry, H.; Rooney, P.; Kearney, J.; Fisher, J.; Ingham, E. Decellularization of Human Donor Aortic and Pulmonary Valved Conduits Using Low Concentration Sodium Dodecyl Sulfate. J. Tissue Eng. Regen. Med. 2018, 12, e841–e853. [Google Scholar] [CrossRef]
- Desai, A.; Vafaee, T.; Rooney, P.; Kearney, J.N.; Berry, H.E.; Ingham, E.; Fisher, J.; Jennings, L.M. In Vitro Biomechanical and Hydrodynamic Characterisation of Decellularised Human Pulmonary and Aortic Roots. J. Mech. Behav. Biomed. Mater. 2018, 79, 53–63. [Google Scholar] [CrossRef]
- Nguyen, D.T.; O’Hara, M.; Graneli, C.; Hicks, R.; Miliotis, T.; Nyström, A.C.; Hansson, S.; Davidsson, P.; Gan, L.M.; Magnone, M.C.; et al. Humanizing Miniature Hearts through 4-Flow Cannulation Perfusion Decellularization and Recellularization. Sci. Rep. 2018, 8, 7458. [Google Scholar] [CrossRef]
- Tenreiro, M.F.; Almeida, H.V.; Calmeiro, T.; Fortunato, E.; Ferreira, L.; Alves, P.M.; Serra, M. Interindividual Heterogeneity Affects the Outcome of Human Cardiac Tissue Decellularization. Sci. Rep. 2021, 11, 20834. [Google Scholar] [CrossRef]
- Dal Sasso, E.; Menabò, R.; Agrillo, D.; Arrigoni, G.; Franchin, C.; Giraudo, C.; Filippi, A.; Borile, G.; Ascione, G.; Zanella, F.; et al. RegenHeart: A Time-Effective, Low-Concentration, Detergent-Based Method Aiming for Conservative Decellularization of the Whole Heart Organ. ACS Biomater. Sci. Eng. 2020, 6, 5493–5506. [Google Scholar] [CrossRef]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. Int. J. Mol. Sci. 2022, 23, 13040. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Kasimir, M.T.; Seebacher, G.; Weigel, G.; Ullrich, R.; Salzer-Muhar, U.; Rieder, E.; Wolner, E. Early Failure of the Tissue Engineered Porcine Heart Valve SYNERGRAFTTM in Pediatric Patients. Eur. J. Cardio-Thorac. Surg. 2003, 23, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Bibevski, S.; Ruzmetov, M.; Fortuna, R.S.; Turrentine, M.W.; Brown, J.W.; Ohye, R.G. Performance of SynerGraft Decellularized Pulmonary Allografts Compared With Standard Cryopreserved Allografts: Results from Multiinstitutional Data. Ann. Thorac. Surg. 2017, 103, 869–874. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Eltayeb, O.; Rodefeld, M.D.; Turrentine, M.W. Performance of SynerGraft Decellularized Pulmonary Homograft in Patients Undergoing a Ross Procedure. Ann. Thorac. Surg. 2011, 91, 416–423. [Google Scholar] [CrossRef]
- Mewhort, H.E.M.; Svystonyuk, D.A.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Guzzardi, D.G.; Park, D.S.; Kang, S.; Hollenberg, M.D.; Fedak, P.W.M. Bioactive Extracellular Matrix Scaffold Promotes Adaptive Cardiac Remodeling and Repair. J. Am. Coll. Cardiol. Basic Trans. Sci. 2017, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, M.; Ivirico, J.L.E.; Kan, H.M.; Bordett, R.; Pandey, R.; Otsuka, T.; Nair, L.S.; Laurencin, C.T. Preparation and Characterization of Amnion Hydrogel and Its Synergistic Effect with Adipose Derived Stem Cells towards IL1β Activated Chondrocytes. Sci. Rep. 2020, 10, 18751. [Google Scholar] [CrossRef]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Hochman-Mendez, C.; Pereira de Campos, D.B.; Pinto, R.S.; Mendes, B.J.D.S.; Rocha, G.M.; Monnerat, G.; Weissmuller, G.; Sampaio, L.C.; Carvalho, A.B.; Taylor, D.A. Tissue-Engineered Human Embryonic Stem Cell-Containing Cardiac Patches: Evaluating Recellularization of Decellularized Matrix. J. Tissue Eng. 2020, 11, 2041731420921482. [Google Scholar] [CrossRef]
- Singelyn, J.M.; Christman, K.L. Modulation of Material Properties of a Decellularized Myocardial Matrix Scaffold. Macromol. Biosci. 2011, 11, 731–738. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.T.; Jia, H.; Swim, M.M.; Iacobazzi, D.; Albertario, A.; Zebele, C.; Holopherne-Doran, D.; Hollander, A.; Madeddu, P.; Caputo, M. Reconstruction of the Pulmonary Artery by a Novel Biodegradable Conduit Engineered with Perinatal Stem Cell-Derived Vascular Smooth Muscle Cells Enables Physiological Vascular Growth in a Large Animal Model of Congenital Heart Disease. Biomaterials 2019, 217, 119284. [Google Scholar] [CrossRef] [PubMed]
- Niwaya, K.; Knott-Craig, C.J.; Santangelo, K.; Lane, M.M.; Chandrasekaran, K.; Elkins, R.C. Advantage of Autograft and Homograft Valve Replacement for Complex Aortic Valve endocarditis. Ann. Thorac. Surg. 1999, 67, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Fabian, O.; Havova, M.; Gebauer, R.; Poruban, R.; Spatenka, J.; Burkert, J.; Rohn, V.; Chaloupecky, V.; Komarek, A.; Kala, T.; et al. Structural Integrity and Cellular Viability of Cryopreserved Allograft Heart Valves in Right Ventricular Outflow Tract Reconstruction: Correlation of Histopathological Changes with Donor Characteristics and Preservation Times. Braz. J. Cardiovasc. Surg. 2022, 37, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bao, M.; Nie, Y. Extracellular Matrix–Based Biomaterials for Cardiac Regeneration and Repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jang, J. 3D Bioprinting and Decellularized ECM-Based Biomaterials for in vitro CV Tissue Engineering. J. 3D Print. Med. 2018, 2, 69–87. [Google Scholar] [CrossRef]
- Kato, N.; Yamagishi, M.; Itatani, K.; Miyazaki, T.; Maeda, Y. Effects of Blood Flow Dynamics on Autologous Pericardial Degeneration in Reconstructed Pulmonary Arteries. Interact Cardiovasc. Thorac. Surg. 2018, 26, 293–300. [Google Scholar] [CrossRef]
- Qian, T.; Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Huang, C.; Wu, Z. Conduits for Right Ventricular Outflow Tract Reconstruction in Infants and Young Children. Front. Surg. 2021, 8, 718940. [Google Scholar] [CrossRef]
- Moroi, M.K.; Bacha, E.A.; Kalfa, D.M. The Ross Procedure in Children: A Systematic Review. Ann. Cardiothorac. Surg. 2021, 10, 420–432. [Google Scholar] [CrossRef]
- van den Eynde, J.; Michel Pompeu, S.; Callahan, C.P.; Dimagli, A.; Vervoort, D.; Kampaktsis, P.N.; Zhigalov, K.; Ruhparwar, A.; Weymann, A. Right Ventricular Outflow Tract Reconstruction with Medtronic Freestyle Valve in the Ross Procedure: A Systematic Review with Meta-Analysis. Artif. Organs 2021, 45, 338–345. [Google Scholar] [CrossRef]
- Murala, J.S.K.; Vela, R.J.; Geoffrion, T.; Chopra, S.; Guhathakurtha, S.; Pezzella, T.; Cherian, K.M. Right Ventricular Outflow Tract Obstruction: A Quest for Ideal Management. Asian Cardiovasc. Thorac. Ann. 2018, 26, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dunne, B.; Suthers, E.; Xiao, P.; Xiao, J.; Litton, E.; Andrews, D. The Freestyle Valve as a Right Ventricle to Pulmonary Artery Conduit. A Systematic Review and Meta-Analysis. Heart Lung Vessel. 2015, 7, 304–310. [Google Scholar]
- Sharabiani, M.T.A.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Peter Tometzki, A.J.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Sarikouch, S.; Theodoridis, K.; Hilfiker, A.; Boethig, D.; Laufer, G.; Andreas, M.; Cebotari, S.; Tudorache, I.; Bobylev, D.; Neubert, L.; et al. Early Insight Into In Vivo Recellularization of Cell-Free Allogenic Heart Valves. Ann. Thorac. Surg. 2019, 108, 581–589. [Google Scholar] [CrossRef]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A Review on Tissue Engineering, Application, and Storage. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Arrizabalaga, J.H.; Nollert, M.U. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 2226–2236. [Google Scholar] [CrossRef]
- Ryzhuk, V.; Zeng, X.X.; Wang, X.; Melnychuk, V.; Lankford, L.; Farmer, D.; Wang, A. Human Amnion Extracellular Matrix Derived Bioactive Hydrogel for Cell Delivery and Tissue Engineering. Mater. Sci. Eng. C 2018, 85, 191–202. [Google Scholar] [CrossRef]
- Syedain, Z.; Reimer, J.; Lahti, M.; Berry, J.; Johnson, S.; Tranquillo, R.T. Tissue Engineering of Acellular Vascular Grafts Capable of Somatic Growth in Young Lambs. Nat. Commun. 2016, 7, 12951. [Google Scholar] [CrossRef]
- Lintas, V.; Fioretta, E.S.; Motta, S.E.; Dijkman, P.E.; Pensalfini, M.; Mazza, E.; Caliskan, E.; Rodriguez, H.; Lipiski, M.; Sauer, M. Development of a Novel Human Cell-Derived Tissue-Engineered Heart Valve for Transcatheter Aortic Valve Replacement: An in Vitro and in Vivo Feasibility Study. J. Cardiovasc. Transl. Res. 2018, 11, 470–482. [Google Scholar] [CrossRef]
- Sarikouch, S.; Horke, A.; Tudorache, I.; Beerbaum, P.; Westhoff-Bleck, M.; Boethig, D.; Repin, O.; Maniuc, L.; Ciubotaru, A.; Haverich, A.; et al. Decellularized Fresh Homografts for Pulmonary Valve Replacement: A Decade of Clinical Experience. Eur. J. Cardio-Thorac. Surg. 2016, 50, 281–290. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Ahmadi Tafti, S.H.; Mokhber-Dezfooli, M.R.; Khorramirouz, R.; Sabetkish, S.; Sabetkish, N.; Rabbani, S.; Tavana, H.; Mohseni, M.J. Aortic Valve Conduit Implantation in the Descending Thoracic Aorta in a Sheep Model: The Outcomes of Pre-Seeded Scaffold. Int. J. Surg. 2016, 28, 97–105. [Google Scholar] [CrossRef]
- Böer, U.; Schridde, A.; Anssar, M.; Klingenberg, M.; Sarikouch, S.; Dellmann, A.; Harringer, W.; Haverich, A.; Wilhelmi, M. The Immune Response to Crosslinked Tissue Is Reduced in Decellularized Xenogeneic and Absent in Decellularized Allogeneic Heart Valves. Int. J. Artif. Organs 2015, 38, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Voges, I.; Bräsen, J.H.; Entenmann, A.; Scheid, M.; Scheewe, J.; Fischer, G.; Hart, C.; Andrade, A.; Pham, H.M.; Kramer, H.H.; et al. Adverse Results of a Decellularized Tissue-Engineered Pulmonary Valve in Humans Assessed with Magnetic Resonance Imaging. Eur. J. Cardio-Thorac. Surg. 2013, 44, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Spinali, K.L.; Schmuck, E.G. Natural Sources of Extracellular Matrix for Cardiac Repair. In Cardiac Extracellular Matrix; Springer: Berlin/Heidelberg, Germany, 2018; pp. 115–130. [Google Scholar]
- Remlinger, N.T.; Gilbert, T.W.; Yoshida, M.; Guest, B.N.; Hashizume, R.; Weaver, M.L.; Wagner, W.R.; Brown, B.N.; Tobita, K.; Wearden, P.D. Urinary Bladder Matrix Promotes Site Appropriate Tissue Formation Following Right Ventricle Outflow Tract Repair. Organogenesis 2013, 9, 149–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woo, J.S.; Fishbein, M.C.; Reemtsen, B. Histologic Examination of Decellularized Porcine Intestinal Submucosa Extracellular Matrix (CorMatrix) in Pediatric Congenital Heart Surgery. Cardiovasc. Pathol. 2016, 25, 12–17. [Google Scholar] [CrossRef]
- Mosala Nezhad, Z.; Poncelet, A.; de Kerchove, L.; Gianello, P.; Fervaille, C.; El Khoury, G. Small Intestinal Submucosa Extracellular Matrix (CorMatrix®) in Cardiovascular Surgery: A Systematic Review. Interact Cardiovasc. Thorac. Surg. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Geiger, R.; Kilo, J.; Zimpfer, D. Cormatrix® for Vessel Reconstruction in Paediatric Cardiac Surgery—A Word of Caution. Interact Cardiovasc. Thorac. Surg. 2022, 34, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Gębczak, K.; Wiatrak, B.; Fortuna, W. Evaluation of PC12 Cells’ Proliferation, Adhesion and Migration with the Use of an Extracellular Matrix (CorMatrix) for Application in Neural Tissue Engineering. Materials 2021, 14, 3858. [Google Scholar] [CrossRef]
- Quarti, A.; Nardone, S.; Colaneri, M.; Santoro, G.; Pozzi, M. Preliminary Experience in the Use of an Extracellular Matrix to Repair Congenital Heart Diseases. Interact Cardiovasc. Thorac. Surg. 2011, 13, 569–572. [Google Scholar] [CrossRef]
- Witt, R.G.; Raff, G.; van Gundy, J.; Rodgers-Ohlau, M.; Si, M.S. Short-Term Experience of Porcine Small Intestinal Submucosa Patches in Paediatric Cardiovascular Surgery. Eur. J. Cardio-Thorac. Surg. 2013, 44, 72–76. [Google Scholar] [CrossRef]
- Yanagawa, B.; Rao, V.; Yau, T.M.; Cusimano, R.J. Initial Experience with Intraventricular Repair Using CorMatrix Extracellular Matrix. Innovations 2013, 8, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.H.; Nathan, M.; Emani, S.; Baird, C.; Pedro, J.; Gauvreau, K.; Harris, M.; Sanders, S.P.; Padera, R.F. Preliminary Experience with Porcine Intestinal Submucosa (CorMatrix) for Valve Reconstruction in Congenital Heart Disease: Histologic Evaluation of Explanted Valves. J. Thorac. Cardiovasc. Surg. 2014, 148, 2216–2225.e1. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, S.H.; Rodriguez Cetina Biefer, H.; Emmert, M.Y.; Falk, V. Use of Extracellular Matrix Materials in Patients with Endocarditis. Thorac. Cardiovasc. Surg. 2014, 62, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Holubec, T.; Caliskan, E.; Sündermann, S.H.; Starck, C.T.; Plass, A.; Bettex, D.; Falk, V.; Maisano, F. The Use of Extracellular Matrix Patches in Cardiac Surgery. J. Card. Surg. 2015, 30, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ruel, M. The Clinical Application Potential of Extracellular Matrix in Cardiac Tissue Engineering. J. Thorac. Cardiovasc. Surg. 2015, 150, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- al Haddad, E.; LaPar, D.J.; Dayton, J.; Stephens, E.H.; Bacha, E. Complete Atrioventricular Canal Repair with a Decellularized Porcine Small Intestinal Submucosa Patch. Congenit Heart Dis. 2018, 13, 997–1004. [Google Scholar] [CrossRef]
- Nelson, J.S.; Heider, A.; Si, M.S.; Ohye, R.G. Evaluation of Explanted CorMatrix Intracardiac Patches in Children with Congenital Heart Disease. Ann. Thorac. Surg. 2016, 102, 1329–1335. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Lordeus, M.; Mankame, O.V.; Valdes-Cruz, L.; Bibevski, S.; Bell, S.M.; Baez, I.; Scholl, F. Hydrodynamic Assessment of Aortic Valves Prepared from Porcine Small Intestinal Submucosa. Cardiovasc. Eng. Technol. 2017, 8, 30–40. [Google Scholar] [CrossRef]
- Bell, D.; Prabhu, S.; Betts, K.; Justo, R.; Venugopal, P.; Karl, T.R.; Alphonso, N. Durability of Tissue-Engineered Bovine Pericardium (CardioCelVR) for a Minimum of 24 Months When Used for the Repair of Congenital Heart Defects. Interact Cardiovasc. Thorac. Surg. 2019, 28, 284–290. [Google Scholar] [CrossRef]
- Wu, J.; Brazile, B.; McMahan, S.R.; Liao, J.; Hong, Y. Heart Valve Tissue-Derived Hydrogels: Preparation and Characterization of Mitral Valve Chordae, Aortic Valve, and Mitral Valve Gels. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1732–1740. [Google Scholar] [CrossRef]
- Iyer, K.S. The Contegra Bovine Jugular Valved Conduit: Living up to Expectations? Ann. Pediatr. Cardiol. 2012, 5, 34–35. [Google Scholar]
- Holmes, A.A.; Co, S.; Human, D.G.; LeBlanc, J.G.; Campbell, A.I. The Contegra Conduit: Late Outcomes in Right Ventricular Outflow Tract Reconstruction. Ann. Pediatr. Card. 2012, 5, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.F.; Qanadli, S.D.; Sekarski, N.; Artemisia, S.; Hurni, M.; Tozzi, P.; Von Segesser, L.K. Bovine Valved Xenograft in Pulmonary Position: Medium-Term Follow-up with Excellent Hemodynamics and Freedom from Calcification. Ann. Thorac. Surg. 2004, 78, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Breymann, T.; Boethig, D.; Goerg, R.; Thies, W.R. The Contegra Bovine Valved Jugular Vein Conduit for Pediatric RVOT Reconstruction: 4 Years Experience with 108 Patients. J. Card. Surg. 2004, 19, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Boethig, D.; Thies, W.R.; Hecker, H.; Breymann, T. Mid Term Course after Pediatric Right Ventricular Outflow Tract Reconstruction: A Comparison of Homografts, Porcine Xenografts and Contegras. Eur. J. Cardio-Thorac. Surg. 2005, 27, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Meyns, B.; van Garsse, L.; Boshoff, D.; Eyskens, B.; Mertens, L.; Gewillig, M.; Fieuws, S.; Verbeken, E.; Daenen, W. The Contegra Conduit in the Right Ventricular Outflow Tract Induces Supravalvular Stenosis. J. Thorac. Cardiovasc. Surg. 2004, 128, 834–840. [Google Scholar] [CrossRef][Green Version]
- Göber, V.; Berdat, P.; Pavlovic, M.; Pfammatter, J.P.; Carrel, T.P. Adverse Mid-Term Outcome Following RVOT Reconstruction Using the Contegra Valved Bovine Jugular Vein. Ann. Thorac. Surg. 2005, 79, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Grabenwöger, M.; Sider, J.; Fitzal, F.; Zelenka, C.; Windberger, U.; Grimm, M.; Moritz, A.; Böck, P.; Wolner, E. Impact of Glutaraldehyde on Calcification of Pericardial Bioprosthetic Heart Valve Material. Ann. Thorac. Surg. 1996, 62, 772–777. [Google Scholar] [CrossRef]
- Watanabe, T.; Kanda, K.; Ishibashi-Ueda, H.; Yaku, H.; Nakayama, Y. Development of Biotube Vascular Grafts Incorporating Cuffs for Easy Implantation. J. Artif. Organs 2007, 10, 10–15. [Google Scholar] [CrossRef]
- Watanabe, T.; Kanda, K.; Ishibashi-Ueda, H.; Yaku, H.; Nakayama, Y. Autologous Small-Caliber “Biotube” Vascular Grafts with Argatroban Loading: A Histomorphological Examination after Implantation to Rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 236–242. [Google Scholar] [CrossRef]
- Yamanami, M.; Yahata, Y.; Uechi, M.; Fujiwara, M.; Ishibashi-Ueda, H.; Kanda, K.; Watanabe, T.; Tajikawa, T.; Ohba, K.; Yaku, H.; et al. Development of a Completely Autologous Valved Conduit with the Sinus of Valsalva Using In-Body Tissue Architecture Technology: A Pilot Study in Pulmonary Valve Replacement in a Beagle Model. Circulation 2010, 122, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Swim, M.M.; Albertario, A.; Iacobazzi, D.; Caputo, M.; Ghorbel, M.T. Amnion-Based Scaffold with Enhanced Strength and Biocompatibility for in Vivo Vascular Repair. Tissue Eng. Part A 2019, 25, 603–619. [Google Scholar] [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D Bioprinting Using Stem Cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Rajamarthandan, S.; Francis, B.; O’Leary-Kelly, M.K.; Sinha, P. Update on Stem Cell Technologies in Congenital Heart Disease. J. Card. Surg. 2020, 35, 174–179. [Google Scholar] [CrossRef]
- Zazzeroni, L.; Lanzoni, G.; Pasquinelli, G.; Ricordi, C. Considerations on the Harvesting Site and Donor Derivation for Mesenchymal Stem Cells-Based Strategies for Diabetes. CellR4 Repair Replace Regen Reprogram 2017, 5, e2435. [Google Scholar]
- Latifi, N.; Lecce, M.; Simmons, C.A. Porcine Umbilical Cord Perivascular Cells for Preclinical Testing of Tissue-Engineered Heart Valves. Tissue Eng. Part C Methods 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Iacobazzi, D.; Rapetto, F.; Albertario, A.; Swim, M.M.; Narayan, S.; Skeffington, K.; Salih, T.; Alvino, V.V.; Madeddu, P.; Ghorbel, M.T.; et al. Wharton’s Jelly-Mesenchymal Stem Cell-Engineered Conduit for Pediatric Translation in Heart Defect. Tissue Eng. Part A 2021, 27, 201–213. [Google Scholar] [CrossRef]
- Tigerprints, T.; Hoskins, E. Tissue-Engineered Living Pulmonary Valve for Young Adult Tissue-Engineered Living Pulmonary Valve for Young Adult Patients Patients. Master’s Thesis, Clemson University, Clemson, SC, USA, 2022. [Google Scholar]
- Cebotari, S.; Lichtenberg, A.; Tudorache, I.; Hilfiker, A.; Mertsching, H.; Leyh, R.; Breymann, T.; Kallenbach, K.; Maniuc, L.; Batrinac, A. Clinical Application of Tissue Engineered Human Heart Valves Using Autologous Progenitor Cells. Circulation 2006, 114, I-132–I-137. [Google Scholar] [CrossRef]
- Park, J.; Anderson, C.W.; Sewanan, L.R.; Kural, M.H.; Huang, Y.; Luo, J.; Gui, L.; Riaz, M.; Lopez, C.A.; Ng, R. Modular Design of a Tissue Engineered Pulsatile Conduit Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Acta Biomater. 2020, 102, 220–230. [Google Scholar] [CrossRef]
- Woods, I.; Black, A.; Molloy, E.J.; Jockenhoevel, S.; Flanagan, T.C. Fabrication of Blood-Derived Elastogenic Vascular Grafts Using Electrospun Fibrinogen and Polycaprolactone Composite Scaffolds for Paediatric Applications. J. Tissue Eng. Regen. Med. 2020, 14, 1281–1295. [Google Scholar] [CrossRef]
- Bracaglia, L.G.; Messina, M.; Winston, S.; Kuo, C.Y.; Lerman, M.; Fisher, J.P. 3D Printed Pericardium Hydrogels to Promote Wound Healing in Vascular Applications. Biomacromolecules 2017, 18, 3802–3811. [Google Scholar] [CrossRef]
- Zhao, L.M.; Wang, L.; Zhang, W.Q.; Wang, R.; Zhang, X.Z.; Lei, X.X.; Liang, Y.; Song, Y.T.; Zhang, Q.Y.; Lin, K.; et al. Promotion of Right Ventricular Outflow Tract Reconstruction Using a Novel Cardiac Patch Incorporated with Hypoxia-Pretreated Urine-Derived Stem Cells. Bioact. Mater. 2022, 14, 206–218. [Google Scholar] [CrossRef]
- Wang, J.N.; Kan, C.D.; Lin, S.H.; Chang, K.C.; Tsao, S.; Wong, T.W. Potential of Autologous Progenitor Cells and Decellularized Porcine Artery Matrix in Construction of Tissue-Engineered Vascular Grafts. Organogenesis 2021, 17, 72–84. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Tan, R.; Cui, J.; Schmull, S. Human-Derived Decellularized Extracellular Matrix Scaffold Incorporating Autologous Bone Marrow Stem Cells from Patients with Congenital Heart Disease for Cardiac Tissue Engineering. Biomed. Mater. Eng. 2022, 33, 407–421. [Google Scholar] [CrossRef]
- Namiri, M.; Kazemi Ashtiani, M.; Abbasalizadeh, S.; Mazidi, Z.; Mahmoudi, E.; Nikeghbalian, S.; Aghdami, N.; Baharvand, H. Improving the Biological Function of Decellularized Heart Valves through Integration of Protein Tethering and Three-Dimensional Cell Seeding in a Bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, e1865–e1879. [Google Scholar] [CrossRef]
- Rapetto, F.; Iacobazzi, D.; Narayan, S.A.; Skeffington, K.; Salih, T.; Mostafa, S.; Alvino, V.V.; Upex, A.; Madeddu, P.; Ghorbel, M.T.; et al. Wharton’s Jelly–Mesenchymal Stem Cell–Engineered Conduit for Pulmonary Artery Reconstruction in Growing Piglets. JACC Basic Transl. Sci. 2022, 7, 207–219. [Google Scholar] [CrossRef]
- Kang, K.; Chuai, J.B.; Xie, B.D.; Li, J.Z.; Qu, H.; Wu, H.; Fang, S.H.; Cui, J.J.; Xiu, L.L.; Han, J.C.; et al. Mesenchymal Stromal Cells from Patients with Cyanotic Congenital Heart Disease Are Optimal Candidate for Cardiac Tissue Engineering. Biomaterials 2020, 230, 119574. [Google Scholar] [CrossRef]
- Saito, J.; Yokoyama, U.; Takayama, T.; Ito, H.; Tadokoro, T.; Sugo, Y.; Kurasawa, K.; Ogawa, M.; Miyagi, E.; Taniguchi, H.; et al. Fabrication of Implantable Human Arterial Graft by Periodic Hydrostatic Pressure. In Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension; Springer: Singapore, 2020; pp. 289–291. ISBN 9789811511851. [Google Scholar]
- Ramasamy, S.; Davoodi, P.; Vijayavenkataraman, S.; Teoh, J.H.; Thamizhchelvan, A.M.; Robinson, K.S.; Wu, B.; Fuh, J.Y.H.; DiColandrea, T.; Zhao, H.; et al. Optimized Construction of a Full Thickness Human Skin Equivalent Using 3D Bioprinting and a PCL/Collagen Dermal Scaffold. Bioprinting 2021, 21, e00123. [Google Scholar] [CrossRef]
- Patel, N.M.; Yazdi, I.K.; Tasciotti, E.; Birla, R.K. Optimizing Cell Seeding and Retention in a Three-Dimensional Bioengineered Cardiac Ventricle: The Two-Stage Cellularization Model. Biotechnol. Bioeng. 2016, 113, 2275–2285. [Google Scholar] [CrossRef]
- Patel, N.M.; Birla, R.K. The Bioengineered Cardiac Left Ventricle. ASAIO J. 2018, 64, 56–62. [Google Scholar] [CrossRef]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current Challenges in Three-Dimensional Bioprinting Heart Tissues for Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Satchell, S.C.; Shah, R.N.; Wertheim, J.A. Kidney Decellularized Extracellular Matrix Hydrogels: Rheological Characterization and Human Glomerular Endothelial Cell Response to Encapsulation. J. Biomed. Mater. Res. A 2018, 106, 2448–2462. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of Heterogeneous Aortic Valve Conduits with Alginate/Gelatin Hydrogels. J. Biomed. Mater. Res. A 2013, 101A, 1255–1264. [Google Scholar] [CrossRef]
- Nachlas, A.L.Y.; Li, S.; Streeter, B.W.; de Jesus Morales, K.J.; Sulejmani, F.; Madukauwa-David, D.I.; Bejleri, D.; Sun, W.; Yoganathan, A.P.; Davis, M.E. A Multilayered Valve Leaflet Promotes Cell-Laden Collagen Type I Production and Aortic Valve Hemodynamics. Biomaterials 2020, 240, 119838. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-Dimensional Printed Trileaflet Valve Conduits Using Biological Hydrogels and Human Valve Interstitial Cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef]
- Lui, C.; Chin, A.F.; Park, S.; Yeung, E.; Kwon, C.; Tomaselli, G.; Chen, Y.; Hibino, N. Mechanical Stimulation Enhances Development of Scaffold-Free, 3D-Printed, Engineered Heart Tissue Grafts. J. Tissue Eng. Regen. Med. 2021, 15, 503–512. [Google Scholar] [CrossRef]
- Kawai, Y.; Tohyama, S.; Arai, K.; Tamura, T.; Soma, Y.; Fukuda, K.; Shimizu, H.; Nakayama, K.; Kobayashi, E. Scaffold-Free Tubular Engineered Heart Tissue from Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in Vivo. Front. Cardiovasc. Med. 2022, 8, 806215. [Google Scholar] [CrossRef]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.H.; Paul, A. Stem Cell-Inspired Secretome-Rich Injectable Hydrogel to Repair Injured Cardiac Tissue. Acta Biomater. 2018, 69, 95–106. [Google Scholar] [CrossRef]
- Huang, N.F.; Serpooshan, V.; Morris, V.B.; Sayed, N.; Pardon, G.; Abilez, O.J.; Nakayama, K.H.; Pruitt, B.L.; Wu, S.M.; Yoon, Y. Big Bottlenecks in Cardiovascular Tissue Engineering. Commun. Biol. 2018, 1, 199. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Anderson, C.; Breuer, C.K. The Potential Role of Regenerative Medicine on the Future Management of Hypoplastic Left Heart Syndrome. J. Cardiovasc. Dev. Dis. 2022, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Dzilic, E.; Doppler, S.; Lange, R.; Krane, M. Regenerative Medicine for the Treatment of Congenital Heart Disease. In Cardiovascular Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 207–221. [Google Scholar]
- Emmert, M.Y.; Fioretta, E.S.; Hoerstrup, S.P. Translational Challenges in Cardiovascular Tissue Engineering. J. Cardiovasc. Transl. Res. 2017, 10, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tang-Quan, K.R.; Mehta, N.A.; Sampaio, L.C.; Taylor, D.A. Whole Cardiac Tissue Bioscaffolds BT. In Cardiac Extracellular Matrix: Fundamental Science to Clinical Applications; Schmuck, E.G., Hematti, P., Raval, A.N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 85–114. ISBN 978-3-319-97421-7. [Google Scholar]
- Reardon, S. First Pig-to-Human Heart Transplant: What Can Scientists Learn? Nature 2022, 601, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, W.; Ruan, Y.; Geng, Q. First Pig-to-Human Heart Transplantation. Innov. 2022, 3, 100223. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, D.K.; Vanderslice, E.J.; VeDepo, M.C.; Jacot, J.G. Engineering Myocardium for Heart Regeneration—Advancements, Considerations, and Future Directions. Front. Cardiovasc. Med. 2020, 7, 586261. [Google Scholar] [CrossRef]
- Heydrick, S.; Roberts, E.; Kim, J.; Emani, S.; Wong, J.Y. Pediatric Cardiovascular Grafts: Historical Perspective and Future Directions. Curr. Opin. Biotechnol. 2016, 40, 119–124. [Google Scholar] [CrossRef]
- Tomov, M.L.; Cetnar, A.; Do, K.; Bauser-Heaton, H.; Serpooshan, V. Patient-specific 3-dimensional–Bioprinted Model for in Vitro Analysis and Treatment Planning of Pulmonary Artery Atresia in Tetralogy of Fallot and Major Aortopulmonary Collateral Arteries. J. Am. Heart Assoc. 2019, 8, e014490. [Google Scholar] [CrossRef]
- Boyd, R.; Parisi, F.; Kalfa, D. State of the Art: Tissue Engineering in Congenital Heart Surgery. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 807–817. [Google Scholar] [CrossRef]


| Advantages | Disadvantages | |
|---|---|---|
| Homografts |
|
|
| Xenografts |
|
|
| Acellular |
|
|
| Recellularised |
|
|
| Advantages | Disadvantages | |
|---|---|---|
| Manual Seeding |
|
|
| Cell Injection |
|
|
| 3D bioprinting |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, A.G.; Salih, T.; Ghorbel, M.T.; Caputo, M.; Biglino, G.; Carrabba, M. Biological Scaffolds for Congenital Heart Disease. Bioengineering 2023, 10, 57. https://doi.org/10.3390/bioengineering10010057
Harris AG, Salih T, Ghorbel MT, Caputo M, Biglino G, Carrabba M. Biological Scaffolds for Congenital Heart Disease. Bioengineering. 2023; 10(1):57. https://doi.org/10.3390/bioengineering10010057
Chicago/Turabian StyleHarris, Amy G., Tasneem Salih, Mohamed T. Ghorbel, Massimo Caputo, Giovanni Biglino, and Michele Carrabba. 2023. "Biological Scaffolds for Congenital Heart Disease" Bioengineering 10, no. 1: 57. https://doi.org/10.3390/bioengineering10010057
APA StyleHarris, A. G., Salih, T., Ghorbel, M. T., Caputo, M., Biglino, G., & Carrabba, M. (2023). Biological Scaffolds for Congenital Heart Disease. Bioengineering, 10(1), 57. https://doi.org/10.3390/bioengineering10010057








