Evaluating the Performance of a Nonelectronic, Versatile Oxygenating Perfusion System across Viscosities Representative of Clinical Perfusion Solutions Used for Organ Preservation
Abstract
1. Introduction
2. Materials and Methods
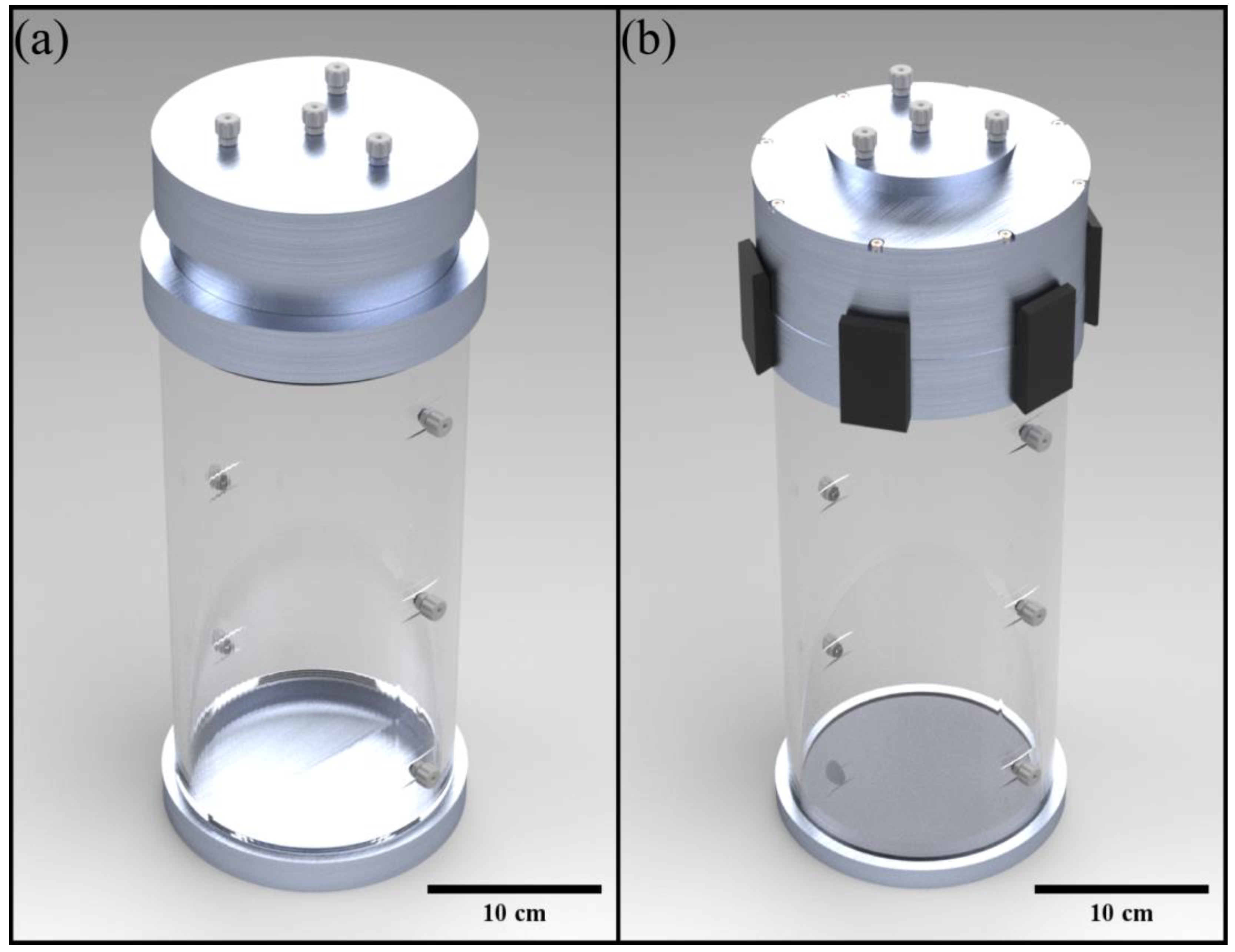
2.1. VOPS Device Design
2.2. VOPS Device Operation and Experimental Setup
2.3. Viscosity Theory and Criterion Determination
2.4. Determining Tuned Pulse Rates
2.5. Test Matrix for VOPS Characterization
3. Results
3.1. Current Device Performance
3.2. Calcium Chloride Dihydrate Viscosity Range
3.3. Current Device Performance
3.3.1. Baseline Data
3.3.2. Data with Tuned Parameters
3.3.3. Study Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1st Order | 2nd Order | 3rd Order | 4th Order | 5th Order | |
|---|---|---|---|---|---|
| b5 | 2373.786 | ||||
| b4 | 698.1803 | −1561.11 | |||
| b3 | 215.5589 | −347.005 | 394.9193 | ||
| b2 | 52.69206 | −76.6822 | 65.46186 | −33.4869 | |
| b1 | 11.27494 | −8.927 | 10.24144 | −1.74879 | 3.106763 |
| b0 | 0.429868 | 1.32782 | 0.84621 | 1.063192 | 1.001567 |
| R Square | 0.7783 | 0.9693 | 0.9971 | 0.9996 | 0.9998 |
| RSME | 0.8483 | 0.3377 | 0.112 | 0.0432 | 0.039 |
References
- U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network, National Data, 2021. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (accessed on 17 August 2022).
- Saidi, R.F.; Hejazii Kenari, S.K. Challenges of organ shortage for transplantation: Solutions and opportunities. Int. J. Organ. Transplant Med. 2014, 5, 87–96. [Google Scholar]
- Watson, C.J.E.; Dark, J.H. Organ transplantation: Historical perspective and current practice. BJA Br. J. Anaesth. 2012, 108 (Suppl. S1), i29–i42. [Google Scholar] [CrossRef] [PubMed]
- Reese, P.P.; Harhay, M.N.; Abt, P.L.; Levine, M.H.; Halpern, S.D. New solutions to reduce discard of kidneys donated for transplantation. J. Am. Soc. Nephrol. 2016, 27, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Skeans, M.A.; Noreen, S.M.; Robinson, A.M.; Miller, E.; Snyder, J.J.; Israni, A.K. 0PTN/SRTR 2017 Annual Data Report: Liver. Am. J. Transplant. 2019, 19 (Suppl. S2), 184–283. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.J.; Harper, S.J.; Saeb-Parsy, K.; Hudson, A.; Gibbs, P.; Watson, C.J.; Praseedom, R.K.; Butler, A.J.; Pettigrew, G.J.; Bradley, J.A. The discard of deceased donor kidneys in the UK. Clin. Transplant. 2014, 28, 345–353. [Google Scholar] [CrossRef]
- Tuttle-Newhall, J.E.; Krishnan, S.M.; Levy, M.F.; McBride, V.; Orlowski, J.P.; Sung, R.S. Organ donation and utilization in the United States: 1998–2007. Am. J. Transplant. 2009, 9, 879–893. [Google Scholar] [CrossRef]
- Volk, M.L.; Reichert, H.A.; Lok, A.S.; Hayward, R.A. Variation in organ quality between liver transplant centers. Am. J. Transplant. 2011, 11, 958–964. [Google Scholar] [CrossRef]
- Olschewski, P.; Gass, P.; Ariyakhagorn, V.; Jasse, K.; Hunold, G.; Menzel, M.; Schöning, W.; Schmitz, V.; Neuhaus, P.; Puhl, G. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology 2010, 60, 337–343. [Google Scholar] [CrossRef]
- Salehi, P.; Mirbolooki, M.; Kin, T.; Tsujimura, T.; Shapiro, A.M.; Churchill, T.A.; Lakey, J.R. Ameliorating injury during preservation and isolation of human islets using the two-layer method with perfluorocarbon and UW solution. Cell Transplant. 2006, 15, 187–194. [Google Scholar] [CrossRef]
- van Suylen, V.; Vandendriessche, K.; Neyrinck, A.; Nijhuis, F.; van der Plaats, A.; Verbeken, E.K.; Vermeersch, P.; Meyns, B.; Mariani, M.A.; Rega, F.; et al. Oxygenated machine perfusion at room temperature as an alternative for static cold storage in porcine donor hearts. Artif. Organs 2022, 46, 246–258. [Google Scholar] [CrossRef]
- Hamaoui, K.; Gowers, S.; Damji, S.; Rogers, M.; Leong, C.L.; Hanna, G.; Darzi, A.; Boutelle, M.; Papalois, V. Rapid sampling microdialysis as a novel tool for parenchyma assessment during static cold storage and hypothermic machine perfusion in a translational ex vivo porcine kidney model. J. Surg. Res. 2016, 200, 332–345. [Google Scholar] [CrossRef]
- Bathini, V.; McGregor, T.; McAlister, V.C.; Luke, P.P.; Sener, A. Renal Perfusion Pump Vs Cold Storage for Donation After Cardiac Death Kidneys: A Systematic Review. J. Urol. 2013, 189, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Büyük, B.; Demirci, T.; Adalı, Y.; Eroğlu, H.A. A new organ preservation solution for static cold storage of the liver. Amniotic fluid. Acta Cir. Bras. 2019, 34, e201900402. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Su, L.; Jiang, S.J. Recipient-Related Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e92773. [Google Scholar] [CrossRef] [PubMed]
- Kargar, B.; Pishvaee, M.S.; Jahani, H.; Sheu, J.B. Organ transportation and allocation problem under medical uncertainty: A real case study of liver transplantation. Transp. Res. Part E Logist. Transp. Rev. 2020, 134, 101841. [Google Scholar] [CrossRef]
- Xu, J.; Buchwald, J.E.; Martins, P.N. Review of Current Machine Perfusion Therapeutics for Organ Preservation. Transplantation 2020, 104, 1792–1803. [Google Scholar] [CrossRef]
- Rudd, D.M.; Dobson, G.P. Early reperfusion with warm, polarizing adenosine–lidocaine cardioplegia improves functional recovery after 6 hours of cold static storage. J. Thorac. Cardiovasc. Surg. 2011, 141, 1044–1055. [Google Scholar] [CrossRef][Green Version]
- O’Callaghan, J.M.; Morgan, R.D.; Knight, S.R.; Morris, P.J. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br. J. Surg. 2013, 100, 991–1001. [Google Scholar] [CrossRef]
- Sellers, M.T.; Gallichio, M.H.; Hudson, S.L.; Young, C.J.; Bynon, J.S.; Eckhoff, D.E.; Deierhoi, M.H.; Diethelm, A.G.; Thompson, J.A. Improved outcomes in cadaveric renal allografts with pulsatile preservation. Clin. Transplant. 2000, 14, 543–549. [Google Scholar] [CrossRef]
- Wight, J.; Chilcott, J.; Holmes, M.; Brewer, N. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol Assess 2003, 7, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.L.; Hosgood, S.A.; Metcalfe, M.S.; Waller, J.R.; Brook, N.R. A Comparison of Renal Preservation by Cold Storage and Machine Perfusion Using a Porcine Autotransplant Model. Transplantation 2004, 78, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Nath, J.; Hodson, J.; Inston, N.; Ready, A. Outcomes of donation after circulatory death kidneys undergoing hypothermic machine perfusion following static cold storage: A UK population-based cohort study. Am. J. Transplant. 2018, 18, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am. J. Transplant. 2016, 16, 3282–3285. [Google Scholar] [CrossRef]
- Portillo, D.J.; Bayliss, L.; Rivas, S.; Pineda, G.; Kaur, S.; Bunegin, L.; Hood, R.L. Characterizing and Tuning Perfusion Parameters Within an Innovative, Versatile Oxygenating Perfusion System. Ann. Biomed. Eng. 2021, 49, 3154–3164. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Neyrinck, A.; Rega, F.; Devos, T.; Pirenne, J. Machine perfusion in organ transplantation: A tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr. Opin. Organ Transplant. 2013, 18, 24–33. [Google Scholar] [CrossRef]
- Salehi, S.; Tran, K.; Grayson, W.L. Advances in Perfusion Systems for Solid Organ Preservation. Yale J. Biol. Med. 2018, 91, 301–312. [Google Scholar]
- Latchana, N.; Peck, J.R.; Whitson, B.A.; Henry, M.L.; Elkhammas, E.A.; Black, S.M. Preservation solutions used during abdominal transplantation: Current status and outcomes. World J. Transplant. 2015, 5, 154–164. [Google Scholar] [CrossRef]
- Mosbah, I.B.; Franco-Gou, R.; Abdennebi, H.B.; Hernandez, R.; Escolar, G.; Saidane, D.; Rosello-Catafau, J.; Peralta, C. Effects of Polyethylene Glycol and Hydroxyethyl Starch in University of Wisconsin Preservation Solution on Human Red Blood Cell Aggregation and Viscosity. Transplant. Proc. 2006, 38, 1229–1235. [Google Scholar] [CrossRef]
- Morariu, A.M.; Vd Plaats, A.; V Oeveren, W.; A’T Hart, N.A.; Leuvenink, H.G.; Graaff, R.; Ploeg, R.J.; Rakhorst, G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: A risk of impaired graft perfusion in organ procurement? Transplantation 2003, 76, 37–43. [Google Scholar] [CrossRef]
- Yan, Q.; Li, Y.; Yan, J.; Zhao, Y.; Liu, Y.; Liu, S. Effects of luteolin on regulatory proteins and enzymes for myocyte calcium circulation in hypothermic preserved rat heart. Exp. Ther. Med. 2018, 15, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Obradović, D.; Aleksić, N.; Mijatov-Ukropina, L. Standardization of liver dimensions for the local population. Med. Pregl. 1991, 44, 266–268. [Google Scholar] [PubMed]
- Standring, S. Gray’s anatomy: The anatomical basis of clinical practice. Am. J. Neuroradiol. 2005, 26, 2703. [Google Scholar]
- Wolf, D.C. Evaluation of the size, shape, and consistency of the liver. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworth: Boston, MA, USA, 1990. [Google Scholar]
- Hron, P. Hydrophilisation of silicone rubber for medical applications. Polym. Int. 2003, 52, 1531–1539. [Google Scholar] [CrossRef]
- Leblanc, G.; Secco, R.; Kostic, M. Viscosity measurement. In The Measurement, Instrumentation, and Sensors; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- White, F.M.; Majdalani, J. Viscous Fluid Flow; McGraw-Hill: New York, NY, USA, 2006; Volume 3. [Google Scholar]
- Jun Kang, Y.; Yeom, E.; Lee, S.J. A microfluidic device for simultaneous measurement of viscosity and flow rate of blood in a complex fluidic network. Biomicrofluidics 2013, 7, 54111. [Google Scholar] [CrossRef]
- Wei, L.; Hata, K.; Doorschodt, B.M.; Büttner, R.; Minor, T.; Tolba, R.H. Experimental small bowel preservation using Polysol: A new alternative to University of Wisconsin solution, Celsior and histidine-tryptophan-ketoglutarate solution? World J. Gastroenterol. WJG 2007, 13, 3684–3691. [Google Scholar] [CrossRef]
- Panisello Rosello, A.; Teixeira da Silva, R.; Castro, C.; Bardallo, R.G.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Roselló Catafau, J.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef]
- Linares-Cervantes, I.; Kollmann, D.; Goto, T.; Echeverri, J.; Kaths, J.M.; Hamar, M.; Urbanellis, P.; Mazilescu, L.; Rosales, R.; Bruguera, C.; et al. Impact of Different Clinical Perfusates During Normothermic Ex Situ Liver Perfusion on Pig Liver Transplant Outcomes in a DCD Model. Transplant. Direct 2019, 5, e437. [Google Scholar] [CrossRef]
- Ringe, B.; Braun, F.; Moritz, M.; Zeldin, G.; Soriano, H.; Meyers, W. Safety and efficacy of living donor liver preservation with HTK solution. Transplant. Proc. 2005, 37, 316–319. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Koshiba, T.; Nakamura, T.; Tsuruyama, T.; Li, Y.; Bando, T.; Wada, H.; Tanaka, K. ET-Kyoto Solution plus Dibutyryl Cyclic Adenosine Monophosphate is Superior to University of Wisconsin Solution in Rat Liver Preservation. Cell Transplant. 2008, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tarif, H. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Pisano, A. From Tubes and Catheters to the Basis of Hemodynamics: Viscosity and Hagen–Poiseuille Equation. In Physics for Anesthesiologists and Intensivists: From Daily Life to Clinical Practice; Springer International Publishing: Cham, Switzerland, 2021; pp. 89–98. [Google Scholar]
- Portillo, D.J.; Gonzalez, J.; Villarreal, C.; Salazar, S.J.; Fasci, A.; Wearden, B.; Oseghale, J.; Khalil, A.; Perillo, T.; Muenchow, L.; et al. Development and Characterization of a Nonelectronic Versatile Oxygenating Perfusion System for Tissue Preservation. Ann. Biomed. Eng. 2022, 50, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Urbanellis, P.; Hamar, M.; Kaths, J.M.; Kollmann, D.; Linares, I.; Mazilescu, L.; Ganesh, S.; Wiebe, A.; Yip, P.M.; John, R.; et al. Normothermic ex vivo kidney perfusion improves early DCD graft function compared with hypothermic machine perfusion and static cold storage. Transplantation 2020, 104, 947–955. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Lu, Y.B.; Tang, G.H.; Tao, W.Q. Experimental study of microchannel flow for non-Newtonian fluid in the presence of salt. Exp. Therm. Fluid Sci. 2016, 74, 91–99. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, B.; Chen, Y. Investigating slime coating in coal flotation using the rheological properties at low CaCl2 concentrations. Int. J. Coal Prep. Util. 2018, 38, 237–249. [Google Scholar] [CrossRef]
- Barman, P.C.; Kairi, R.R.; Das, A.; Islam, R. An Overview of Non-Newtonian Fluid. Int. J. Appl. Sci. Eng. 2016, 4, 97–101. [Google Scholar] [CrossRef]
- García Raurich, J.; Ruiz, Q.P.; Herrador, A.M. Effect of sodium chloride and calcium chloride on thickeners used in the control of oropharyngeal dysphagia. Nutr. Clínica Y Dietética Hosp. 2019, 39, 63–72. [Google Scholar]
- Kim, J.O.; Bau, H.H. Instrument for simultaneous measurement of density and viscosity. Rev. Sci. Instrum. 1989, 60, 1111–1115. [Google Scholar] [CrossRef]
- Telis, V.R.N.; Telis-Romero, J.; Mazzotti, H.B.; Gabas, A.L. Viscosity of Aqueous Carbohydrate Solutions at Different Temperatures and Concentrations. Int. J. Food Prop. 2007, 10, 185–195. [Google Scholar] [CrossRef]
- Laliberté, M. Model for Calculating the Viscosity of Aqueous Solutions. J. Chem. Eng. Data 2007, 52, 321–335. [Google Scholar] [CrossRef]
- Monbaliu, D.; Brassil, J. Machine perfusion of the liver: Past, present and future. Curr. Opin. Organ Transplant. 2010, 15, 160–166. [Google Scholar] [CrossRef]
- Vaziri, N.; Thuillier, R.; Favreau, F.D.; Eugene, M.; Milin, S.; Chatauret, N.P.; Hauet, T.; Barrou, B. Analysis of machine perfusion benefits in kidney grafts: A preclinical study. J. Transl. Med. 2011, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Lee, S.H.; Korneszczuk, K.; Culberson, C.R.; Southard, J.H.; Berthiaume, F.; Zhang, J.X.; Clemens, M.G.; Lee, C.Y. Improved Preservation of Warm Ischemic Livers by Hypothermic Machine Perfusion with Supplemented University of Wisconsin Solution. J. Investig. Surg. 2008, 21, 83–91. [Google Scholar] [CrossRef]
- Jochmans, I.; Nicholson, M.L.; Hosgood, S.A. Kidney perfusion: Some like it hot others prefer to keep it cool. Curr. Opin. Organ Transplant. 2017, 22, 260–266. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Tatum, R.; O’Malley, T.J.; Bodzin, A.S.; Tchantchaleishvili, V. Machine perfusion of donor organs for transplantation. Artif. Organs 2021, 45, 682–695. [Google Scholar] [CrossRef]




| Preservation Solution | Viscosity | Temperature |
|---|---|---|
| PBS | 1.0 cP [39] | 25 °C |
| Celsior | 1.3 cP [40] | 5 °C |
| IGL-2 | 1.4, 1.7 cP [41,42] | 4 °C, N/A |
| STEEN | 1.5, 4.5 cP [43] | 37, 4 °C |
| HTK | 1.8, 2.0 cP [40,44] | 5 °C |
| Polysol | 1.8 cP [40] | 5 °C |
| BMPS | 2.4, 2.6 cP [41,42] | 4 °C, N/A |
| Blood * | 3.5–5.0 cP [45] | N/A |
| ET-K | 4.0 cP [46] | 4.5 °C |
| UW | 5.5, 5.7, 6.2 cP [40,44,46] | 4.5, 5, 1·°C |
| Viscosity Desired | Mass Percentage Required |
|---|---|
| 1 cP | 0% |
| 3 cP | 31.53% |
| 5 cP | 37.47% |
| 6.5 cP | 39.99% |
| Length of Tubing | Vascular Resistance | Oxygen Pressure | Viscosity |
|---|---|---|---|
| 6.1 m | 0.22 mmHg/mL/min | 27.6 kPa | 1 cP |
| 55.2 kPa | 3 cP | ||
| 82.7 kPa | 5 cP | ||
| 110.3 kPa | 6.5 cP |
| Mean Flow Rate (mL/min) | Difference | Peak Perfusion Pressure (mmHg) | Difference | |
|---|---|---|---|---|
| 27.6 kPa | 3.5 | 45.7% ↑ | 37.2 | 52.2% ↑ |
| 55.2 kPa | 6.8 | 44.1% ↑ | 59.1 | 42.8% ↑ |
| 82.7 kPa | 10.9 | 46.8% ↑ | 86.2 | 45.6% ↑ |
| 110.3 kPa | 11.8 | 28.8% ↑ | 89.9 | 16.1% ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, J.M.; Villarreal, C.; Fasci, A.; Rocco, D.D.; Salazar, S.; Khalil, A.; Wearden, B.; Oseghale, J.; Garcia, M.; Portillo, D.J.; et al. Evaluating the Performance of a Nonelectronic, Versatile Oxygenating Perfusion System across Viscosities Representative of Clinical Perfusion Solutions Used for Organ Preservation. Bioengineering 2023, 10, 2. https://doi.org/10.3390/bioengineering10010002
Gonzalez JM, Villarreal C, Fasci A, Rocco DD, Salazar S, Khalil A, Wearden B, Oseghale J, Garcia M, Portillo DJ, et al. Evaluating the Performance of a Nonelectronic, Versatile Oxygenating Perfusion System across Viscosities Representative of Clinical Perfusion Solutions Used for Organ Preservation. Bioengineering. 2023; 10(1):2. https://doi.org/10.3390/bioengineering10010002
Chicago/Turabian StyleGonzalez, Jose M., Carorina Villarreal, Anjelyka Fasci, David Di Rocco, Sophia Salazar, Anis Khalil, Brandt Wearden, Jessica Oseghale, Mariana Garcia, Daniel J. Portillo, and et al. 2023. "Evaluating the Performance of a Nonelectronic, Versatile Oxygenating Perfusion System across Viscosities Representative of Clinical Perfusion Solutions Used for Organ Preservation" Bioengineering 10, no. 1: 2. https://doi.org/10.3390/bioengineering10010002
APA StyleGonzalez, J. M., Villarreal, C., Fasci, A., Rocco, D. D., Salazar, S., Khalil, A., Wearden, B., Oseghale, J., Garcia, M., Portillo, D. J., & Hood, R. L. (2023). Evaluating the Performance of a Nonelectronic, Versatile Oxygenating Perfusion System across Viscosities Representative of Clinical Perfusion Solutions Used for Organ Preservation. Bioengineering, 10(1), 2. https://doi.org/10.3390/bioengineering10010002






