Application of Tissue Engineering in Manufacturing Absorbable Membranes to Improve the Osteopromoting Potential of Collagen
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Surgery
2.3. Histological and Microtomographic Processing
2.4. Histological and Histometric Analysis
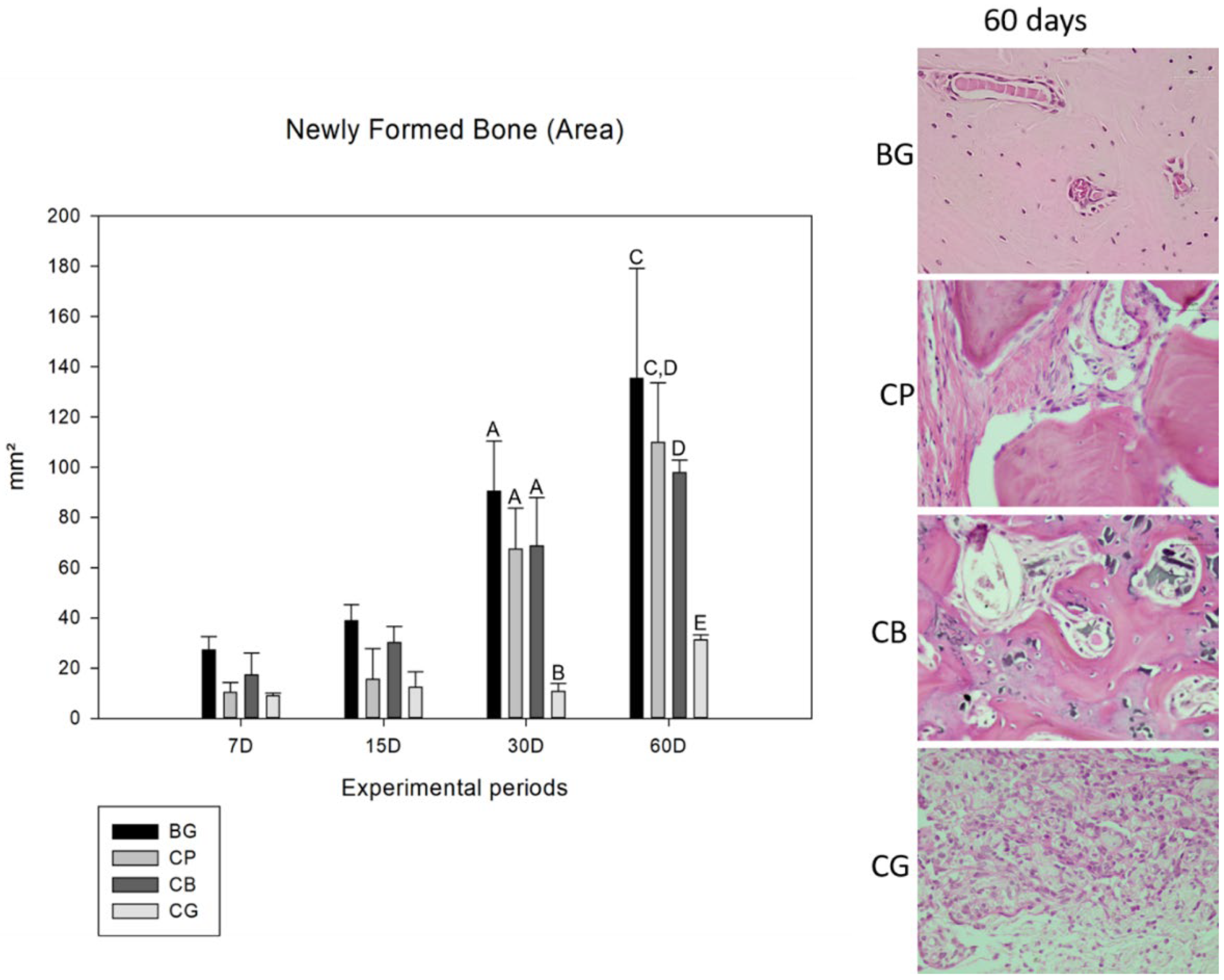
2.4.1. Neoformed Bone Area
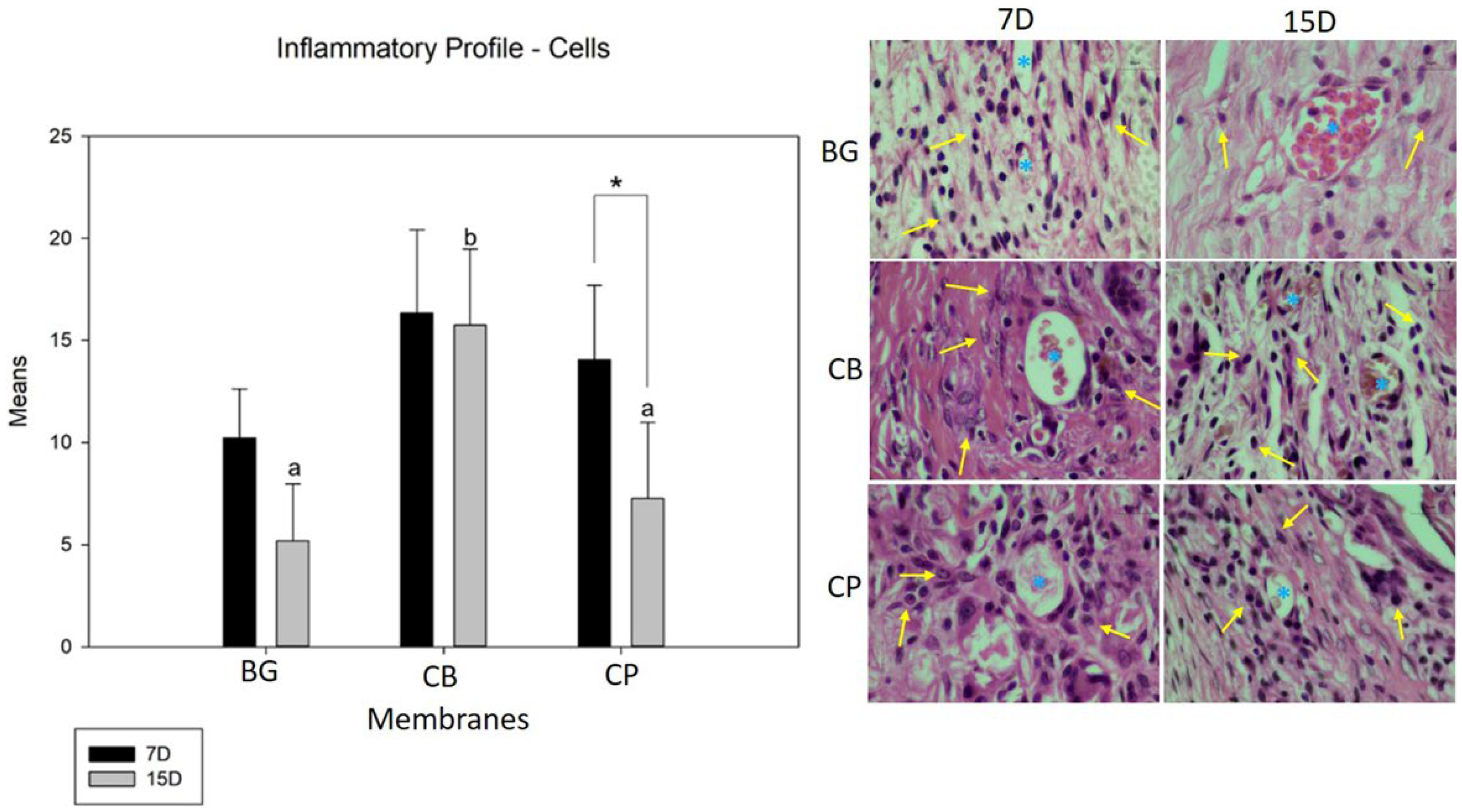
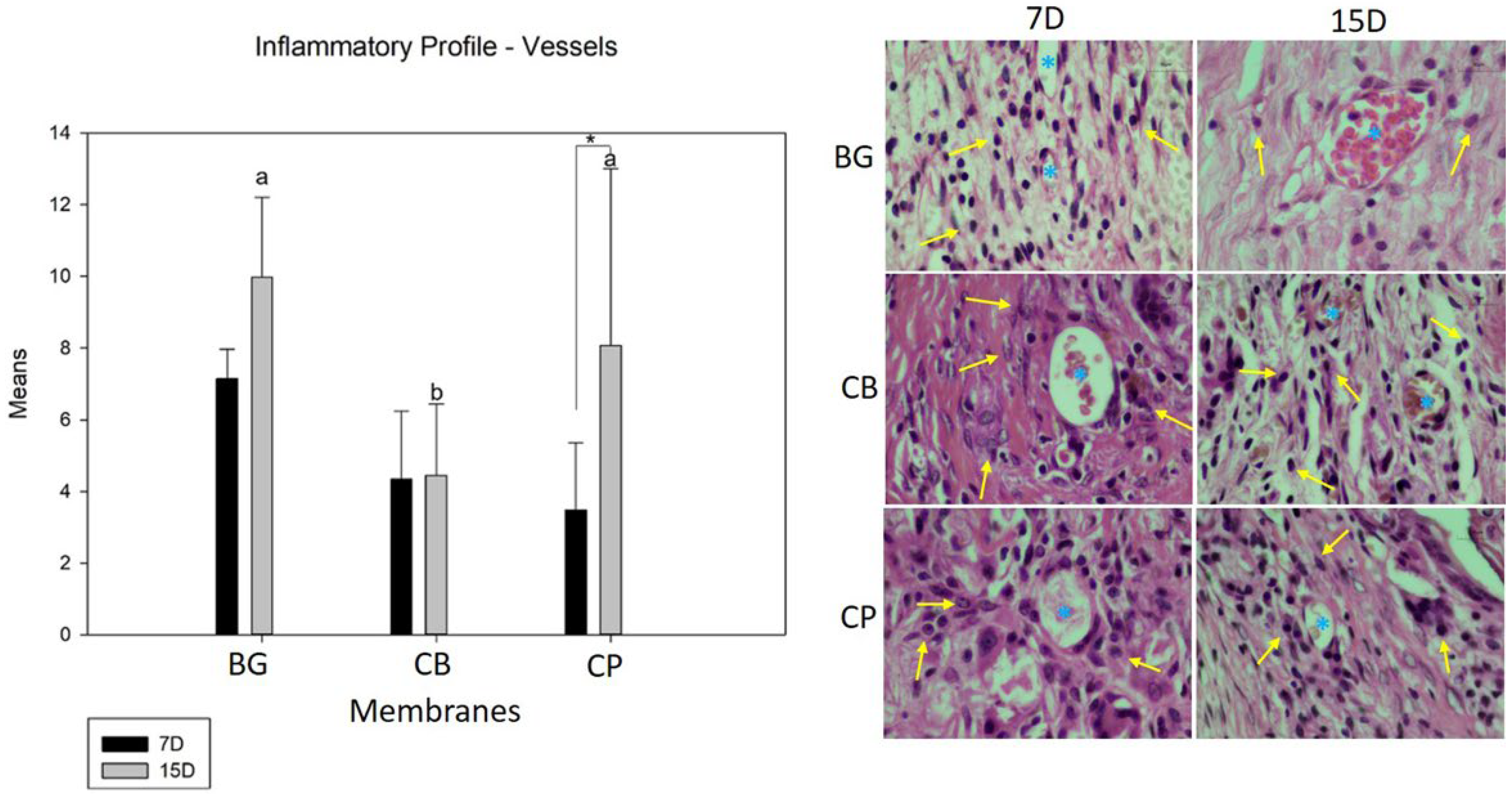
2.4.2. Inflammatory Profile
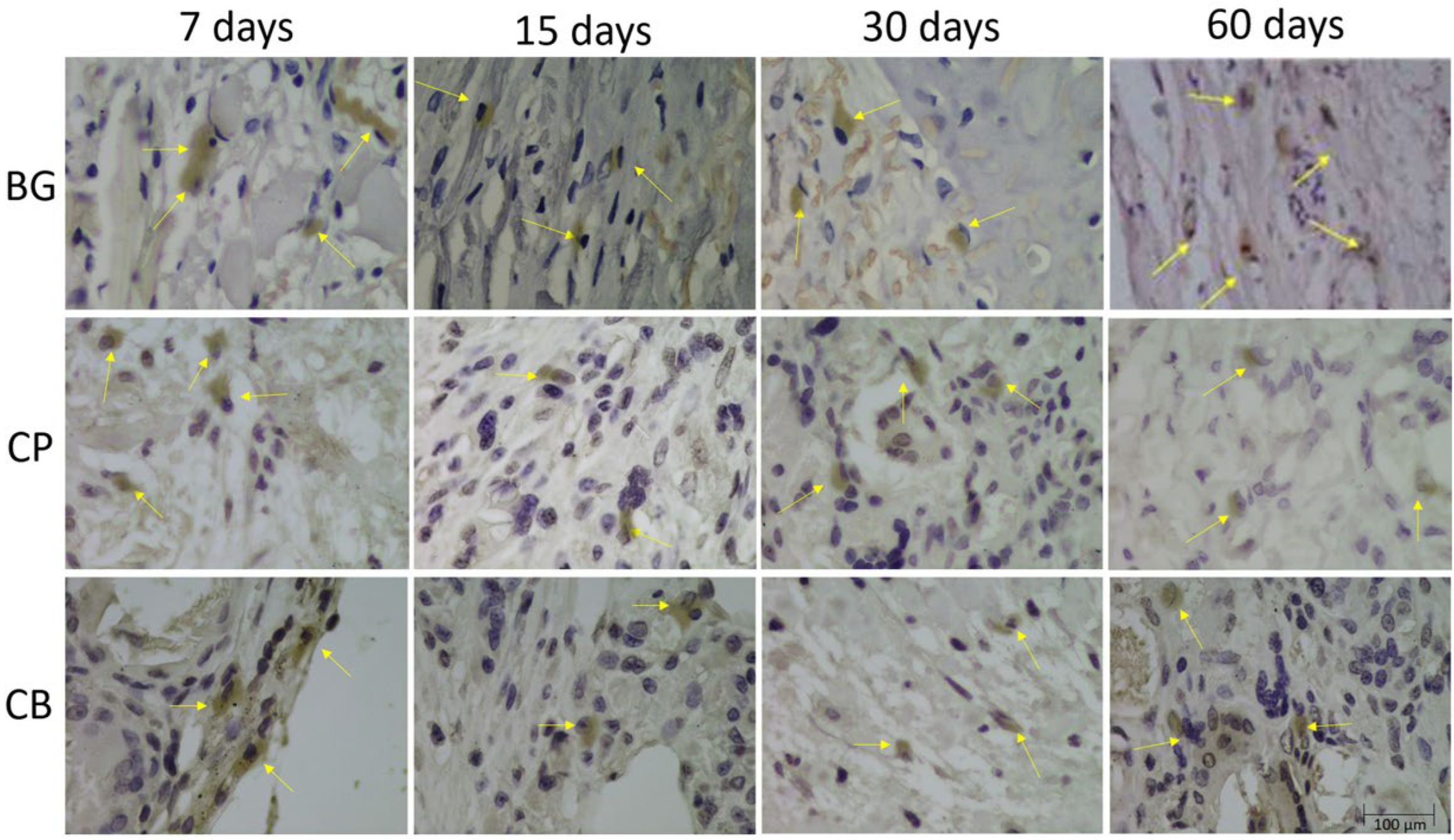
2.5. Immunohistochemical Analysis
2.6. Microtomographic Analysis
2.7. Statistical Analysis
3. Results
3.1. Histological Analysis
3.2. Histometric Analysis
3.3. Inflammatory Profile (Cells and Blood Vessels)
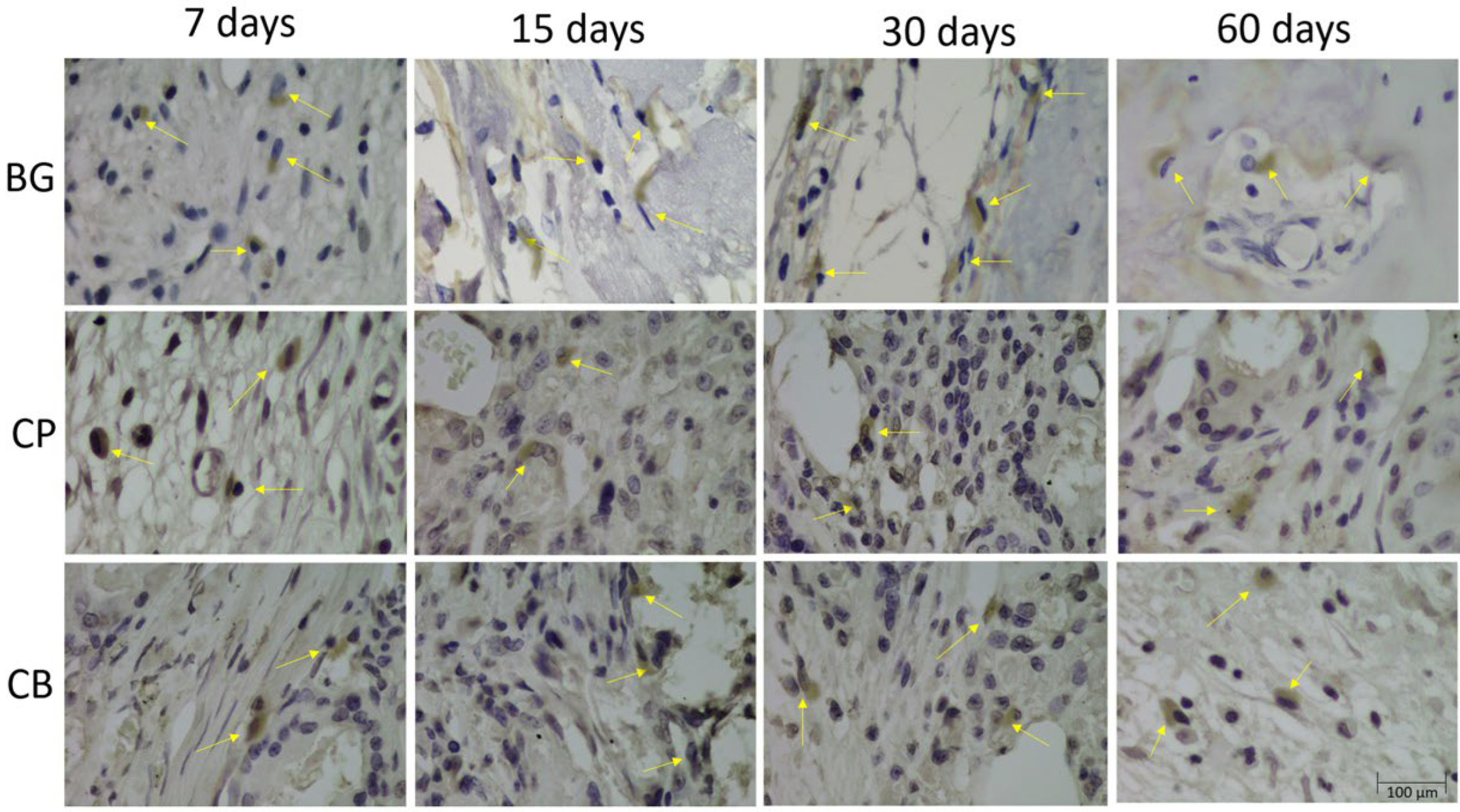
3.4. Immunohistochemical Analysis
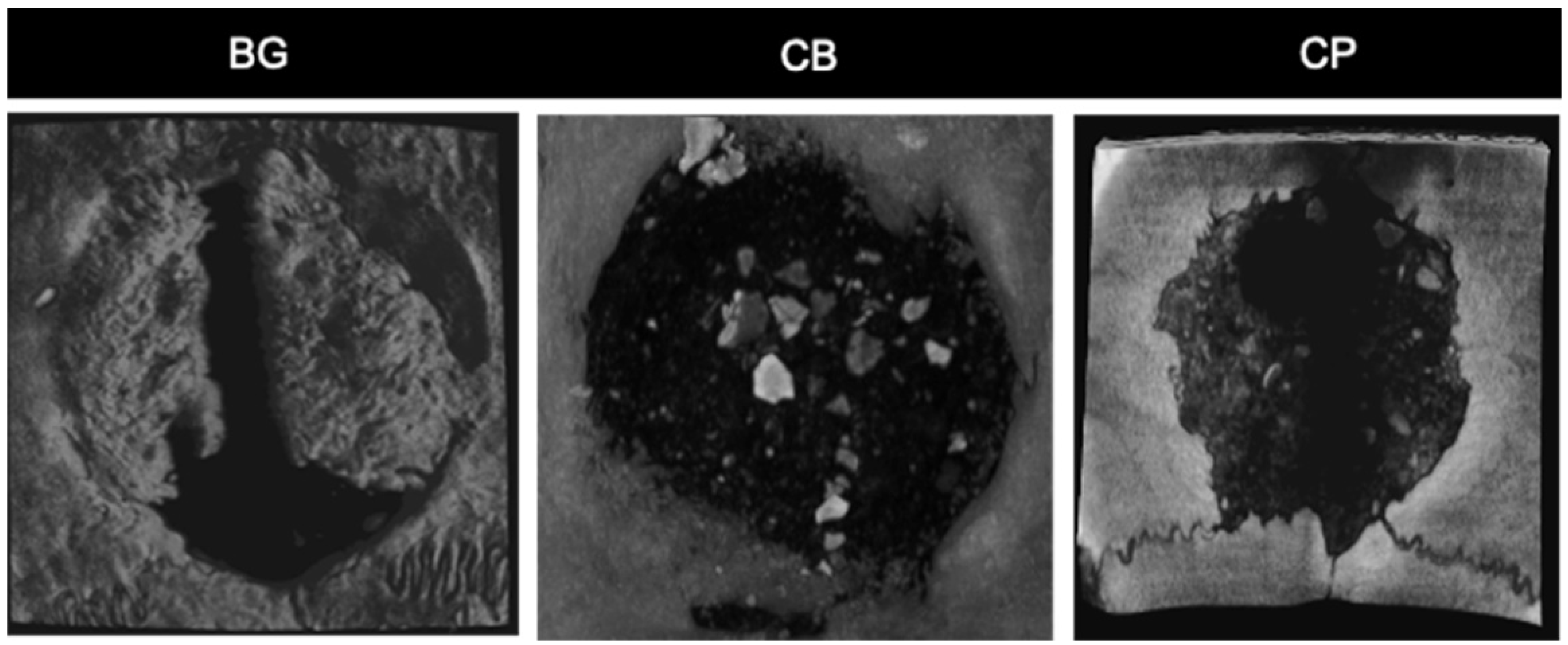
3.5. Microtomography Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donos, N.; Karring, T. Nikolaos Donos Lambros Kostopoulos Thorkild Karring Alveolar Ridge Augmentation Using a Resorbable Copolymer Membrane and Autogenous Bone Grafts an Experimental Study in the Rat. Clin. Oral Implants Res. 2002, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; van Leeuwen, A.; Yuan, H.; Bos, R.R.M.; Grijpma, D.W.; Kuijer, R. Evaluation of Novel Resorbable Mem-Branes for Bone Augmentation in a Rat Model. Clin. Oral Implants Res. 2016, 27, e8–e14. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural Graft Tissues and Synthetic Biomaterials for Periodontal and Alveolar Bone Reconstructive Applications: A Review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Hadad, H.; Rodrigues De Vasconcelos, I.; Teixeira Colombo, L.; Capalbo Da Silva, R.; Flavia Piquera Santos, A.; Cunha Cervantes, L.C.; Poli, P.P.; Signorino, F.; Maiorana, C.; et al. Functional Biomaterials Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement. J. Funct. Biomater. 2019, 10, 21. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Jung, R.E. Bone Augmentation by Means of Barrier Membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Hassumi, J.S.; Mulinari-Santos, G.; da Fabris, A.L.S.; Jacob, R.G.M.; Gonçalves, A.; Rossi, A.C.; Freire, A.R.; Faverani, L.P.; Okamoto, R. Alveolar Bone Healing in Rats: Micro-CT, Immunohistochemical and Molecular Analysis. J. Appl. Oral Sci. 2018, 26, e20170326. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 29, 115. [Google Scholar] [CrossRef]
- Carpio, L.; Loza, J.; Lynch, S.; Genco, R. Guided Bone Regeneration Around Endosseous Implants with Anorganic Bovine Bone Mineral. A Randomized Controlled Trial Comparing Bioabsorbable Versus Non-Resorbable Barriers. J. Periodontol. 2000, 71, 1743–1749. [Google Scholar] [CrossRef]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of Rabbit Calvarial Defects Using Biphasic Calcium Phosphate and a Strontium Hydroxyapatite-Containing Collagen Membrane. Clin. Oral Implants Res. 2016, 27, e206–e214. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Handelsman, M.; Ochsenbein, C.; Albrektsson, T. Guided Tissue Regeneration for Implants Placed into Extraction Sockets: A Study in Dogs. J. Periodontol. 1991, 62, 703–709. [Google Scholar] [CrossRef]
- Becker, W.; Lynch, S.E.; Lekholm, U.; Becker, B.E.; Caffesse, R.; Donath, K.; Sanchez, R. A Comparison of EPTFE Membranes Alone or in Combination with Platelet-Derived Growth Factors and Insulin-like Growth Factor-I or Demineralized Freeze-Dried Bone in Promoting Bone Formation around Immediate Extraction Socket Implants. J. Periodontol. 1992, 63, 929–940. [Google Scholar] [CrossRef]
- AlKanan, A.; Greenwell, H.; Patel, A.; Hill, M.; Shumway, B.; Lowy, J. Ridge preservation Comparing the Clinical and Histologic Healing of Membrane vs No-Membrane Approach to Buccal Overlay Grafting. Int. J. Restaur. Periodontal Dent. 2019, 39, 643–650. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of Peri-Implantitis with Resective Surgery. A 3-Year Clinical Trial on Rough Screw-Shaped Oral Implants. Part II: Radiographic Outcome. Clin. Oral Implants Res. 2006, 18, 179–187. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignedelli, E.; Corinaldesi, G. Hitological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar]
- Urban, I.A.; Monje, A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac. Surg. Clin. 2019, 31, 331–333. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal Regeneration of Human Infrabony Defects. I. Clinical Measures. J. Periodontol. 1993, 64, 254–260. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef]
- Behfarnia, P.; Khorasani, M.; Birang, R.; Abbas, F. Histological and Histomorphometric Analysis of Animal Experimental Dehiscence Defect Treated with Three Bio Absorbable GTR Collagen Membrane. Dent. Res. J. 2012, 9, 574–581. [Google Scholar] [CrossRef]
- Raz, P.; Brosh, T.; Ronen, G.; Tal, H. Tensile Properties of Three Selected Collagen Membranes. Biomed. Res. Int. 2019, 2019, 5163603. [Google Scholar] [CrossRef]
- Dos Santos Voloski, A.P.; de Figueiredo Soveral, L.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan Improves Wound Healing in Silver Catfish (Rhamdia quelen). Fish Shellfish Immunol. 2019, 93, 575–579. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Liu, D.; Sun, L.L.; Li, B.F.; Hou, H. Physicochemical and Biocompatibility Properties of Type I Collagen from the Skin of Nile Tilapia (Oreochromis Niloticus) for Biomedical Applications. Mar. Drugs 2019, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Tihista, S.; Echavarría, E. Effect of Omega 3 Polyunsaturated Fatty Acids Derived from Fish Oil in Major Burn Patients: A Prospective Randomized Controlled Pilot Trial. Clin. Nutr. 2018, 37, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ferrairo Danieletto-Zanna, C.; Ferreira Bizelli, V.; Del, G.A.; Ramires, A.; Francatti, T.M.; Sérgio, P.; de Carvalho, P.; Paula, A.; Bassi, F. Osteopromotion Capacity of Bovine Cortical Membranes in Critical Defects of Rat Calvaria: Histological and Immunohistochemical Analysis. Int. J. Biomater. 2020, 2020, 6426702. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sreekumar, G.; Sastry, T.; Chamundeeswari, M. Collagen as a potential biomaterial in biomedical applications. Rev. Adv. Mater. Sci. 2018, 53, 29–39. [Google Scholar] [CrossRef]
- Bassi, A.P.F.; Bizelli, V.F.; Freitas de Mendes Brasil, L.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; de Almeida Lucas, F. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier Membranes for Tissue Regeneration in Dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Iordache, F.; Trusca, R.; Ciocan, L.T.; Ficai, A.; Andronescu, E. Nano-Hydroxyapatite vs. Xenografts: Synthesis, Characterization, and In Vitro Behavior. Nanomaterials 2021, 11, 2289. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen Type I: A Versatile Biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Piattelli, A.; Scarano, A.; Coraggio, F.; Matarasso, S. Early Tissue Reactions to Polylactic Acid Resorbable Membranes: A Histological and Histochemical Study in Rabbit. Biomaterials 1998, 19, 889–896. [Google Scholar] [CrossRef]
- Becker, W.; Dahlin, C.; Lekholm, U.; Bergstrom, C.; van Steenberghe, D.; Higuchi, K.; Becker, B.E. Five-Year Evaluation of Implants Placed at Extraction and with Dehiscences and Fenestration Defects Augmented with EPTFE Membranes: Results from a Prospective Multicenter Study. Clin. Implant Dent. Relat. Res. 1999, 1, 27–32. [Google Scholar] [CrossRef]
- Wang, J.; Qu, Y.; Chen, C.; Sun, J.; Pan, H.; Shao, C.; Tang, R.; Gu, X. Fabrication of Collagen Membranes with Different Intrafibrillar Mineralization Degree as a Potential Use for GBR. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109959. [Google Scholar] [CrossRef]
- Bizelli, V.F.; Ramos, E.U.; Veras, A.S.C.; Teixeira, G.R.; Faverani, L.P.; Bassi, A.P.F. Calvaria Critical Size Defects Regeneration Using Collagen Membranes to Assess the Osteopromotive Principle: An Animal Study. Membranes 2022, 12, 461. [Google Scholar] [CrossRef]
- Bassi, A.P.F.; Bizelli, V.F.; Francatti, T.M.; Rezende de Moares Ferreira, A.C.; Carvalho Pereira, J.; Al-Sharani, H.M.; de Almeida Lucas, F.; Faverani, L.P. Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria. Membranes 2021, 11, 124. [Google Scholar] [CrossRef]
- Salamanca, E.; Tsai, C.Y.; Pan, Y.H.; Lin, Y.T.; Huang, H.M.; Teng, N.C.; Lin, C.T.; Feng, S.W.; Chang, W.J. In Vitro and In Vivo Study of a Novel Porcine Collagen Membrane for Guided Bone Regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef]
- Pripatnanont, P.; Chankum, C.; Meesane, J.; Thonglam, J. Soft Tissues and Materials Physical and Biological Performances of a Semi-Resorbable Barrier Membrane Based on Silk Fibroin-Glycerol-Fish Collagen Material for Guided Bone Regeneration. J. Biomater. Appl. 2021, 36, 930–942. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, J.C.S.; Baggio, A.M.P.; Benetti, L.P.; Delamura, I.F.; Ramos, E.U.; Bizelli, V.F.; Bassi, A.P.F. Application of Tissue Engineering in Manufacturing Absorbable Membranes to Improve the Osteopromoting Potential of Collagen. Bioengineering 2023, 10, 15. https://doi.org/10.3390/bioengineering10010015
de Oliveira JCS, Baggio AMP, Benetti LP, Delamura IF, Ramos EU, Bizelli VF, Bassi APF. Application of Tissue Engineering in Manufacturing Absorbable Membranes to Improve the Osteopromoting Potential of Collagen. Bioengineering. 2023; 10(1):15. https://doi.org/10.3390/bioengineering10010015
Chicago/Turabian Stylede Oliveira, Júlio César Silva, Ana Maira Pereira Baggio, Luan Pier Benetti, Izabela Fornazari Delamura, Edith Umasi Ramos, Vinícius Ferreira Bizelli, and Ana Paula Farnezi Bassi. 2023. "Application of Tissue Engineering in Manufacturing Absorbable Membranes to Improve the Osteopromoting Potential of Collagen" Bioengineering 10, no. 1: 15. https://doi.org/10.3390/bioengineering10010015
APA Stylede Oliveira, J. C. S., Baggio, A. M. P., Benetti, L. P., Delamura, I. F., Ramos, E. U., Bizelli, V. F., & Bassi, A. P. F. (2023). Application of Tissue Engineering in Manufacturing Absorbable Membranes to Improve the Osteopromoting Potential of Collagen. Bioengineering, 10(1), 15. https://doi.org/10.3390/bioengineering10010015







