Micro-Slab Coil Design for Hyperpolarized Metabolic Flux Analysis in Multiple Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Hyperpolarization Experiments
2.2. Acquisition and Processing of MR Signals
2.3. Calculation of the Average Distance between the Micro-Solenoid Coil and Origin of Samples
2.4. Cell Culture
2.5. Analysis of Intracellular Level of NAD+ and NADH
2.6. Analysis of Extracellular Level of Lactate
2.7. Drug Treatment
2.8. Immunoblot Analysis
2.9. Flow Cytometry Analysis
3. Results and Discussion
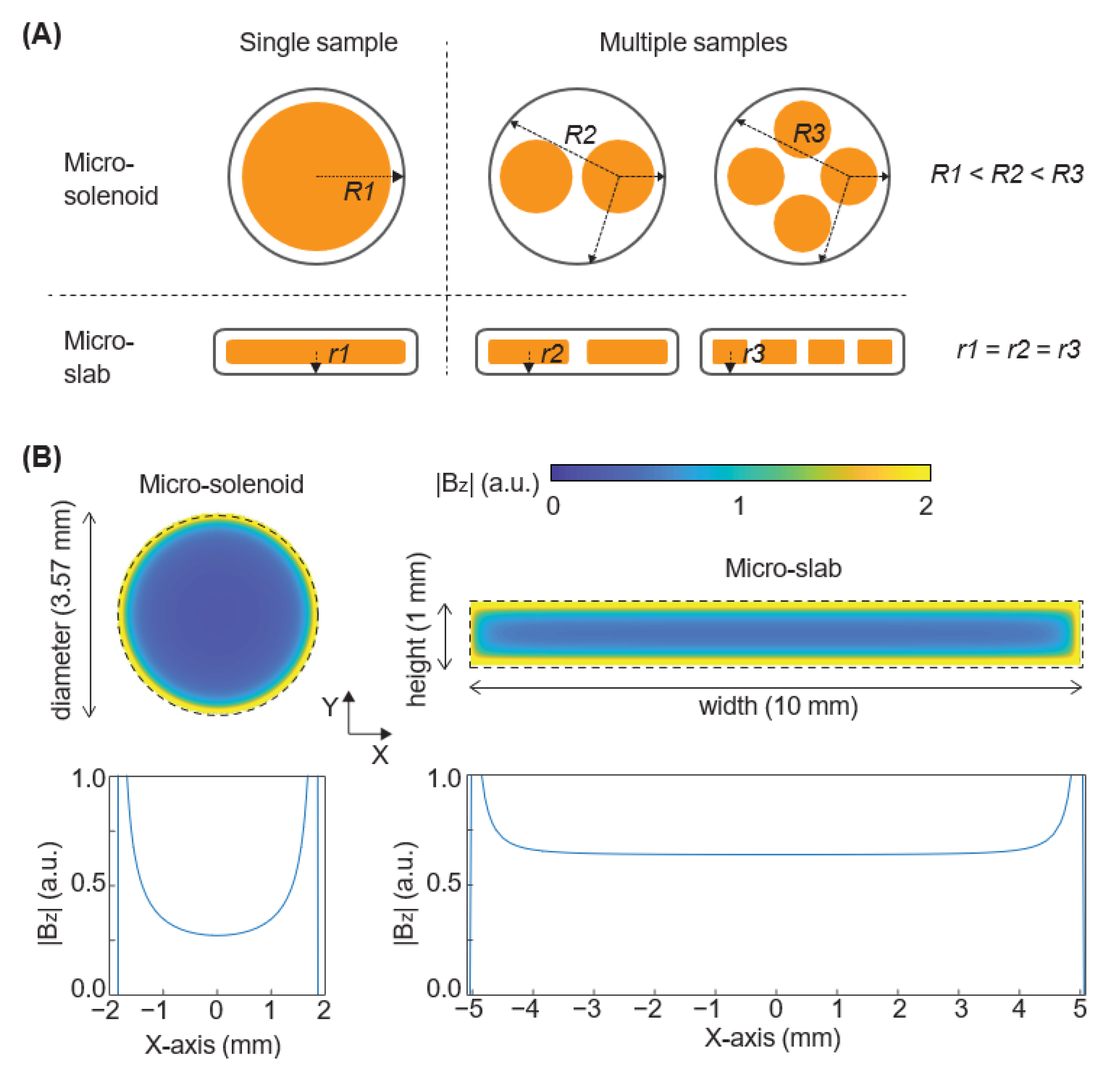
3.1. Micro-Slab Coil Design Achieves a Higher Detection Sensitivity and Generates a More Homogeneous Magnetic Field Than Micro-Solenoid Coil Design
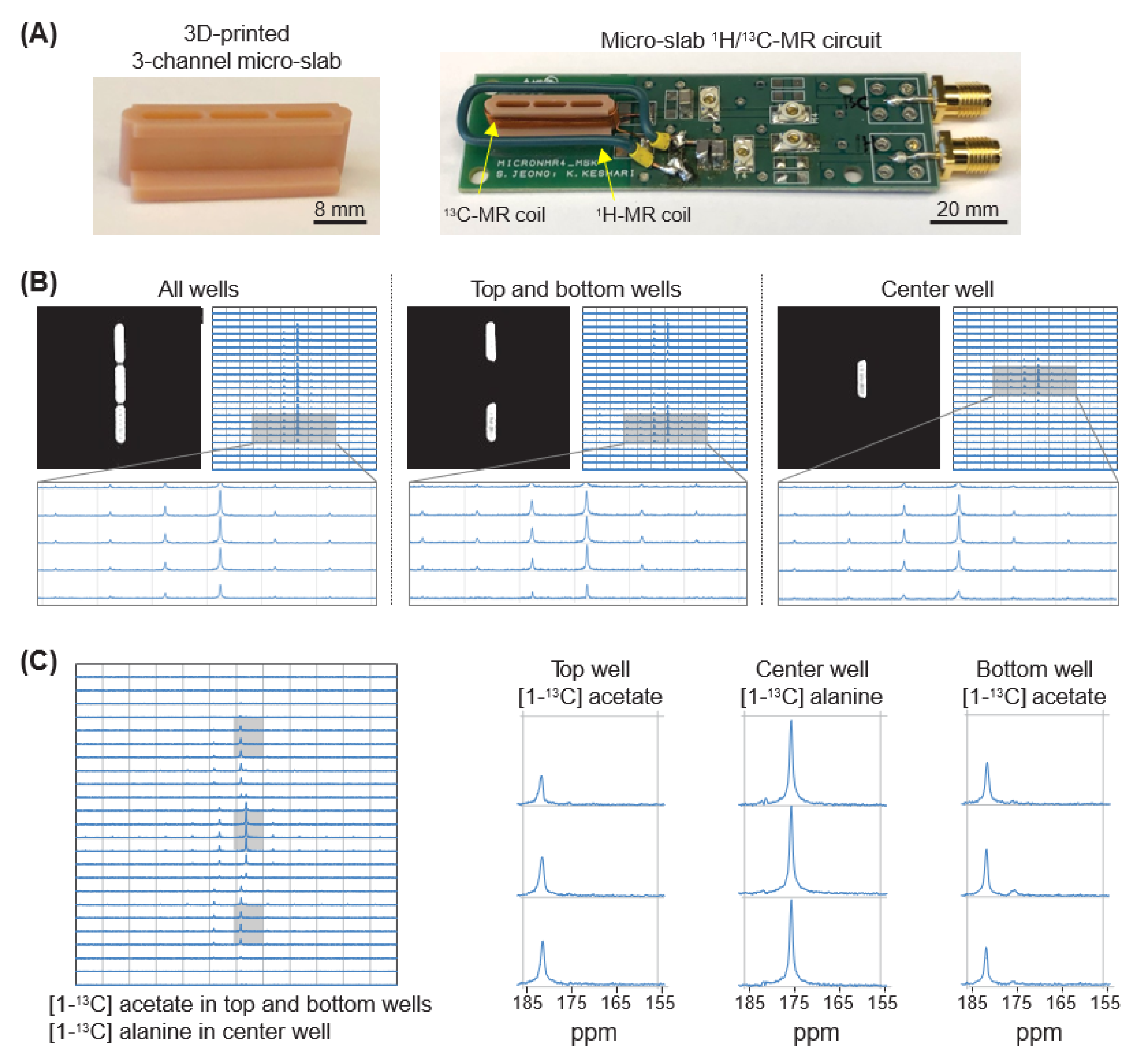
3.2. Micro-Slab Coil with Three Separate Wells Enables Simultaneous Mr Analysis of Three Different Samples
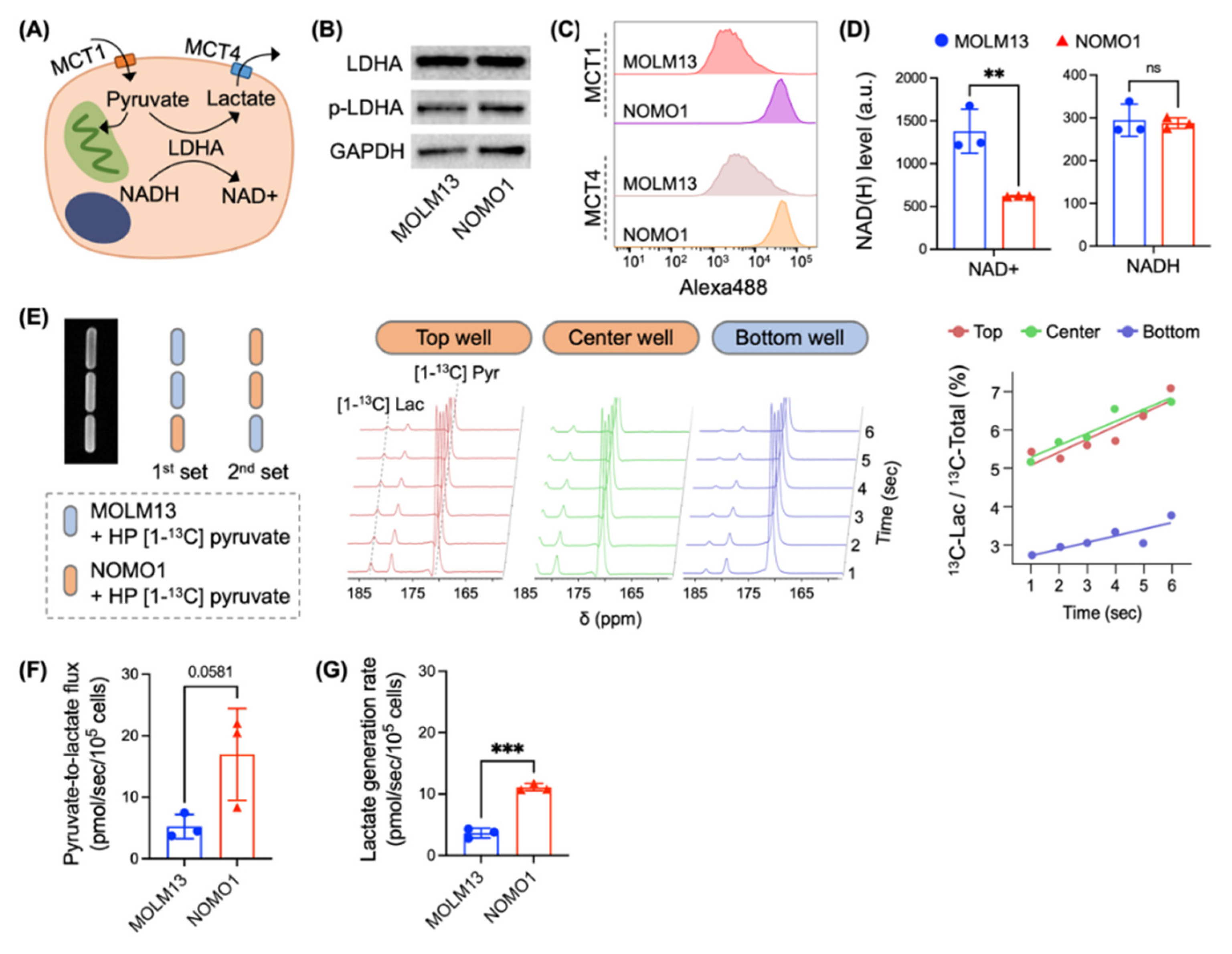
3.3. Micro-Slab MR Coil in Conjunction with Hyperpolarized Probes Enables Quantitative Analysis of Metabolic Flux in Different Biological Samples
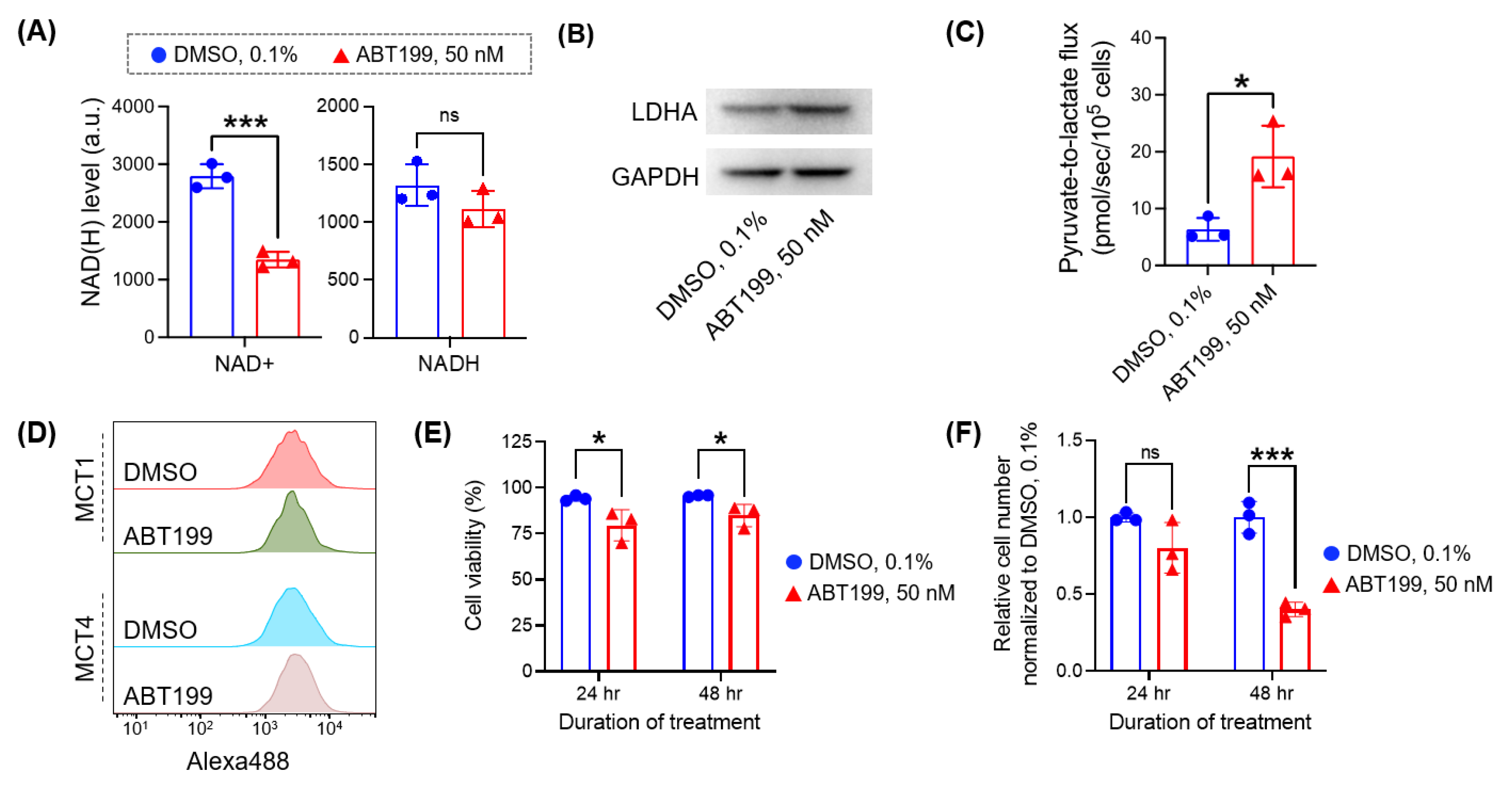
3.4. Micro-Slab MR Coil with HP [1-13C] Pyruvate Enables the Rapid Assessment of Pyruvate-to-Lactate Flux in Cancer Cells Treated with Different Doses of Drug
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Tanaka, K.; Akhavan, D.; Babic, I.; Gini, B.; Matsutani, T.; Iwanami, A.; Liu, F.; Villa, G.R.; Gu, Y.; et al. mTOR Complex 2 Controls Glycolytic Metabolism in Glioblastoma through FoxO Acetylation and Upregulation of c-Myc. Cell Metab. 2013, 18, 726–739. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Ma, J.; Peng, H.; Wang, F.; Zha, X.; Wang, Y.; Jing, Y.; Yang, H.; Chen, R.; et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. USA 2011, 108, 4129–4134. [Google Scholar] [CrossRef]
- Akalin, A.; Garrett-Bakelman, F.; Kormaksson, M.; Busuttil, J.; Zhang, L.; Khrebtukova, I.; Milne, T.; Huang, Y.; Biswas, R.; Hess, J.; et al. Base-Pair Resolution DNA Methylation Sequencing Reveals Profoundly Divergent Epigenetic Landscapes in Acute Myeloid Leukemia. PLOS Genet. 2012, 8, e1002781. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; VasanthaKumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Keshari, K.R. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell 2022, 185, 2678–2689. [Google Scholar] [CrossRef]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef]
- Andronesi, O.C.; Kim, G.S.; Gerstner, E.; Batchelor, T.; Tzika, A.A.; Fantin, V.R.; Heiden, M.G.V.; Sorensen, A.G. Detection of 2-Hydroxyglutarate in IDH -Mutated Glioma Patients by In Vivo Spectral-Editing and 2D Correlation Magnetic Resonance Spectroscopy. Sci. Transl. Med. 2012, 4, 116ra4. [Google Scholar] [CrossRef]
- Maher, E.A.; Marin-Valencia, I.; Bachoo, R.M.; Mashimo, T.; Raisanen, J.; Hatanpaa, K.J.; Jindal, A.; Jeffrey, F.M.; Choi, C.; Madden, C.; et al. Metabolism of [U-13C] glucose in human brain tumors in vivo. NMR Biomed. 2012, 25, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; de Graaf, R.A. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjær-Larsen, J.H.; Bowen, S.; Petersen, J.R.; Rybalko, O.; Vinding, M.S.; Ullisch, M.; Nielsen, N.C. Cryogen-free dissolution dynamic nuclear polarization polarizer operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med. 2019, 81, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Miloushev, V.Z.; Granlund, K.L.; Boltyanskiy, R.; Lyashchenko, S.K.; DeAngelis, L.M.; Mellinghoff, I.K.; Brennan, C.W.; Tabar, V.; Yang, T.J.; Holodny, A.I.; et al. Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor Patients. Cancer Res. 2018, 78, 3755–3760. [Google Scholar] [CrossRef] [PubMed]
- Granlund, K.L.; Tee, S.-S.; Vargas, H.A.; Lyashchenko, S.K.; Reznik, E.; Fine, S.; Laudone, V.; Eastham, J.A.; Touijer, K.A.; Reuter, V.E.; et al. Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab. 2019, 31, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Eskandari, R.; Ray, C.; Granlund, K.L.; Dos Santos-Cunha, L.; Miloushev, V.Z.; Tee, S.S.; Jeong, S.; Aras, O.; Chen, Y.-B.; et al. Hyperpolarized MRI Visualizes Warburg Effects and Predicts Treatment Response to mTOR Inhibitors in Patient-Derived ccRCC Xenograft Models. Cancer Res. 2019, 79, 242–250. [Google Scholar] [CrossRef]
- Cho, A.; Eskandari, R.; Granlund, K.L.; Keshari, K.R. Hyperpolarized [6-13C, 15N3]-arginine as a probe for in vivo arginase activity. ACS Chem. Biol. 2019, 14, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.; Kurhanewicz, J.; Bok, R.; Xu, D.; Joun, D.; Zhang, V.; Nelson, S.J.; Hurd, R.E.; Vigneron, D.B. Feasibility of using hyperpolarized [1-13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magn. Reson. Imaging 2008, 26, 721–726. [Google Scholar] [CrossRef]
- Mayer, D.; Yen, Y.-F.; Josan, S.; Park, J.M.; Pfefferbaum, A.; Hurd, R.E.; Spielman, D.M. Application of hyperpolarized [1-13 C]lactate for the in vivo investigation of cardiac metabolism. NMR Biomed. 2012, 25, 1119–1124. [Google Scholar] [CrossRef]
- Salamanca-Cardona, L.; Shah, H.; Poot, A.J.; Correa, F.M.; Di Gialleonardo, V.; Lui, H.; Miloushev, V.Z.; Granlund, K.L.; Tee, S.S.; Cross, J.R.; et al. In vivo imaging of glutamine metabolism to the oncometabolite 2-hydroxyglutarate in IDH1/2 mutant tumors. Cell Metab. 2017, 26, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Eskandari, R.; Park, S.M.; Alvarez, J.; Tee, S.S.; Weissleder, R.; Kharas, M.G.; Lee, H.; Keshari, K.R. Real-time quantitative analysis of metabolic flux in live cells using a hyperpolarized micromagnetic resonance spectrometer. Sci. Adv. 2017, 3, e1700341. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.S.; DiGialleonardo, V.; Eskandari, R.; Jeong, S.; Granlund, K.L.; Miloushev, V.; Poot, A.J.; Truong, S.; Alvarez, J.A.; Aldeborgh, H.N.; et al. Sampling Hyperpolarized Molecules Utilizing a 1 Tesla Permanent Magnetic Field. Sci. Rep. 2016, 6, 32846. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Eskandari, R.; Miloushev, V.Z.; Keshari, K.R. A non-synthetic approach to extending the lifetime of hyperpolarized molecules using D2O solvation. J. Magn. Reson. 2018, 295, 57–62. [Google Scholar] [CrossRef]
- Lees, H.; Millan, M.; Ahamed, F.; Eskandari, R.; Granlund, K.L.; Jeong, S.; Keshari, K.R. Multi-sample measurement of hyperpolarized pyruvate-to-lactate flux in melanoma cells. NMR Biomed. 2021, 34, e4447. [Google Scholar] [CrossRef] [PubMed]
- Webb, A. Increasing the Sensitivity of Magnetic Resonance Spectroscopy and Imaging. Anal. Chem. 2011, 84, 9–16. [Google Scholar] [CrossRef]
- Guo, L.; Zhi, S.; Sun, X.; Lei, C.; Zhou, Y. Ultrasensitive detection of bioanalytes based on signal amplification of coil-integrated giant magnetoimpedance biosystems. Sens. Actuators B Chem. 2017, 247, 1–10. [Google Scholar] [CrossRef]
- Mohmmadzadeh, M.; Baxan, N.; Badilita, V.; Kratt, K.; Weber, H.; Korvink, J.; Wallrabe, U.; Hennig, J.; von Elverfeldt, D. Characterization of a 3D MEMS fabricated micro-solenoid at 9.4T. J. Magn. Reson. 2011, 208, 20–26. [Google Scholar] [CrossRef]
- Khelifa, M.; Mounier, D.; Yaakoubi, N. Design of High Performance Scroll Microcoils for Nuclear Magnetic Resonance Spectroscopy of Nanoliter and Subnanoliter Samples. Sensors 2020, 21, 170. [Google Scholar] [CrossRef]
- Kentgens, A.P.M.; Bart, J.; Van Bentum, P.J.M.; Brinkmann, A.; Van Eck, E.R.H.; Gardeniers, H.; Janssen, J.W.G.; Knijn, P.; Vasa, S.; Verkuijlen, M.H.W. High-resolution liquid- and solid-state nuclear magnetic resonance of nanoliter sample volumes using microcoil detectors. J. Chem. Phys. 2008, 128, 052202. [Google Scholar] [CrossRef]
- Boidot, R.; Végran, F.; Meulle, A.; Le Breton, A.; Dessy, C.; Sonveaux, P.; Lizard−Nacol, S.; Feron, O. Regulation of Monocarboxylate Transporter MCT1 Expression by p53 Mediates Inward and Outward Lactate Fluxes in Tumors. Cancer Res. 2012, 72, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Curry, J.M.; Ko, Y.-H.; Lin, Z.; Tuluc, M.; Cognetti, D.; Birbe, R.C.; Pribitkin, E.; Bombonati, A.; Pestell, R.G. Oncogenes and inflammation rewire host energy metabolism in the tumor microenvironment: RAS and NFκB target stromal MCT4. Cell Cycle 2013, 12, 2580–2597. [Google Scholar] [CrossRef] [PubMed]
- Sasca, D.; Szybinski, J.; Schüler, A.; Shah, V.; Heidelberger, J.; Haehnel, P.S.; Dolnik, A.; Kriege, O.; Fehr, E.-M.; Gebhardt, W.H.; et al. NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood 2019, 133, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Klingbeil, O.; Tarumoto, Y.; Somerville, T.D.; Huang, Y.-H.; Wei, Y.; Wai, D.C.; Low, J.K.; Milazzo, J.P.; Wu, X.S.; et al. A Transcription Factor Addiction in Leukemia Imposed by the MLL Promoter Sequence. Cancer Cell 2018, 34, 970–981. [Google Scholar] [CrossRef]
- Radoul, M.; Najac, C.; Viswanath, P.; Mukherjee, J.; Kelly, M.; Gillespie, A.M.; Chaumeil, M.M.; Eriksson, P.; Santos, R.D.; Pieper, R.O.; et al. HDAC inhibition in glioblastoma monitored by hyperpolarized13C MRSI. NMR Biomed. 2018, 32, e4044. [Google Scholar] [CrossRef]
- Di Gialleonardo, V.; Aldeborgh, H.N.; Miloushev, V.; Folkers, K.M.; Granlund, K.; Tap, W.D.; Lewis, J.S.; Weber, W.A.; Keshari, K.R. Multinuclear NMR and MRI Reveal an Early Metabolic Response to mTOR Inhibition in Sarcoma. Cancer Res. 2017, 77, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Spielman, D.M.; Josan, S.; Jang, T.; Merchant, M.; Hurd, R.E.; Mayer, D.; Recht, L.D. Hyperpolarized13C-lactate to13C-bicarbonate ratio as a biomarker for monitoring the acute response of anti-vascular endothelial growth factor (anti-VEGF) treatment. NMR Biomed. 2016, 29, 650–659. [Google Scholar] [CrossRef]
- Day, S.E.; Kettunen, M.I.; Gallagher, F.A.; Hu, D.-E.; Lerche, M.; Wolber, J.; Golman, K.; Ardenkjaer-Larsen, J.H.; Brindle, K.M. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat. Med. 2007, 13, 1382–1387. [Google Scholar] [CrossRef]
- Corbet, C.; Bastien, E.; Draoui, N.; Doix, B.; Mignion, L.; Jordan, B.F.; Marchand, A.; Vanherck, J.-C.; Chaltin, P.; Schakman, O.; et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat. Commun. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Rodriguez-Blanco, G.; Sumpton, D.; Cloix, C.; Mullin, M.; Mackay, G.M.; O’Neill, K.; Lemgruber, L.; Luo, X.; Tait, S.W.G. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 2020, 11, 616. [Google Scholar] [CrossRef]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015, 7, 279ra40. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C.; et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Alkhatabi, H.A.; Zohny, S.F.; Mohammed, M.R.S.; Choudhry, H.; Rehan, M.; Ahmad, A.; Ahmed, F.; Khan, M.I. Venetoclax−Resistant MV4−11 Leukemic Cells Activate PI3K/AKT Pathway for Metabolic Reprogramming and Redox Adaptation for Survival. Antioxidants 2022, 11, 461. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Ruan, T.; Wong, C.; Deh, K.; Abolarin, A.; Correa, A.; Keshari, K.R.; Jeong, S. Micro-Slab Coil Design for Hyperpolarized Metabolic Flux Analysis in Multiple Samples. Bioengineering 2023, 10, 14. https://doi.org/10.3390/bioengineering10010014
Lee G, Ruan T, Wong C, Deh K, Abolarin A, Correa A, Keshari KR, Jeong S. Micro-Slab Coil Design for Hyperpolarized Metabolic Flux Analysis in Multiple Samples. Bioengineering. 2023; 10(1):14. https://doi.org/10.3390/bioengineering10010014
Chicago/Turabian StyleLee, Geonhui, Thomas Ruan, Claudia Wong, Kofi Deh, Alli Abolarin, Alexander Correa, Kayvan R. Keshari, and Sangmoo Jeong. 2023. "Micro-Slab Coil Design for Hyperpolarized Metabolic Flux Analysis in Multiple Samples" Bioengineering 10, no. 1: 14. https://doi.org/10.3390/bioengineering10010014
APA StyleLee, G., Ruan, T., Wong, C., Deh, K., Abolarin, A., Correa, A., Keshari, K. R., & Jeong, S. (2023). Micro-Slab Coil Design for Hyperpolarized Metabolic Flux Analysis in Multiple Samples. Bioengineering, 10(1), 14. https://doi.org/10.3390/bioengineering10010014





