Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises
Abstract
1. Introduction
2. Cardiac Cells
3. Cardiac Extracellular Matrix
4. Regenerative Medicine: Current Therapies for Cardiac Diseases
4.1. Gene Therapy
4.2. Cell Therapy
4.3. Fabrication of Biomimetic Cardiac Tissues
4.3.1. Hydrogels
4.3.2. Microfabrication
4.3.3. Electrospinning
4.3.4. Bioprinting
4.3.5. Decellularized Bioscaffolds
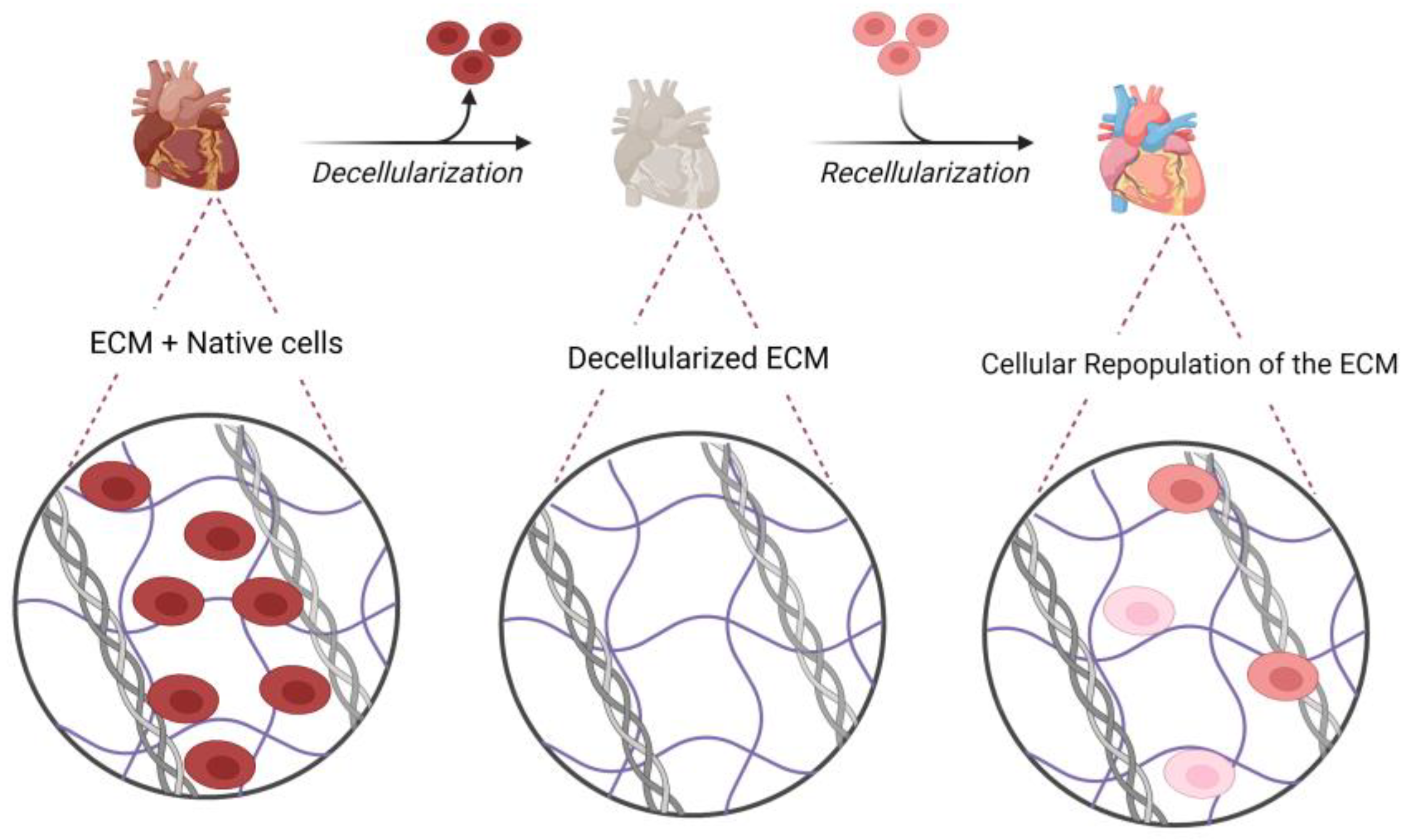
5. Decellularization and Recellularization Methods
5.1. Decellularization Agents
5.2. Techniques for Applying Decellularization Agents
5.3. Evaluation of Decellularized ECM
5.4. Recellularization and Cell Sources
5.5. Bioreactors for Recellularization
5.6. Immunogenicity and Its Implications
6. Whole-Heart Engineering
6.1. Development of Decellularization Protocols in Rodent Models
6.2. Development of Decellularization Protocols in Human-Sized Models
6.3. Development of Decellularization Protocols for Human Tissue
7. Hurdles Should Be Addressed for Whole-Heart Engineering
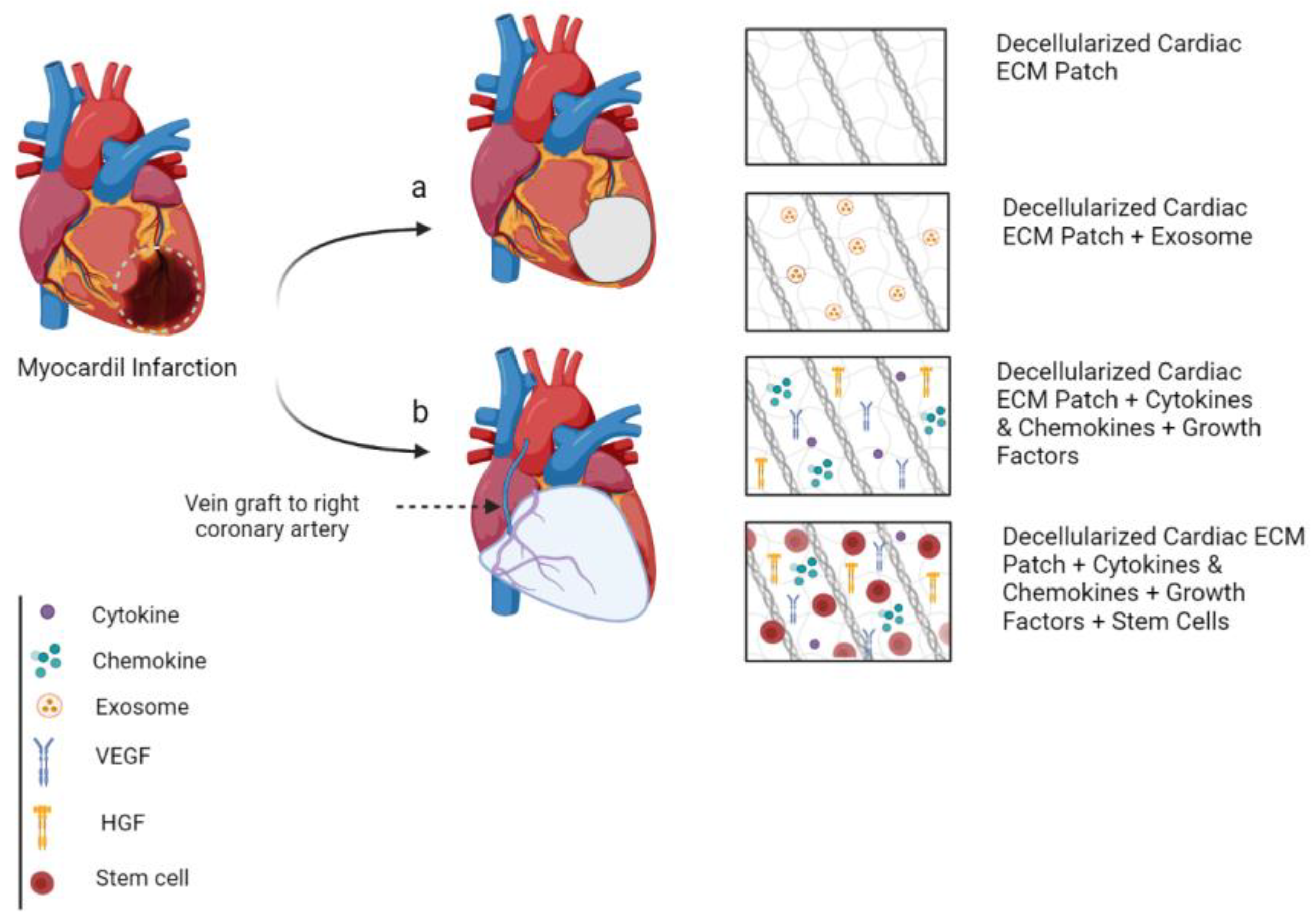
8. Cardiac Patch
8.1. Small-Scale Natural Myocardial ECM Patch
8.1.1. Development of Natural Myocardial ECM Patch in a Rodent Model
8.1.2. Development of Natural Myocardial ECM Patch in Porcine Model
8.1.3. Development of Natural Myocardial ECM Patch from Human Tissue
8.1.4. Drawbacks of Small-Scale Natural CECM Patch
8.2. Large-Scale Natural Myocardial ECM Patch
9. The Role of Cardiac Tissue Engineering in Clinic
10. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenreiro, M.F.; Louro, A.F.; Alves, P.M.; Serra, M.J. Next generation of heart regenerative therapies: Progress and promise of cardiac tissue engineering. Npj Regen. Med. 2021, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G.J.C.r. Can we engineer a human cardiac patch for therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Parikh, R.B.; Sampaio, L.C. Bioengineering hearts: Simple yet complex. Curr. Stem Cell Rep. 2017, 3, 35–44. [Google Scholar] [CrossRef]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv. Healthc. Mater. 2019, 8, 1801217. [Google Scholar] [CrossRef]
- Johnson, T.D.; Hill, R.C.; Dzieciatkowska, M.; Nigam, V.; Behfar, A.; Christman, K.L.; Hansen, K.C. Quantification of decellularized human myocardial matrix: A comparison of six patients. Proteom.–Clin. Appl. 2016, 10, 75–83. [Google Scholar] [CrossRef]
- Gray, S.J.; Samulski, R.J. Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin. Biol. Ther. 2008, 8, 911–922. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Ylä-Herttuala, S. Current status of cardiovascular gene therapy. Mol. Ther. 2007, 15, 1233–1247. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Baker, A.H. Cardiovascular gene therapy: Past, present, and future. Mol. Ther. 2017, 25, 1095–1106. [Google Scholar] [CrossRef]
- Lehrman, S. Virus treatment questioned after gene therapy death. Nature 1999, 401, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood J. Am. Soc. Hematol. 2013, 122, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Zentilin, L.; Giacca, M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ. Res. 2014, 114, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Nabel, E.G.; Plautz, G.; Nabel, G.J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science 1990, 249, 1285–1288. [Google Scholar] [CrossRef]
- Cannatà, A.; Ali, H.; Sinagra, G.; Giacca, M. Gene therapy for the heart lessons learned and future perspectives. Circ. Res. 2020, 126, 1394–1414. [Google Scholar] [CrossRef]
- Madonna, R.; Van Laake, L.W.; Botker, H.E.; Davidson, S.M.; De Caterina, R.; Engel, F.B.; Eschenhagen, T.; Fernandez-Aviles, F.; Hausenloy, D.J.; Hulot, J.-S. ESC Working Group on Cellular Biology of the Heart: Position paper for Cardiovascular Research: Tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 2019, 115, 488–500. [Google Scholar] [CrossRef]
- Madonna, R. Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzas, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, R.J.; Owen, T.J.; Pandey, P.; Harding, S.E. Future potential of engineered heart tissue patches for repairing the damage caused by heart attacks. Expert Rev. Med. Devices 2020, 17, 1–3. [Google Scholar] [CrossRef]
- Hsiao, L.-C.; Carr, C.; Chang, K.-C.; Lin, S.-Z.; Clarke, K. Stem cell-based therapy for ischemic heart disease. Cell Transplant. 2013, 22, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Huang, R.; Sun, A.; Qian, J.; Liu, X.; Ge, L.; Zhang, Y.; Zhang, S.; Niu, Y.; Wang, Q. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur. J. Heart Fail. 2009, 11, 691–698. [Google Scholar] [CrossRef]
- Hsiao, L.-C.; Lin, Y.-N.; Shyu, W.-C.; Ho, M.; Lu, C.-R.; Chang, S.-S.; Wang, Y.-C.; Chen, J.-Y.; Lu, S.-Y.; Wu, M.-Y. First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 961920. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P. Stem cell therapy for heart failure: Are arrhythmias a real safety concern? Circulation 2009, 119, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.N.; Bolli, R.; Hare, J.M. Clinical studies of cell therapy in cardiovascular medicine: Recent developments and future directions. Circ. Res. 2018, 123, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.J.C. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Sanganalmath, S.K.; Bolli, R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013, 113, 810–834. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; LeCapitaine, N. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef]
- Heeschen, C.; Lehmann, R.; Honold, J.r.; Assmus, B.; Aicher, A.; Walter, D.H.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004, 109, 1615–1622. [Google Scholar] [CrossRef]
- Golpanian, S.; El-Khorazaty, J.; Mendizabal, A.; DiFede, D.L.; Suncion, V.Y.; Karantalis, V.; Fishman, J.E.; Ghersin, E.; Balkan, W.; Hare, J.M. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J. Am. Coll. Cardiol. 2015, 65, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Climent, A.M.; Badimon, L.; Bolli, R.; Charron, D.; Fuster, V.; Janssens, S.; Kastrup, J.; Kim, H.-S. Global position paper on cardiovascular regenerative medicine. Eur. Heart J. 2017, 38, 2532–2546. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.J.N.m. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Scuderi Scuderi, G.J.; Butcher, J.J.F.i.c.; Biology, d. Naturally engineered maturation of cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.; Bursac, N.; Langer, R.; Merok, J.; Vunjak-Novakovic, G.; Freed, L. Tissue engineering of functional cardiac muscle: Molecular, structural, and electrophysiological studies. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H168–H178. [Google Scholar] [CrossRef]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Su, T.; Lu, F.; Chang, Q.; Gao, J. Recent Advances in Macroporous Hydrogels for Cell Behavior and Tissue Engineering. Gels 2022, 8, 606. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Feinberg, A.W.; Feigel, A.; Shevkoplyas, S.S.; Sheehy, S.; Whitesides, G.M.; Parker, K.K. Muscular thin films for building actuators and powering devices. Science 2007, 317, 1366–1370. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019, 176, 913–927.e918. [Google Scholar] [CrossRef]
- Feiner, R.; Engel, L.; Fleischer, S.; Malki, M.; Gal, I.; Shapira, A.; Shacham-Diamand, Y.; Dvir, T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016, 15, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Montgomery, M.; Liang, Y.; Liu, H.; Radisic, M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc. Natl. Acad. Sci. USA 2012, 109, E3414–E3423. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Matsuda, R.; Toda, M.; Shimamoto, K. Three-dimensional reconstruction of the human capillary network and the intramyocardial micronecrosis. Am. J. Physiol. -Heart Circ. Physiol. 2011, 300, H754–H761. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, P.J.; Rosa, S.; Ricotti, L.; Abecasis, B.; Almeida, H.; Monteiro, L.; Nunes, J.; Carvalho, F.S.; Serra, M.; Luchkin, S. Flexible nanofilms coated with aligned piezoelectric microfibers preserve the contractility of cardiomyocytes. Biomaterials 2017, 139, 213–228. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.A.; Sheehy, S.P.; Chantre, C.O.; Zimmerman, J.F.; Pasqualini, F.S.; Liu, X.; Goss, J.A.; Campbell, P.H.; Gonzalez, G.M.; Park, S.-J. A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2018, 2, 930–941. [Google Scholar] [CrossRef]
- Castilho, M.; van Mil, A.; Maher, M.; Metz, C.H.; Hochleitner, G.; Groll, J.; Doevendans, P.A.; Ito, K.; Sluijter, J.P.; Malda, J. Melt electrowriting allows tailored microstructural and mechanical design of scaffolds to advance functional human myocardial tissue formation. Adv. Funct. Mater. 2018, 28, 1803151. [Google Scholar] [CrossRef]
- Eberli, D. Regenerative Medicine and Tissue Engineering: Cells and Biomaterials; BoD–Books on Demand: Norderstedt, Germany, 2011. [Google Scholar]
- Sharma, D.; Ferguson, M.; Kamp, T.J.; Zhao, F. Constructing biomimetic cardiac tissues: A review of scaffold materials for engineering cardiac patches. Emergent Mater. 2019, 2, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.; Kaunas, R.; Gaharwar, A.K. Nanoengineered ionic–covalent entanglement (NICE) bioinks for 3D bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater. 2018, 7, 1800672. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, J.; Ahearne, M.J.S.R. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 14933. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Khaboushan, A.S.; Daghigh, F.; Kajbafzadeh, A.M.; Zolbin, M.M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Badylak, S.F. Regenerative medicine and developmental biology: The role of the extracellular matrix. Anat. Rec. Part B New Anat. Off. Publ. Am. Assoc. Anat. 2005, 287, 36–41. [Google Scholar] [CrossRef]
- Arenas-Herrera, J.; Ko, I.; Atala, A.; Yoo, J.J.B.m. Decellularization for whole organ bioengineering. Biomed. Mater. 2013, 8, 014106. [Google Scholar] [CrossRef]
- Cartmell, J.; Dunn, M. Effect of chemical treatments on tendon cellularity and mechanical properties. J. Biomed. Mater. Res. 2000, 49, 134–140. [Google Scholar] [CrossRef]
- Lovekamp, J.J.; Simionescu, D.T.; Mercuri, J.J.; Zubiate, B.; Sacks, M.S.; Vyavahare, N.R.J.B. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials 2006, 27, 1507–1518. [Google Scholar] [CrossRef]
- Robertson, M.J.; Dries-Devlin, J.L.; Kren, S.M.; Burchfield, J.S.; Taylor, D.A. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS ONE 2014, 9, e90406. [Google Scholar] [CrossRef]
- Tadevosyan, K.; Iglesias-García, O.; Mazo, M.M.; Prósper, F.; Raya, A. Engineering and Assessing Cardiac Tissue Complexity. Int. J. Mol. Sci. 2021, 22, 1479. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Barad, L.; Novak, A.; Reiter, I.; Itskovitz-Eldor, J.; Binah, O.; Popescu, L. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: Comparative ultrastructure. J. Cell. Mol. Med. 2011, 15, 2539–2551. [Google Scholar] [CrossRef]
- Chen, H.-C.; Hu, Y.-C. Bioreactors for tissue engineering. Biotechnol. Lett. 2006, 28, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Pellegata, A.F.; Tedeschi, A.M.; De Coppi, P.J.F.i.b. Whole organ tissue vascularization: Engineering the tree to develop the fruits. Front. Bioeng. Biotechnol. 2018, 6, 56. [Google Scholar] [CrossRef]
- Constant, S.L.; Bottomly, K.J.A.r.o.i. Induction of Th1 and Th2 CD4plus T cell responses: The alternative approaches. Annu. Rev. Immunol. 1997, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Stamer, D.; Kyriakides, T. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A.J.N.m. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Momtahan, N.; Sukavaneshvar, S.; Roeder, B.L.; Cook, A.D. Strategies and processes to decellularize and recellularize hearts to generate functional organs and reduce the risk of thrombosis. Tissue Eng. Part B Rev. 2015, 21, 115–132. [Google Scholar] [CrossRef]
- Ng, S.L.; Narayanan, K.; Gao, S.; Wan, A.C. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials 2011, 32, 7571–7580. [Google Scholar] [CrossRef]
- Akhyari, P.; Aubin, H.; Gwanmesia, P.; Barth, M.; Hoffmann, S.; Huelsmann, J.; Preuss, K.; Lichtenberg, A.J.T.E.P.C.M. The quest for an optimized protocol for whole-heart decellularization: A comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng. Part C Methods 2011, 17, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Witzenburg, C.; Raghupathy, R.; Kren, S.M.; Taylor, D.A.; Barocas, V.H.J.J.o.b. Mechanical changes in the rat right ventricle with decellularization. J. Biomech. 2012, 45, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.; Koshy, S.T.; Jhamb, G.; Woodford, C.; Thompson, C.M.; Levy, A.S.; Rush, J.W.; Guillemette, J.G.; Lillicrap, D.; Jervis, E.J.T.C.J.o.C.E. Cardiac decellularisation with long-term storage and repopulation with canine peripheral blood progenitor cells. Can. J. Chem. Eng. 2012, 90, 1457–1464. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.-W.; Mohamed, M.; Hogan, M.; Salazar, B.; Patel, N.M.; Birla, R.K. Establishing the framework for fabrication of a bioartificial heart. ASAIO J. 2015, 61, 429–436. [Google Scholar] [CrossRef]
- Garry, M.G.; Kren, S.M.; Garry, D.J. Neonatal cardiac scaffolds: Novel matrices for regenerative studies. JoVE (J. Vis. Exp.) 2016, 5, e54459. [Google Scholar] [CrossRef]
- Nguyen, D.T.; O’Hara, M.; Graneli, C.; Hicks, R.; Miliotis, T.; Nyström, A.-C.; Hansson, S.; Davidsson, P.; Gan, L.-M.; Magnone, M.C.J.S.r. Humanizing miniature hearts through 4-flow cannulation perfusion decellularization and recellularization. Sci. Rep. 2018, 8, 7458. [Google Scholar] [CrossRef]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Buica, T.P.; Gavriliuc, O.I.; Baderca, F.; Hoinoiu, T.; Paunescu, V. Innovative biotechnology for generation of cardiac tissue. Appl. Sci. 2021, 11, 5603. [Google Scholar] [CrossRef]
- Park, S.M.; Yang, S.; Rye, S.-M.; Choi, S.W. Effect of pulsatile flow perfusion on decellularization. BioMedical Eng. OnLine 2018, 17, 15. [Google Scholar] [CrossRef]
- Dal Sasso, E.; Menabò, R.; Agrillo, D.; Arrigoni, G.; Franchin, C.; Giraudo, C.; Filippi, A.; Borile, G.; Ascione, G.; Zanella, F. RegenHeart: A time-effective, low-concentration, detergent-based method aiming for conservative decellularization of the whole heart organ. ACS Biomater. Sci. Eng. 2020, 6, 5493–5506. [Google Scholar] [CrossRef]
- Alexanian, R.A.; Mahapatra, K.; Lang, D.; Vaidyanathan, R.; Markandeya, Y.S.; Gill, R.K.; Zhai, A.J.; Dhillon, A.; Lea, M.R.; Abozeid, S. Induced cardiac progenitor cells repopulate decellularized mouse heart scaffolds and differentiate to generate cardiac tissue. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118559. [Google Scholar] [CrossRef] [PubMed]
- Hochman-Mendez, C.; Mesquita, F.C.; Morrissey, J.; da Costa, E.C.; Hulsmann, J.; Tang-Quan, K.; Xi, Y.; Lee, P.-F.; Sampaio, L.C.; Taylor, D.A. Restoring anatomical complexity of a left ventricle wall as a step toward bioengineering a human heart with human induced pluripotent stem cell-derived cardiac cells. Acta Biomater. 2022, 141, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kirita, Y.; Kami, D.; Kitani, T.; Ozaki, C.; Itakura, Y.; Toyoda, M.; Gojo, S. Novel detergent for whole organ tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3364–3373. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, J.M.; Czajka, C.A.; Patel, U.B.; Freytes, D.O.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng. Part C Methods 2010, 16, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Weymann, A.; Loganathan, S.; Takahashi, H.; Schies, C.; Claus, B.; Hirschberg, K.; Soós, P.; Korkmaz, S.; Schmack, B.; Karck, M. Development and Evaluation of a Perfusion Decellularization Porcine Heart Model–Generation of 3-Dimensional Myocardial Neoscaffolds. Circ. J. 2011, 75, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Weymann, A.; Patil, N.P.; Sabashnikov, A.; Jungebluth, P.; Korkmaz, S.; Li, S.; Veres, G.; Soos, P.; Ishtok, R.; Chaimow, N. Bioartificial heart: A human-sized porcine model–the way ahead. PLoS ONE 2014, 9, e111591. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, H.; Yagi, H.; Tajima, K.; Okamoto, K.; Yoshitake, A.; Aeba, R.; Kudo, M.; Kashima, I.; Kawaguchi, S.; Hirano, A. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-F.; Chau, E.; Cabello, R.; Yeh, A.T.; Sampaio, L.C.; Gobin, A.S.; Taylor, D.A. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater. 2017, 49, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, M.J.; Knutson, C.C.; Momtahan, N.; Cook, A.D. Extracellular matrix from whole porcine heart decellularization for cardiac tissue engineering. In Decellularized Scaffolds and Organogenesis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 95–102. [Google Scholar]
- Akhyari, P.; Oberle, F.; Hülsmann, J.; Heid, H.; Lehr, S.; Barbian, A.; Nakanishi, S.; Aubin, H.; Jenke, A.; Lichtenberg, A. Characterization of the epicardial adipose tissue in decellularized human-scaled whole hearts: Implications for the whole-heart tissue engineering. Tissue Eng. Part A 2018, 24, 682–693. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Khorramirouz, R.; Ghorbani, F.; Beigi, R.S.H.; Hashemi, J.; Kajbafzadeh, A.-M. Preparation and characterization of human size whole heart for organ engineering: Scaffold microangiographic imaging. Regen. Med. 2019, 14, 939–954. [Google Scholar] [CrossRef]
- Remlinger, N.T.; Wearden, P.D.; Gilbert, T.W. Procedure for decellularization of porcine heart by retrograde coronary perfusion. JoVE (J. Vis. Exp.) 2012, 70, e50059. [Google Scholar] [CrossRef] [PubMed]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng. Part C Methods 2013, 19, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J. Bioengineering human myocardium on native extracellular matrix. Circ. Res. 2016, 118, 56–72. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Cabello, R.; Elgalad, A.; Parikh, R.; Wood, R.P.; Myer, K.A.; Yeh, A.T.; Lee, P.-F. Decellularization of whole human heart inside a pressurized pouch in an inverted orientation. JoVE (J. Vis. Exp.) 2018, 141, e58123. [Google Scholar] [CrossRef] [PubMed]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Murry, C.E. Cardiac regeneration using pluripotent stem cells—Progression to large animal models. Stem Cell Res. 2014, 13, 654–665. [Google Scholar] [CrossRef]
- Taylor, D.A.; Frazier, O.H.; Elgalad, A.; Hochman-Mendez, C.; Sampaio, L.C. Building a total bioartificial heart: Harnessing nature to overcome the current hurdles. Artif. Organs 2018, 42, 970–982. [Google Scholar] [CrossRef]
- Hülsmann, J.; Aubin, H.; Wehrmann, A.; Lichtenberg, A.; Akhyari, P. The impact of left ventricular stretching in model cultivations with neonatal cardiomyocytes in a whole-heart bioreactor. Biotechnol. Bioeng. 2017, 114, 1107–1117. [Google Scholar] [CrossRef]
- Hülsmann, J.; Aubin, H.; Sugimura, Y.; Lichtenberg, A.; Akhyari, P. Electrophysiological stimulation of whole heart constructs in an 8-pole electrical field. Artif. Organs 2018, 42, E391–E405. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Fink, C.; Remmers, U.; Scholz, H.; Wattchow, J.; Weil, J.; Zimmermann, W.; Dohmen, H.H.; Schäfer, H.; Bishopric, N. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart muscle model system. FASEB J. 1997, 11, 683–694. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Fink, C.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 2000, 68, 106–114. [Google Scholar] [CrossRef]
- Mei, X.; Cheng, K. Recent development in therapeutic cardiac patches. Front. Cardiovasc. Med. 2020, 7, 610364. [Google Scholar] [CrossRef]
- Chan, G.; Mooney, D.J. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008, 26, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Borazjani, A.; Tahai, M.; de Jongh Curry, A.L.; Simionescu, D.T.; Guan, J.; To, F.; Elder, S.H.; Liao, J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J. Biomed. Mater. Res. Part A 2010, 94, 1100–1110. [Google Scholar] [CrossRef]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Baruch Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Riabov Bassat, D. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, C.; Martinez-Fernandez, A.; Beraldi, R.; Nelson, T.J. Embryonic decellularized cardiac scaffold supports embryonic stem cell differentiation to produce beating cardiac tissue. Int. Sch. Res. Not. 2014, 2014, 625164. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Nemeno, J.; Yang, W.; Yoon, J.; Lee, S.; Lee, J. Natural cardiac extracellular matrix sheet as a biomaterial for cardiomyocyte transplantation. Transplant. Proc. 2015, 47, 751–756. [Google Scholar]
- Silva, A.; Rodrigues, S.; Caldeira, J.; Nunes, A.; Sampaio-Pinto, V.; Resende, T.; Oliveira, M.; Barbosa, M.; Thorsteinsdóttir, S.; Nascimento, D. Three-dimensional scaffolds of fetal decellularized hearts exhibit enhanced potential to support cardiac cells in comparison to the adult. Biomaterials 2016, 104, 52–64. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Bai, A.; Jiang, W.; Li, X.; Wang, X.; Mao, Y.; Lu, C.; Qian, R.; Guo, F. Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials 2016, 105, 52–65. [Google Scholar] [CrossRef]
- Hong, X.; Yuan, Y.; Sun, X.; Zhou, M.; Guo, G.; Zhang, Q.; Hescheler, J.; Xi, J. Skeletal extracellular matrix supports cardiac differentiation of embryonic stem cells: A potential scaffold for engineered cardiac tissue. Cell. Physiol. Biochem. 2018, 45, 319–331. [Google Scholar] [CrossRef]
- Hochman-Mendez, C.; Pereira de Campos, D.B.; Pinto, R.S.; Mendes, B.J.d.S.; Rocha, G.M.; Monnerat, G.; Weissmuller, G.; Sampaio, L.C.; Carvalho, A.B.; Taylor, D.A. Tissue-engineered human embryonic stem cell-containing cardiac patches: Evaluating recellularization of decellularized matrix. J. Tissue Eng. 2020, 11, 2041731420921482. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.L.; de Carvalho, P.H.; Gomes, D.A.; Goes, A.M. Characterization of decellularized heart matrices as biomaterials for regular and whole organ tissue engineering and initial in-vitro recellularization with ips cells. J. Tissue Sci. Eng. 2012, 11, 002. [Google Scholar] [CrossRef] [PubMed]
- Eitan, Y.; Sarig, U.; Dahan, N.; Machluf, M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: In vitro cell support, remodeling, and biocompatibility. Tissue Eng. Part C Methods 2010, 16, 671–683. [Google Scholar] [CrossRef]
- Wainwright, J.M.; Hashizume, R.; Fujimoto, K.L.; Remlinger, N.T.; Pesyna, C.; Wagner, W.R.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs 2012, 195, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kc, P.; Zhang, G. In vivo assessment of decellularized porcine myocardial slice as an acellular cardiac patch. ACS Appl. Mater. Interfaces 2019, 11, 23893–23900. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; To, F.; Butler, J.R.; Claude, A.; McLaughlin, R.M.; Williams, L.N.; de Jongh Curry, A.L.; Liao, J. Myocardial scaffold-based cardiac tissue engineering: Application of coordinated mechanical and electrical stimulations. Langmuir 2013, 29, 11109–11117. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Uriarte, J.J.; Prat-Vidal, C.; Gálvez-Montón, C.; Roura, S.; Llucià-Valldeperas, A.; Soler-Botija, C.; Farré, R.; Navajas, D.; Bayes-Genis, A. In vitro comparative study of two decellularization protocols in search of an optimal myocardial scaffold for recellularization. Am. J. Transl. Res. 2015, 7, 558. [Google Scholar]
- Blazeski, A.; Kostecki, G.M.; Tung, L. Engineered heart slices for electrophysiological and contractile studies. Biomaterials 2015, 55, 119–128. [Google Scholar] [CrossRef]
- Schwan, J.; Kwaczala, A.T.; Ryan, T.J.; Bartulos, O.; Ren, Y.; Sewanan, L.R.; Morris, A.H.; Jacoby, D.L.; Qyang, Y.; Campbell, S.G. Anisotropic engineered heart tissue made from laser-cut decellularized myocardium. Sci. Rep. 2016, 6, 32068. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kc, P.; Copeland, K.M.; Liao, J.; Zhang, G. A thin layer of decellularized porcine myocardium for cell delivery. Sci. Rep. 2018, 8, 16206. [Google Scholar] [CrossRef] [PubMed]
- Sarig, U.; Au-Yeung, G.C.; Wang, Y.; Bronshtein, T.; Dahan, N.; Boey, F.Y.; Venkatraman, S.S.; Machluf, M. Thick acellular heart extracellular matrix with inherent vasculature: A potential platform for myocardial tissue regeneration. Tissue Eng. Part A 2012, 18, 2125–2137. [Google Scholar] [CrossRef]
- Wang, B.; Tedder, M.E.; Perez, C.E.; Wang, G.; de Jongh Curry, A.L.; To, F.; Elder, S.H.; Williams, L.N.; Simionescu, D.T.; Liao, J. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012, 23, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.B.; Simionescu, A.; Simionescu, D.T. The acellular myocardial flap: A novel extracellular matrix scaffold enriched with patent microvascular networks and biocompatible cell niches. Tissue Eng. Part C Methods 2013, 19, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Sarig, U.; Nguyen, E.B.-V.; Wang, Y.; Ting, S.; Bronshtein, T.; Sarig, H.; Dahan, N.; Gvirtz, M.; Reuveny, S.; Oh, S.K. Pushing the envelope in tissue engineering: Ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng. Part A 2015, 21, 1507–1519. [Google Scholar] [CrossRef]
- Sarig, U.; Sarig, H.; de-Berardinis, E.; Chaw, S.-Y.; Nguyen, E.B.; Ramanujam, V.S.; Thang, V.D.; Al-Haddawi, M.; Liao, S.; Seliktar, D. Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater. 2016, 44, 209–220. [Google Scholar] [CrossRef]
- Kc, P.; Shah, M.; Liao, J.; Zhang, G. Prevascularization of decellularized porcine myocardial slice for cardiac tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 2196–2204. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Tafti, S.H.A.; Sabetkish, S.; Hassannejad, Z.; Kajbafzadeh, A.-M. Coronary-Based Right Heart Flap Recellularization by Rat Neonatal Whole Cardiac Cells: A Viable Sheep Cardiac Patch Model for Possible Management of Heart Aneurysm. Regen. Eng. Transl. Med. 2022, 8, 425–436. [Google Scholar] [CrossRef]
- Oberwallner, B.; Brodarac, A.; Choi, Y.H.; Saric, T.; Anić, P.; Morawietz, L.; Stamm, C. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J. Biomed. Mater. Res. Part A 2014, 102, 3263–3272. [Google Scholar] [CrossRef]
- Di Meglio, F.; Nurzynska, D.; Romano, V.; Miraglia, R.; Belviso, I.; Sacco, A.M.; Barbato, V.; Di Gennaro, M.; Granato, G.; Maiello, C. Optimization of human myocardium decellularization method for the construction of implantable patches. Tissue Eng. Part C Methods 2017, 23, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.-H.; Stamm, C. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur. J. Cardio-Thorac. Surg. 2015, 47, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; De Oñate, L.; Fernández-Santos, M.E.; Oria, R.; Tarantino, C.; Climent, A.M.; Marco, A.; Samitier, M.; Martínez, E.; Valls-Margarit, M. Myocardial commitment from human pluripotent stem cells: Rapid production of human heart grafts. Biomaterials 2016, 98, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Rhee, J.-W.; Wu, J.C. Adult stem cell therapy and heart failure, 2000 to 2016: A systematic review. JAMA Cardiol. 2016, 1, 831–841. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Zeng, L.; Hu, Q.; Wang, X.; Mansoor, A.; Lee, J.; Feygin, J.; Zhang, G.; Suntharalingam, P.; Boozer, S.; Mhashilkar, A. Bioenergetic and functional consequences of bone marrow–derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 2007, 115, 1866–1875. [Google Scholar] [CrossRef]
- Huang, S.; Yang, Y.; Yang, Q.; Zhao, Q.; Ye, X. Engineered circulatory scaffolds for building cardiac tissue. J. Thorac. Dis. 2018, 10, S2312. [Google Scholar] [CrossRef]
- Radisic, M.; Malda, J.; Epping, E.; Geng, W.; Langer, R.; Vunjak-Novakovic, G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 2006, 93, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Korecky, B.; Hai, C.; Rakusan, K. Functional capillary density in normal and transplanted rat hearts. Can. J. Physiol. Pharmacol. 1982, 60, 23–32. [Google Scholar] [CrossRef]
- Ruzza, A.; Czer, L.S.; Arabia, F.; Vespignani, R.; Esmailian, F.; Cheng, W.; De Robertis, M.A.; Trento, A. Left ventricular reconstruction for postinfarction left ventricular aneurysm: Review of surgical techniques. Tex. Heart Inst. J. 2017, 44, 326. [Google Scholar] [CrossRef]
- Beck, C.S. Operation for aneurysm of the heart. Ann. Surg. 1944, 120, 34. [Google Scholar] [CrossRef]
- Likoff, W.; Bailey, C.P. Ventriculoplasty: Excision of myocardial aneurysm: Report of a successful case. J. Am. Med. Assoc. 1955, 158, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Levinsky, L.; Arani, D.; Raza, S.; Kohn, R.; Schimert, G. Dacron patch enlargement of anterior wall of left ventricle after aneurysmectomy with concomitant infarctectomy. J. Thorac. Cardiovasc. Surg. 1979, 77, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Svystonyuk, D.A.; Mewhort, H.E.; Hassanabad, A.F.; Heydari, B.; Mikami, Y.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Wagner, K.T.; Tarraf, S.A. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury. Sci. Rep. 2020, 10, 9459. [Google Scholar] [CrossRef]
- Di Franco, S.; Amarelli, C.; Montalto, A.; Loforte, A.; Musumeci, F. Biomaterials and heart recovery: Cardiac repair, regeneration and healing in the MCS era: A state of the “heart”. J. Thorac. Dis. 2018, 10, S2346. [Google Scholar] [CrossRef] [PubMed]
- Banyasz, T.; Lozinskiy, I.; Payne, C.E.; Edelmann, S.; Norton, B.; Chen, B.; Chen-Izu, Y.; Izu, L.T.; Balke, C.W. Transformation of adult rat cardiac myocytes in primary culture. Exp. Physiol. 2008, 93, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Peters, N.S.; Severs, N.J.; Rothery, S.M.; Lincoln, C.; Yacoub, M.H.; Green, C.R. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 1994, 90, 713–725. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.-Z.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Hillebrandt, K.H.; Everwien, H.; Haep, N.; Keshi, E.; Pratschke, J.; Sauer, I.M. Strategies based on organ decellularization and recellularization. Transpl. Int. 2019, 32, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.G.; Lee, E.L.; Backman, D.; Buczek-Thomas, J.A.; Emani, S.; Wong, J.Y. Engineering myocardial tissue patches with hierarchical structure–function. Ann. Biomed. Eng. 2015, 43, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; Fast, V.G.; Sethu, P. Biomimetic cardiac tissue model enables the adaption of human induced pluripotent stem cell cardiomyocytes to physiological hemodynamic loads. Anal. Chem. 2016, 88, 9862–9868. [Google Scholar] [CrossRef]
- Liau, B.; Christoforou, N.; Leong, K.W.; Bursac, N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials 2011, 32, 9180–9187. [Google Scholar] [CrossRef]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.-Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef]
- Monteiro, L.M.; Vasques-Nóvoa, F.; Ferreira, L.; Pinto-do-Ó, P.; Nascimento, D.S. Restoring heart function and electrical integrity: Closing the circuit. NPJ Regen. Med. 2017, 2, 9. [Google Scholar] [CrossRef]
- Le, T.; Chong, J. Cardiac progenitor cells for heart repair. Cell Death Discov. 2016, 2, 16052. [Google Scholar] [CrossRef]
- Jung, J.-H.; Fu, X.; Yang, P.C. Exosomes generated from iPSC-derivatives: New direction for stem cell therapy in human heart diseases. Circ. Res. 2017, 120, 407–417. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C. Embryonic stem cell–derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Mayourian, J.; Ceholski, D.K.; Gorski, P.A.; Mathiyalagan, P.; Murphy, J.F.; Salazar, S.I.; Stillitano, F.; Hare, J.M.; Sahoo, S.; Hajjar, R.J. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ. Res. 2018, 122, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Santoso, M.R.; Mahmoudi, M.; Shukla, P.; Wang, L.; Bennett, M.; Goldstone, A.B.; Wang, M.; Fukushi, M.; Ebert, A.D. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ. Res. 2017, 121, e22–e36. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ozpinar, E.W.; Su, T.; Tang, J.; Shen, D.; Qiao, L.; Hu, S.; Li, Z.; Liang, H.; Mathews, K. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12, eaat9683. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.E.S.; Ali, M.A.; Ghanem, H.G.; Shehata, A.I.; Abdelgawad, A.A.; Handal, H.R.; Talaat, K.A.; Ashaal, A.E.; El-Shal, A.S. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J. Gene Med. 2017, 19, e2995. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef]
- Cui, H.; Liu, C.; Esworthy, T.; Huang, Y.; Yu, Z.-x.; Zhou, X.; San, H.; Lee, S.-j.; Hann, S.Y.; Boehm, M.; et al. 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv. 2020, 6, eabb5067. [Google Scholar] [CrossRef]
| Animal Model | Decellularization Agent | Cell Source | Major Result |
|---|---|---|---|
| Rat [79] | 1% SDS; 1% Triton-X100 | Neonatal rat CMs; fibrocytes; endothelial cells; smooth muscle cells; rat aortic endothelial cells | Sacromeric-α-actin, cardiac α-myosin heavy chain, and Connexin-43 were expressed after the recellularization of the whole heart with cardiac cells. However, the implanted tissue-engineered heart produced only 2% of adult rat heart function, insufficient pumping for life. |
| Mouse [81] | 1% SDS; 1% Triton-X100 | Human embryonic stem cells (hESCs); human mesendodermal cells (hMECs) derived from hESCs | Differentiation of the hESCs and hMECs, which were differentiated from hESCs in the decellularized scaffold after recellularization, was approved by upregulated expression of cardiac markers such as cTnT and Nkx2.5 upon differentiation. Part of the stem cells was differentiated to CD31 positive cells. There is no beating in the recellularized scaffold. |
| Rat [82] | SDS; sodium deoxycholate (SDC); glycerol+ saponin | Mouse myoblast cell line (C2C12) | Introducing a new protocol and comparing it with three published protocols for rat whole-heart decellularization in terms of remaining residual DNA content, preservation of ECM, and viability of C2C12 myoblasts after reseeding into the ECM. Results showed that none of the scaffolds were entirely free of cellular components and could not keep the ECM structure. |
| Rat [83] | 1% SDS | - | The mechanical characteristics of the right ventricles of the hearts were examined after the decellularization process, and the presented decellularized cECM had notably higher stiffness compared to the native myocardium tissue. |
| Rat [84] | 1% SDS; 1% Triton-X100 | Canine blood outgrowth endothelial cells | Rat hearts were decellularized and cryopreserved at −80 °C with 10% DMSO in PBS, and the recellularization process with canine ECs completed after 1 year. On day 9 of the culture, seeded cells in the lumen showed viability and well-attached morphology. Long intervals between decellularization and reseeding might be a predictor of viability for commercial scales. |
| Rat [71] | 1% SDS; 1% Triton-X100 | RAECs | This is the first study to re-endothelialize whole decellularized hearts through both arterial and venous beds and cavities. Scaffold vessel re-endothelialization with RAECs was increased in the combination of brachiocephalic artery (BA) + inferior vena cava (IVC) delivery strategy in comparison with single-route strategies. |
| Mouse [85] | 0.02% Trypsin; 0.05% ethylenediaminetetraacetic acid (EDTA); 0.05% NaN3; 1% SDS; 3% Triton X-100; 4% deoxycholic acid (DCA) | Human iPSC-derived cardiac progenitor cells | The hearts beat on day 20 of the recellularization of a whole mouse heart with cardiac multipotent stem cells derived from human iPSCs (Y1-iPSCs). Differentiation into cardiac myocytes, smooth muscle cells, and ECs on the ECM was confirmed, and it was presented that ECM provokes the proliferation of cardiac myocytes. |
| Rat [94] | sodium lauryl ester sulfate (SLES); 1% TritonX-100; 500 U/mL DNase | - | A novel detergent, sodium lauryl ether sulfate (SLES), was used to compare with SDS for decellularization. The resulting ECMs from SLES-treatment were better preserved in comparison with ECMs from SDS-treatment. Mesenteric transplantation of both ECM revealed SLES did not elicit an immune response, unlike SDS. |
| Rat [86] | 80% glycerol; 0.9% NaCl; 0.05% NaN3, 25 mM; EDTA, 4.2% sodium deoxycholate;1% SDS; 3% Triton X-100 | Neonatal rat cardiac cells | Cardiac markers (α-actinin, cTnI, connexin 43), endothelial marker (vWF), and proliferation marker (ki67) were positive after reseeding whole decellularized rat hearts with neonatal rat cardiac cells. Within 2 days after repopulation, the heart began beating. This model only accounts for about 36% of the mass of an adult rat heart. |
| Neonatal mouse [87] | 1% SDS; 1% Triton-X100 | Neonatal murine cardiomyocyte | Reseeding of the decellularized neonatal mouse heart with neonatal murine cardiomyocyte showed positive cells for both NKX2.5 and α-actinin, expressed by cardiac progenitor cells and differentiated CMs, respectively. Even after these cells undergo prolonged culture continue to express cardiomyocyte markers. So, the neonatal matrices have enormous potential to be a novel construct for repopulation. |
| Rat [90] | 1% SDS | - | Comparing two groups of rat hearts, one decellularized with pulsatile perfusion, the other with non-pulsatile perfusion showed significantly lower residual DNA content in the scaffolds developed by the pulsatile perfusion, which is attributed to the higher hemodynamic energy of pulsatile perfusion. There are no significant differences between the groups in collagen and GAG contents. |
| Rat [88] | 1% SDS; endonuclease in Hank’s balanced salt solution (25 U/mL); ultra-pure water; collagenase IV solution | HEK293; primary human cardiac FBs; human iPS-derived cardiac progenitor cells (iPS-CPCs) | Four-Flow cannulation is adjusted for whole heart decellularization and recellularization through cannulating the ascending aorta (AA), superior vena cava (SV), pulmonary artery (PA), and pulmonary vein (PV). After the recellularization process, HEK293 cells seen around the vascular structures and seeded primary hcFBs were able to migrate and attach to the scaffolds. Growth of cell patches can be seen macroscopically when the h-iPS-CPCs were perfused to the scaffold. |
| Rat [91] | Protease inhibition; antioxidation; 0.5% SDS, 1% Triton-X100 | - | By applying protease inhibition, antioxidation, and excitation−contraction uncoupling in simultaneous perfusion/submersion modality, as a novel decellularization method, detergent concentration and detergent exposure time were reduced. |
| Mouse [92] | 1% SDS; 1% Triton-X100 | Induced cardiac progenitor cells (iCPCs) | After 3 weeks of repopulation of the whole decellularized heart with iCPCs, cells began migrating, colonizing, and finally differentiating in a scaffold, as demonstrated by the detection of cardiac actin-positive CMs, SMA-positive smooth muscle cells, and CD31+. Electrically functional cardiomyocyte clusters have appeared in the scaffold under field stimulation. |
| Rat [88] | 1% SDS | Human mesenchymal stem cells (hMSCs) | The detergent exposure time was decreased by using an electric field in the decellularization protocol. To repopulate the decellularized ECM, hMSCs differentiated iCMs with 5-azacytidine (5-aza), and these cells were similar to CMs and arranged in fibers. |
| Rabbit [93] | Hypertonic (500 mM) saline; hypotonic (20 mM) saline; 1% SDS | Human iPSC-derived ECs; hiPSCs-derived cardiac cells (CCs) | The whole decellularized rabbit heart was reendothelialized by ECs and recellularized by CCs. The cells (ECs and CCs) recovered 92.6% ± 18.4% of LV wall thickness after 60 days. Cardiomyocyte maturation was confirmed by decreasing the percentage of cells positive for progenitor markers. Recellularized hearts exhibited visible beating. Results revealed maintaining vessel patency after transplanting this heart to the femoral artery bed of a pig and starting perfusion. |
| Animal Model | Decellularization Agent | Cell Source | Major Result |
|---|---|---|---|
| Porcine [95] | 0.02% Trypsin/0.05% EDTA; 0.05% NaN3 solution; 3% Triton X-100/0.05% EDTA; 4% DCA solution | White leghorn chicken embryonic CMs | Using pulsatile retrograde aortic perfusion for 10 h resulted in whole porcine decellularized heart with intact ECM component. Lyophilized cECM sheets were repopulated with 500,000 cells/cm2 white leghorn chicken embryonic CMs. The development of organized chicken cardiomyocyte sarcomere structure was supported by cECM. |
| Porcine [96] | 4% SDS | - | The Langendorff perfusion decellularization model was improved by adding a heat exchanger to keep the temperature of perfusion fluids at 37 °C. A pressure transducer can control the roller pump through a computer system. The decellularized scaffolds produced by this system were cell-free, while their ECM components were preserved. |
| Porcine [103] | 0.02% Trypsin/0.05% ethylenediaminetetraacetic acid; 0.05% NaN3 solution; 3% Triton X-100/0.05% ethylenediaminetetraacetic acid; 4% DCA solution | - | By increasing the perfusion speeds greater than 2 L/min, exposure time of the tissue to detergent was minimized. The loss of cardiac muscle bundles in decellularized scaffolds was confirmed, while ECM substances and micro-architecture remained. |
| Porcine [104] | Protocol I: 0.02% Trypsin/0.05% EDTA/0.05% NaN3 for 3 days and 3% Triton X-100/0.05% EDTA/0.05% NaN3 for 4 days; Protocol II: 0.02% Trypsin/0.05% EDTA/0.05% NaN3 for 1 day and 3% Triton X-100/0.05% EDTA/0.05% NaN3 for 6 days; Protocol III: 0.02% Trypsin/0.05% EDTA/0.05% NaN3 for 7 days; Protocol IV: 3% Triton X-100/0.05% EDTA/0.05% NaN3 for 7 days | - | Comparing three decellularization protocols showed that using Trypsin only and Triton only could not fully remove nuclear materials. In Triton only group, collagen network was saved more as same as elastin, which could contribute to the increase in the compressive modulus in this group. These data revealed that, to characterize the structural and mechanical properties of decellularized scaffolds, noninvasive and nondestructive methods, such as multiphoton microscopy (MPM) combined with image correlation spectroscopy (ICS), could be used. |
| Porcine [97] | 4% SDS | Human umbilical cord- derived endothelial cells (HUVEC); neonatal rat cardiomyocyte | After reseeding the decellularized porcine hearts with neonatal rat cardiac cells and HUVEC, viable cardiac cells were detected, and coronary vasculature was reendothelialized. The electrical activity of the heart remained for up to 3 weeks in perfusion bioreactor system. |
| Porcine [98] | 1% SDS; 1% Triton X-100 | Porcine mesenchymal stem cells (pMSCs) | Two groups of whole porcine hearts: decellularized and recellularized with pMSCs, were transplanted into pigs under systemic anticoagulation treatment with heparin. On day 3, hearts from both groups were harvested. Porcine MSCs were not found in vessel lumen, and a coronary thrombosis was detected in both groups. |
| Porcine [99] | Hypertonic (500 mM NaCl); hypotonic (20 mM NaCl; 1% SDS | - | Porcine hearts were decellularized with two unique retrograde methods, inverting the heart and venting the apex. The inverted method offers a patent coronary vascular architecture with a higher coronary perfusion efficiency that can help ECM to rid of more native cells. Moreover, improved ECM preservation and correspondingly better retention of the heart shape following decellularization are also obtained from inverted method. |
| Ovine [101] | 0.75% SDS; 0.25% DCA | Neonatal rat primary cardiac FBs | After whole-heart decellularization, total protein content in EAT, as regulator of cardiac anatomy and function, was strongly reduced, and large amounts of lipids were detected in EAT, indicated by lipid staining. However, there is no donor material in other regions of heart. Incubation cardiac FBs with decellularized EAT showed a significantly diminished viability versus incubating with native EAT or unconditioned culture medium. Overall, incomplete removal of donor material can cause cytotoxic effects. |
| Porcine [100] | 0.5%. SDS; 1% Triton X-100 | - | Detergent exposure time decreased by using automated pressure-controlled bioreactor. The decellularization process is standardized by helping automated systems and effective liquid perfusing through coronary arteries achieved by pressure-controlled system. Cell components of the whole porcine heart were completely removed (98%) with only 6 h of detergent exposure in this modified bioreactor. |
| Ovine [102] | 1% SDS; 1% Triton-X100 | - | After decellularization process of whole ovine heart, different parts of the heart, such as auricle, aortic valve, left and right ventricular myocardia and chordae tendineae, were examined separately due to having a variable composition of ECM. Collagen and GAGs contents in chordae tendineae were decreased. The microvascular angiography indicated the natural 3D architecture of coronary tree was preserved. Harvested, subcutaneously implanted dECM into the omentum of Sprague–Dawley (host) rat, after 2 months, showed good vascularization and repopulation of the graft, indicated by the existence of CD31+, CD34+, and smooth muscle cells. |
| Whole Heart | Decellularization Agent | Cell Source | Major Result |
|---|---|---|---|
| Human [105] | 1%SDS | hCPC; human bone-marrow mesenchymal cells (hMSCs); HUVECs; H9c1 rat CMs; HL-1 CMs | After 21 days of repopulating of whole human heart with various cells, CMs genes were expressed by hCPCs and hMSCs, but they did not show cardiomyocyte morphology. Endocardium and vasculature were covered by HUVECs. Well-organized H9c2 and HL1 cardiomyocytes into nascent muscle bundles exhibited mature calcium dynamics and electrical coupling |
| Human [106] | 1% SDS; 1% Triton-X100 | CMs human BJ fibroblast RNA-induced pluripotent stem cells (BJ RiPS)-derived CMs; human-induced pluripotent stem cell-derived CMs | After recellularization of whole decellularized heart with human BJ RiPS-derived CMs, most of the seeded cardiomyocytes expressed cardiac markers, including sarcomeric α-actinin, cardiac troponin T, and MHC. Under biomimetic culture, the repopulated scaffolds showed electrical conductivity, developing left ventricular pressure, and metabolic function. Cadaveric and decellularized human myocardium were subcutaneously implanted into the Sprague–Dawley rats and harvested after 2 weeks. CD68+ mononuclear cells in 2 groups were detected. M2 macrophages appear in greater quantities in decellularized human myocardium in comparison with cadaveric humans in proinflammatory response |
| Human [107] | Hypertonic (500 mM NaCl); hypotonic (20 mM NaCl; 1% SDS | - | In keeping the aortic valve closed to improve myocardial decellularization perfusion system, pressurized pouch can help by generating pressure gradients across the aortic valve |
| Decellularization Agent | Cell Source | Major Result | |
|---|---|---|---|
| Mouse [120] | 0.25% SDS; 0.5 mg/mL DNase | Mouse embryonic ventricular cells; mouse ESC-derived progenitors | To optimize the differentiation and maturation of embryonic stem cells, mouse embryonic ventricular cells, and mouse ESC-derived progenitors were seeded on embryonic decellularized cECM, and ESCs were differentiated, proved by expression of endothelial, cardiac, and smooth muscle markers causing spontaneous beating after 20 h and 24 days of culture, respectively. |
| Rat [121] | 0.25% Triton X-100/10 mmol/l NH4OH | Neonatal rat CMs | Seeding neonatal rat cardiac cells on decellularized heart scaffold sheets with 10 µm thickness produced higher proliferation rates, cardiac genes, and protein expression compared with those cultured without the ECM sheets. |
| Rat [126] | 1% SDS; 1% Triton X-100 | iPS | Seeding iPS cells on the decellularized cardiac scaffold confirmed proper cell scaffold attachment, cell survival, and cell differentiation and growth; furthermore, this phenomenon is characterized by decreased expression of pluripotency markers (Oct-4 and SSEA-4) after 7 days of culture. |
| Rat and mouse [122] | 10 mM Tris HCl/0.1% EDTA; 0.2% SDS/10 mM Tris HCl; DNAse (50 U/mL) | Immortalized adult Lin-Sca1þ cardiac progenitor cells (iCPCSca-1); neonatal rat CMs | After repopulating both fetal and adult ECM matrices with cardiac progenitors and neonatal CMs, these cells migrated into the scaffolds with excellent viability. Fetal scaffolds showed better repopulation efficiency, migration, and colonization rates of seeded cells rather than adult ECM matrices. |
| Rat [123] | 1% SDS; 1% Triton X-100/ 0.5% EDTA | Human-induced pluripotent stem cell-derived CMs; human-induced pluripotent stem cell-derived CD90+ cells | Decellularized cECM supported the maturation of human iPSC-derived cardiac cells in vitro. This construct showed normal electrical properties in response to the pharmaceutical agents. After grafting this patch on the acute rat MI model, the recellularized decellularized cECM reduced the infarct size, increased the wall thickness, and promoted vascularization. |
| Mouse [124] | 0.05% Trypsin/0.02% EDTA, 1.1% NaCl, and 0.7% NaCl; 0.1% SDS; 1% Triton X-100 | Murine embryonic stem cells (mESC); murine embryonic stem cell-derived CMs | Shortage of organ donation and immunogenicity offered skeletal ECM (sECM) as a substitute for engineered cardiac tissue (ECT) strategies since the microstructure and morphological properties of sECM were similar to decellularized cECM. SECM granted the adherence, survival, proliferation, and differentiation of murine embryonic stem cells into functional cardiac microtissue with both stimulated electrical responses and normal adrenergic responses, which showed synchronized contraction within 6 days of repopulation. |
| Rat [125] | 1% SDS; 1% Triton X-100 | Human embryonic stem cell-derived CMs | Isolated decellularized left atrial (LA) and decellularized left ventricular (LV) cECM were repopulated by human embryonic stem cell-derived CMs. The myoglobin levels in the recellularized LA and LV were almost 57% and 55%, respectively, of the level in cadaveric heart tissue; this confirmed a four- to five-fold increase in myoglobin levels in both the rLA and rLV than the level in hESC-CMs cultured alone. Both tissue groups presented synchronous depolarization in non-adjacent regions, and connexin 43 was expressed by CMs. The elastic properties of the dECM scaffolds were restored, similar to that in cadaveric tissues. |
| Animal Model | Decellularization Agent | Cell Source | Major Result |
|---|---|---|---|
| Porcine [118] | 0.1% SDS/0.01% Trypsin; 1 mM phenylmethylsulfonylfluoride; 20 mg/mL RNase A/0; 2 mg/mL DNase | Porcine bone marrow mononuclear cells | After decellularization of myocardial slices with 2000µm thickness, the scaffolds were cultured with a combination of undifferentiated and differentiated bone marrow mononuclear cells toward cardiac phenotype. Attachment, viability, infiltration, and proliferation of seeded cells were guaranteed by dECM. Cardiomyocyte-like phenotype was maintained, and possible endothelialization within the scaffold was observed. By means of recellularization, stiffer mechanical response was recovered. |
| Porcine [127] | 1.1% NaCl/0.02% EDTA and 0.7% NaCl/0.02% EDTA; 0.05% Trypsin/ 0.02% EDTA; 1% Triton X-100 and 0.1% ammonium hydroxide | Sheep cardiac fibroblast; neonatal rat cardiac myocytes; rat bone marrow-derived MSCs | Porcine myocardium tissues with 3000 µm thickness were decellularized and seeded with cardiac fibroblast, resulting in the scaffold shrinkage and ECM remodeling. Reseeded scaffolds with CMs started beating few days after initial seeding, and functional cardiac markers were expressed. The seeded MSCs remained viable for 24 days in culture. |
| Porcine [135] | 1.1% NaCl and 0.7% NaCl; 0.05% Trypsin/0.02% EDTA; 1% Triton X-100/ 1% ammonium hydroxide | Rat MSCs; human umbilical vein endothelial cells | Left ventricular tissue slabs containing the LAD coronary artery and its adjacent veins were dissected. Trypsin/Triton-based perfusion procedure resulted in a nonimmunogenic and cell-supportive dECM, which was found to be more effective than stirring, sonication, or sodium dodecyl sulfate/Triton-based procedures to achieve thick dECM. The dECM scaffold with 14,600 ± 1900 µm thickness supported the attachment and long-term cell survival of rat MSCs. Monolayer of HUVECs was formed in the inner lumen of the intact vasculature of dECM. |
| Porcine [136] | 0.1% SDS/0.01% Trypsin;1 mM phenylmethylsulfonylfluoride; 20 mg/mL RNase A/0; 2 mg/mL DNase | - | By using supporting system and a rotating bioreactor, treated porcine myocardium slices with 0.1% SDS and 0.01% Trypsin solution by a frame-pin resulted in dECM scaffold with 2270 ± 380µm thickness, which is free from cells and α-Gal porcine antigens. Decellularized ECM showed stiffer tensile properties than native porcine myocardium tissue. |
| Porcine [128] | 0.02% Trypsin/0.05% EDTA/0.05% NaN3; 3% Triton X-100/0.05% EDTA/0.05% NaN3; 4% deoxycholic acid | - | Comparing porcine decellularized ECM cardiac patch with Dacron patch for reconstruction of a full-thickness RVOT in a rat model showed that the Dacron patch was encapsulated by dense fibrous tissue and a few cell infiltrations while the decellularized cECM patch remodeled into dense, cellular connective tissue that spread small islands of CMs, leading to improvement in heart function. |
| Porcine [130] | 0.1% SDS/0.01% Trypsin; 1 mM phenylmethylsulfonylfluoride and 20 mg/mL RNase A/0.2 mg/mL DNase | Rat MSCs | Porcine myocardium tissues with 3000 µm thickness were decellularized and reseeded with rat MSCs and exposed to 5-aza treatment. After 2-day culture with coordinated mechanical (20% strain) and electrical stimulation (5 V, 1 Hz), MSCs differentiated to CM-like phenotype can express sarcomeric α-actinin, myosin heavy chain, cardiac troponin T, connexin-43, and N-cadherin. |
| Porcine [137] | 30 mM EDTA; 1% SDS; 0.1 M NaOH | Rat dermal FBs; neonatal rat CMs | After decellularization of dissected flap with a 10,000 µm thickness, 5000 µm-diameter punch biopsies were taken and lyophilized. Rat dermal FBs were seeded onto the dry scaffold samples; they showed high cell viability. Following CMs seeded on dECM with 1000 µm-diameter, cells started expressing cardiac markers: sarcomeric α-actinin, myosin heavy chain, actin, and connexin43. |
| Porcine [131] | Protocol 1: 1% SDS; 1% Triton X-100; 0.1 mg/ ml DNase I Protocol2: 1.1% NaCl and 0.7% NaCl; 0.05% Trypsin/0.02% EDTA; 1% Triton X-100/ 1% ammonium hydroxide | Adipose tissue-derived progenitor cells (ATDPCs) | Porcine myocardium tissues with 3000 µm thickness were decellularized with two decellularization protocols: 1. detergent-based and 2. Trypsin and acid with Triton X-100. After one week of reseeding dECM with ATDPCs, receded scaffold from protocol 1 contained higher cell density, and differentiated cells expressed the cardiac markers GATA4, connexin43, and cardiac troponin. |
| Rat and porcine [132] | SDS; Triton X-100 | Neonatal rat ventricular cells (NRVCs) | Rat or pig ventricular tissue was sectioned into 300 µm-thick slices, decellularized, spread on coverslips, and reseeded with NRVCs. The ECM can promote cell elongation and alignment, resulting in fabrication of an anisotropic, functional tissue that could be electrically paced. |
| Porcine [138] | 1.1% NaCl and 0.7% NaCl; 0.05% Trypsin/0.02% EDTA; 1% Triton X-100/ 1% ammonium hydroxide | Bone marrow-derived MSCs; human umbilical vein endothelial cells; human ESC-derived CMs | Comparing the repopulation of thick decellularized porcine myocardium with 15,000 µm thickness by mixture of hMSCs and HUVECs using a perfusion bioreactor or static culture conditions showed higher cell infiltration through bioreactor, which can fabricate functional vascularized construct. Three days after initial human ESC-derived CMs seeding, synchronous beating was observed. |
| Porcine [139] | 1.1% NaCl/0.02% EDTA and 0.7% NaCl/0.02% EDTA; 0.05% Trypsin/ 0.02% EDTA; 1% Triton X-100 and 0.1% ammonium hydroxide | - | Porcine myocardial sections with 1500 µm thickness were decellularized and implanted in acute and chronic rat MI models. In both MI models, cECM were vascularized and induced a constructive remodeling process, as explained by increased M2/M1 macrophage phenotypic ratio. CECM promoted recruiting progenitor (GATA4+, c-kit+) and myocyte (MYLC+, TRPI+) after implanting. Recruited progenitors expressed both early and late CMs differentiation markers. |
| Porcine [132] | 1% Triton X-100, 1% SDS, and 0.5% Trypsin | Rat myocardial fibroblast (rMFs); rat neonatal CMs | Porcine myocardial slices with 2000 µm thickness were decellularized with different protocols. Compared to decellularization with Trypsin and Triton X-100, the SDS-based treatment has resulted in better ECM with the preserved component and microstructure. Reseeding of three different decellularized scaffolds with rMFs and rCMs showed the following: in SDS group, rMFs appeared to be more uniformly aligned. The long axis of the CMs in the SDS group tended to align in parallel, which contrasts the random orientation in Trypsin group. Regarding beating magnitudes, the Trypsin-treated tissue had the largest, the SDS-treated tissue had a modest projection magnitude, and there was no beating in the Triton-treated group. |
| Porcine [133] | 10 mM Tris/0.1% EDTA; 0.5% SDS; DMEM containing 10% fetal bovine serum; 0.1% peracetic acid/4% ethanol | Neonatal rat ventricular myocytes (NRVMs); human ESC-derived CMs; human-induced pluripotent stem cell-derived CMs (hESC-CMs) | After decellularization of thin myocardial sheets (150 µm thickness) prepared by laser cutter, NRVMs seeded on the decellularized porcine myocardium slice (dPMS) produced synchronously beating scaffolds and showed a striated pattern of organized sarcomeres. Another group that was seeded with hESC-CMs resulted in beating scaffolds with measurable intracellular calcium transients and maximum twitch stress of 1.7 N/mm2. Likewise, seeded dPMS with hiPSC-CM reached maximum peak stress of 6.5 mN/mm2 and twitch kinetics similar to the previously reported values regarding adult human right ventricular trabecula. |
| Porcine [140] | 1% SDS; 0.01% Triton X-100 | Human mesenchymal stem cells (hMSCs); rat adipose-derived stem cells(rASCs) | Cardiac cryosections were taken in different sizes (300, 600, and 900 μm thickness) and decellularized. When hMSCs and rASCs were cultured on top of the dPMS from one side (lateral cell seeding) and both sides (bilateral cell seeding), these dPMS supported cell attachment with high viability and induced endothelial differentiation and maturation of hMSCs and rASCs after 1 and 5 days. However, seeded bilateral 600 μm dPMS group could significantly enhance seeding efficiency, infiltration, and growth of cells. |
| Porcine [134] | 1% SDS; 0.01% Triton X-100 | Rat Adipose-derived stem cells (rASCs); pig adipose-derived stem cells (pASCs) | Thin layers (300 µm thickness) of decellularized porcine myocardium repopulated with rASCs and pASCs. Both rat and pig ASCs showed high viability and similar patterns of proliferation and infiltration within the dPMS. ASCs were delivered to the infarcted myocardium (rat model) using dPMS. After 1 week, a higher number of transplanted cells were presented in the infarcted area than in direct injection, which led to increased vascular formation within the patch. |
| Porcine [129] | 1% SDS | - | Two different thicknesses (300 and 600 μm) of dPMS were patched into the infarcted rat heart. After implantation, implanted dPMS was strongly attached to host myocardium and prevented thinning of the left ventricular (LV). Neovascularization into scaffold began soon after implantation, and a large number of host cells were recruited into the implanted dPMS. In treated rats with dPMSs, higher density of M2 macrophages was observed as compared to the MI group without treatment. Four weeks after surgery, contraction of the LV wall and cardiac functional parameters made significant progress. |
| Ovine [141] | 1% SDS | Green fluorescent protein (GFP+) rat neonatal cardiac cells | Right heart with attached pedicle and coroners was dissected and decellularized through perfusion-based method. Then, decellularized cardiac flaps were repopulated with GFP+ rat neonatal cardiac cells under dynamic culture conditions using a perfusion bioreactor. Confluent coverage of fibroblast, cardiomyocyte, endothelial, and SMCs exhibited on the recellularized myocardial flap were indicated by positive immunohistochemistry staining for CD34, Desmin, α-SMA, Vimentin, connexin43. |
| Animal Model | Decellularization Agent | Cell Source | Major Result |
|---|---|---|---|
| Human and porcine [142] | 10 mM Tris/0.1% EDTA; 0.5% SDS | Human umbilical cord blood-derived MSCs; murine iPSC-derived CMs; murine neonatal CMs | Human and porcine myocardial sections with 300 µm thickness were decellularized and cell-seeded. Murine iPSC-derived CMs showed less cell attachment, proliferation, and infiltration on the human dECM compared to MSCs. In standard culture, murine neonatal CMs contracted synchronously as it continued after seeding onto the matrix, and their beating could make strong contractions to move the whole cardiac slices |
| Human [145] | 10 mM Tris/0.1% EDTA; 0.5% SDS | Murine ESCs; murine-induced pluripotent stem cells; murine mesenchymal stromal cells | A 300 µm thick decellularized cECM prepared from human myocardium was compared with another matrix such as Matrigel or Geltrex to show their positive effect on differentiation of stem cells. Promoting cell attachment, viability, proliferation, and cardiac lineage commitment of seeded ESCs and iPSCs seeded on decellularized cECM was proved by positive immunohistochemistry staining for cardiac troponin T, heavy-chain cardiac myosin plus, mRNA expression for myosin heavy polypeptide 6, cardiac troponin T2, and NK2 homeobox 5 being significantly increased. Matrigel and Geltrex were not able to induce cardiac-specific markers. There is no evidence to show that MSCs differentiated from CM |
| Human [143] | 1% SDS | Human ESC-derived CM-like cells; human-induced pluripotent stem cell-derived CM-like cells (CLC) | Human decellularized cECM with 400 µm thickness, when cultured with hPSC-derived CLCs, could lead to differentiation and maturation of hPSC-derived CLCs toward CMs, as illustrated by positive immunofluorescence staining for alpha-sarcomeric actinin, Troponin T, MYH6, NKX2.5, and CX43. Moreover, after 10 days of culture (as more evidence for differentiation of hPSC-derived CLCs), levels of expression of different ion channels determinant for calcium homeostasis and heart contractile function enhanced Significantly. |
| Human [144] | Protocol I: lysis buffer (10 mM Tris, 0.1% wt/vol EDTA, pH 7.4); 0.5% SDS Protocol II: 10 mM Tris buffer and 0.1% wt/vol EDTA; 0.5% SDS; 50 U/mL DNase and 1 U/mL RNase Protocol III: protease inhibitors (aprotinin, 10 KIU/mL, 0.1% w/v EDTA); 10 mM Tris-HCl, 0.1% SDS Protocol IV: 1% SDS; 1% Triton-X100 Protocol IV: 1% SDS and 1% Triton-X100 together | Human cardiac primitive cells | 350μm-thick sections were cut from the human myocardium and decellularized with five protocols. The best result was detected from the one by 1% SDS and 1% Triton for 24 h. Reseeded dECM with human cardiac primitive cells has supported the differentiation of seeded cells toward CMs and SMCs, as explained by distinct gene expression for CMs (MEF2C, ACTC1) and SMCs (GATA6, ACTA2). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarzadeh, A.; Sobhani, S.; Soltani Khaboushan, A.; Kajbafzadeh, A.-M. Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises. Bioengineering 2023, 10, 106. https://doi.org/10.3390/bioengineering10010106
Akbarzadeh A, Sobhani S, Soltani Khaboushan A, Kajbafzadeh A-M. Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises. Bioengineering. 2023; 10(1):106. https://doi.org/10.3390/bioengineering10010106
Chicago/Turabian StyleAkbarzadeh, Aram, Soheila Sobhani, Alireza Soltani Khaboushan, and Abdol-Mohammad Kajbafzadeh. 2023. "Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises" Bioengineering 10, no. 1: 106. https://doi.org/10.3390/bioengineering10010106
APA StyleAkbarzadeh, A., Sobhani, S., Soltani Khaboushan, A., & Kajbafzadeh, A.-M. (2023). Whole-Heart Tissue Engineering and Cardiac Patches: Challenges and Promises. Bioengineering, 10(1), 106. https://doi.org/10.3390/bioengineering10010106







