Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy
Abstract
1. Introduction
2. Materials and Methods
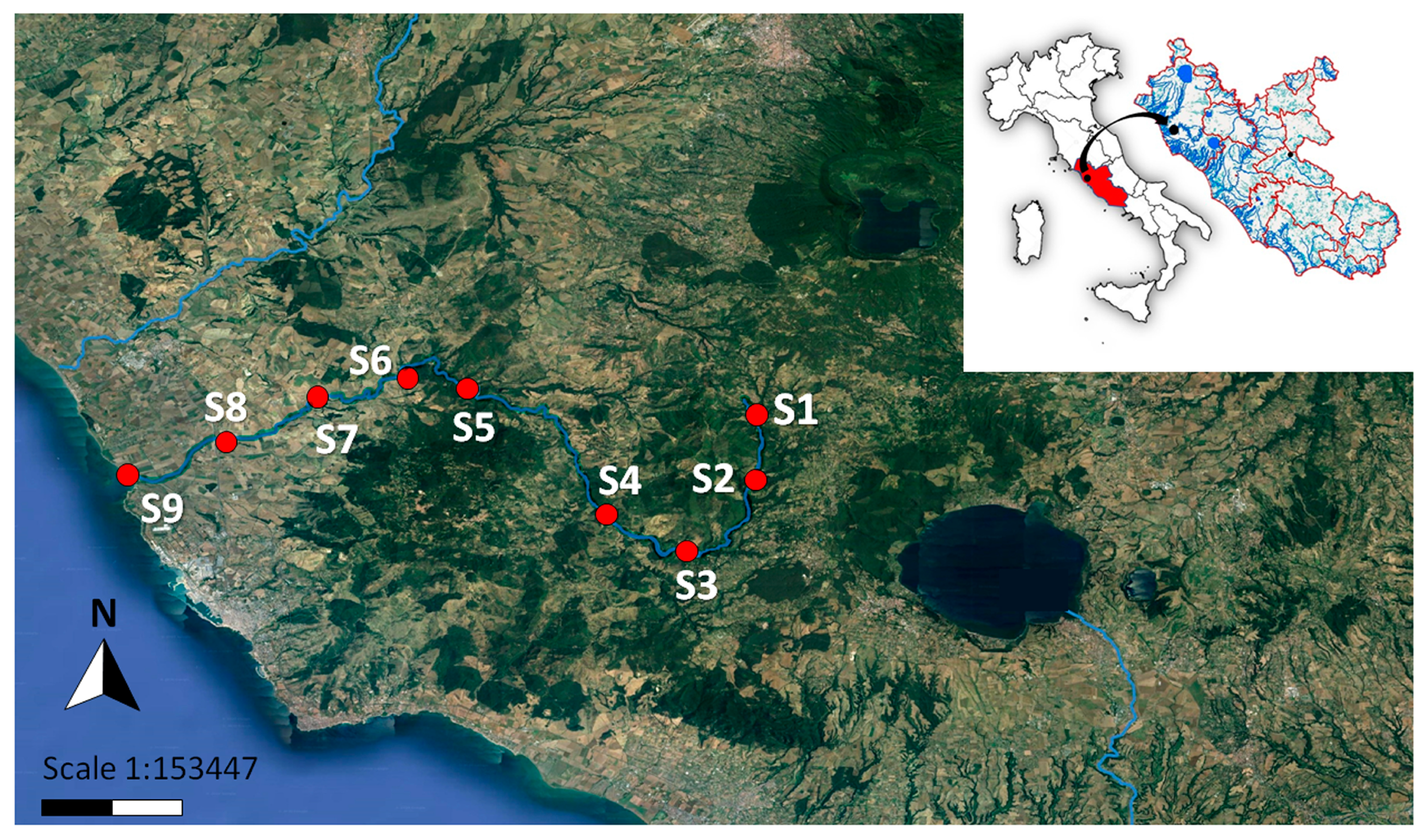
2.1. Study Area
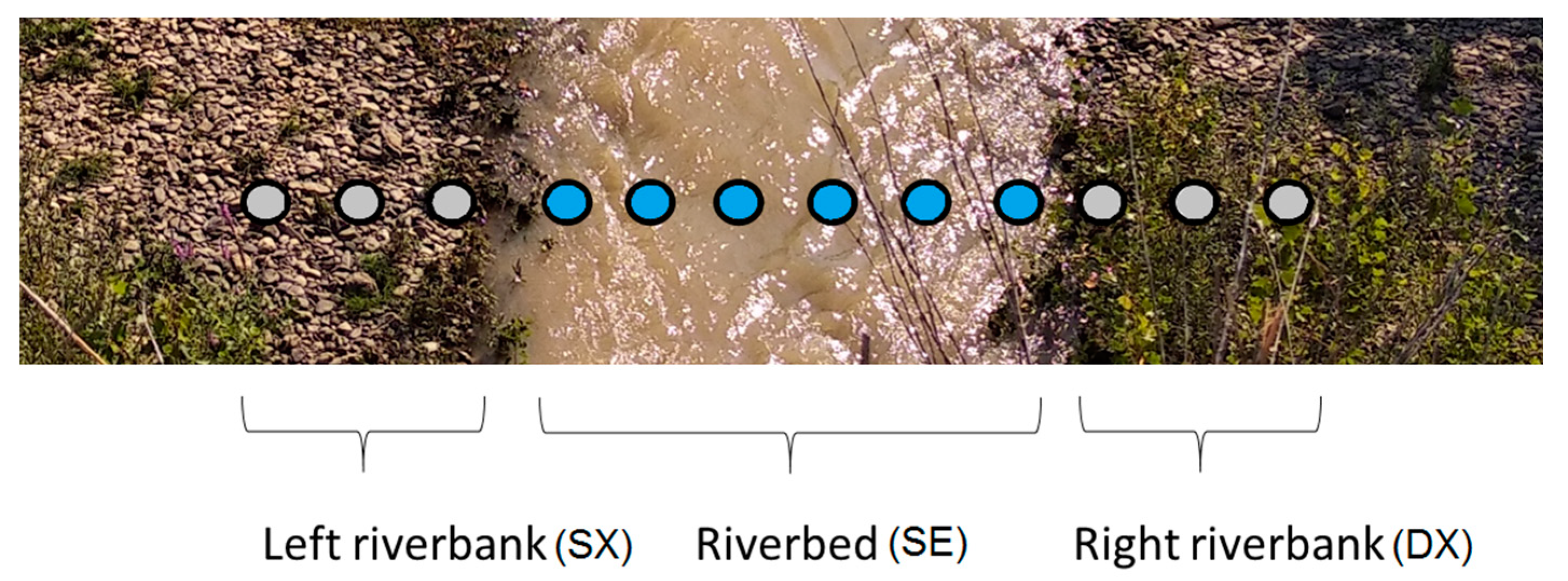
2.2. Sampling of Water, Sediments and Macroinvertebrates
2.3. Visual Detection of Plastics in Water, Sediments and Macroinvertebrates
2.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis and Polymer Identification
2.5. Quality Indices
2.6. Statistical Analyses
3. Results
3.1. Quantitative and Qualitative Description of Plastics in River Mignone
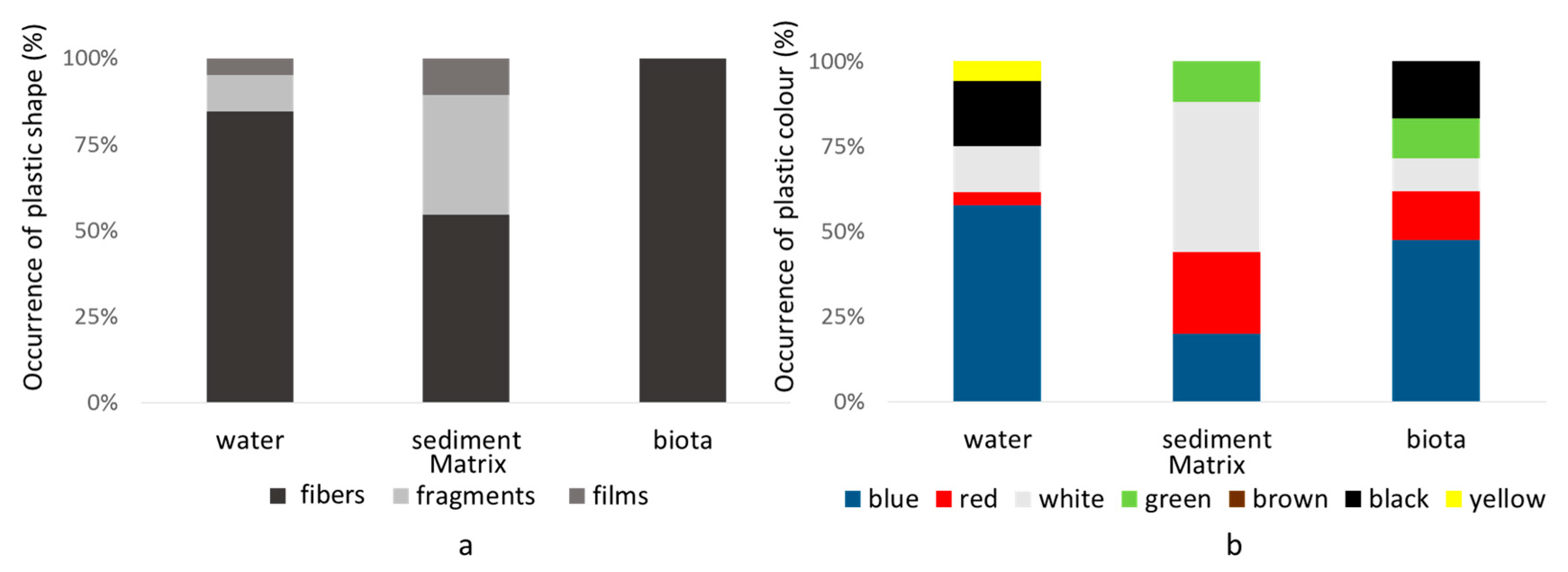
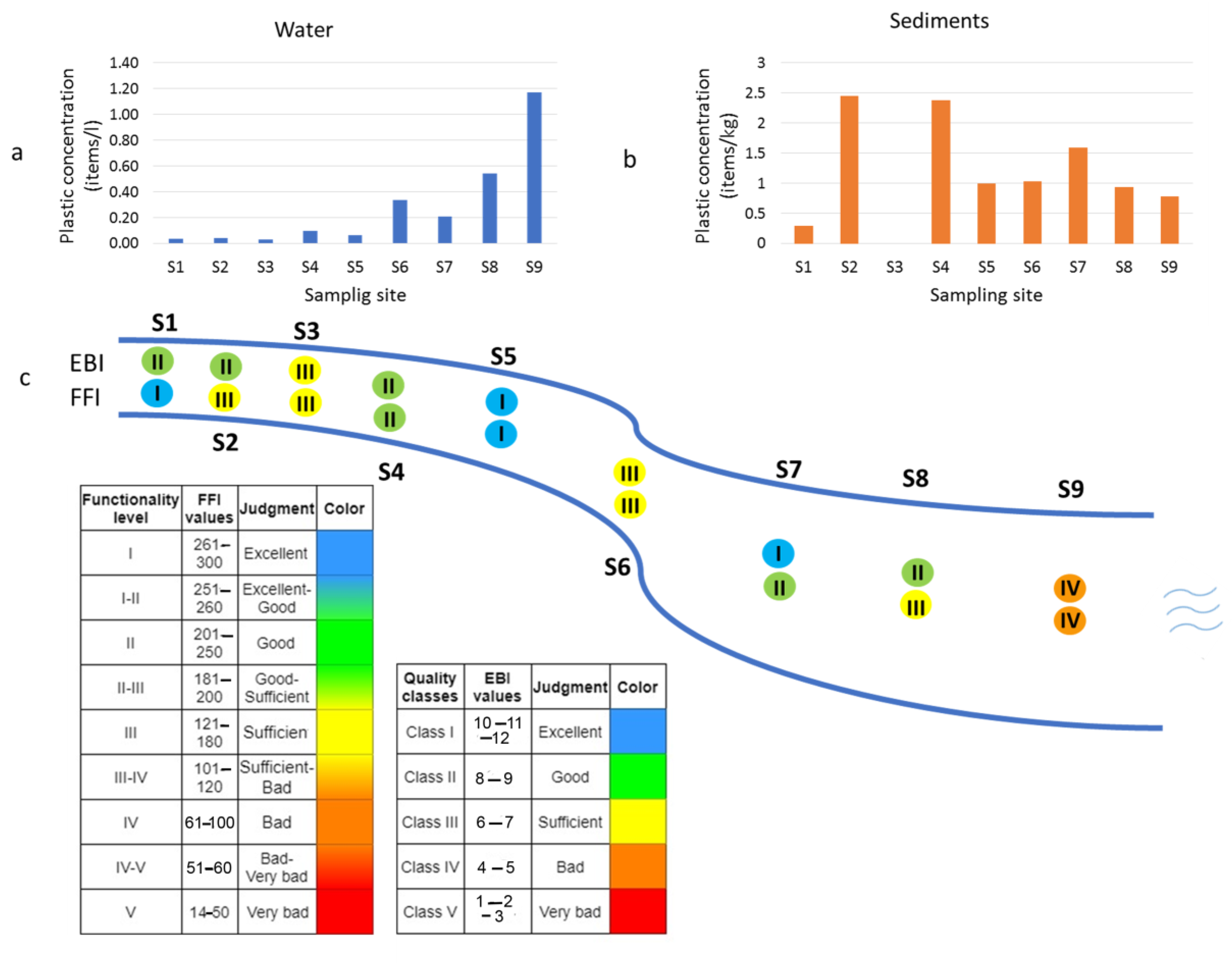
3.1.1. Plastic Items in Water
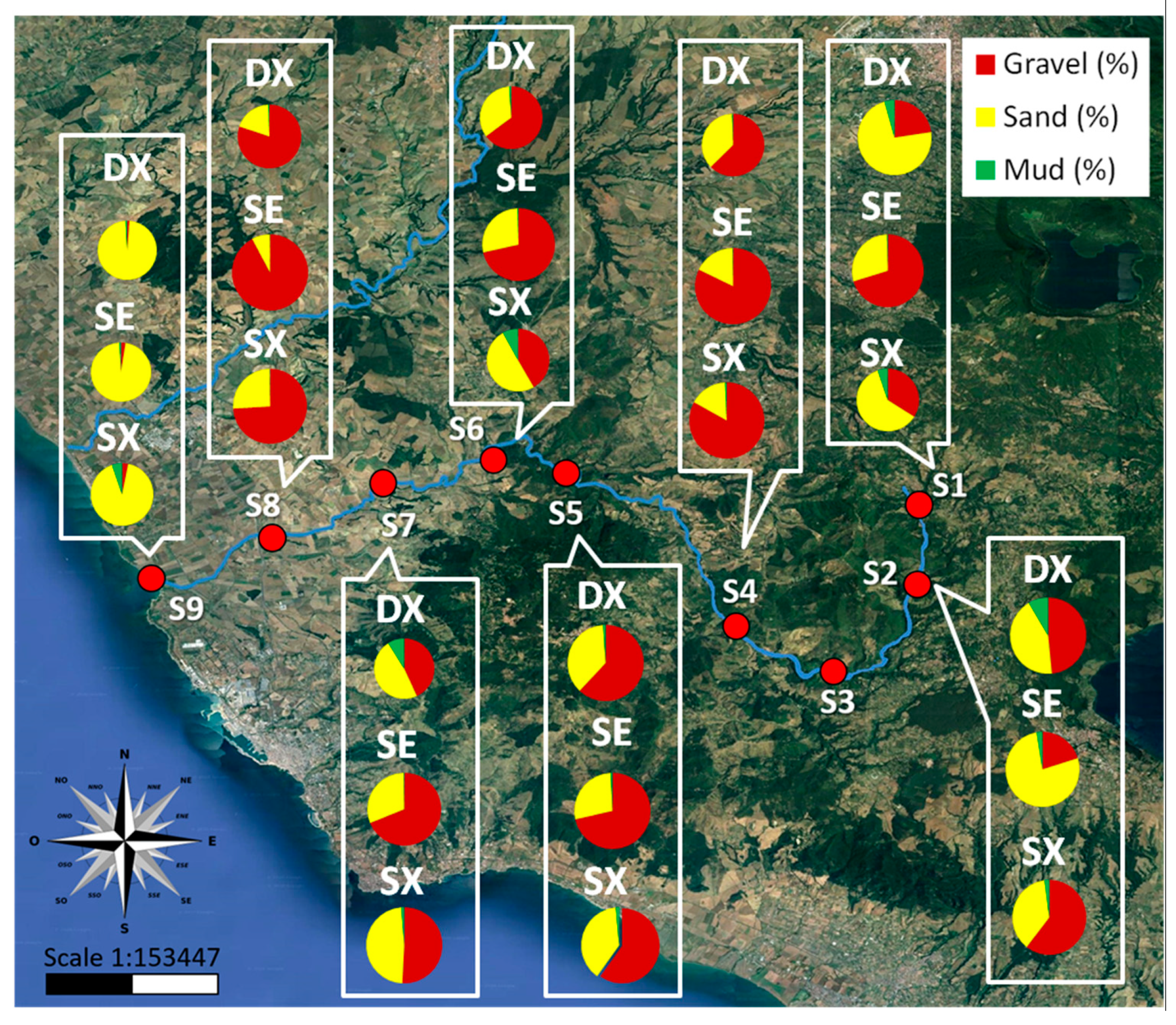
3.1.2. Plastic Items in Sediments
3.1.3. Plastic Items in Macroinvertebrates
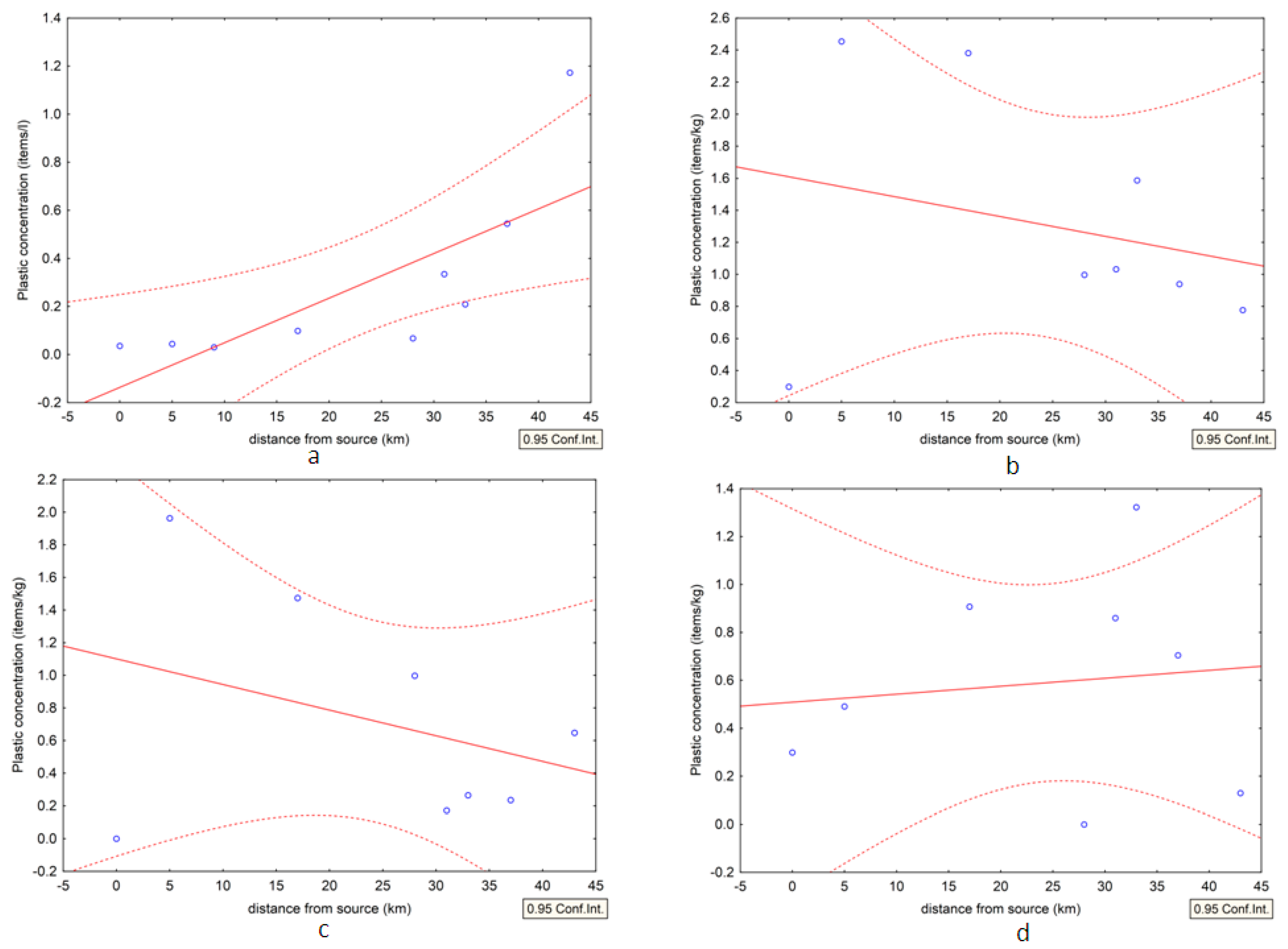
3.2. Analysis of the Distribution of Plastics
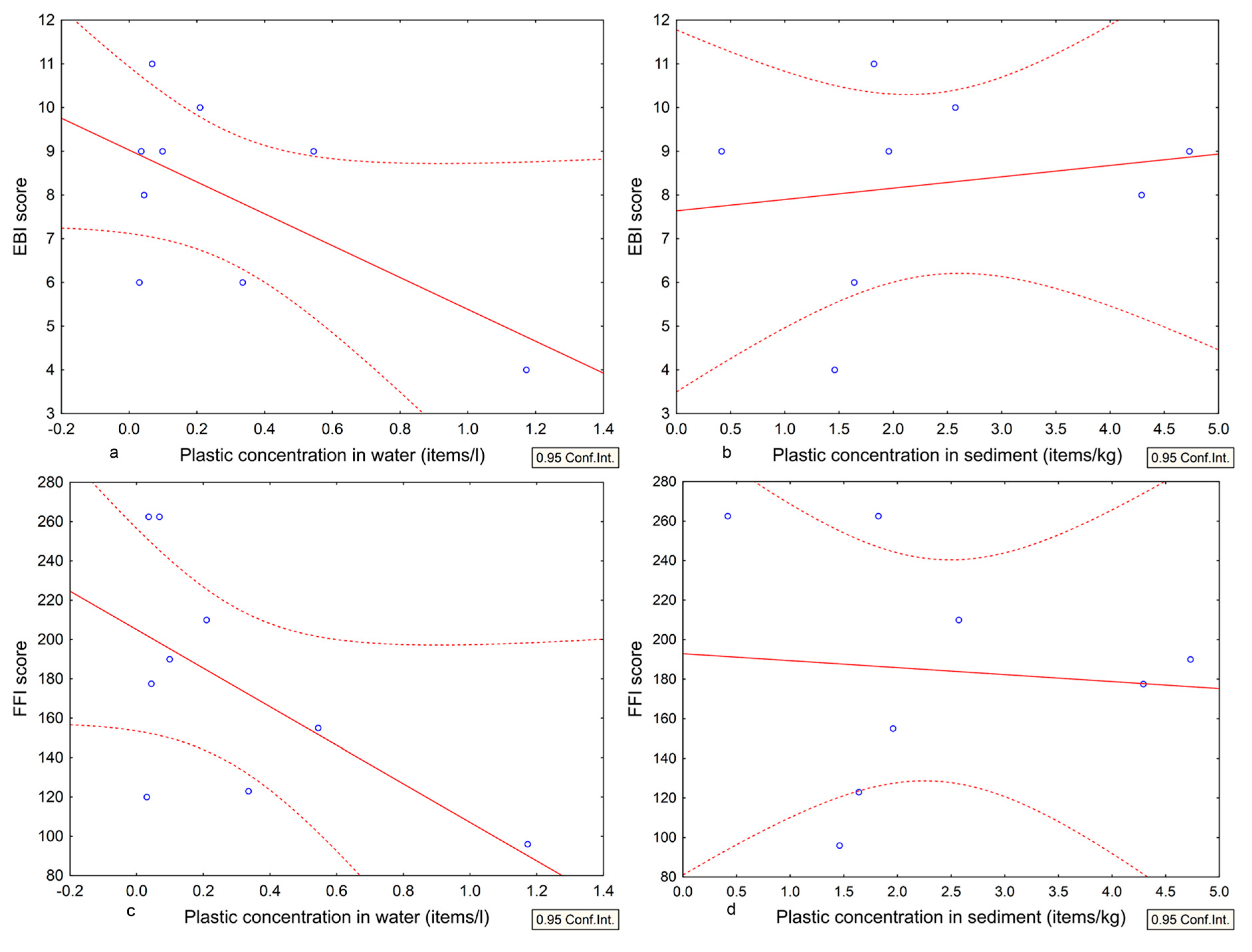
3.3. Comparison between the Results of Plastic Occurrence and Quality Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.-A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro- (nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC. Trend. Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Cera, A.; Cesarini, G.; Scalici, M. Microplastics in freshwater: What are the news from the world? Diversity 2020, 12, 276. [Google Scholar] [CrossRef]
- Atwood, E.C.; Falcieri, F.M.; Piehl, S.; Bochow, M.; Matthies, M.; Franke, J.; Carniel, F.; Sclavo, M.; Laforsch, C.; Siegert, F. Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: Comparing remote sensing and hydrodynamic modeling with in situ sample collections. Mar. Pollut. Bull. 2019, 138, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto River in southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Constant, M.; Ludwig, W.; Kerhervé, P.; Sola, J.; Charrière, B.; Sanchez-Vidal, A.; Canals, M.; Heussner, S. Microplastic fluxes in a large and a small Mediterranean river catchments: The Têt and the Rhône, Northwestern Mediterranean Sea. Sci. Total Environ. 2020, 716, 136984. [Google Scholar] [CrossRef]
- Simon-Sánchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef]
- Blašković, A.; Guerranti, C.; Fastelli, P.; Anselmi, S.; Renzi, M. Plastic levels in sediments closed to Cecina river estuary (Tuscany, Italy). Mar. Pollut. Bull. 2018, 135, 105–109. [Google Scholar] [CrossRef]
- Guerranti, C.; Cannas, S.; Scopetani, C.; Fastelli, P.; Cincinelli, A.; Renzi, M. Plastic litter in aquatic environments of Maremma Regional Park (Tyrrhenian Sea, Italy): Contribution by the Ombrone river and levels in marine sediments. Mar. Pollut. Bull. 2017, 117, 366–370. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson., R. Occurrence, fate, and effect of microplastics in freshwater systems. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–132. [Google Scholar]
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastics in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Xiong, X.; Ruan, Y.; Zhou, H.; Wu, C.; Lam, P.K.S. The hydro-fluctuation belt of the Three Gorges Reservoir: Source or sink of microplastics in the water? Environ. Pollut. 2019, 248, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Schüttrumpf, H. Erosion behavior of different microplastic particles in comparison to natural sediments. Environ. Sci. Technol. 2019, 53, 13219–13227. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Shogren, A.J.; Tank, J.L.; Risteca, P.; Kelly, J.J. Microplastic deposition velocity in streams follows patterns for naturally occurring allochthonous particles. Sci. Rep. 2019, 9, 3740. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Ockelford, A.; Cundy, A.; Ebdon, J.E. Storm response of fluvial sedimentary microplastics. Sci. Rep. 2020, 10, 1865. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities Legis. 2000, 327, 1–73. [Google Scholar]
- Nel, H.A.; Dalu, T.; Wasserman, R.J. Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci. Total Environ. 2018, 612, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.M.; Al Najjar, T.; Taupp, T.; Koop, J.H.E. PVC and PET microplastics in caddisfly (Lepidostoma basale) cases reduce case stability. Environ. Sci. Pollut. Res. 2020, 27, 22380–22389. [Google Scholar] [CrossRef]
- Ghetti, P.F.; The Extended Biotic Index (EBI). Macroinvertebrates in the Quality Control of Freshwater Environments; Provincia Autonoma di Trento: Trento, Italy, 2017; (Italian). [Google Scholar]
- Siligardi, M.; Avolio, F.; Baldaccini, G.; Bernabei, S.; Bucci, M.S.; Cappelletti, C.; Chierici, E.; Ciutti, F.; Sansoni, G.; Floris, B.; et al. Indice di Funzionalità Fluviale I.F.F.; APAT: Roma, Italy, 2007. [Google Scholar]
- Morgana, J.G.; Prato, S.; Antonelli, D. Caratterizzazione della Qualità Ambientale del Fiume Mignone Tramite il Calcolo di Indici Biotici Basati sulla Struttura delle Comunità di Macroinvertebrati Bentonici; ENEA—Dipartimento Ambiente Centro Ricerche Casaccia: Roma, Italy, 1999. [Google Scholar]
- Remote Sensing Project. Available online: http://www.pcn.minambiente.it/mattm/progetto-piano-straordinario-di-telerilevamento/ (accessed on 4 September 2020). (Italian).
- Traversetti, L.; Scalici, M. Assessing the influence of source distance and hydroecoregion on the invertebrate assemblage similarity in central Italy streams. Knowl. Manag. Aquat. Ecosyst. 2014, 414, 2. [Google Scholar] [CrossRef]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés D’eau Douce: Systématique, Biologie, Écologie; CNRS editions: Paris, France, 2010; Volume 15. [Google Scholar]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef]
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Co.: Austin, TX, USA, 1974; p. 182. [Google Scholar]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Manz, W.; Koop, J.H.E. Microplastics of different characteristics are incorporated into the larval cases of the freshwater caddisfly Lepidostoma Basale. Aquat. Biol. 2019, 28, 67–77. [Google Scholar] [CrossRef]
- Gallitelli, L.; Cera, A.; Cesarini, G.; Pietrelli, L.; Scalici, M. Microplastics effects on macroinvertebrates: Preliminary indoor evidences on case making caddisflies and burrowing mayflies. (unpublished; manuscript in preparation).
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tancioni, L.; Celauro, D.; Colombari, P.T.; Gibertini, G.; Maio, G.; Sarrocco, S.; Scalici, M. La carta della biodiversità ittica del Lazio: Analisi preliminare dei risultati. Ital. J. Freshwater Ichthyol. 2017, 1. Available online: http://www.aiiad.it/ijfi/index.php/ijfi/article/view/14 (accessed on 1 September 2020).
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Illies, J.; Botosaneanu, L. Problèmes et méthodes de la classification et de la zonation écologique des eaux courantes, considerées surtout du point de vue faunistique. Mitt. Int. Ver. Theor. Angew. Limnol. 1963, 12, 1–57. [Google Scholar] [CrossRef]
- Guerranti, C.; Perra, G.; Martellini, T.; Giari, L.; Cincinelli, A. Knowledge about microplastics in Mediterranean tributary river ecosystems: Lack of data and research needs on such a crucial marine pollution source. J. Mar. Sci. Eng. 2020, 8, 216. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; de Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Schmidt, L.K.; Bochow, M.; Imhof, H.K.; Oswald, S.E. Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy—Study of an urban watercourse traversing the city of Berlin, Germany. Environ. Pollut. 2018, 239, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, P.L.; Belontz, S.L.; Ryan, K.; Walzac, M.J. Factors controlling the distribution of miroplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 2020, 54, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Falahudin, D.; Cordova, M.R.; Sun, X.; Yogaswara, D.; Wulandari, I.; Hindarti, D.; Arifin, Z. The first occurrence, spatial distribution and characteristics of microplastic particles in sediments from Banten Bay, Indonesia. Sci. Total Environ. 2020, 705, 135304. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Le Tilly, V.; Bourseau, P.; Bellegou, H.; César, G.; Sire, O.; Bruzaud, F. Microplastics elutriation for sandy sediments: A granulometric approach. Mar. Pollut. Bull. 2016, 107, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Y.; Zhong, S.; Liu, J.; Qin, Y.; Gao, P. Microplastics in freshwater and wild fishes from Lijiang River in Guangxi, Southwest China. Sci. Total Environ. 2021, 755, 142428. [Google Scholar] [CrossRef]
- Fernández Severini, M.D.; Buzzi, N.S.; Forero López, A.D.; Colombo, C.V.; Chatelain Sartor, G.L.; Rimondino, G.N.; Truchet, D.M. Chemical composition and abundance of microplastics in the muscle of commercial shrimp Pleoticus muelleri at an impacted coastal environment (Southwest Atlantic). Mar. Pollut. Bull. 2020, 161, 111700. [Google Scholar] [CrossRef]
- Shahul Hamid, F.; Sanam Bhatti, M.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Nguyen, Q.A.T.; Nguyen, H.N.Y.; Strady, E.; Nguyen, Q.T.; Trinh-Dang, M. Characteristics of microplastics in shoreline sediments from a tropical and urbanized beach (Da Nang, Vietnam). Mar. Pollut. Bull. 2020, 161, 111768. [Google Scholar] [CrossRef]
- Neto, J.G.B.; Rodriguez, F.L.; Ortega, I.; dos S. Rodrigues, L.; Lacerda, A.L.d.F.; Coletto, J.L.; Kessler, F.; Cardoso, L.G.; Madureira, L.; Proietti, M.C. Ingestion of plastic debris by commercially important marine fish in southeast-south Brazil. Environ. Pollut. 2020, 267, 115508. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Kojima, F.; Miwa, Y.; Ito, N.; Nihei, Y.; Fukunaga, Y.; Yashima, Y.; Itsubo, N. Rapid sampling of suspended and floating microplastics in challenging riverine and coastal water environments in Japan. Water 2020, 12, 1903. [Google Scholar] [CrossRef]

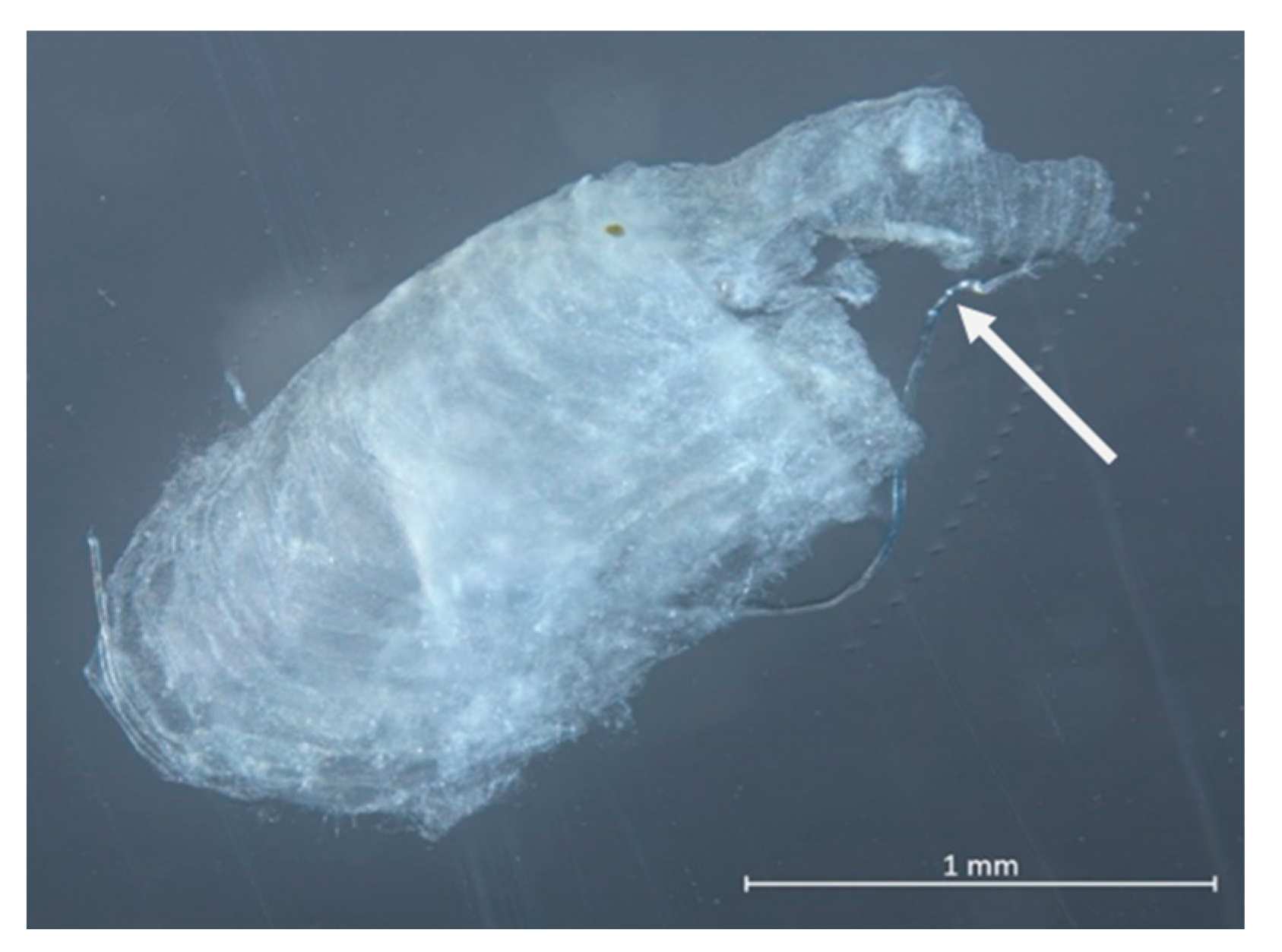
| Matrix | Plastic Concentration | Study Area | Reference |
|---|---|---|---|
| Water | 1–84 items/m3 | River Po mouth (Italy) | Atwood et al., 2019 [5] |
| 0.5–18 items/m3 | Ofanto River (Italy) | Campanale et al., 2020 [6] | |
| 2.4–88.4 items/m3 | Rhône River (France) | Constant et al., 2020 [7] | |
| 0.8–618 items/m3 | Têt River (France) | Constant et al., 2020 [7] | |
| 2.1–4.9 items/m3 | Ebro River delta (Spain) | Simon-Sánchez et al., 2019 [8] | |
| Sediment | 72–191 items/kg | Cecina River estuary (Italy) | Blašković et al., 2018 [9] |
| 33–798 items/kg | Têt River (France) | Constant et al., 2020 [7] | |
| 45–1069 items/kg | Ombrone River (Italy) | Guerranti et al., 2017 [10] | |
| 1306–2798 items/kg | Ebro River delta (Spain) | Simon-Sánchez et al., 2019 [8] |
| Sampling Site | Station Label | Latitude | Longitude |
|---|---|---|---|
| Vejano | S1 | 42°12′36.82″ N | 12°5′39.02″ E |
| Mola di Oriolo | S2 | 42°10′16.81″ N | 12°5′35.24” E |
| Canale Monterano | S3 | 42°7′58.09″ N | 12°3′12.13″ E |
| Rota | S4 | 42°9′13.69″ N | 12°0′34.98″ E |
| Monte Romano | S5 | 42°13′30.07″ N | 11°55′50.29″ E |
| Casale Santa Maria | S6 | 42°13′52.36″ N | 11°53′42.10″ E |
| La Farnesiana | S7 | 42°13′5.43″ N | 11°50′34.27″ E |
| Fontana Matta | S8 | 42°11′44.79″ N | 11°47′32.91″ E |
| Foce | S9 | 42°10′35.36″ N | 11°44′7.25″ E |
| Station | Plastic Concentration in Water (Items L−1) | Plastic Concentration in Sediment (Items Kg−1) | Mean Number of Plastic Items in Caddisfly Cases (Items Case−1) | |||
|---|---|---|---|---|---|---|
| Total | Riverbed | Riverbank | ||||
| Left | Right | |||||
| S1 | 0.04 | 0.2987 | 0 | 0 | 0.2987 | n.a. 1 |
| S2 | 0.04 | 2.4543 | 1.9634 | 0.1636 | 0.3272 | n.a. 1 |
| S3 | 0.03 | not sampled | not sampled | not sampled | not sampled | n.a. 1 |
| S4 | 0.10 | 2.3815 | 1.4743 | 0.0000 | 0.9072 | 7.5 |
| S5 | 0.07 | 0.9977 | 0.9977 | 0.0000 | 0 | 16 |
| S6 | 0.34 | 1.0322 | 0.1720 | 0.1720 | 0.6881 | 1.0 |
| S7 | 0.21 | 1.5868 | 0.2645 | 0.7934 | 0.5289 | n.a. 1 |
| S8 | 0.54 | 0.9395 | 0.2349 | 0.0000 | 0.7046 | 1.0 |
| S9 | 1.17 | 0.7779 | 0.6483 | 0.1297 | 0 | n.a. 1 |
| mean | 0.28 | 1.3086 | 0.7194 | 0.1573 | 0.4319 | 6.4 |
| total | 2.54 | 10.4687 | 5.7551 | 1.2587 | 3.4549 | 25.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallitelli, L.; Cesarini, G.; Cera, A.; Sighicelli, M.; Lecce, F.; Menegoni, P.; Scalici, M. Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy. Hydrology 2020, 7, 90. https://doi.org/10.3390/hydrology7040090
Gallitelli L, Cesarini G, Cera A, Sighicelli M, Lecce F, Menegoni P, Scalici M. Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy. Hydrology. 2020; 7(4):90. https://doi.org/10.3390/hydrology7040090
Chicago/Turabian StyleGallitelli, Luca, Giulia Cesarini, Alessandra Cera, Maria Sighicelli, Francesca Lecce, Patrizia Menegoni, and Massimiliano Scalici. 2020. "Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy" Hydrology 7, no. 4: 90. https://doi.org/10.3390/hydrology7040090
APA StyleGallitelli, L., Cesarini, G., Cera, A., Sighicelli, M., Lecce, F., Menegoni, P., & Scalici, M. (2020). Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy. Hydrology, 7(4), 90. https://doi.org/10.3390/hydrology7040090







