3.1. Hydrochemical Characterization
A summary of basic statistical analysis of the various hydrochemical parameters for the three distinct geologic units of the study area is presented in
Table 4.
Table 4 also includes contributions of these units to the levels of hydrochemical parameters together with the WHO [
13] guidelines for comparative studies. Discussions of the characterization of the hydrochemical parameters are based on the categorization of samples with respect to the existing geological units.
For the determination of suitability of water for various purposes, pH plays a very significant role. The pH values in the samples from the various units range from 6.91 to 7.80, 6.83 to 7.25, and 6.83 to 7.81 for the Afram formation (AFM), Bimbila formation (BFM), and Chereponi formation (CFM) respectively. The respective mean values are 7.17, 7.05 and 7.01 (
Table 4). All the samples from the three regions have pH values falling within the acceptable WHO [
13] guidelines for drinking water and domestic purposes. It therefore suggests that, out of the 28 boreholes for the entire study area, contributions to the pH levels from the various geologic units are: Nine (
n = 9) from AFM, ten (
n = 10) from BFM and nine (
n = 9) from CFM. The pH values are almost homogenous in the entire study area suggesting similar geochemical processes. However, nearly all the samples from the CFM (7 samples) are slightly acidic and majority from AFM (7 samples) and BFM (7 samples) are slightly alkaline. Generally, the pH in the entire area can be characterized as slightly acidic to slightly alkaline.
The EC of water is indicative of the water’s purity. The purer the water, the lower the EC, as exemplified by distilled water which is almost an insulator due to very low EC, but seawater is a very efficient electrical conductor. In this study, the EC values are in the range of 576–1456 μS/cm for AFM, 863–1398 μS/cm for BFM, and 746–2180 μS/cm for CFM (
Table 4). All the samples from the three units have EC values within the WHO limits for drinking water with contributions from the various geologic units indicated in
Table 4. Groundwater in the area is thus characterized as suitable for drinking in terms of EC levels.
Consumption of hard water is generally safe. It has no known adverse health effect, having health benefits including the fulfilment of dietary needs of essential minerals such as calcium and magnesium [
49,
50,
51]. The total hardness (TH) values in the study area range from 40.7 to 204.89 mg/L for AFM, 55 to 175.84 mg/L for BHF, and 82.03 to 310.3 mg/L for CFM with mean values of 108.7 mg/L, 118.63 mg/L and 159.47 mg/L respectively (
Table 4). This range of values in TH is an indication of moderate contents of calcium and magnesium in groundwater of the area since TH is represented by the co-participation of both cations [
52]. Majority of the total samples (89%) are within the WHO [
13] acceptable limits for drinking water. Out of the 11% of the samples falling outside the WHO limits, 67% and 33% of this number are contributions from CFM and AFM respectively. This indicates that, all the samples from BFM are within the WHO limits. However, the dominance of the higher TH values from CFM could be coming from the sandstones of the Chereponi formation. Generally, the TH can be characterized as good for drinking and domestic purposes.
Calcium is identified as one of the most abundant substances in water. Water described as “hard” contains high levels of dissolved minerals, specifically calcium and magnesium. Even though hard drinking water is not a health hazard, as it generally contributes to human dietary needs of calcium and magnesium, high levels of these substances in water contribute to inefficient and expensive operation of water-using appliances. Calcium and magnesium contents in groundwater of the area studied indicate the following range of values: AFM (7.9 to 47.8 mg/L Calcium and 5.1 to 25.8 mg/L Magnesium), BFM (7.8 to 38.8 mg/L Calcium and 5.3 to 23.8 mg/L Magnesium), and CFM (13 to 102.9 mg/L Calcium and 11.2 to 35.1 mg/L Magnesium). All the water samples have concentrations of calcium and magnesium falling within the WHO acceptable limits. The respective contributions to the contents of calcium and magnesium from the various geologic units are as indicated in
Table 4. From
Table 4, generally, the calcium concentrations appear to be enriched more than the magnesium concentrations. This may be due to partial dissolution of carbonate minerals in rocks of the study area [
53]. These substances are thus characterized as suitable for drinking and domestic uses.
High sodium levels can be associated with the dissolution of soluble salts such as halite, or ion exchange [
54]. High intake of sodium has been reported to cause problems of blood pressure and arteriosclerosis, and very low intakes may also cause dehydration and general numbness [
55]. The concentrations of sodium in the groundwater samples of the study area show values in the range of 88.2 to 250 mg/L for AFM, 96.2 to 266.7 mg/L for BFM, and 76.7 to 413.8 mg/L for CFM. Though sodium is characterized as good for drinking as majority of the samples (64%) have concentrations of sodium falling within the WHO acceptable limits for drinking water, 36% of the samples have concentration of sodium higher than the acceptable limit. Contribution to the values of sodium outside the WHO limits indicates the AFM recording the highest number of samples (18%) followed by BFM and CFM contributing 11% and 7% respectively.
The values of potassium in water samples from AFM, BFM, and CFM are 1–5.2 mg/L, 0.8–6.3 mg/L, and 1.3–5.4 mg/L respectively (
Table 4). All the values are within the acceptable limits for drinking water. This signifies that, contributions from all the geologic formations are within the WHO acceptable limits. The water is said to be good for drinking with respect to potassium levels. Generally, levels of potassium in drinking water are not much of health concerns. Though may cause some health effects in susceptible individuals, intake of potassium from drinking water is mostly well below the level that may cause adverse effect [
56].
Bicarbonate is present in all body fluids and organs and plays a very significant role in the acid-base balances in the human body. Groundwater in the study area has bicarbonate values ranging from 251 to 623.6 mg/L for AFM, 401 to 495 mg/L for BFM and 345 to 831 mg/L for CFM with respective mean values of 453.09 mg/L, 455.21 mg/L, and 487.7 mg/L (
Table 4). All the samples have values exceeding the WHO threshold value. This implies the water is not suitable for drinking in terms of bicarbonate concentration. The possible sources of bicarbonate could be the presence of organic matter in the aquifer. This is oxidized to produce carbon dioxide which promotes dissolution of minerals [
57]. The bicarbonate concentration may also result from the interaction of precipitation with rocks in the area. Atmospheric reaction of precipitation and carbon dioxide forming weak carbonic acid is introduced into the soil system as the precipitated water infiltrates through the weathered material. Carbonic acid contributes to the dissolution of feldspar particularly in the sandstones of the geologic formations resulting in the release of HCO
3− into groundwater [
53].
Some of the main physiological effects resulting from the consumption of considerable amounts of sulphate include dehydration and gastrointestinal irritation [
58]. Sulphate may also be a contributory factor to the corrosion of water distribution systems [
59]. The sulphate values in groundwater within the three units (AFM, BFM, and CFM) range from 5.8 to 180.1 mg/L, 4.71 to 190 mg/L, and 7.1 to 62 mg/L respectively. All the samples are within the WHO standard limits for drinking and domestic purposes. Contributions to the concentrations of sulphate from the various geologic units are as indicated below (
Table 4). The water is suitable for drinking and domestic purposes in terms of sulphate concentration in groundwater of the study area.
The respective chloride concentration in the three geologic environments: AFM, BFM and CFM range from 4.8 to 226 mg/L, 7.9 to 120 mg/L and 5 to 792 mg/L. Twenty-seven of the total samples are within the WHO limits for drinking water. The lone sample with chloride level beyond the WHO limit might have resulted from the influence of poor insanitary conditions or runoff of chemical fertilizers from farmlands.
On the other hand, nitrate concentration ranges from 0.01 to 23.23 mg/L for AFM, 0.01 to 10.1 mg/L for BFM and 0.01 to 16.86 mg/L for CFM. All the samples have NO3− values within the permissible limit. Generally, nitrates occur in trace contents in surface water but may occur in high levels in groundwater. Groundwater in the area can be characterized as good for drinking in terms of NO3− levels.
Twenty-seven of the total samples (representing 96%) have marginal phosphate levels falling within the range of 0 to 0.008 mg/L with only one sample (4%) having phosphate level of 0.94 mg/L, from the BFM beyond the WHO threshold value (
Table 4). The high value could be a local influence of anthropogenic activities such as the use of detergent for washing of clothes and utensils.
Generally, fluoride concentrations in groundwater vary with the type of rock water interacts with during its flow, and usually do not exceed 10 mg/L. Elevated levels of fluoride are associated with dental and skeletal fluorosis with fluoride deficiency leading to dental caries. The fluoride concentrations in the study area range from 0.2 to 2.51 mg/L, 0.2 to 4.2 mg/L, and 0.5 to 4.2 mg/L, respectively, for AFM, BFM, and CFM (
Table 4). A majority of the groundwater samples (67.86%) are within the WHO limits for drinking water. The remaining 32.14% of the samples have fluoride values beyond the WHO threshold limit with the BFM contributing the greatest portion (
Table 4). It is possible that the fluoride might have been released from the sandstones of the Oti-Pendjari group as evidenced by the fact that fluoride is more common in the sandstone units than in the mudstones or the siltstones [
23].
3.2. Hydrochemical Facies
In order to understand and identify the dominant ionic constituents and water types in the aquifer of the study area, the hydrochemical data were subjected to various conventional graphical plots. The idea of plotting data in various diagrams as corroborated by Tadesse et al. [
8] is to confirm the effectiveness of the data for drinking and irrigation waters quality assessment.
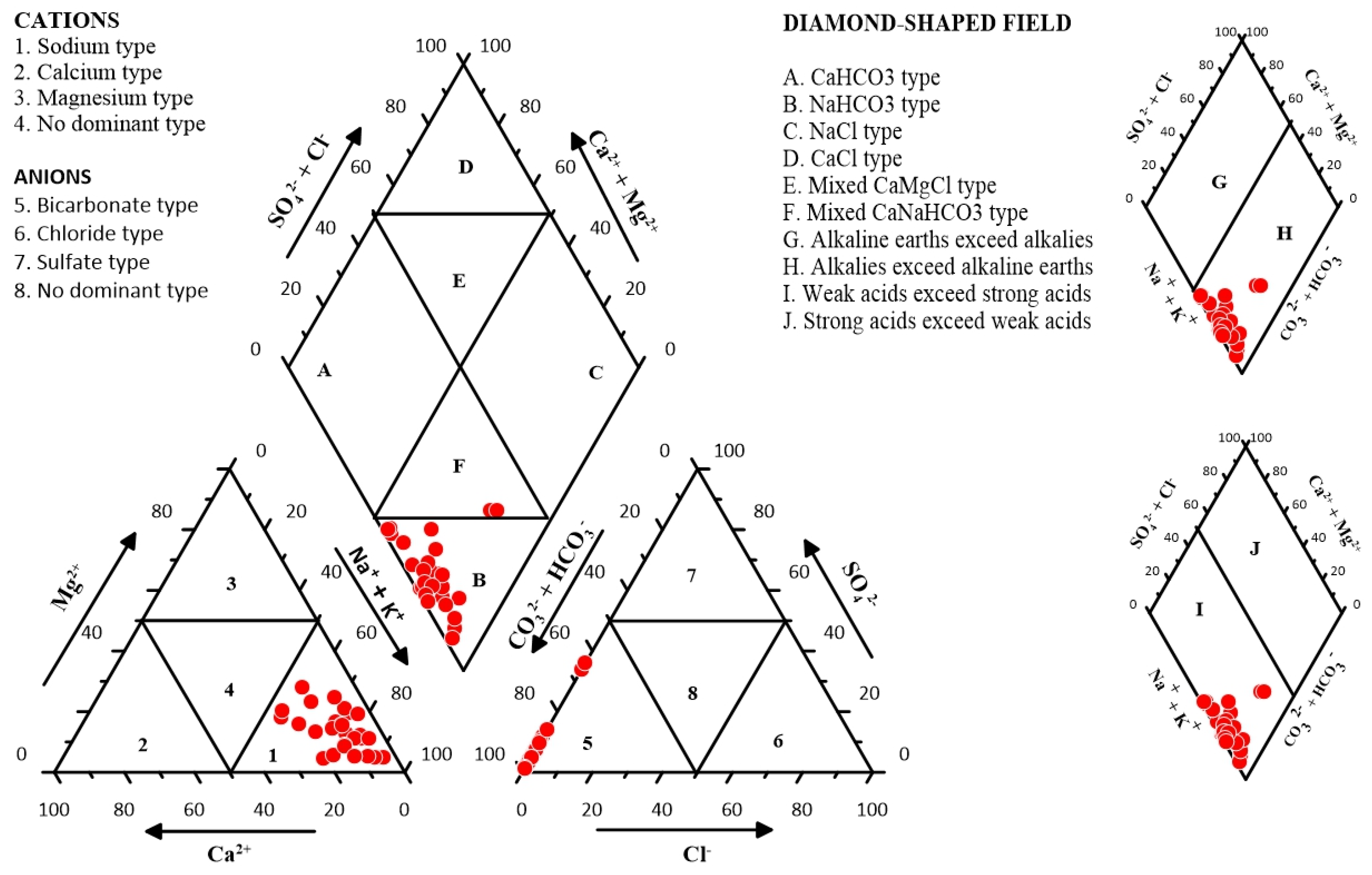
A convenient and widely used method to classify water types on the basis of ionic constituents has been proposed by Piper [
41]. By plotting the hydrochemical data on a trilinear diagram, the relative abundance of chemical constituents and water types can be identified.
Figure 3 shows all the 28 samples falling in zone 1 indicating sodium dominance for the cations, and bicarbonate dominance in zone 5 for the anions. Two types of water can be identified, Na-HCO
3 type with 26 samples falling in Zone B and a mixed Ca-Na-HCO
3 type in Zone F with only 2 samples. It can be determined from the graph that alkalies exceed alkaline earths. Na and K are common constituents of minerals such as bentonite, biotite, muscovite and illite which form the bedrock of the area under study. The samples also demonstrate that weak acids exceed strong acids in the groundwater of the study area.
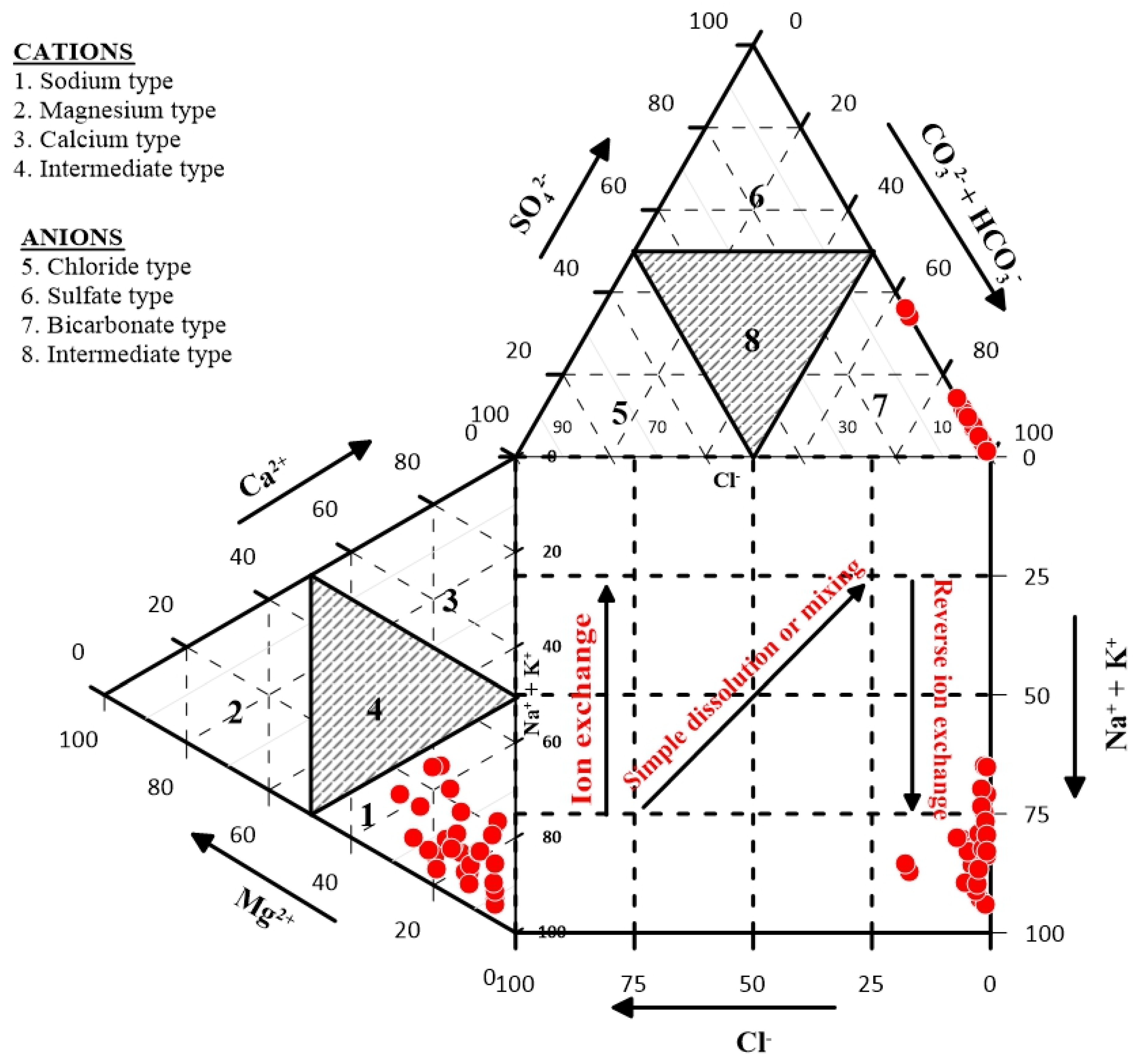
Durov [
42] plot showed similar hydrochemical facies as the Piper [
41] plot, whereby the cations field shows Na
+ + K
+ enrichment with HCO
3− dominance in the anions field (
Figure 4). The plot also suggests reverse ion exchange to be the main hydrochemical processes affecting groundwater chemistry in the study area. This may have been influenced by the dissolution of phyllosilicates such as micas and clay minerals which are common constituents of the geology of the study area (
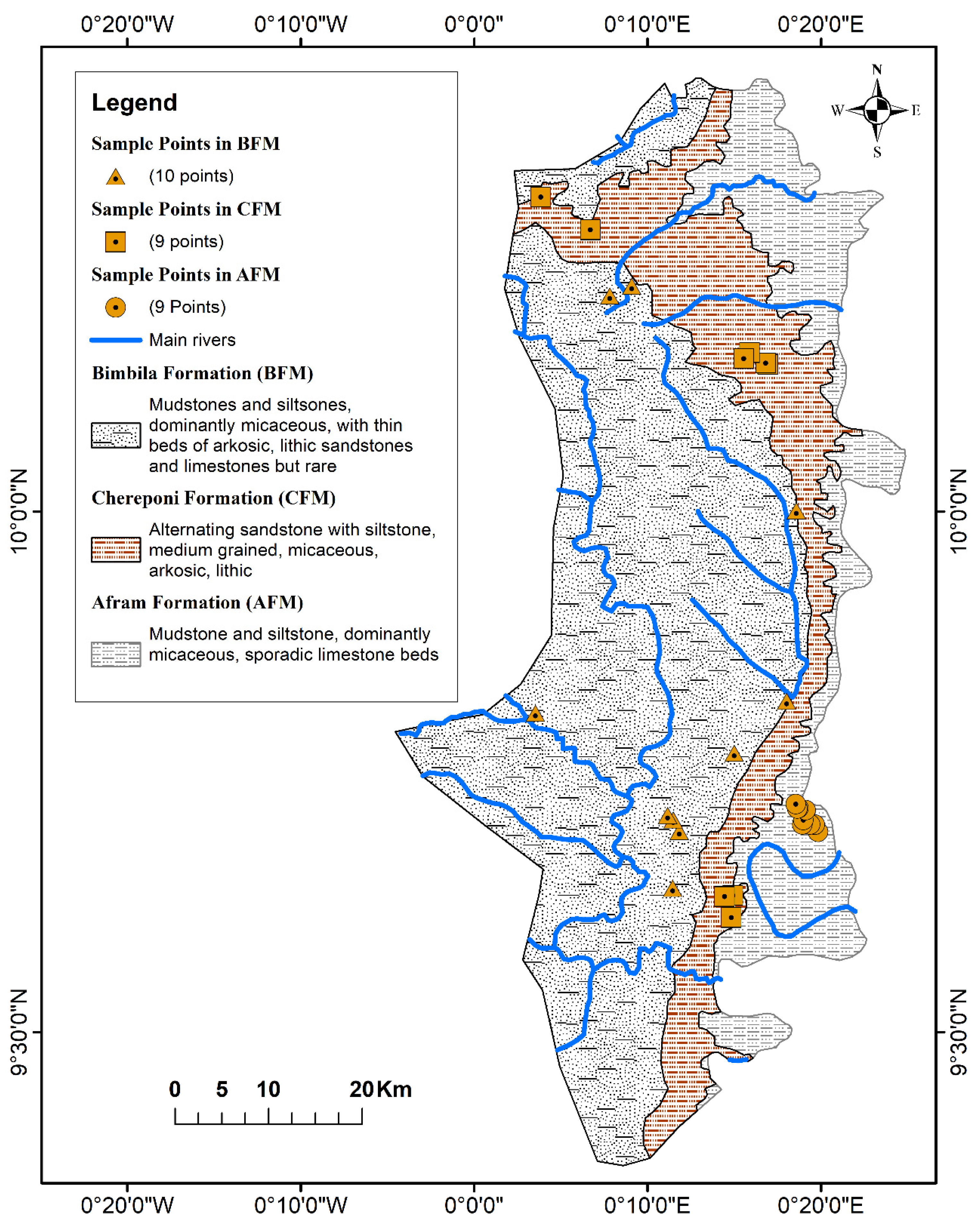
Figure 2), and the subsequent replacement of alkaline earths with the alkalis.
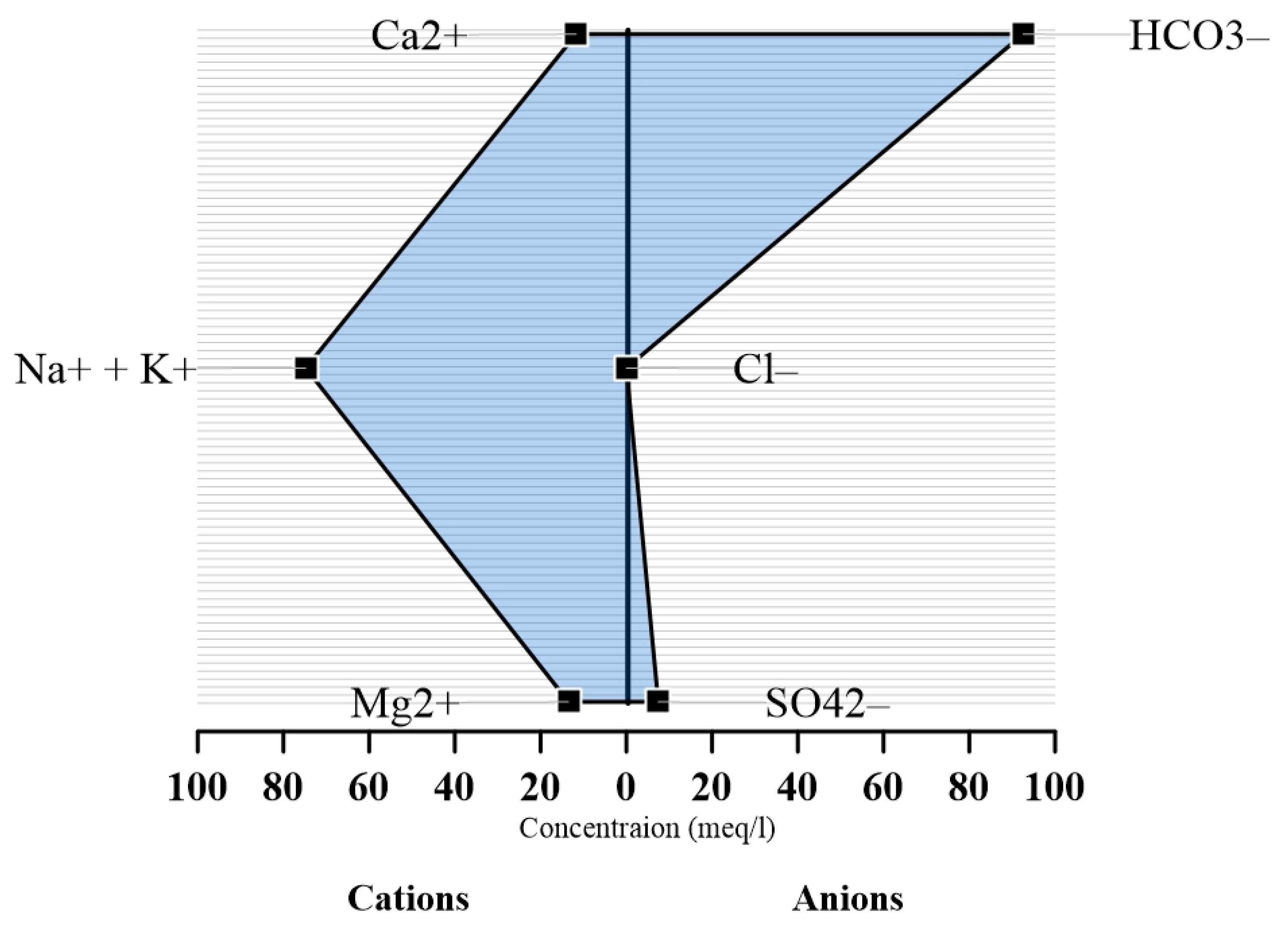
A pattern diagram for representing hydrochemical data using four parallel horizontal axes and one vertical axis was first suggested by Stiff [
61]. In this diagram, as described by Singh and Kumar [
60], ionic concentrations expressed in meq/L of the major cations (Na
+, K
+, Ca
2+, Mg
2+) are plotted to the left of a vertical zero axis and major anions (Cl
−, SO
42−, HCO
3−, NO
3−, PO
43−) plotted to the right yielding points which when connected, form an irregular polygonal pattern. A distinctive shape is thus defined for waters of similar quality. In this present study, average concentrations of the cations (Ca
2+, Na
+ + K
+, Mg
2+) and anions (SO
42−, Cl
−, HCO
3−) were instead considered for the plot. From the Stiff diagram (
Figure 5), the abundance of cations and anions is consistent with the results obtained from both Piper and Durov diagrams which showed sodium and bicarbonate being the dominant cation and anion respectively.
3.4. Assessment of Groundwater Quality for Irrigation Purposes
The suitability of groundwater for irrigation has been assessed through the use of USSL [
28] and Wilcox [
44] diagrams (
Figure 7 and
Figure 8), together with some irrigation water indices. A statistical summary of the irrigation assessment indices is shown in
Table 7.
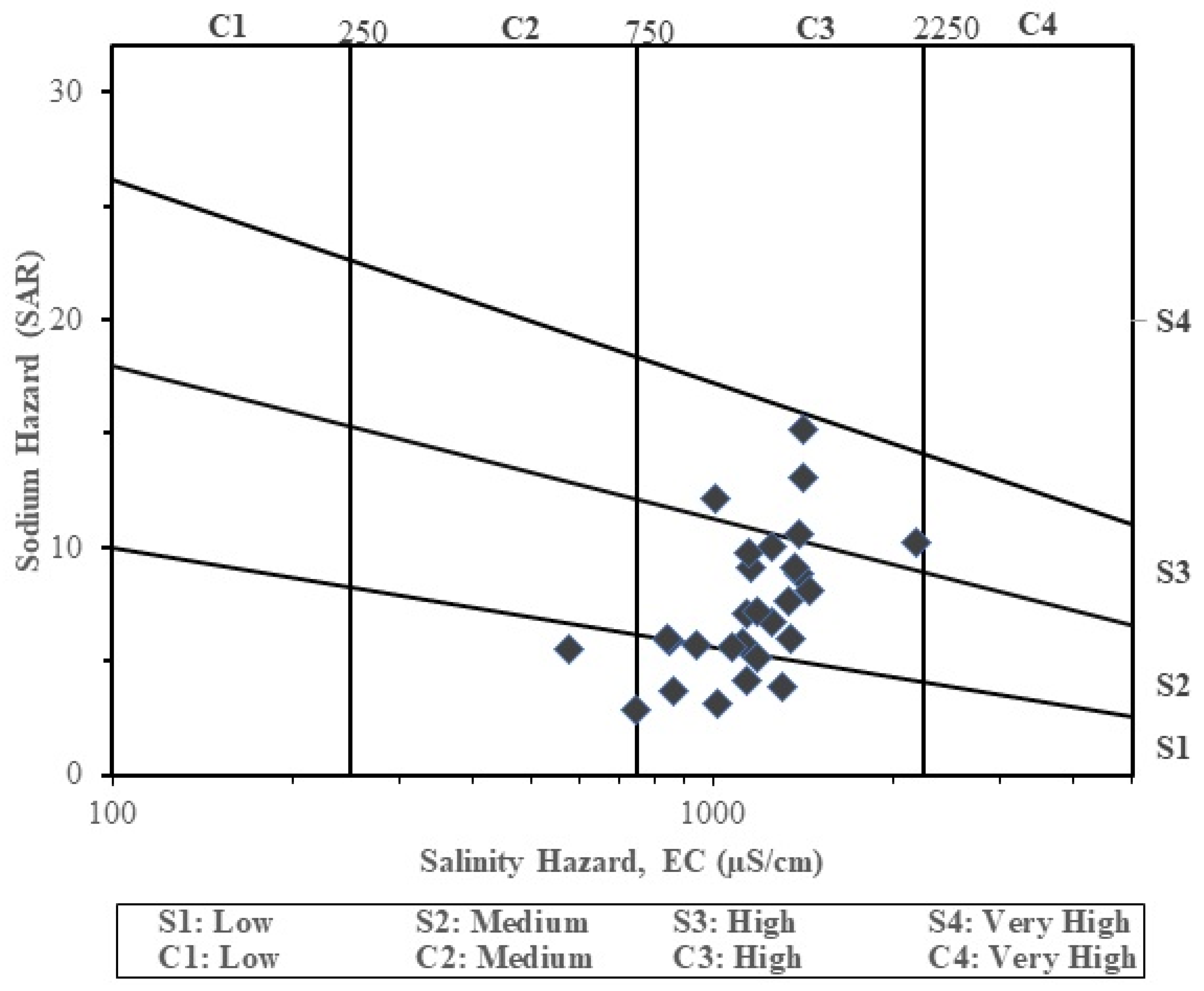
The USSL [
28] developed a relation between SAR and EC used for the determination of irrigation water suitability. In this present study, most of the groundwater samples (26 samples) plotted in the C3-S1-S2-S3 column (
Figure 7), indicating high salinity and low to high sodium hazard respectively. Groundwater belonging to these groups can be used for irrigation activities with salinity control. Very few of the samples (2 samples), however, fell within the C2–S1 category, indicating medium salinity (C2) and low sodium hazards (S1). Groundwater belonging to this category can be used for irrigation activities without any serious salinity control. A high amount of salt in irrigation water can alter the osmotic pressure in the root zone, which will result in limiting the amount of water taken by the plant and consequently hindering the plant growth [
31].
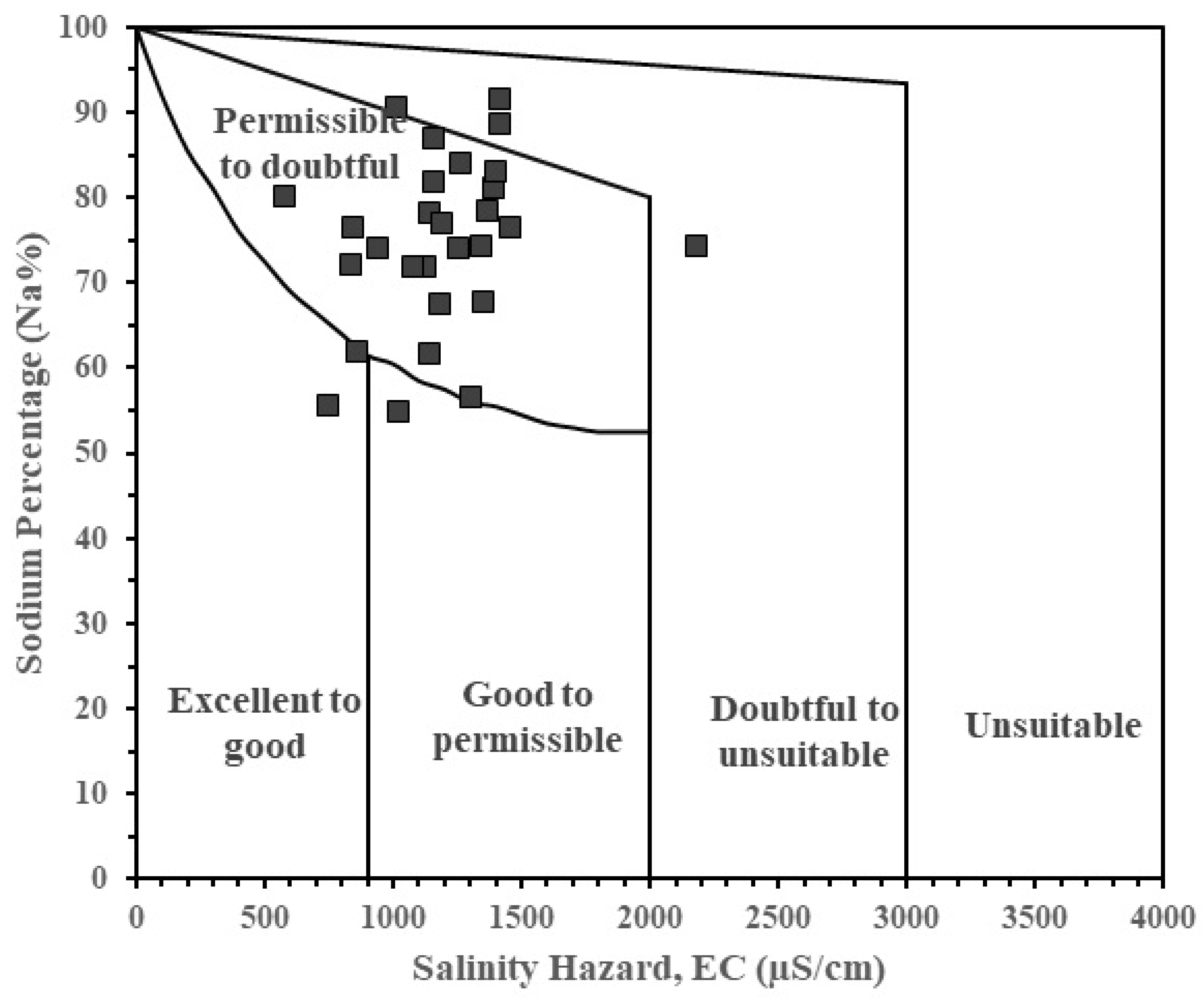
Similarly, the computed %Na versus EC values have also been plotted on the Wilcox diagram (
Figure 8). Results from the Wilcox diagram show most of the groundwater samples falling within the “permissible to doubtful” category followed by “doubtful to unsuitable” category, with two samples falling within the “excellent to good, whiles only one sample plotted within good to permissible category.
For irrigation purposes, EC values of water can have great influence on the level of salinity hazard to crops. With excess salinity, the osmotic activity of plants is reduced thereby interfering with the absorption of water and nutrients from the soil [
62]. The values of EC in this study range from 576 to 2180 µS/cm. Based on irrigation water classification (
Table 7), it can be observed that, out of the 28 analyzed samples, while 7% of the samples are within the range 250 to 750 µS/cm described as good for irrigation, the remaining samples (93%) have EC values within the 750 to 2250 µS/cm range which is described as permissible water. By this irrigation suitability index (EC), the two types of groundwater identified in the study area suggest that, groundwater under the good water category is not hazardous and thus, needs no restriction in its use for irrigation and can be used for almost all crops and on all types of soils without soil or cropping problems arising. However, groundwater under the permissible category needs slight restriction in its use for irrigation, as very little salinity hazard may develop but under normal irrigation practices, it is permissible for use except in extreme cases of soils of low permeability [
8]. The EC results are corroborated by the SAR-EC plots as observed in the USSL diagram in
Figure 7 for which 26 samples representing 92.9% falling within the C3-column depicting high salinity hazard and two samples falling within the C2-column depicting medium salinity hazard.
Sodium adsorption ratio is an important irrigation water index for determining the suitability of groundwater for irrigation. It is a measure of sodium or alkali hazard to crops. Irrigation water with high SAR value is suggestive of high Na
+ and low Ca
2+. Ion exchange favours the abundance of Na
+ thereby destroying the soil structure arising from the dispersion of clay particles [
62]. The SAR values range between 2.88 to 15.15 with a mean value of 2.88 (
Table 7). It can be observed that 78.6% of all the samples have SAR value less than 10 and the remaining 21.4% have values in the range of 10 to 18. Two water types can thus be realized based on the range of SAR values, with excellent water being the dominant type followed by the good water type. The SAR classification system to assess the suitability of groundwater for irrigation purpose can also be determined with the USSL diagram. The results as presented in
Figure 7 show 11 samples falling in the S1 field, indicating low sodium hazards, 12 samples falling in the S2 field, indicating medium sodium hazard, and five samples falling in S3 field, an indication of high sodium hazard.
Generally, hardness of water causes building up of scales in irrigation pipes thereby limiting the effective operation and performance of the entire irrigation system. TH values range from 40.7 to 310.3 meq/L (
Table 7). Based on the TH values of groundwater in the area, five types of water can be recognized (
Table 7): Soft water with 18% of samples falling within this category, moderately hard water, hard water, and very hard water with the respective representation of groundwater samples as 50%, 29% and 4%.
Magnesium ratio or magnesium hazard (MR or MH) is a measure of the effect of magnesium in irrigation water proposed by Paliwal [
47]. Excess magnesium content in groundwater results in the dispersion of clay particles thereby damaging the soil structure. Groundwater with MR value less than 50 is considered suitable for irrigation, while MR value greater than 50 is considered unsuitable for irrigation. The present study shows MR values ranging from 17.24 to 83.42. It can be observed that 39.3% of all the sampled water have MR values below 50, indicating that, the water is suitable for irrigation. However, the remaining samples (60.7%) have MR values greater than 50, an indication that, the water is unsuitable for irrigation.
Wilcox’s [
44] percentage of sodium (%Na) irrigation water parameter is widely used for assessing the suitability of water for irrigation purposes. With this parameter, Na
+ is expressed as soluble sodium percentage (%Na), which is computed using the formula as presented in
Table 3. The present study shows %Na values in the range of 55.00% to 91.47%. Three types of water can be recognized using this approach: permissible with three samples, doubtful with sixteen samples, and unsuitable water having nine samples falling within this category.