Isotopic Discrimination of Aquifer Recharge Sources, Subsystem Connectivity and Flow Patterns in the South Fork Palouse River Basin, Idaho and Washington, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Wells, Sample Collection and Field Parameters
2.2. Groundwater Sample Analyses
2.3. Precipitation Isotope Data
2.4. Snow Isotope Data
2.5. Model of Geologic Layers
2.6. Principal Component Analysis
2.7. Inverse Modeling and Source Water Mixing
3. Results
3.1. Hydrochemistry Variation
3.2. Precipitation and Surface Water Isotope Signals
3.3. Groundwater Source Discrimination
3.4. Source Water Mixing in the Central Area
3.5. Source Water Homogenization and Transport to the West
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foxworthy, B.L.; Washburn, R.L. Ground Water in the Pullman Area, Whitman County, Washington; US Government Printing Office: Washington, DC, USA, 1963; Volume 1655, pp. 1–78.
- Robischon, S. Palouse Basin Aquifer Committee—Annual Water Use Report 2016; Palouse Basin Aquifer Committee: Moscow, ID, USA, 2016; pp. 1–19.
- Beall, A.; Fiedler, F.; Boll, J.; Cosens, B. Sustainable water resource management and participatory system dynamics: Case study—Developing the Palouse Basin Participatory Model. Sustainability 2011, 3, 720–742. [Google Scholar] [CrossRef]
- Reidel, S.P.; Tolan, T.L.; Hooper, P.R.; Beeson, M.H.; Fecht, K.R.; Bentley, R.D.; Anderson, J.L. The Grande Ronde Basalt, Columbia River Basalt Group; Stratigraphic descriptions and correlations in Washington, Oregon, and Idaho. In Geological Society of America Special Papers; Geological Society of America: Boulder, CO, USA, 1989; Volume 239, pp. 21–54. ISBN 978-0-8137-2239-9. [Google Scholar]
- Tolan, T.; Lindsey, K.; Nielson, M.; Loper, S. Geologic Framework of Selected sediment and Columbia River Basalt units in the Columbia Basin Ground Water Management Area of Adams, Franklin, Grant, and Lincoln Counties, Washington; Columbia Basin Ground Water Management Area, GSI Water Solutions and Franklin Consevation District: Portland, OR, USA, 2007.
- Dhungel, R.; Fiedler, F. Water balance to recharge calculation: Implications for watershed management using systems dynamics approach. Hydrology 2016, 3, 13. [Google Scholar] [CrossRef]
- Candel, J.; Brooks, E.; Sánchez-Murillo, R.; Grader, G.; Dijksma, R. Identifying groundwater recharge connections in the Moscow (USA) sub-basin using isotopic tracers and a soil moisture routing model. Hydrogeol. J. 2016, 24, 1739–1751. [Google Scholar] [CrossRef]
- Bush, J.H.; Garwood, D.L.; Dunlap, P. Geology and geologic history of the Moscow-Pullman basin, Idaho and Washington, from late Grande Ronde to late Saddle Mountains time. In Exploring the Geology of the Inland Northwest; Lewis, R.S., Schmidt, K.L., Eds.; The Geological Society of America Field Guide; Geological Society of America: Boulder, CO, USA, 2016; pp. 151–174. ISBN 978-0-8137-0041-0. [Google Scholar]
- Bush, J.H.; Dunlap, P.; Reidel, S.P.; Kobayashi, D. Geologic cross sections across the Moscow-Pullman Basin, Idaho and Washington; Idaho Geologic Survey: Boise, ID, USA, In Press; 3 sheets, scale 1:24,000.
- Crosby, J.W.; Chatters, R.M. New techniques of water sampling for carbon 14 analysis. J. Geophys. Res. 1965, 70, 2839–2844. [Google Scholar] [CrossRef]
- Douglas, A.A.; Osiensky, J.L.; Keller, C.K. Carbon-14 dating of ground water in the Palouse Basin of the Columbia River basalts. J. Hydrol. 2007, 334, 502–512. [Google Scholar] [CrossRef]
- Bush, J.H.; Pierce, J.L.; Potter, G.N. Bedrock Geologic Map of the Moscow East Quadrangle, Latah County, Idaho; Idaho Geologic Survey: Boise, ID, USA, 2010; 1 sheet, scale 1:24,000. [Google Scholar]
- Bennett, B. Recharge Implications of Strategic Pumping of the Wanapum Aquifer System in the Moscow Sub-Basin. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2009. [Google Scholar]
- Fiedler, A. Well Interference Effects in the Grande Ronde Aquifer System in the Moscow-Pullman Area of Idaho and Washington. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2009. [Google Scholar]
- Owsley, D. Characterization of Grande Ronde Aquifers in the Palouse Basin Using Large Scale Aquifer Tests. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2009. [Google Scholar]
- Dijksma, R.; Brooks, E.S.; Boll, J. Groundwater recharge in Pleistocene sediments overlying basalt aquifers in the Palouse Basin, USA: Modeling of distributed recharge potential and identification of water pathways. Hydrogeol. J. 2011, 19, 489–500. [Google Scholar] [CrossRef]
- Lum II, W.E.; Smoot, J.L.; Ralston, D.R. Geohydrology and numerical model analysis of ground-water flow in the Pullman-Moscow area, Washington and Idaho; United States Geologic Survey: Tacoma, WA, USA, 1990; pp. 1–79.
- Ackerman, D.J. Transmissivity of the Snake River Plain Aquifer at the Idaho National Engineering Laboratory, Idaho; United States Geologic Survey: Idaho Falls, ID, USA, 1991; pp. 1–35.
- Hernandez, H.P. Observations of Recharge to the Wanapum Aquifer System in the Moscow Area, Latah County, Idaho. Master’s Thesis, University of Idaho, Moscow, ID, USA, 2007. [Google Scholar]
- Kopp, W.P. Hydrogeology of the Upper Aquifer of the Pullman-Moscow Basin at the University of Idaho Aquaculture Site. Ph.D. Thesis, University of Idaho, Moscow, ID, USA, 1994. [Google Scholar]
- Li, T. Hydrogeologic Characterization of a Multiple Aquifer Fractured Basalt System. Ph.D. Thesis, University of Idaho, Moscow, ID, USA, 1991. [Google Scholar]
- Révész, K.M.; Doctor, D.H. Automated Determination of the Stable Carbon Isotopic Composition (δ13C) of Total Dissolved Inorganic Carbon (DIC) and Total Nonpurgeable Dissolved Organic Carbon (DOC) in Aqueous Samples; United States Geologic Survey: Reston, VA, USA, 2014; pp. 1–38.
- IAEA/WMO Global Network of Isotopes in Precipitation—GNIP Database. 2019. Available online: http://www.iaea.org/water (accessed on 16 February 2018).
- Welker, J.M. ENSO effects on δ18O, δ2H and d-excess values in precipitation across the U.S. using a high-density, long-term network (USNIP): Precipitation isotopes across the USA. Rapid Commun. Mass Spectrom. 2012, 26, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Welker, J.M. Isotopic (δ18O) characteristics of weekly precipitation collected across the USA: An initial analysis with application to water source studies. Hydrol. Process. 2000, 14, 1449–1464. [Google Scholar] [CrossRef]
- Poage, M.A.; Chamberlain, C.P. Empirical relationships between elevation and the stable isotope composition of precipitation and surface waters: Considerations for studies of paleoelevation change. Am. J. Sci. 2001, 301, 1–15. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.K.; Butcher, C.N.; Smith, J.L.; Allen-King, R.M. Nitrate in tile drainage of the semiarid Palouse Basin. J. Environ. Qual. 2008, 37, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.J.; Keller, C.K.; Brooks, E.S.; Smith, J.L.; Huyck Orr, C.; Evans, R.D. Water and nitrogen movement through a semiarid dryland agricultural catchment: Seasonal and decadal trends. Hydrol. Process. 2017, 31, 1889–1899. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 121–245. ISBN 978-1-4665-9174-5. [Google Scholar]
- Moravec, B.G.; Keller, C.K.; Smith, J.L.; Allen-King, R.M.; Goodwin, A.J.; Fairley, J.P.; Larson, P.B. Oxygen-18 dynamics in precipitation and streamflow in a semi-arid agricultural watershed, Eastern Washington, USA. Hydrol. Process. 2009, 24, 446–460. [Google Scholar] [CrossRef]
- Western Regional Climate Center Cooperative Climatological Data Summaries, NOAA Cooperative Stations, Pullman Experimental Station—Climate Summary. Available online: https://wrcc.dri.edu/cgi-bin/cliMAIN.pl?wa6784 (accessed on 9 January 2019).
- National Water and Climate Center NWCC Report Generator—Idaho SNOTEL Moscow Mountain Site, Average Precipitation Accumulation (1981–2010). Available online: https://wcc.sc.egov.usda.gov/reportGenerator/ (accessed on 9 January 2019).
- Moxley, N. Stable Isotope Analysis of Surface Water and Precipitation in the Palouse Basin: Hydrologic Tracers of Aquifer Recharge. Master’s Thesis, Washington State University, Pullman, WA, USA, 2012. [Google Scholar]
- Peters, K.E.; Curry, D.J.; Kacewicz, M. An overview of basin and petroleum system modeling: Definitions and concepts. In Basin Modeling: New Horizons in Research and Applications; AAPG Hedberg Series; American Association of Petroleum Geologists: Danvers, MA, USA, 2012; pp. 1–16. ISBN 978-0-89181-903-5. [Google Scholar]
- Abatzoglou, J.T.; Rupp, D.E.; Mote, P.W. Seasonal climate variability and change in the Pacific Northwest of the United States. J. Clim. 2013, 27, 2125–2142. [Google Scholar] [CrossRef]
- Sánchez-Murillo, R.; Brooks, E.S.; Elliot, W.J.; Gazel, E.; Boll, J. Baseflow recession analysis in the inland Pacific Northwest of the United States. Hydrogeol. J. 2014, 23, 287–303. [Google Scholar] [CrossRef]
- Meixner, T.; Manning, A.H.; Stonestrom, D.A.; Allen, D.M.; Ajami, H.; Blasch, K.W.; Brookfield, A.E.; Castro, C.L.; Clark, J.F.; Gochis, D.J.; et al. Implications of projected climate change for groundwater recharge in the western United States. J. Hydrol. 2016, 534, 124–138. [Google Scholar] [CrossRef]
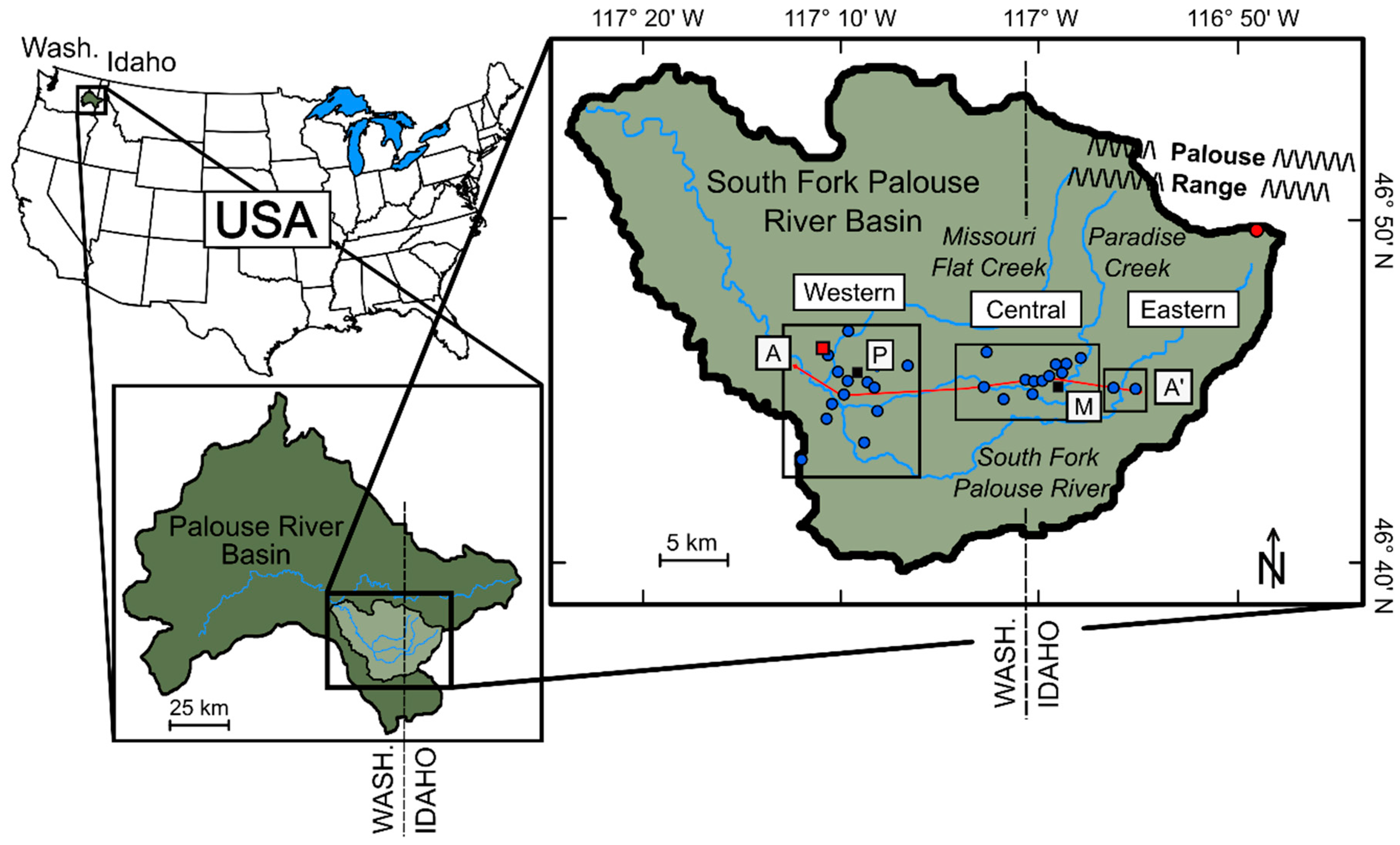

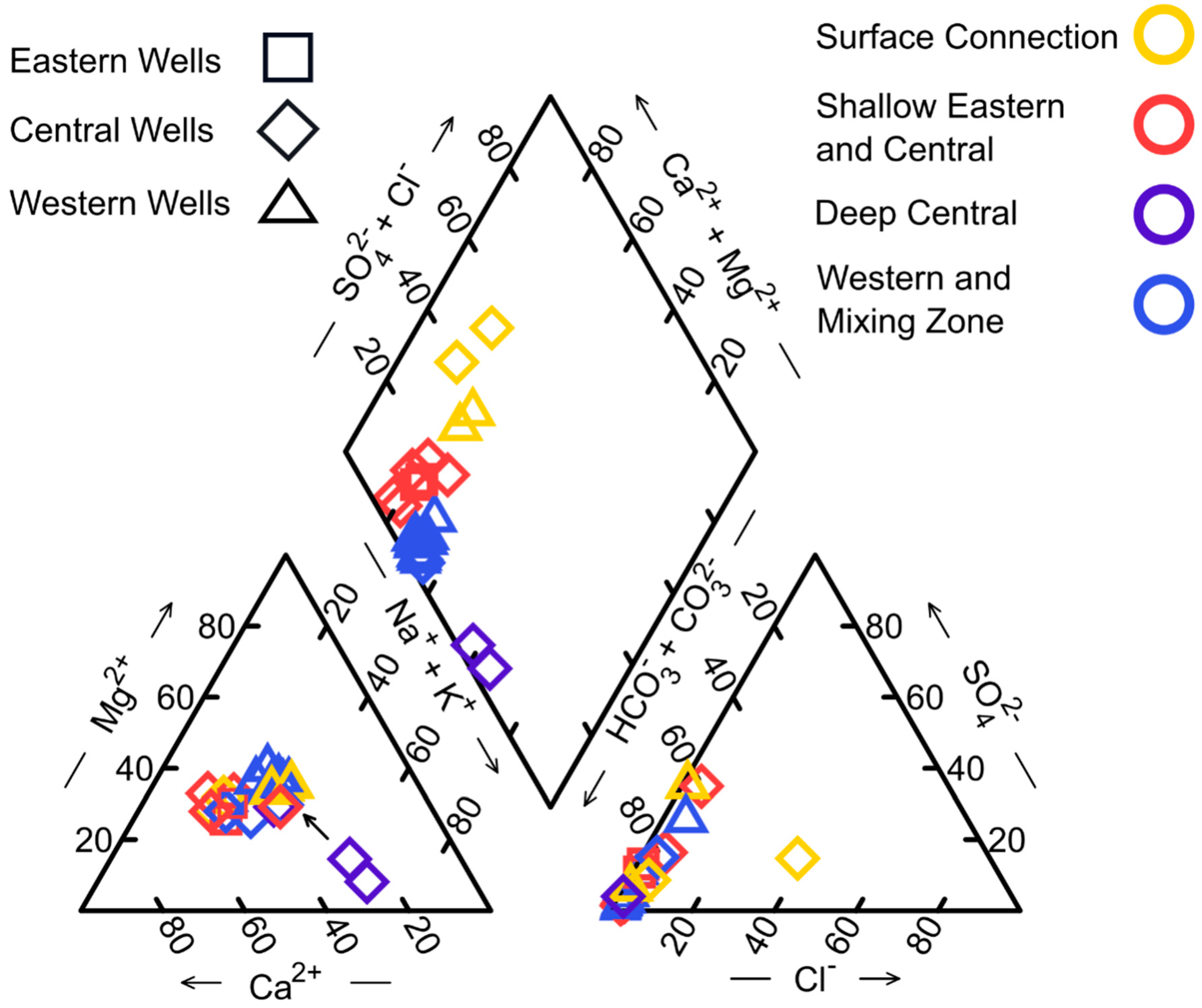
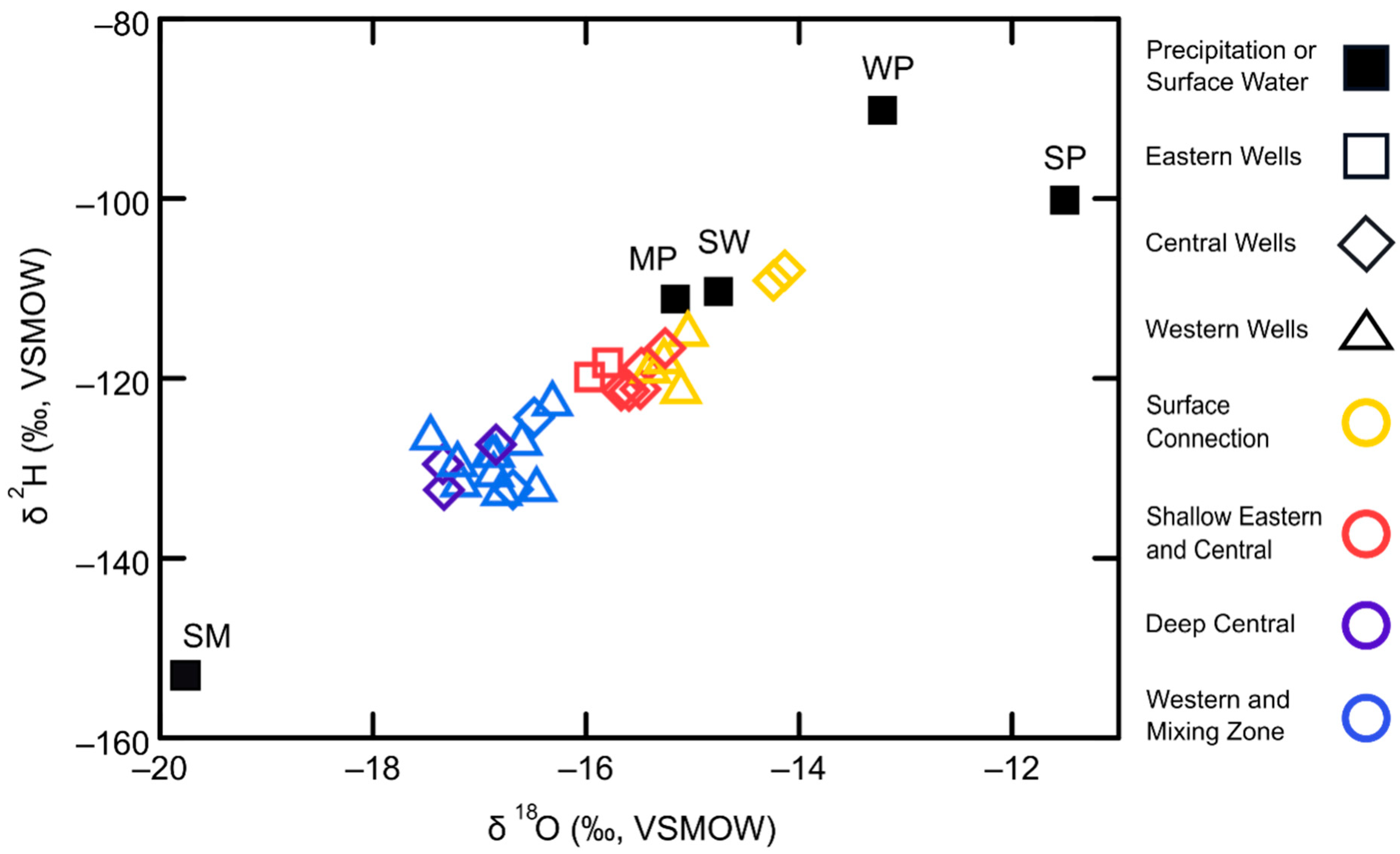
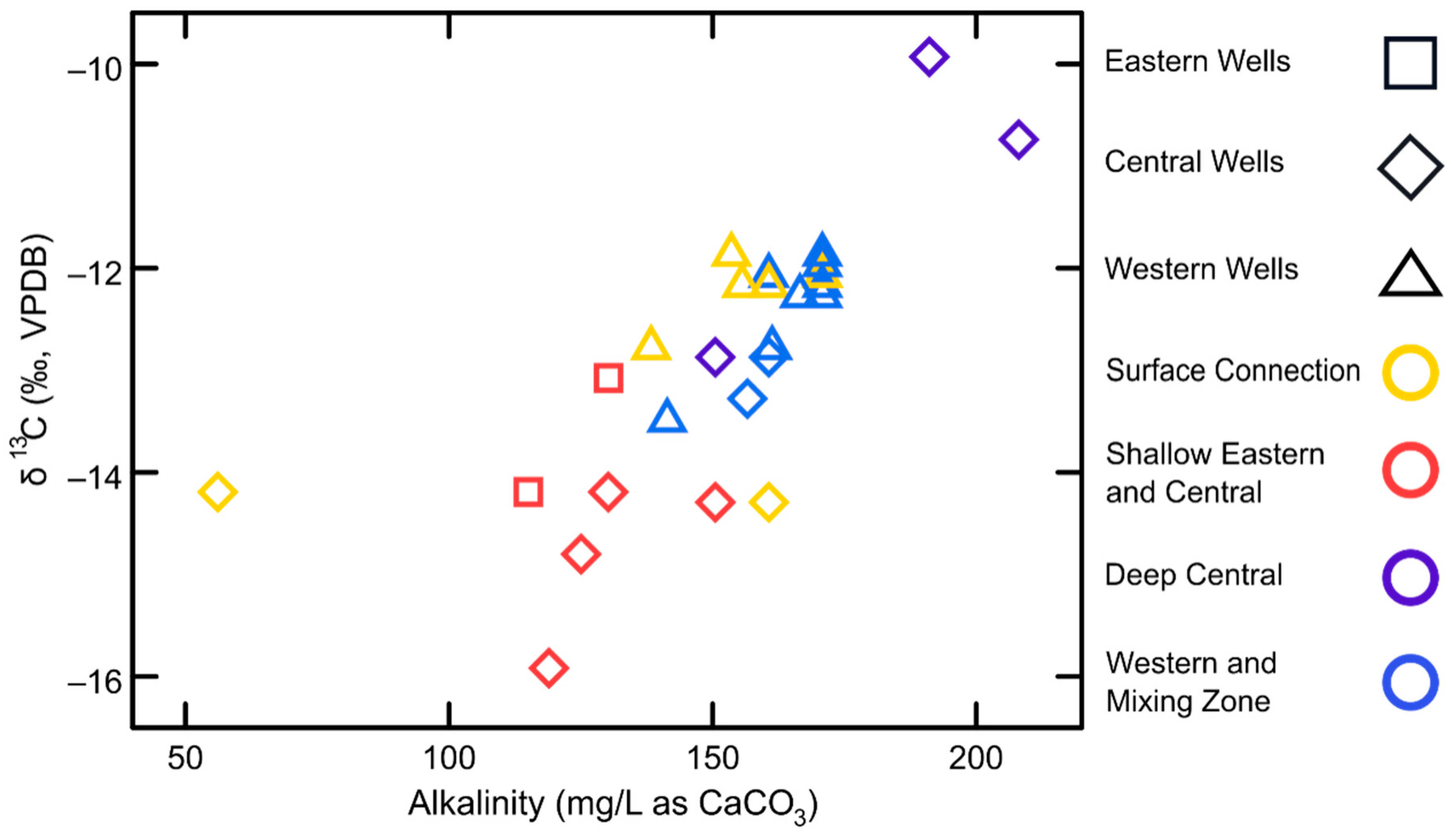
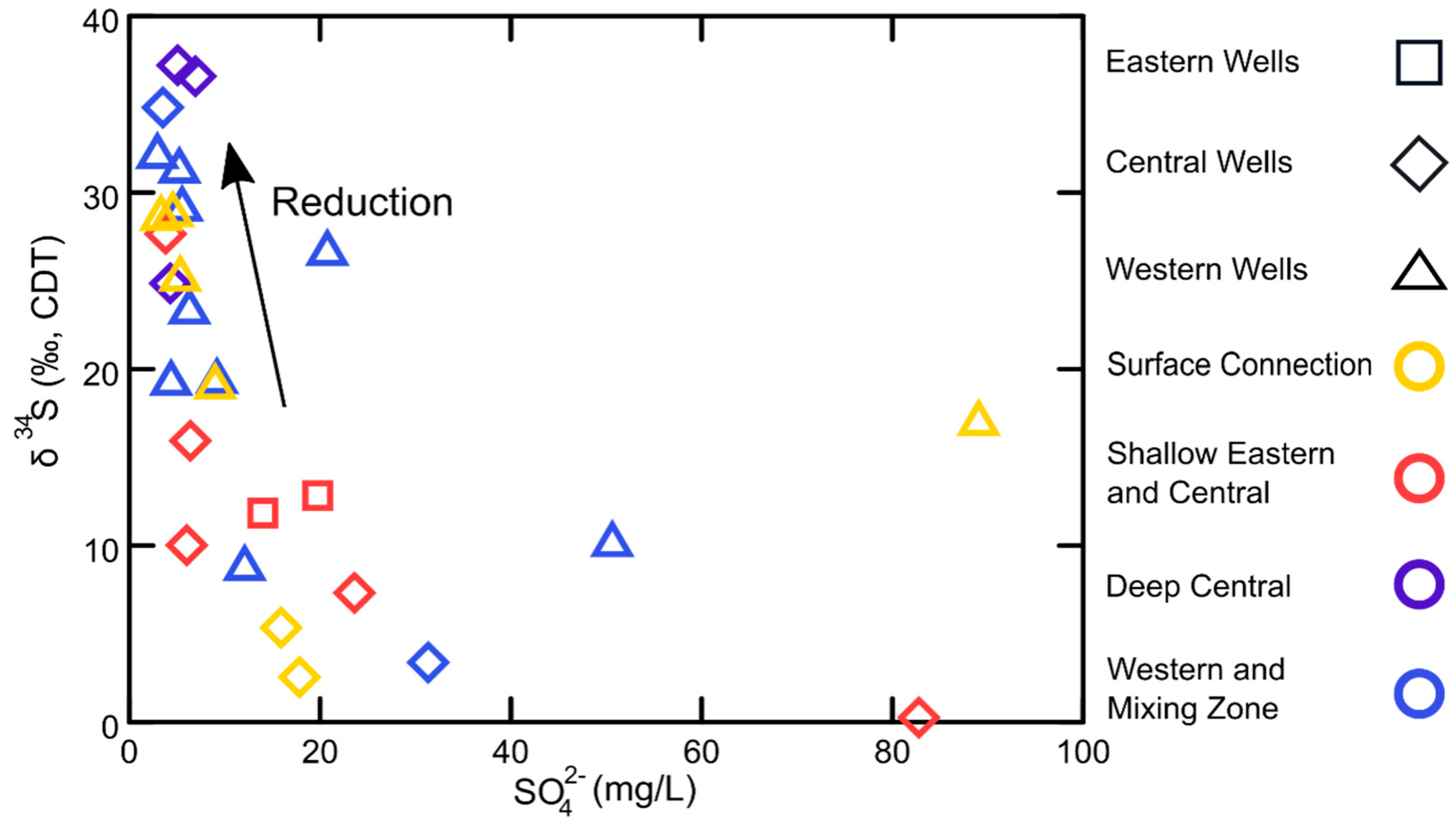
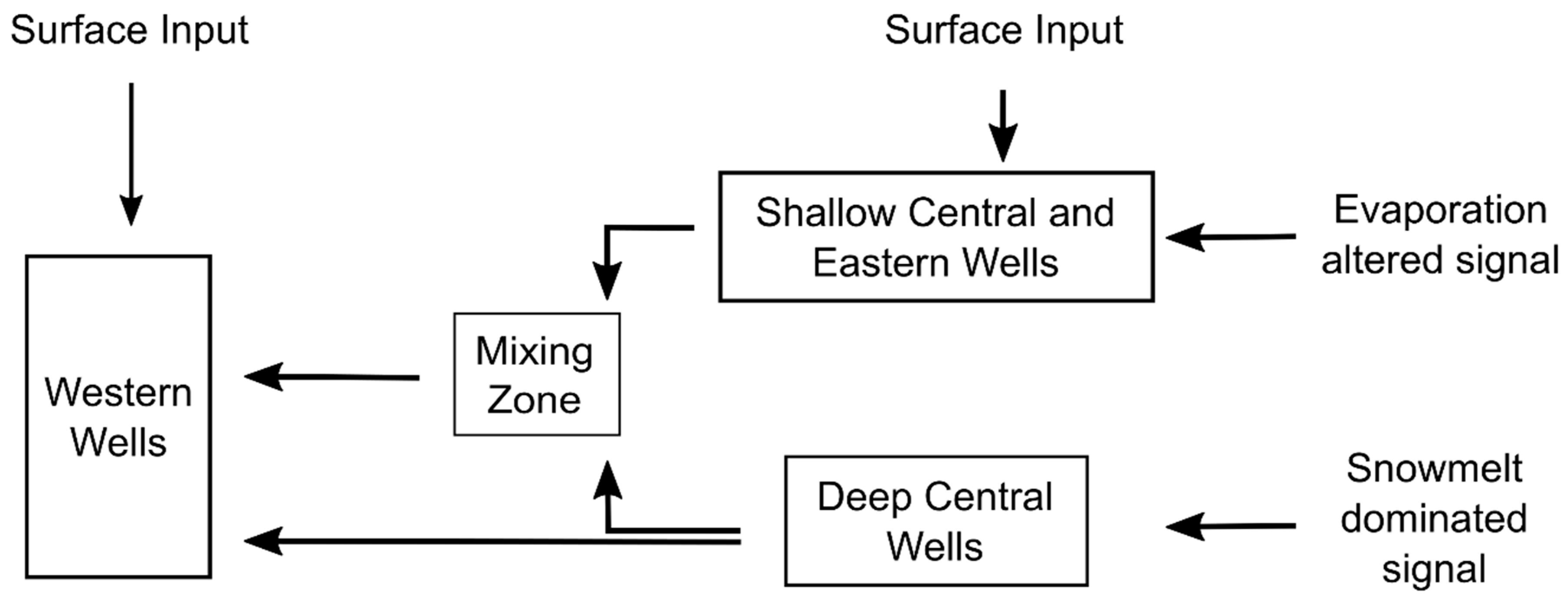
| Study Group | Subgroup Identifier | Well Identifier | Location (Lat./Long., NAD83) | Well Elev. (m NAVD88) | Well Depth (m) | Screened Depth (m) |
|---|---|---|---|---|---|---|
| Eastern | Shallow | WE1 | 46.7241, −116.9428 | 794 | 82 | 20–44; 69–82 |
| WE2 | 46.7256, −116.9555 | 797 | 150 | 32–83 | ||
| Central | Surface Connection | UGC1 | 46.7374, −117.0619 | 771 | 93 | 72–93 |
| LC1 | 46.7297, −117.0278 | 796 | 106 | 86–90; 95–101 | ||
| Shallow | LC2 | 46.7193, −117.0376 | 838 | 100 | 96–100 | |
| WC1 | 46.7349, −117.0025 | 783 | 174 | 12–73 | ||
| WC2 | 46.7351, −117.0023 | 783 | 173 | 21–72 | ||
| UGC2 | 46.7351, −117.0249 | 778 | 224 | 209–228 | ||
| WC3 | 46.7533, −117.0646 | 796 | 78 | 66–78 | ||
| Mixing Zone | LC3 | 46.7435, −116.9724 | 797 | 105 | 73–105 | |
| GC1 | 46.7346, −117.0324 | 779 | 382 | 193–205; 215–222; 239–244; 369–375 | ||
| Deep | DGC1 | 46.7404, −117.0132 | 798 | 444 | 320–444 | |
| DGC2 | 46.7410, −116.9954 | 788 | 399 | 334–398 | ||
| DGC3 | 46.7370, −117.0210 | 782 | 407 | 201–236; 297–335; 366–407 |
| Study Group | Subgroup Identifier | Well Identifier | Location (Lat./Long., NAD83) | Well Elev. (m NAVD88) | Well Depth (m) | Screened Depth (m) |
|---|---|---|---|---|---|---|
| Western | Surface Connection | GW1 | 46.7357, −117.1763 | 715 | 70 | 12–70 |
| GW2 | 46.7134, −117.1824 | 746 | 216 | 205–216 | ||
| GW3 | 46.7475, −117.1730 | 739 | 158 | 72–158 | ||
| UGW1 | 46.7301, −117.1711 | 725 | 84 | 20–31; 63–84 | ||
| UGW2 | 46.6969, −117.1500 | 753 | 122 | 9–122 | ||
| Mixing Zone/Deep | GW4 | 46.7589, −117.1673 | 741 | 108 | 90–108 | |
| GW5 | 46.7260, −117.1137 | 771 | 92 | 79–91 | ||
| GW6 | 46.7358, −117.1765 | 715 | 219 | 83–107 | ||
| GW7 | 46.7228, −117.1765 | 766 | 243 | 100–243 | ||
| GW8 | 46.6925, −117.2416 | 780 | 183 | 122–183 | ||
| GW9 | 46.6925, −117.2414 | 780 | 183 | 131–183 | ||
| GW10 | 46.7342, −117.1568 | 773 | 214 | 121–214 | ||
| GW11 | 46.7291, −117.1698 | 736 | 678 | 164–176; 203–215; 225–234; 274–286; 307–553 | ||
| GW12 | 46.7320, −117.1497 | 794 | 247 | 165–212; 229–244 |
| Analyte | Collection | Filter | Preservation | Analysis Method |
|---|---|---|---|---|
| Cations (mg/L) | Acid-washed HDPE, 125 mL | 0.45 µm | 1 mL HNO3 | ICP-MS |
| Anions (mg/L) | Nalgene, 125 mL | 0.45 µm | Chilled | IC |
| Alkalinity | Nalgene, 125 mL | 0.45 µm | Chilled | Titration: inflection pt. |
| δ13C (VPDB, ‰) | Glass/polyseal, 240 mL | 0.20 µm | Chilled | Gas-ratio MS |
| δ34S (CDT, ‰) | 3 L carboy | 0.45 µm | Chilled | Continuous Flow IRMS |
| δ2H, δ18O (VSMOW, ‰) | Glass/polycone, 60 mL | Unfiltered | Chilled | Cavity ring-down spec. |
| Analyte | Correlation | First Component Contribution (%) |
|---|---|---|
| SO4 (mg/L) | −0.50 | 6% |
| Alkalinity (mg/L as CaCO3) | 0.75 | 14% |
| Temperature (°C) | 0.68 | 11% |
| δ2H (‰) | −0.85 | 18% |
| δ18O (‰) | −0.81 | 16% |
| δ13C (‰) | 0.79 | 15% |
| δ34S (‰) | 0.86 | 18% |
| Study Group | Subgroup Identifier | Well Identifier | δ18O | δ2H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Shallow | Mixing Zone | Deep | Surface | Shallow | Mixing Zone | Deep | |||
| Eastern | Shallow | WE1 | 45% | 55% | 40% | 60% | ||||
| WE2 | 40% | 60% | 30% | 70% | ||||||
| Central | Shallow | LC2 | 55% | 45% | 25% | 75% | ||||
| WC1 | 50% | 50% | 25% | 75% | ||||||
| WC2 | 55% | 45% | 35% | 65% | ||||||
| UGC2 | 55% | 45% | 25% | 75% | ||||||
| WC3 | 65% | 35% | 45% | 55% | ||||||
| Mixing Zone | LC3 | 45% | 55% | 25% | 75% | |||||
| GC1 | 30% | 70% | 0% | 100% | ||||||
| Western | Surface Connection | GW1 | 50% | 50% | 20% | 80% | ||||
| GW2 | 60% | 40% | 10% | 90% | ||||||
| GW3 | 25% | 75% | 25% | 75% | ||||||
| GW4 | 10% | 90% | 0% | 100% | ||||||
| UGW1 | 40% | 60% | 0% | 100% | ||||||
| UGW2 | 20% | 80% | 0% | 100% | ||||||
| Mixing Zone/Deep | GW5 | 0% | 100% | 0% | 100% | |||||
| GW6 | 100% | 0% | 0% | 100% | ||||||
| GW7 | 55% | 45% | 0% | 100% | ||||||
| GW8 | 50% | 50% | 0% | 100% | ||||||
| GW9 | 65% | 35% | 0% | 100% | ||||||
| GW10 | 0% | 100% | 20% | 80% | ||||||
| GW11 | 50% | 50% | 0% | 100% | ||||||
| GW12 | 0% | 100% | 0% | 100% | ||||||
| Study Group | Subgroup Identifier | Well Identifier | δ13C and Alkalinity | Sulfate and Reduction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Surface | Shallow | Mixing Zone | Deep | Conc. | Conc. | ORP | |||
| Eastern | Shallow | WE1 | 70% | 30% | … | ↑ | ↓ | ||
| WE2 | 85% | 15% | … | ↑ | ↓ | ||||
| Central | Shallow | LC2 | 40% | 60% | … | … | … | ||
| WC1 | 40% | 60% | … | ↑ | … | ||||
| WC2 | ― | ― | ↑ | ↑ | … | ||||
| UGC2 | 0% | 100% | ↑ | … | ↓ | ||||
| WC3 | 30% | 70% | … | … | ↓ | ||||
| Mixing Zone | LC3 | ― | ― | ↑ | ↑ | … | |||
| GC1 | ― | ― | ↑ | … | ↓ | ||||
| Western | Surface Connection | GW1 | 20% | 80% | … | … | ↓ | ||
| GW2 | 50% | 50% | … | … | ↓ | ||||
| GW3 | 35% | 65% | … | … | ↓ | ||||
| GW4 | 30% | 70% | … | ↑ | … | ||||
| UGW1 | ― | ― | ↑ | ↑ | ↓ | ||||
| UGW2 | 55% | 45% | … | … | ↓ | ||||
| Mixing Zone/Deep | GW5 | 45% | 55% | … | … | … | |||
| GW6 | 0% | 100% | ↑ | … | ↓ | ||||
| GW7 | 60% | 40% | … | … | ↓ | ||||
| GW8 | 0% | 100% | ↑ | … | ↓ | ||||
| GW9 | ― | ― | … | … | ↓ | ||||
| GW10 | 0% | 100% | … | … | ↓ | ||||
| GW11 | ― | ― | … | … | ↓ | ||||
| GW12 | 0% | 100% | ↑ | ↑ | ↓ | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duckett, K.A.; Langman, J.B.; Bush, J.H.; Brooks, E.S.; Dunlap, P.; Welker, J.M. Isotopic Discrimination of Aquifer Recharge Sources, Subsystem Connectivity and Flow Patterns in the South Fork Palouse River Basin, Idaho and Washington, USA. Hydrology 2019, 6, 15. https://doi.org/10.3390/hydrology6010015
Duckett KA, Langman JB, Bush JH, Brooks ES, Dunlap P, Welker JM. Isotopic Discrimination of Aquifer Recharge Sources, Subsystem Connectivity and Flow Patterns in the South Fork Palouse River Basin, Idaho and Washington, USA. Hydrology. 2019; 6(1):15. https://doi.org/10.3390/hydrology6010015
Chicago/Turabian StyleDuckett, Kyle A., Jeff B. Langman, John H. Bush, Erin S. Brooks, Pamela Dunlap, and Jeffrey M. Welker. 2019. "Isotopic Discrimination of Aquifer Recharge Sources, Subsystem Connectivity and Flow Patterns in the South Fork Palouse River Basin, Idaho and Washington, USA" Hydrology 6, no. 1: 15. https://doi.org/10.3390/hydrology6010015
APA StyleDuckett, K. A., Langman, J. B., Bush, J. H., Brooks, E. S., Dunlap, P., & Welker, J. M. (2019). Isotopic Discrimination of Aquifer Recharge Sources, Subsystem Connectivity and Flow Patterns in the South Fork Palouse River Basin, Idaho and Washington, USA. Hydrology, 6(1), 15. https://doi.org/10.3390/hydrology6010015






