Abstract
The present study examines the water quality in the Quaternary Mio-Plio-Quaternary aquifer of the mining basin of Gafsa using a hydrochemical approach and multivariate statistical methods, to assess groundwater mineralization processes. Results from the analysis of groundwater quality collected during the winter (January 2020) and summer (June 2021) seasons reveal a pronounced stability in geochemical parameters, emphasizing a noteworthy consistency in water composition between the two seasons, with the dominance of the Na-Ca-Mg-SO4-Cl facies, in addition to the fact that all year round these concentrations are beyond their respective WHO limits. Despite the intensive extractive and transformation phosphate industry, the prolonged interaction of water with geological formations is the primary factor controlling their high mineralization. This results from the dissolution of carbonates (calcite, dolomite), gypsum, and halite. The results of the PCA represent two correlation classes. Class 1 comprises major elements sulfate, chloride, sodium, magnesium, and calcium strongly correlated with electrical conductivity (EC) and total dissolved solids (TDS). This correlation is indicative of the water mineralization process. Class 2 includes major elements nitrate and potassium weakly correlated with (TDS) and (EC) As regards heavy metals, their concentrations fall consistently below their respective potability standards established by the WHO across all water sampling points. Meanwhile, fluoride (F-) concentrations exhibited values ranging from (1.6 mg·L−1 to 2.9 mg·L−1) in the winter of January 2020 and (1 to 2.9 mg·L−1) in the summer of June 2021, surpassing its WHO limit (1.5 mg·L−1) in almost all water samples. These findings allow us to conclude that the high mineralization of these waters is acquired due to the dissolution of carbonates (calcite, dolomite), gypsum, and halite due to their prolonged interaction with the geological formations. The deterioration of groundwater quality in the Gafsa mining basin associated with phosphate extraction and processing activities appears to be primarily due to the intensive exploitation of deep-water resources.
1. Introduction
Groundwater is the second largest freshwater resource on the planet and meets more than one-third of global drinking water demand [1]. However, aquifers are at risk of contamination owing to extensive pumping and agricultural and industrial activities. Intensive pesticide and fertilizer applications, wastewater discharge, industrial effluences, and excessive groundwater abstraction are some of the activities that lead to groundwater contamination. These activities have resulted in the deterioration of water resources in various regions worldwide [2]. In most arid and semiarid regions worldwide, the availability of sufficient freshwater has become a limiting factor for development [3,4]. Water scarcity has always been a dominant problem in North Africa and the Middle East. It has been accentuated by rain shortages that have caused a decrease in groundwater resources [5,6]. Overexploitation and changes in land usage have provoked not only the reduction of available water but also the deterioration of water quality [7,8,9]. The degree and severity of soil salinization escalated rapidly during the latter part of the 20th century [8]. The Sahara oases of Northern Africa are no exception [8,9,10]. Generally, groundwater chemistry is primarily controlled by natural processes such as aquifer lithology, residence time, and water rock interactions in unsaturated and saturated zones [1,9]. However, anthropogenic processes, such as agricultural and industrial activities and urban development, have several harmful consequences, such as the overexploitation of groundwater resources [10], the return flow of irrigation water [11], and saltwater intrusion [8,12,13], which may contribute to the degradation of water quality. Many hydrogeological approaches have been used to understand these interactions and predict groundwater dynamics [3,13,14,15]. On the other hand, fluoride is found ubiquitously in natural waters, with varying concentrations across different sources. In groundwater, fluoride concentrations can vary widely depending on the geological characteristics of the rocks [16]. Flouride-rich waters are encountered in three major geological terrains: sedimentary basins, crystalline basement areas, and volcanic regions [16]. In groundwater, the fluoride content depends on various factors, including the porosity of rocks and soils through which water circulates, speed of circulation, temperature during the interaction between water and rocks, composition of salts encountered by water before reaching aquifers, and chemical capacity of water to solubilize fluoride [8,17]. Mining operations generally significantly impact the amount and quality of water resources in a neighborhood. During exploitation, dewatering leads to the drawdown of the water table, which results in the disappearance of specific springs or a decrease in their flow. After mine closure, rebound degrades groundwater quality [4,8,18]. Numerous studies have examined the groundwater chemistry in the Gafsa mining basin in Southwest Tunisia [19,20]. All these studies conducted on the hydrochemistry of water are concentrated mainly at specific sites in the basin. This means that these studies have yet to focus on the hydrochemistry of water in the entire basin, especially around industrial sites. However, this study focuses on water hydrochemistry in mining as the whole basin, especially those close to industrial sites (Moulares, Redeyef, M’etlaoui, and M’dhilla).
Chemical analyses conducted as part of the Gafsa North aquifer system investigation by [21] indicated that the Mio-plio-Quaternary waters are of the sodium sulfate type. The salinization of these waters is primarily attributed to the dissolution of gypsum and, to a lesser extent, halite. The geological condition of the region can explain this. According to [22], the influence of geological features, notably the Gafsa Fault, has emerged as a pivotal factor affecting the quality of groundwater, specifically its salinity levels in the vicinity.
The results from [23] study in the Northern Gafsa Basin, specifically in Gafsa city, revealed concentrations of Ca2+, Mg2+, Na+, K+, Cl−, SO42−, NO3−, and HCO3− that surpassed the standards set by the (WHO). The applied analysis of significant elements has substantiated an anthropogenic origin, predominantly linked to agricultural activities, for these major elements. These elements contributed significantly to the pronounced mineralization observed in the study area.
A study conducted by [24] in the same area revealed that groundwater in Northern Gafsa exceeds the standards set by the (WHO) for sulfate, calcium, and chloride concentrations, with dominant calcic sulfated facies. These results indicate that the chemical quality of water is primarily influenced by the geological nature of the aquifer, with these elements playing a significant role in the observed high salinity levels.
None of the potassium and nitrate values from either campaign exceeded the WHO standards, a finding consistent with the observations of [19,25,26]. This suggests that these elements may not significantly contribute to winter mineralization in the mining basin.
Refs. [27,28], investigated the adverse impacts of a phosphate industrial zone on groundwater in the Jorf Lasfar region of Morocco. Their study revealed a significant increase in the measured electrical conductivity of groundwater. The calcium and potassium concentrations met the compatibility standards set by the WHO. However, the chloride, sodium, nitrate, and magnesium concentrations exceeded the water quality standards. The increased conductivity was primarily attributed to chloride and sodium ions.
The study conducted by [29] in the phosphate region of central Jordan, in the Al Hisa province, revealed a significant concentration of chloride, magnesium, and sulfide, surpassing the standards set by the WHO. This investigation highlights the saline nature of the waters in the Al-Hisa zone, with water salinity attributed to the dissolution of soluble elements in the effluent waters. In addition, the concentrations of potassium and nitrate in the same phosphate region were below the standards established by the WHO. These observations are consistent with the findings of the present study.
This study aimed to identify and interpret the factors responsible for mineralization and the degradation of water quality in the mining basin of Gafsa. Therefore, it is essential to assess the processes and phenomena controlling the reactions of aquifer systems that may be exposed to environmental or climate changes. Hydrochemical techniques (Piper, Schoeller–Berkalov representations) were used to determine the dominant facies of groundwater in the study area, better understand the relationship between these elements, and specify the factors affecting mineralization. Statistical calculations (NPCA) using Python language were performed to better understand the link between the quantitative variability of cations and anions.
2. Materials and Methods
2.1. Presentation of the Study Area
2.1.1. Geology
The mining basin of Gafsa, situated in the southwest of Tunisia, encompasses most of the country’s phosphate mining activities [8,30]. To its north lies the Gafsa Range, while to the east, it is bordered by the Guettar and Jebel Chamsi regions. To the south, it is delineated by Chott El Gharsa, and to the west lies Algeria [8,30]. Geologically, the outcrops of this region consist mainly of Quaternary to Mio-Pliocene formations, including the sands of the Beglia Formation and the sandy/silty clays and conglomerates of the Segui Formation in the regions of Redeyef and Metlaoui [8,30].
The Palaeogene (mainly Eocene) and Early/Late Cretaceous formations constitute the geological outcrops in the Metlaoui, Gafsa, and Sehib Ranges. According to [8,31,32] the geological units of the Metlaoui Group, which hosts the phosphate series of the Gafsa-Metlaoui basin, are attributed to the Eocene epoch. This primarily includes three formations: The Thelja Formation, characterized by dolomitic deposits alternating with gypsum and marl. The Chouabine Formation comprises four phosphate units (A, B, C, and D). The Kef-Eddour Formation consists of marl–phosphate alternations framed by two limestone bars.
The lithostratigraphic column (Figure 1), spanning the Cretaceous to the Quaternary, exhibits two primary stratigraphic sequences. The first sequence comprises marine Cretaceous and Paleogene deposits from formations including Sidi Khalif, Melloussi, Boudinar, Bouhedma, Sidi Aïch, Orbabta, Zebbeg, Aleg, Abiod, El Haria, Metlaoui, and Jeb’s. In contrast, the second sequence consists of continental Neogene and Quaternary deposits from the Sehib, Beglia, and Segui formations. These two sequences are divided by a significant sedimentary hiatus extending from the Middle Eocene to the Early Miocene [33].
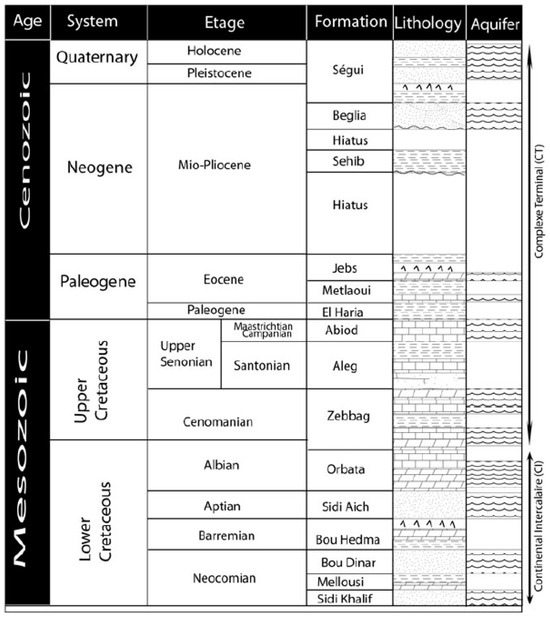
Figure 1.
Lithostratigraphic column of the study area [8].
All sampling points are located within the Mio-Plio-Quaternary.
2.1.2. Phosphate Industry and Environment
The phosphate industry is considered one of Tunisia’s most polluting (air, soil, freshwater, seawater, sediments, soil, etc.), activities [8,34]. The Phosphate Company of Gafsa (CPG) operates in eight open-pit mines (Kef Schfaier, Kef Eddour Central, Kef Eddour West, Metloui, Jallabia, M’dhilla, Redeyef and Moulares) and nine processing plants (laundries) (Figure 2). Most of the phosphate production (around 90%) is processed by the (CPG) and the Groupe Chimique Tunisien (GCT) in the Gafsa basin (M’dhilla) and other phosphoric acid-producing plants operating in the south of Tunisia (Gabes, Skhira, Sfax). These plants produce vast amounts of highly toxic effluents [35,36]; loaded with fluorides, radionuclides, metallic trace elements (MTE), sulfites, and phosphoric acid [36,37,38], these effluents are discharged into the hydrographic network (Wadi Bayech, EL Kbir, Wadi El Melah, Wadi Moulares, Wadi Magroun, and Wadi Tebedditt).
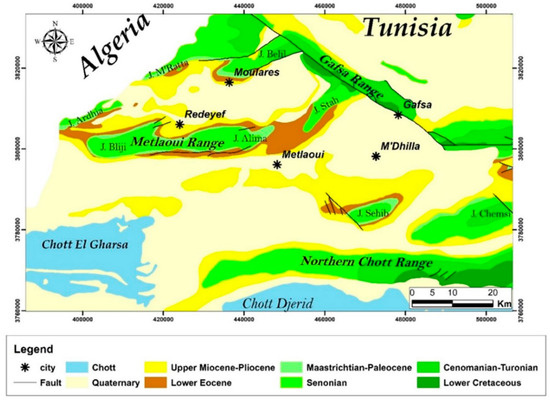
Figure 2.
Geologic map of the Gafsa mining basin [19], showing the location of the phosphate laundries and the water sampling points.
2.1.3. Sampling and Methods
Groundwater samples were collected during wet (January 2020) and dry (June 2021) seasons from seven boreholes (80–250 m deep) exploiting the so-called Complexe Terminal (CT) Mio-Plio-Quaternary aquifer system (Figure 1). Borehole positions (34° 40–34° 20 N latitude and 8° 00–8° 40 E longitude), were located using a GPS.
Water samples were conditioned in polyethylene containers and kept in a cooler for 24 h. In the laboratory, samples were vacuum filtered through 0.45 µm Millipore filters MilliporeSigma, Darmstadt, Germany, a Merck company and stored at 4° C before being analyzed.
Temperature (T) and hydrogen potential (pH) were measured in situ using an ISO-SCAN PH meter. The electrical conductivity (EC) and total dissolved salt (TDS) were measured using a conductivity meter (WTW Model INOLAB Cond7310P).
Water analysis was performed at the laboratory of the Centre International des Technologie de l’Environnement (CITET), using Dionex chromatography (DX ICS-1100) for the analysis of anions (SO42−, Cl−, F−, NO3−) and an inductively coupled plasma atomic emission spectrometer (ICP-AES; Optima 7300 DV) for the analysis of cations (Ca2+, Mg2+, K+, Na2+) and trace elements (F−, Fe, Mn, Al, Zn, Cr, Pb, Cd). The total alkalinity (HCO3−) was determined by titration with 0.01 or 0.1 M HCl.
The accuracy of complete chemical analysis is also checked and ionically balanced by calculating the cation–anion equilibrium in terms of equivalents per milligram (mg·L−1). The ionic balance for all samples is within the ±5% interval.
Multivariate statistical techniques (PCA) and hydrochemical representations (Piper and Schoeller–Berkaloff) are used for results processing.
3. Results
Water samples collected from boreholes during both winters (January 2020) and summer (June 2021) seasons were subjected to the in situ measurements of physico-chemical parameters (EC, TDS, pH, T) as well as the determination of major constituents (anions and cations) and trace elements.
3.1. Physico-Chemical Parameters
The results presented in Table 1 reveal relatively consistent temperature values, ranging from 24 to 27 °C, during January 2020 and June 2021. pH levels exhibited variability, with values ranging between 7.17 (Borehole 6) and 7.76 (Borehole 7) in January 2020 and between 7.02 (Borehole 6) and 7.83 (Borehole 2) in June 2021. In terms of electrical conductivity (EC), values ranged from 3700 μs·cm−1 (Borehole 6) to 7100 μs·cm−1 (Borehole 7) in January 2020 and from 4000 μs·cm−1 (Borehole 6) to 7200 μs·cm−1 (Borehole 7) in June 2021. Notably, EC does not appear to undergo significant seasonal fluctuations.

Table 1.
Physico-chemical parameters and geochemical characteristics of groundwater in the Gafsa mining basin.
Regarding total dissolved solids (TDS), measured values spanned from 3986 mg·L−1 (Borehole 6) to 6184 mg·L−1 (Borehole 2) in January, and from 4394 mg·L−1 (Borehole 5) to 6603 mg·L−1 (Borehole 2) in June. This range suggests a prevalent brackish quality in the water throughout the measured period.
3.2. Major Constituents (Cations and Anions)
Table 1 summarizes the major ionic species (cations and anions) concentrations present in the studied waters. The ionic balance (IB) values for all samples during the two campaigns were lower than 5%.
Na+ content in the water varies between 700 mg·L−1 (Borehole 6) and 1400 mg·L−1 (Borehole 7) for January 2020 and between 780 mg·L−1 (Borehole 6) and 1250 mg·L−1 (Borehole 7) for June 2021. The maximum Na+ concentration is observed in the Plio-Quaternary aquifer of the Métlaoui area (1420 mg·L−1 in Borehole Bh2).
Ca++ contents vary between 325 mg·L−1 (Boreholes 6 and 7) and 479 mg·L−1 (Borehole2) for January 2020 and between 310 mg·L−1 (Borehole 7) and 488 mg·L−1 (Borehole 2) for June 2021. Mg++ contents fluctuate between 174 mg·L−1 (Borehole 5) and 250 mg·L−1 (Borehole 2) for January 2020 between 200 mg·L−1 (Borehole 5) and 264 mg·L−1 (Borehole 2) for June 2021. K+ content varies between 3.9 mg·L−1 (Borehole 3) and 10.54 mg·L−1 (Borehole 7) in January 2020 and between 4.4 mg·L−1 (Borehole 3) and 9.7 mg·L−1 (Borehole 7) in June 2021. SO42− contents vary between 1500 mg·L−1 (Borehole 6) and 1899 mg·L−1 (Borehole 1) for January 2020 and between 1648 mg·L−1 (Borehole 6) and 2100 mg·L−1 (Borehole 2) for June 2021. Cl− concentrations vary between 946 mg·L−1 (Borehole 5) and 1899 mg·L−1 (Borehole 2) for January 2020 and between 1040 mg·L−1 (Borehole 3) and 1900 mg·L−1 (Borehole 2) for June 2021. HCO3− contents range from 210 mg·L−1 (Borehole 6) to 438 mg·L−1 (Borehole 2) for January 2020 and between 210 mg·L−1 (Borehole 7) and 394 mg·L−1 (Borehole 2) for June 2021. NO3− contents vary between 10.1 mg·L−1 (Borehole 1) and 60 mg·L−1 (Borehole 6) for January 2020 and between 14.4 mg·L−1 (Borehole 5) and 48.3 mg·L−1 (Borehole 6) for June 2021.
The results of Table 1 show that these waters have the following general pattern: [Na+] > [Ca2+] > [Mg2+] >> [K+] and [SO42−] > [Cl−] >> [HCO3−] > [NO3−]. Compared to the WHO standards, these results show that the investigated waters are enriched in sodium (>200 mg·L−1), calcium (>200 mg·L−1), magnesium (>50 mg·L−1), chlorine (>200 mg·L−1), sulfate (>500 mg·L−1), and bicarbonate (>15 mg·L−1) with a deficit in potassium (<20 mg·L−1) and nitrate (<50 mg·L−1), except the slight excess in nitrate (60 mg·L−1) in the borehole 6 of Redeyef in January 2020.
3.3. Trace Elements
The results presented in Table 2 enable a comparison of the concentrations of heavy metals and fluoride in two distinct sampling campaigns, January 2020 and June 2021, and thus, to evaluate any significant variation in water quality. Table 2 shows that there is no significant variation in the concentration levels of heavy metals (Fe, Mn, Al, Zn, Cr, Pb) between the two sampling campaigns of January 2020 and June 2021. For example, the levels of iron vary between 0.041 µg·L−1 and 0.099 µg·L−1 (BH6) in January 2020 and between 0.011 µg·L−1 (BH1) and 0.092 µg·L−1 (BH7) in June 2021. The levels of fluoride do not show significant variation between the two campaigns. Compared to WHO standards, the results from (Table 2) indicate that the examined waters are not enriched in heavy metals. However, fluorine contents exceed the WHO standards in almost all samples.

Table 2.
Trace metals and fluoride contents in the groundwater of the Gafsa mining basin.
4. Discussion
The pH levels observed in the analyzed waters during both the winter (January 2020) and summer seasons (June 2021) demonstrate significant consistency, highlighting the marked hydrochemical stability of the Gafsa mining basin and consistently align with the circum-neutral range of 7.1 to 7.8 sensu Bocoum (2004), which is in agreement with previous studies conducted on groundwaters in the same basin [25,26,39]. It is worth noting, however, that such pH values indicate the intervention of carbonates as buffering minerals. In such an environment, buffering provides a notably stable pH level [39].
Electrical conductivity (EC) exhibits significant variability, ranging from 3700 to 7200 µS·cm−1 during winter (Figure 3a) and from 4100 to 7200 µS·cm−1 during summer seasons (Figure 3b). However, no significant variation is observed between the two seasons. Total dissolved solids (TDS) do not exhibit noteworthy variations, ranging from 3986 to 6184.5 mg·L−1 (Figure 3a) during winter seasons and from 4393.9 mg·L−1 to 6603.2 mg·L−1 (Figure 3b) during summer seasons. Nonetheless, no notable difference was observed between the two seasons. Figure 3a,b reveals a high concentration of total dissolved solids (TDS) during both winter and summer seasons in the areas of Métlaoui (Borehole 2) and M’dhillla (Borehole 7). In contrast, a lower concentration of (TDS) is observed in the Moulares area (Boreholes 3 and 4) and the Redyef area (Boreholes 5 and 6). (Borehole 2) in the Métlaoui area and (Borehole 7) in the M’dhilla area are near the Gafsa Phosphate Company, which could potentially explain these elevated TDS values attributed to the overexploitation of the aquifer by the phosphate industry.
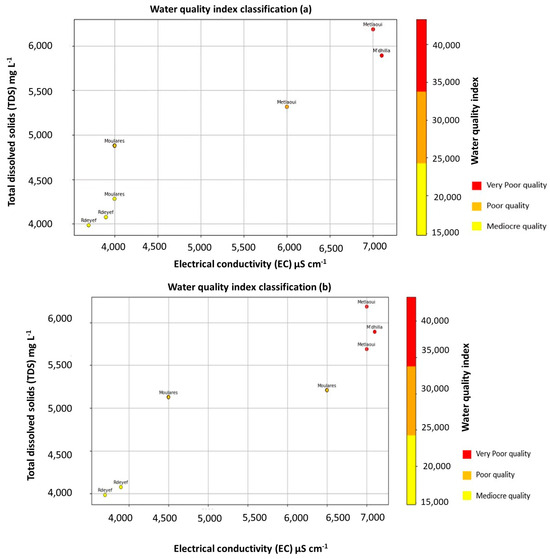
Figure 3.
Index water quality of mining basin of Gafsa for (“January 2020 (a); June 2021 (b)”).
These TDS values exceed the WHO standard (1000 mg·L−1). However, falling within the 1000 to 10,000 mg·L−1 range, sense Rodier (1996) and [21,22,40,41,42], these waters may be classified as brackish. Similar values have been reported by [25].
Based on the measurements of electrical conductivity and dissolved solids concentrations (Table 1a,b), the classification of the water quality index in the Gafsa mining basin for two distinct seasons, winter and summer, is illustrated (Figure 3a,b). According to this graphical representation, the water quality in the mining basin is systematically categorized into three classes, each assigned a specific color (red, orange, yellow) (Figure 3a,b). Specifically, the observations disclose the following water quality assessments: (i) Mediocre water quality is evident in the Moulares and Redeyef (Figure 3a,b); (ii) poor water quality is identified in the Metlaoui zone (Figure 3a,b); (iii) inferior water quality is observed in the M’thilla and Metlaoui (Figure 3a,b). This classification presents the nuanced variations in water quality in the mining basin.
4.1. Hydrochemical Characteristics of the Investigated Waters
The concentrations of major elements observed in the two seasons exhibit significant elevations, particularly in sodium (700 to 1400 mg·L−1) in January 2020 (Table 1a) and from 780 to 1420 mg·L−1 in June 2021 (Table 1b), chloride (946 to 1899 mg·L−1) in January 2020 (Table 1a) and 1040 to 1900 mg·L−1 in June 2021 (Table 1b), sulfate (1500 to 1899 mg·L−1) in January 2020 and 1630 to 2100 mg·L−1 in June 2021, calcium (325 to 497 mg·L−1) in January 2020 (Table 1a), and 310 to 488 mg·L−1 in June 2021 (Table 1b), and magnesium (174 to 300 mg·L−1) in January 2020 (Table 1a) and 200 to 264 mg·L−1 in June 2021 (Table 1b). However, it is essential to note that no significant variation is observed in these physico-chemical parameters between the winter and summer seasons. These values consistently surpass their respective WHO standards (Table 1).
Heightened high sodium levels were observed in the study by [19] in the Moulares Redeyef mining area, specifically around the Tebedditt region. The ionic composition is prominently characterized by SO42− (0.54–1.75 g/L), Cl− (0.36–0.43 g/L), Na+ (0.19–0.28 g/L), Ca2+ (0.18–0.35 g/L), and Mg2+ ranging between 0.11 and 0.24 g/L. This phenomenon can be attributed to various factors, including the hydrodynamic characteristics of the aquifer and intricate water–rock interactions involving processes such as dissolution and infiltration.
Moreover, discernible human influences, mainly from mining and agricultural activities, significantly contribute to the observed compositional trends. These anthropogenic factors amplify the impact on groundwater quality, necessitating careful consideration of environmental management strategies for the Gafsa mining basin.
The high concentrations of sulfate and chloride in this study are further supported by the study conducted by [25,26], which indicates that the concentrations of sodium, chloride, calcium, and sulfate ions surpass the standards set by the World Health Organization (WHO). This phenomenon was attributed to the dissolution of halite and gypsum.
The drawing of water samples onto the Piper diagram (Figure 4), of two seasons (January 2020 (a) and June 2021 (b)) shows that on the triangle of anions, water samples are located next to the SO4− Cl− lines and far from the pole HCO3. note however that for the samples Bh1, Bh3, Bh4, Bh5, and Bh6 the SO42− content is slightly higher than that of Cl−, and vice versa for Bh2 and Bh7 these two boreholes are richer in NaCl than the others, If we take into consideration the cation triangle, and assuming that potassium contents are accessory water samples are highly concentrated in Na, Ca and Mg, with Na > Ca > Mg. Thereby, the waters analyzed belong to the Na-Ca-Mg-SO4-Cl chemical facies. The chemical particularities of (Bh1, Bh2, Bh3, Bh4, Bh5, Bh6, and Bh2, Bh7) depend on many natural factors, such as the lithology of the aquifer, the quality of recharge waters, and the types of interaction between water and aquifer.
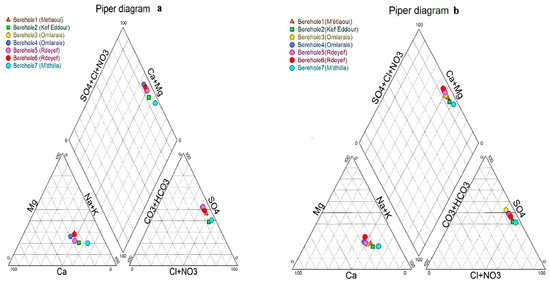
Figure 4.
Plot of the groundwater samples on the Piper diagram: (a) January 2020; (b) June 2021.
The representation of the ion concentrations on the Scholler–Berkalov diagram (Figure 5) of two seasons (January 2020 (a) and June 2021 (b)) demonstrates that all the analyzed waters display identical profiles and that there is a cationic dominance of sodium, calcium, and magnesium in all examined samples and are highly loaded in Na+, Ca2+, Mg2+, SO42− and Cl−. We can conclude that the groundwater in the mining basin is heavily loaded and oversaturated with calcite, halite, dolomite, and gypsum (Hamed et al., 2008) [25]. Figure 5a,b also show that all the aforementioned ions, in addition to bicarbonates (HCO3−) in the analyzed groundwater, exceed their respective WHO guidelines. The only exception is nitrate (NO3−) concentrations, which remain below the recommended limit, with the exception of sample Bh6, where the concentration (60 mg/L) slightly exceeds the WHO standard of 50 mg/L.
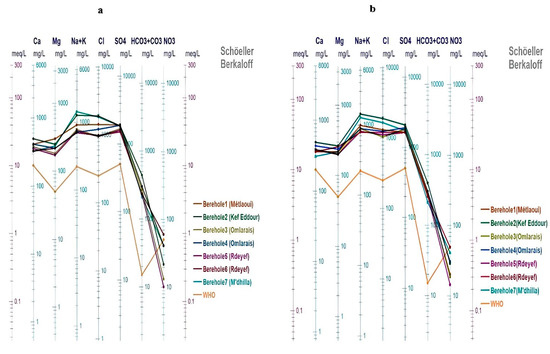
Figure 5.
Plot of the characteristics of the studied groundwater samples on the Schoeller–Berkalov diagram, (a) January 2020), (b) June 2021).
The sulfated, calcic, magnesium, sodium, and chlorinated facies result from the presence of sulfate, calcium, sodium, magnesium, and chloride in the soil due to the leaching of these ions by rainwater during infiltration and/or the dissolution of evaporates.
Comparing our results with other studies on water hydrochemistry in phosphate mining areas in various countries worldwide is paramount for a better understanding of the underlying factors contributing to the deterioration of water quality.
Based on internal research and the findings of our study, it can be stated that the dynamic interaction between water and rock primarily influences the groundwater quality in the Moulares Redeyef basin. This complex relationship, integral to the hydrogeological dynamics of the aquifer, manifests through processes such as dissolution, and infiltration. The distinct geological characteristics of the study area play a pivotal role in driving these phenomena, exerting a substantial influence on the composition of groundwater resources. Notably, the etiology and geological attributes of the region function as foundational elements initiating shifts in water quality. This primary factor lays the groundwork for subsequent considerations, facilitating a comprehensive understanding of the geological intricacies that underscore the multifaceted challenges within the Moulares Redeyef basin. As we delve into the ensuing sections, we will expound upon additional contributing factors, including anthropogenic influences and intensified industrial activities, enriching the narrative with a holistic exploration of the diverse elements shaping the groundwater landscape in Phosphatic mining regions.
Phosphate extraction activities in the Gafsa mining basin pose significant risks to human health. The contamination of groundwater with harmful pollutants, as found by Hamed et al., 2014 [42], can lead to serious health conditions. For instance, fluoride presence in drinking water can lead to fluorosis, a condition characterized by dental and skeletal damage following prolonged exposure to elevated fluoride concentrations, as noted by Umer 2023 [27]. Additionally, elevated sulfate levels in water can cause gastrointestinal issues, such as diarrhea and intestinal irritation, as they disrupt the body’s fluid balance and irritate intestinal tissues, as found by Backer 2000 [43].
The contamination of local water sources with these pollutants has a direct and adverse impact on the health of the regional population, who are exposed to fluoride and sulfates through their drinking water Guissouma and Tarhouni 2015, Guissouma et al. 2017 [44,45]. Therefore, it is essential to implement stringent management and monitoring strategies to safeguard water quality in the region. This includes optimizing extraction techniques and employing effective water treatment processes to mitigate these health risks.
To reduce the impact of the phosphate industry on groundwater contamination, several strategies should be adopted. First, improving waste management practices, particularly in the storage and treatment of phosphate residues, is crucial. Advanced containment and treatment technologies, such as filtration and neutralization systems, can significantly reduce the risk of contaminants leaching into groundwater. Additionally, establishing vegetated buffer zones around industrial sites can filter runoff water and reduce pollutant transfer to aquifers. Continuous monitoring of groundwater contamination levels and rapid response protocols for pollution incidents are also necessary. Finally, promoting research into more environmentally sustainable phosphate production methods and encouraging the adoption of eco-friendly practices can help minimize the industry’s overall environmental impact.
4.2. Statistical Parameters and Correlation Matrix
The results of the PCA of two seasons, winter and summer, are summarized in Table 3. The binary relationship and the correlation coefficients between the different variables are given by the correlation matrix (Table 4).

Table 3.
Statistical parameters of physico-chemical characteristics of groundwaters ((a) January 2020, (b) June 2021).

Table 4.
Correlation matrix of the physico-chemical characteristics of the groundwater in the mining basin of Gafsa ((a) January 2020; (b) June 2021).
The PCA was performed to analyze water sampled on the entire study area to highlight the possible associations between the different variables and the possible existence of the sub-populations of anions and cations. PCA is a dimensionality reduction method that allows the identification and visualization of underlying structures in multivariate data. It transforms the original variables into a set of new variables known as principal components, which explain the maximum possible variance in the data.
In our analysis, we applied PCA to simplify the interpretation of the relationships between the different studied variables, reducing data complexity while retaining essential characteristics. This approach enabled us to better understand variation patterns and evaluate the main sources of contamination and the chemical elements responsible for the mineralization of water in the phosphate basin.
The statistical characterizing of the distribution of the major elements was calculated for each of the physicochemical variables (Table 3)
The coefficient of variation (CV) and the standard deviation ratio divided by the arithmetic mean are parameters used to measure the dispersion of a series of observations of a variable around its arithmetic mean. If the value of the CV is less than 50%, the observed variable has a homogeneous distribution around its arithmetic mean, whereas if the value of the CV is greater than 50%, the observed variable has a heterogeneous distribution around its arithmetic mean [29].
The results indicate a homogeneous geographical distribution of physicochemical parameters (EC and TDS) and major ions, with a coefficient of variation (CV) below 50% (Table 3). However, the nitrate anion (NO3−) exhibits a heterogeneous geographical distribution, with a CV exceeding 50% (Table 3a) during the two sampling periods.
A correlation matrix in which 10 parameters characterizing the analyzed waters of two seasons, January 2020 and June 2021, are involved is presented in Table 4. Significant associations between the various parameters, reflecting the links between the studied variables, are given by this matrix. The PCA shows that the major ions are well correlated with EC and TDS. However, the strong correlation between the contents of major ions reveals that the total mineralization of water is mainly due to these elements that increase the salt load. On the other hand, the nitrate ion (NO3−) (Table 4 contents are the least correlated with the salt load.
Table 4 shows a strong positive correlation between sodium and chloride (R = 0.93 and 0.96; Table 4a and Table 4b, respectively), in the mining basin. This correlation reflects a proportional evolution between these two major elements in all sampling points. Therefore, these elements appear to share a common origin of mineralization, likely stemming from the dissolution of halite [38].
The good correlation between SO42− and Ca2+ (R = 0.61 and 0.84; Table 4a and Table 4b, respectively), shows that the two chemical compounds have common origins, e.g., the dissolution of gypsum (CaSO4, 2H2O), or anhydrite (CaSO4).
A good correlation (R = 0.97 and 0.93; Table 4a and Table 4b, respectively), is noticed between Ca2+ and HCO3−.
Calcium and magnesium exhibit an average correlation (R = 0.41 and 0.50; Table 4a and Table 4b, respectively). Furthermore, the dominance of calcium over magnesium is evident at all sampling points (Table 1a,b).
Eigenvalues and Variance
Table 5 shows the eigenvalues, the expressed variances for each factor, and their accumulation. Factor F1, with an expressed variance of 57.5% for the winter season of January 2020 and 52% for the summer season of June 2021, is the most important of all, followed by factors F2 and F3, with, respectively, 18.75% and 16% for January 2020 of the expressed variance and 23% and 16.57% for June 2021. The cumulative variance expressed is 92.23% for the three factors for January 2020 and 91.51% for June 2021.

Table 5.
Eigenvalues and variance expressed by the main axes.
These factorial axes chosen for this statistical analysis are, therefore, representative of the variance of the whole data set. The factorial plans F1–F2 and F1–F3 represent cumulative variances equal to 76.1% and 74.8% and 74.8% and 68.5%, respectively. These two plans not only represent almost the same cumulative variance but also integrate enough representativeness to highlight the general trends. Since the different results are almost the same, only those of the F1–F2 design have been presented because this design more clearly distinguishes the trends.
The analysis of the results of the PCA of the space of the variables of the factorial plan F1–F2 shows that the factor F1, the most important (57.4% and 51.9%; Figure 6a and Figure 6b, respectively), is determined by Ca2+, Na+, Mg2+, SO42−, HCO3−, TDS, CE, and Cl−, (Figure 6a,b). The F1 factor, therefore, groups together the bulk of the chemical elements and the electrical conductivity. The proximity of these variables in the community circle shows that they control the phenomenon of putting the mineral elements into a solution. These variables highlight a slow process of dissolution of the ions. Thus, the F1 factor seems to account for the conditions of acquisition of the water chemistry. It explains the phenomenon of mineralization residence time. Most of the variables are positively correlated with this phenomenon showing that the main phenomenon at the origin of the mineralization of the groundwater in the region is dissolution. This dissolution is due to the contact during a sufficient residence time of the water with the surrounding geological formations. Factor F2 (Figure 6), accounting for 18.7% and 22.9% of the inertia of the sample point cloud, is influenced by the elements NO3− and K+. These results show that the F2 factor is not related to the hydrolysis phenomenon but rather to the redox phenomenon.
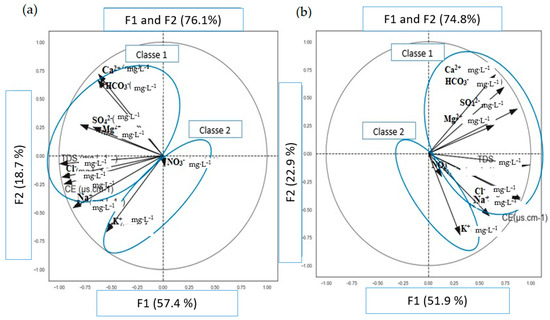
Figure 6.
Variables space of the factorial plane (“F1–F2 ((a) January 2020; (b) June 2021”).
The PCA community circle (Figure 6) serves as a graphical representation in the statistical unit space, depicting the distribution of water points based on the various F1–F2 factors, revealing two primary groupings of water points.
These two factors, F1–F2, can also be grouped into two classes. Class 1 encompasses the major elements that are well correlated with electrical conductivities (EC) and total dissolved solids (TDS) sulfate, chloride, sodium, magnesium, and calcium), as depicted in (Figure 6). Conversely, Class 2 includes major elements with a weaker correlation with (TDS) and (EC) (nitrate, potassium), as shown in (Figure 5).
The concentrations of Pb, Mn, Zn, Cr, Fe, and Al in the analyzed water samples fell for winter (January 2020) and summer (June 2021) within the regulatory limits established by the WHO (2011) during the two sampling campaigns. Conversely, fluoride (F−) concentrations exhibited values ranging from (1.6 mg·L−1 to 2.9 mg·L−1) in the winter of January 2020 and (1 to 2.9 mg·L−1) in the summer of June 2021 (Table 2) surpassing its WHO limit (1.5 mg·L−1) in almost all water samples. According to (Table 2), it is observed that the M’étlaoui zone (Borehole 1) and the M’dhilla zone (Borehole 7) are characterized by a high concentration of fluoride, with respective values of (2.1 and 2.9 mg·L−1) for January 2020 and (2.4 to 2.9 mg·L−1) for June 2021 season. On the other hand, a lower concentration is observed in the Moulares and Redeyef zones (Table 2). These elevated fluoride concentrations in these zones are attributed to high TDS values. These boreholes are near the phosphate industry, explaining the high TDS concentration resulting from overexploitation of the aquifer by the phosphate industry.
These findings align with [46], who investigated fluoride concentrations in drinking water and revealed that the fluoride concentration in drinking water in the Metlaoui and Redeyef regions ranged from 2.2 to 3.39 mg·L−1. Furthermore, [44,45] conducted comprehensive fluoride health risk assessments and revealed that the fluoride concentration in groundwater in the Gafsa regions is (2.22; 1.27; 2.22 mg·L−1) surpassing its WHO limit. They highlighted that groundwater in Southern Tunisia, notably in Gafsa, naturally contains elevated fluoride levels due to geological factors. In contrast, northern and central areas exhibit lower concentrations, falling short of meeting WHO water quality standards.
The elevated levels of fluoride in drinking water have become a focal point of extensive scientific investigation at both national and international levels. That is why a water purification program for the mining basin is strongly recommended, especially for domestic use and particularly as drinking water.
5. Conclusions
In conclusion, this study highlights the groundwater quality in the Mio-Plio-Quaternary aquifer of the Gafsa mining basin, using a hydrochemical approach and multivariate statistical methods based on PCA to assess the mineralization processes of these waters.
The hydrochemical study of the Gafsa basin groundwaters, conducted during both the winter and summer seasons, has shown that these waters are characterized all year round by the dominance of the Na-Ca-Mg-SO4-Cl facies.
The interpretation of major ion analyses indicates that mineralization is influenced by the prolonged interaction of water with geological formations. This highlights the predominant role of dissolution (calcite, dolomite, gypsum, and halite) and infiltration in the high mineralization of groundwater at a regional scale.
The results of the PCA represent two correlation classes. Class 1 comprises major elements strongly correlated with electrical conductivity (EC) and total dissolved solids (TDS)—sulfate, chloride, sodium, magnesium, and calcium. Thus, these strong correlations among these major elements are attributed to high mineralization in the waters of the basin. Class 2 includes major elements with a weaker correlation with (TDS) and (EC)—nitrate and potassium. These two elements do not play a role in the mineralization phenomenon.
The levels of heavy metals in the groundwater of the Gafsa mining basin do not exceed the WHO limits. However, the presence of fluoride in this basin’s groundwater exceeds the WHO limits [47]
These observations suggest a high degree of mineralization, rendering the investigated waters unsuitable for human consumption. These implications underscore the importance of carefully considering alternative water sources and implementing robust purification processes in the studied region. Furthermore, this study provides valuable insights into groundwater management in similar areas, highlighting the importance of implementing appropriate management strategies to ensure water quality and protect public health.
Author Contributions
Conceptualization, N.N., R.S., E.A.L.M. and F.S.; methodology, N.N., F.S., A.I., T.B.A., O.S., D.T., E.A.L.M. and R.S.; software, N.N., D.T., F.S., O.S. and A.I., validation, N.N., R.S., A.I. and T.B.A., formal analysis; N.N., F.S., D.T. and A.I.; investigation, N.N., F.S., T.B.A., A.I., O.S., D.T., E.A.L.M. and R.S.; resources, E.A.L.M. and R.S.; data curation, N.N., R.S., A.I. and T.B.A.; writing—original draft preparation, N.N., F.S., A.I., O.S., D.T., E.A.L.M. and R.S.; writing—review and editing, E.A.L.M. and R.S.; supervision, E.A.L.M. and R.S., project administration, N.N., E.A.L.M. and R.S.; funding acquisition, E.A.L.M. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the institutions involved in developing this research project. They also appreciate the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT) for the financial support (Grant No. A1-S-38139).
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to express their gratitude to the International Center for Environmental Technologies of Tunis (CITET), for their collaboration and assistance.
Conflicts of Interest
Authors Nada Nasri, Fouad Souissi, Amina Ismailia, Olfa Smida and Radhia Souissi were employed by the company Technopark of Sidi Thabet. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PCA: principal component analysis; WHO: World Health Organization; EC: electrical conductivity; TDS: total dissolved solids; GPC: Gafsa Phosphate Company; TGC: Tunisian Chemical Group.
References
- Li, P.; Wu, J.; Tian, R.; He, S. Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Environ. Earth Sci. 2018, 37, 222–237. [Google Scholar] [CrossRef]
- Pandey, A. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar] [CrossRef]
- Chkir, N.; Trabelsi, R.; Bahir, M.; Hadj Ammar, F.; Zouari, K.; Chaamchati, H.; Manteito, J.P. Vulnérabilité des ressources en eaux des aquifères côtiers en zones semi-arides–Etude comparative entre les bassins d’Essaouira (Maroc) et de la Jeffara (Tunisie). Comun. Geológicas 2008, 95, 107–121. [Google Scholar]
- Navarro, M.C.; Perez, C.; Martinez, M.J.; Vidal, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. Environ. Pollut. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Qadir, M.; Charma, B.; Karajeh, F. Non-conventional water resources and opportunities for water augmentation to achieve food security in water scarce countries. Agric. Water Manag. 2007, 87, 2–22. [Google Scholar] [CrossRef]
- Ramadam, E. Sustainable water resources management in arid environment: The case of Arabian Gulf. Int. J. Waste Resour. 2015, 5, 3–6. [Google Scholar]
- Salameh, E. Over-exploitation of groundwater resources and their environmental and socio-economic implications: The case of Jordan. Water Int. 2008, 33, 55–68. [Google Scholar] [CrossRef]
- Hamed, Y. The hydro geochemical characterization of groundwater in Gafsa-Sidi Boubaker region (Southwestern Tunisia). Arab. J. Geosci. 2011, 6, 697–710. [Google Scholar] [CrossRef]
- Ncibi, K.; Chaar, H.; Hadji, R.; Baccari, N.; Sebei, A.; Khelifi, F.; Abbes, M.; Hamed, Y. A GIS-based statistical model for assessing groundwater susceptibility index in shallow aquifer in Central Tunisia (Sidi Bouzid basin). Arab. J. Geosci. 2020, 13, 98. [Google Scholar] [CrossRef]
- Gasmi, N.; Bouissou, S.; Piriou, F. Comparison of Potential Dual Formulations Developed with Different Elements. In Electric and Magnetic Fields; Nicolet, A., Bellman, R., Eds.; Springer: Boston, MA, USA, 1995. [Google Scholar] [CrossRef]
- Tarki, M.; Ben Hammadi, M.; El Mejri, H.; Dassi, L. Assessment of hydrochemical processes and groundwater hydrodynamics in a multilayer aquifer system under long-term irrigation condition: A case study of Nefzaoua basin, Southern Tunisia. Appl. Radiat. Isot. 2016, 110, 138–149. [Google Scholar] [CrossRef]
- Dassi, L. Investigation by multivariate analysis of groundwater composition in a multilayer aquifer system from North Africa: A multi-tracer approach. Appl. Geochem. 2011, 26, 1386–1398. [Google Scholar] [CrossRef]
- Naseem, S.; Erum, B.; Ahmed, P.; Rafique, T. Impact of seawater intrusion on the geochemistry of groundwater of Gwadar District, Balochistan and its appraisal for drinking water quality. Environ. Monit. Assess. 2018, 43, 281–293. [Google Scholar] [CrossRef]
- Sharaf, M.; Amin, M. Major elements hydrochemistry and groundwater quality of Wadi Fatimah, West Central Arabian Shield, Saudi Arabia. Arab. J. Geosci. 2013, 6, 2633–2653. [Google Scholar] [CrossRef]
- Benmarce, K.; Hadji, R.; Hamed, Y.; Zahri, F.; Zighmi, K.; Hamad, A.; Gentilucci, M.; Ncibi, K.; Besser, H. Hydrogeological and water quality analysis of thermal springs in the Guelma region of North- Eastern Algeria: A study using hydrochemical, statistical, and isotopic approaches. J. Afr. Earth Sci. 2023, 205, 105011. [Google Scholar] [CrossRef]
- WHO. Fluoride in Drinking-Water, Background Document for Development of Who Guidelines For Drinking-Water Quality; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- WHO. Fluor et Santé, Série de Monographie, Genève, 59; WHO: Geneva, Switzerland, 1972. [Google Scholar]
- Cidu, R.; Ridou, R.; Fanfani, L.; Luca, F. Impact of past mining activity on SW Sardinia (Italy) groundwater quality. J. Geochem. Explor. 2009, 100, 125–132. [Google Scholar] [CrossRef]
- Hamdi, M.; Goïta, K.; Karaouli, F.; Zagrarni, M.F. Hydrodynamic groundwater modeling and hydro chemical conceptualization of the mining area of Moulares Redeyef (southwestern of Tunisia): New local insights. Phys. Chem. Earth Parts A/B/C 2021, 121, 102974. [Google Scholar] [CrossRef]
- Mokadem, N.; Hamed, Y.; Ben Sâad, A.; Gargouri, I. Atmospheric pollution in North Africa (ecosystems–atmosphere interactions): A case study in the mining basin of El Guettar–M’Dilla (southwestern Tunisia). Atmos. Pollut. Res. 2014, 7, 2071–2079. [Google Scholar] [CrossRef]
- Yermani, M.; Zouari, K.; Michelot, J.L.; Mamou, A.; Moumni, L. Approche géochimique du fonctionnement de la nappe profonde de Gafsa Nord (Tunisie centrale). Hydrol. Sci. J. 2003, 48, 95–108. [Google Scholar] [CrossRef][Green Version]
- Farhat, H.; Moumni, L. Etude Hydrogéologique de la Nappe de Gafsa Nord; DGRE: Tunis, Tunisia, 1989. [Google Scholar]
- Malik, N.; Slim, N.; Shimi, N. Etude de la vulnérabilité des eaux souterraines de la ville de Gafsa (Sud-Ouest de la Tunisie): Effets anthropiques et conséquences. Alger. J. Environ. Sci. Technol. 2019, 5, 1127–1134. [Google Scholar]
- Majdoub, R.; Dridi, L.; M’nasri, S. Caractérisation de la nappe profonde Gafsa nord suite à la surexploitation des eaux souterraines. Larhyss J. 2014, 17, 179–192. [Google Scholar]
- Hamed, Y.; Dassi, L.; Ahmadi, R.; Dhia, H.B. Geochemical and isotopic study of the multilayer aquifer system in the Moulares-Redayef basin, southern Tunisia / Etude géochimique ET isotopique du système aquifère multicouche du bassin de Moulares-Redayef, sud tunisien. Hydrol. Sci. J. 2008, 53, 1241–1252. [Google Scholar] [CrossRef]
- Hamed, Y.; Dassi, L.; Tarki, M.; Ahmadi, R.; Mehdi, K.; Dhia, H.B. Groundwater origins and mixing pattern in the multilayer aquifer system of the Gafsa-south mining district: A chemical and isotopic approach. Environ. Earth Sci. 2010, 63, 1355–1368. [Google Scholar] [CrossRef]
- Umer, M.F. A Systematic Review on Water Fluoride Levels Causing Dental Fluorosis. Sustainability 2023, 15, 12227. [Google Scholar] [CrossRef]
- El Hasnaoui, B.; Younsi, A.; Mountadar, M.; Garmes, H.; Mouhab, I. Impacts négatifs d’une zone industrielle sur les eaux souterraines et sur le cheptel (Cas du Jorf Lasfar, Maroc): Approches pluridisciplinaires. Déchets Sci. Tech. 2011, 59, 2945. [Google Scholar]
- Jiries, A.; El-Hasan, T.; Al-Hweiti, M.; Seiler, K.-P. Evaluation of the Effluent Water Quality Produced at Phosphate Mines in Central Jordan. Mine Water Environ. 2004, 23, 133–137. [Google Scholar] [CrossRef]
- Khelifi, F.; Besser, H.; Ayadi, Y.; Liu, G. Evaluation of potentially toxic elements’ (PTEs) vertical distribution in sediments of Gafsa–Metlaoui mining basin (Southwestern Tunisia) using geochemical and multivariate statistical analysis approaches. Environ. Earth Sci. 2019, 78, 53. [Google Scholar] [CrossRef]
- Burollet, P.F. Contribution à l‘étude Stratigraphique de la Tunisie Centrale. Ann. Mines Geol. 1956, 18, 352. [Google Scholar]
- Sassi, S. La Sedimentation Phosphatee au Paléocène dans le Sud et le Centre Ouest de la Tunisie. Ph.D. Thesis, Université de Paris Sud, Orsay, France, 1974. [Google Scholar]
- Dlala, M.; Hfaiedh, M. Le séisme du 7 novembre 1989 à Metlaoui (Tunisie méridionale): Une tectonique active en compression. Comptes Rendus L‘Académie Sci. Série 2 HFAIEDH Mécanique Phys. Chim. Sci. L‘Univers Sci. Terre 1993, 317, 1297–1302. [Google Scholar]
- Hamed, Y. Caractérisation Hydrogéologique, Hydrochimique et Isotopique du Système Aquifère de Moularès-Tamerza. Ph.D. Thesis, University of Sfax, Sfax, Tunisia, 2009. [Google Scholar]
- Chraiti, R.; Raddaoui, M.; Hafiane, A. Effluent Water Quality at Phosphate Mines in M’Dhilla, Tunisia and its Potential Environmental Effects. Mine Water Environ. 2016, 35, 462–468. [Google Scholar] [CrossRef]
- Salem, M.; Souissi, R.; Souissi, F.; Abbes, N.; Moutte, J. Phosphoric acid purification sludge: Potential in heavy metals and rare earth elements. Waste Manag. 2019, 83, 46–56. [Google Scholar] [CrossRef]
- Smida, O.; Souissi, R.; Marzougui, S.; Souissi, F. Geochemical Assessment and Mobility of Undesired Elements in the Sludge of the Phosphate Industry of Gafsa-Metlaoui Basin, (Southern Tunisia). Minerals 2021, 11, 1075. [Google Scholar] [CrossRef]
- Souissi, R.; Souissi, F.; Chakroun, H.K.; Bouchardon, J.L. Mineralogical and geochemical characterization of mine tailings and Pb, Zn, and Cd mobility in a carbonate setting (Northern Tunisia). Environ. Earth Sci. 2013, 32, 16–27. [Google Scholar] [CrossRef]
- Bocoum, M. Méthodes D’analyses des Sols. Document de Travail; Institut National de Pédologie: Dakar, Sénégal, 2004; 55p. [Google Scholar]
- Rodier, J. L‘Analyse de l‘Eau, Eaux Naturelles, Eaux Résiduaires, Eaux de Mer; Edition Dunod: Malakoff, France, 1996; 1434p. [Google Scholar]
- Banton, O.; Bangoy, L.M.; Chevalier, S.; Houenou, P.; Lafrance, P.; Rivard, C. Hydrogéologie: Multi Science Environnementale des Eaux Souterraines; Presses de l’Université du Québec/AUPELF: Quebec, Canada, 1997; 460p. [Google Scholar]
- Hamed, Y.; Gentilucci, M.; Mokadem, N.; Khalil, R.; Ayadi, Y.; Hadji, R.; Elaloui, E. Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis. Hydrology 2024, 11, 84. [Google Scholar] [CrossRef]
- Backer, L.C. Assessing the acute gastrointestinal effects of ingesting naturally occurring, high levels of sulfate in drinking water. Crit. Rev. Clin. Lab. Sci. 2000, 37, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ben Nasr, K.; Walha, C.; Charcosset, R. Ben Amar, Removal of fluoride ions using cuttlefish bones. J. Fluor. Chem. 2011, 132, 57–62. [Google Scholar] [CrossRef]
- Guissouma, W.; Tarhouni, J. Fluoride in Tunisian Drinking Tap Water. J. Water Resour. Prot. 2015, 7, 860. [Google Scholar] [CrossRef]
- Essouli, O.F. Impact de la Décharge Publique du lac Mbeubeuss sur la Ressource en eau de L’aquifère des Sables Quaternaires de Thiaroye (Dakar, Sénégal). Ph.D. Thesis, Département de Géologie, Faculté des Sciences de Sénégal, Dakar, Senegal, 2005. [Google Scholar]
- Guissouma, W.; Hakami, O.; Al-Rajab, A.J.; Tarhouni, J. Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere 2017, 177, 102–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).