Abstract
Microplastics, plastic particles smaller than 5 mm, pose a significant environmental threat due to their persistence and distribution in aquatic ecosystems. Research on the dynamics of microplastics within freshwater systems, particularly concerning their transport and deposition along river corridors, remains insufficient. This study investigated the occurrence and deposition of microplastics at the water–sediment interface of the White River near Muncie, Indiana. Sediment samples were collected from three sites: White River Woods (upstream), Westside Park (midstream), and Morrow’s Meadow (downstream). The microplastic concentrations varied significantly, with the highest concentration recorded upstream, indicating a strong influence from agricultural runoff. The types of microplastics identified were predominantly fragments (43.1%), fibers (29.6%), and films (27.3%), with fragments being consistently the most abundant at all sampling sites. A polymer analysis with selected particles using Fourier-transform infrared (FTIR) spectroscopy revealed that the most common polymers were polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET). The hydrodynamic conditions played a crucial role in the deposition and transport of microplastics. The statistical analysis demonstrated a strong positive correlation between the microplastic concentration and flow velocity at the downstream site, suggesting that lower flow velocities contribute to the accumulation of finer sediments and microplastics. Conversely, the upstream and midstream sites exhibited weaker correlations, indicating that other environmental and anthropogenic factors, such as land use and the sediment texture, may influence microplastic retention and transport. This study provides valuable insights into the complex interactions between river dynamics, sediment characteristics, and microplastic deposition in freshwater systems. These findings contribute to the growing body of knowledge on freshwater microplastic pollution and can help guide mitigation strategies aimed at reducing microplastic contamination in riverine ecosystems.
1. Introduction
Microplastic pollution, characterized by plastic particles less than 5 mm in length [,], has emerged as a significant environmental and human health concern due to its ubiquity and persistence in ecosystems and the food chain [,]. These particles originate from both primary sources, such as the manufacture of pellets for the production of large plastic items, and secondary sources, such as the fragmentation of larger plastic articles due to weathering and mechanical degradation [,,]. Rivers play a crucial role in transporting these pollutants from terrestrial to marine environments, acting as key vectors in the global dispersal of microplastics [,].
Despite increased attention to the prevalence and impact of microplastics in marine environments, research into their dynamics within freshwater systems, particularly regarding their transport and deposition along river corridors, remains a formidable challenge [,]. Existing research has focused predominantly on surface water sampling, with limited consideration given to the complexities of microplastics’ vertical distribution and interactions with sediments, which act as potential sinks for denser particles and those prone to being attached to sediments [,]. This gap highlights the need for further investigation into the microplastic dynamics at the water–sediment interface, which is crucial for a deeper understanding of the fate and transport of this contaminant and in developing effective mitigation strategies.
Microplastics are subject to similar transport processes as sediment particles in rivers, such as being transported at a high flow velocity and deposited at a low flow velocity []. However, the complexity of microplastics’ morphology makes their transportation more complicated than that of typical sediments. For example, fibers can be readily trapped by sediments despite their low density [].
Due to the temporal and spatial variability of river flows, the deposition of microplastics in sediments can be complex. Nonetheless, hotspots of microplastics are generally expected to occur in areas that experience the deposition of fine sediment, which indicates low energy []. A stream experiment demonstrated that the accumulation of plastic litter, including pellets, fragments, and fibers, followed the same patterns as that of natural particles [,]. For example, flooding resuspension can transform rivers from sinks to sources of plastic pollution [].
Most microplastics in a riverine system can typically be traced back to their contributing area in the upper watershed. As rivers receive point and non-point source pollutants, the abundance of microplastics is impacted by the land use and anthropogenic practices in the watershed. Elevated concentrations of microplastics have been observed in locations of intense agricultural practices, near combined sewer overflows (CSOs) or wastewater effluents, and in proximity to urban areas. The management of microplastics is thus dependent on the control of their release sources [].
The microplastic contamination of waterways in the agriculture-heavy U.S. Midwest has been widely reported []. However, the extent to which the combined effects of plastic sources and river flow mechanisms impact microplastic transport and deposition in the U.S. Midwest is still an open question. This research explores the potential processes affecting microplastic transport and deposition at the water–sediment interface, using the White River in Muncie, Indiana, as the study site. The study design encompasses three sites: a zone upstream of the City of Muncie dominated by agricultural runoff, a midstream zone dominated by urban runoff and point source pollutants, and a zone downstream of the city after passing a municipal wastewater treatment facility. By examining the factors that affect microplastics’ movement, such as the flow velocity and sediment properties, this study offers insights into their environmental fate and the broader consequences for aquatic ecosystems. The results of this study augment our existing body of knowledge and could inform mitigation strategies for this contaminant of emerging concern. The specific research objectives include (1) to investigate the impact of the flow velocity on the concentration of microplastics within the river; (2) to examine the variation in the microplastics’ concentrations at different points downstream of the city; and (3) to determine the predominant types within river sediments.
2. Materials and Methods
2.1. Study Area and Sample Collection
The White River near Muncie is located in East Central Indiana, in the upstream area of the Eastern Corn Belt Plains ecoregion of the United States (Figure 1). The area is dominated by agricultural land, and the water quality has been impacted by various point and non-point source pollutants []. Microplastics have been detected in water bodies and organisms in this area [,,]. Despite the close connection and importance of river sediments in trapping and releasing plastic particles, microplastics in river sediments have not been reported in the region to date.
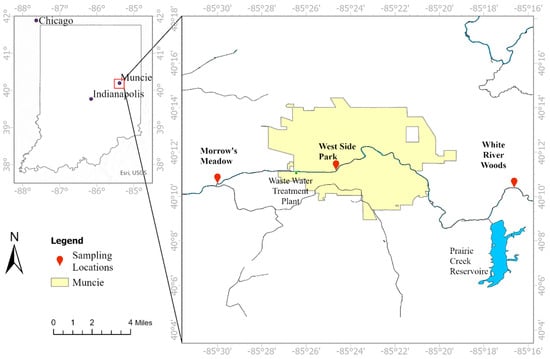
Figure 1.
Sampling locations along the White River near Muncie. Sampling sites include the White River Woods (upstream); West Side Park, which crosses the urban sector of Muncie (midstream); and Morrow’s Meadow (downstream). The upstream zone is dominated by agricultural land.
Sediment samples were collected from the White River near Muncie, Indiana. Sampling was conducted consistently on three separate sunny days in May 2023 to minimize variations caused by weather. Three sampling sites—White River Woods (WRW, upstream), Westside Park (WSP, midstream), and Morrow’s Meadow (MM, downstream)—were strategically chosen to represent varying levels of anthropogenic impact (Figure 1). Specifically, the WRW site (upstream) is dominated by agricultural fields. The WSP site is located near downtown Muncie and receives urban runoff and also has a recreational park nearby. The MM site (downstream) is located about 3 miles downstream of the Muncie Wastewater Treatment Plant, with a high school and a recreational park nearby.
A Wildco (Saginaw, MI, USA) standard Ponar bottom stainless steel grab sampler was employed to collect 12 cross-sectional sediment samples from each site, with intervals of 10 feet separating each collection point (Figure 2). Following collection, the samples were immediately placed in aluminum foil pans, transported to the laboratory, and dried at 70 °C for 48 h. The samples were covered with aluminum caps during drying to reduce airborne contamination. Once dried, larger debris such as wood and large plastic pieces were removed, and the samples were manually crushed []. The grain size of each sediment sample was determined with a Tyler Company Ro-Tap Test Sieve Shaker (model 15694; Mentor, OH, USA).
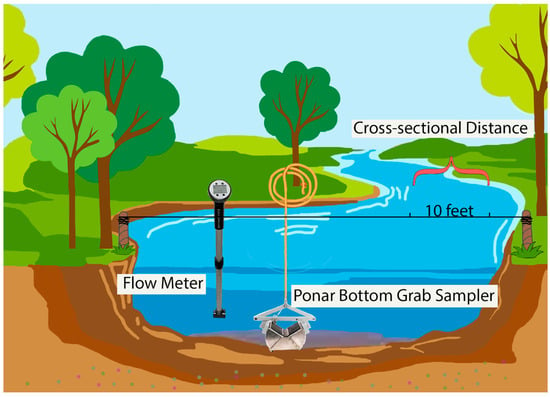
Figure 2.
Water–sediment interface sampling in the White River. This diagram illustrates the cross-sectional setup for sediment sampling in a riverine system using a Ponar bottom grab sampler, positioned at 10-foot intervals for systematic collection. A flow meter is included to measure the water velocity.
2.2. Laboratory Analysis
Sieve Grain Size Analysis
Sieve grain size analysis was conducted to categorize the sediment samples based on the particle size []. The sieves were arranged in series with progressively smaller mesh sizes (Table 1). Five hundred grams of dry soil was weighed. The sieves and the collecting pan were weighed beforehand, and each sieve was meticulously cleaned to avoid cross-contamination. The sieves were then stacked in ascending order of mesh size, with the larger openings placed at the top. The soil sample was carefully distributed into the top sieve and covered with a lid. The entire assembly was placed on the mechanical sieve shaker and agitated vigorously for 20 min. Following shaking, the weight of each individual sieve and the collecting pan was measured to determine the quantity of soil retained. The data from the sieve analysis were then used to classify the soil particles into categories such as gravel, sand, silt, and clay, following the Unified Soil Classification System (USCS) (Table 2).

Table 1.
Sieves utilized in grain size analysis test.

Table 2.
Sediment classification based on particle size range (USCS).
2.3. Microplastic Extraction
Sample Digestion and Density Separation
Fenton’s reagent, consisting of 30% H2O2 with Fe2+ as the catalyst, was prepared following the method of Radford et al. (2021) to remove organic matter adhering to microplastics []. The Fe2+ solution was prepared by diluting 5 mL of 0.1 N sulfuric acid into 500 mL of deionized (DI) water, resulting in a pH of ~3. Approximately 10 g of iron sulfate heptahydrate (FeSO4•7H2O) was then added. One part of the Fe2+ solution was mixed with 3 parts of 30% H2O2 to proceed with the Fenton process. In a fume hood, 100 g sediment samples were treated with 200 mL Fenton reagent in a 500 mL beaker and allowed to stand for 24 h. The digested samples were filtered through a Pall 47 mm 0.45 μm MetricelTM filter to prepare for the next phase of microplastic extraction. A two-stage density separation process was used to isolate microplastics from freshwater sediments. Initially, the digested samples were combined with a NaCl (density ≈ 1.2 g/cm3) solution, stained with Rose Bengal to distinguish microplastics, stirred, and left to settle for 24 h. The supernatant was centrifuged for 30 min and the residue subjected to a second density separation with ZnCl2 (1.55 g/cm3) solution to capture high-density polymers. In the second density separation, the suspension was centrifuged at 3900 rpm for 15 min. Finally, the microplastic-containing supernatant was vacuum-filtered, and the filter paper was subsequently dried in a desiccator for 24 h [,].
2.4. Data Collection and Statistical Analysis
The separated microplastics were analyzed on dry filter paper using a fluorescence stereomicroscope (SMZ25, Nikon Corporation, Tokyo, Japan). The particle morphology, color, and size were evaluated. Microplastics in sediments were quantified per unit mass of dried sediment. Following visual sorting, 24 selected particles underwent Fourier transform infrared (FTIR) spectroscopy analysis using a Perkin Elmer Frontier FTIR spectrometer equipped with an attenuated total reflectance (ATR) diamond tip, to confirm the polymer types. Microplastic particles were picked from the filter paper using high-precision forceps under a Bausch & Lomb StereoZoom 5 Microscope (Rochester, NY, USA) and transferred to the diamond tip of the FTIR spectrometer [,]. The anvil was lowered steadily onto the microplastic particle and the percentage transmittance was then recorded.
Key statistical methods included the construction of normality plots revealing the distribution characteristics of different sediment types, scatter plots examining the correlations between the flow velocity and microplastic concentrations, and a multivariate ANOVA assessing differences in the microplastic concentrations across locations. The analysis considered factors including the sediment texture and river dynamics, providing insights into the environmental implications of microplastic contamination. All statistical analyses were performed using Excel, the Statistical Package for Social Sciences (SPSS), and Origin Pro on a Windows-based PC.
2.5. Quality Control Measures
To ensure accurate microplastic quantification, all containers and equipment were pre-cleaned using DI water. To mitigate airborne contamination, the samples and apparatus were covered with aluminum foil during both field and laboratory work. Personal protective gear, including nitrile gloves and cotton lab coats, was worn consistently. Before use, all liquid solutions were filtered through 0.45 μm membrane filters to remove potential microplastics. A laboratory blank with deionized water was used to check for potential laboratory contamination during processing, and no contaminants were found in the blank.
3. Results
3.1. Sediment Analysis
The analysis of the grain size distribution for sediments from the White River showed distinct differences across the three examined locations. The sediment at White River Woods (WRW) was characterized by a coarse profile compared to those at West Side Park (WSP) and Morrow’s Meadow (MM) (Figure 3). The WRW sediments had larger grain sizes, indicating the greater presence of gravel and coarse sand. In contrast, the sediments at WSP and MM had a higher proportion of silt- and clay-sized particles. These variations in the grain size distribution suggest differing sedimentological processes and sources at each location.
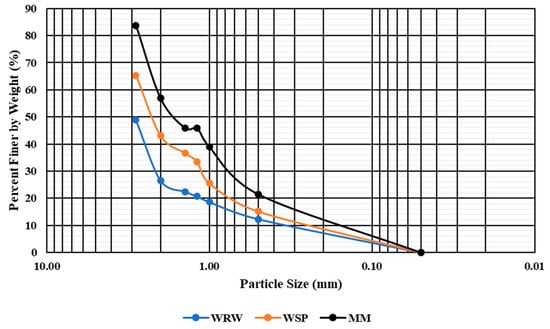
Figure 3.
Grain size distribution curve for White River sediments. The particle size in millimeters is plotted on a logarithmic scale on the x-axis, with the percent value of particles finer than by weight on the y-axis.
The sediment composition by weight revealed variations in the grain size distribution across all the samples collected from the three sites. The gravel content by weight was moderate, ranging from 20 to 40% with an average of 30.9%. In contrast, sand showed a near-normal distribution, with mean content by weight of 50.4%. The percentages of silt and clay were low but varied significantly across the locations, underscoring the heterogeneity of sediment deposition along the river (Figure 4).
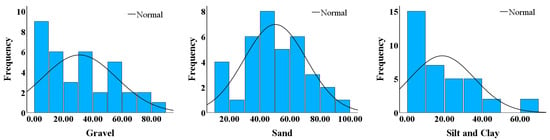
Figure 4.
Normality plots of sediment grain size distribution by weight from samples collected across WRW, WSP, and MM.
3.2. Microplastic Distribution
Prevalence and Implications of Microplastic Types in Aquatic Ecosystems
The White River sediments contained an average of 2499 microplastic particles per kilogram of sediment. Fragments were the predominant microplastic type, constituting approximately 43.1% of the microplastics identified, followed by fibers (29.6%) and films (27.3%) (Figure 5).
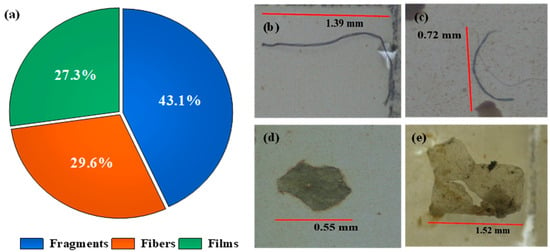
Figure 5.
Microplastic types identified in White River sediment. Proportional distribution of microplastic types (a). Photos of microplastic fibers (b,c); fragment (d); and film (e).
3.3. Flow Velocity and Microplastic Variability in River Samples
The relationship between the flow velocity and microplastic concentration at each sampling point along the White River showed varied levels of correlation (Figure 6). Specifically, WRW and WSP exhibited non-significant relationships (r = 0.35, p = 0.10; r = 0.27, p = 0.10, respectively), while MM demonstrated a significant relationship (r = 0.94, p < 0.001). The disparity in the correlation coefficient (r) across the different sites provides insights into the complex dynamics governing microplastic pollution in riverine environments and highlights the importance of site-specific conditions in microplastic accumulation.
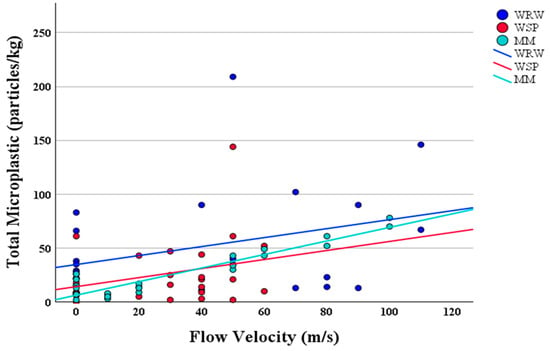
Figure 6.
Correlation of microplastic concentration and flow velocity. Each point represents the microplastic concentration of the sample at a given flow velocity, with linear trend lines indicating the correlation trend for each site.
3.4. Dynamics of Microplastics with Downstream Flow
Notable differences in microplastic pollution were observed across the three sampling locations (Figure 7). Specifically, WRW exhibited a significantly higher mean concentration of fiber particles (M = 34.67 particles/Kg) compared to WSP (M = 12.00 particles/Kg), with a mean difference (MD) of 22.67 and a standard error (SE) of 8.30 (p = 0.03) (Table 3). Conversely, no significant differences in the fiber particle amount were observed between MM and either WSP (p = 1.00) or WRW (p = 0.07). In terms of films, WRW again showed a higher mean concentration (M = 35.08 particles/Kg) compared to MM (M = 7.17 particles/Kg), with a significant mean difference of 27.92 (SE = 10.82, p = 0.04). However, the differences in the film counts between MM and WSP and between WSP and WRW were not statistically significant.
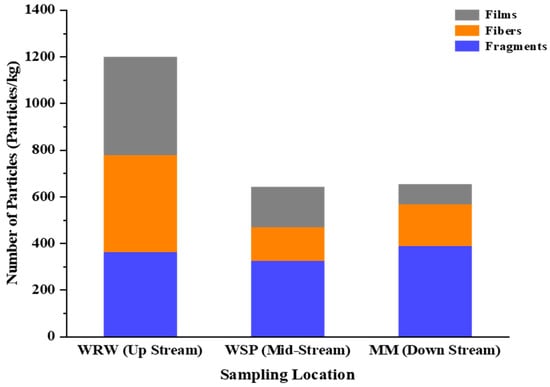
Figure 7.
Distribution of microplastic particles by river sampling location.

Table 3.
Pairwise comparisons of MP particles across different river sample locations.
3.5. Polymer Type Identification
Polyethylene (PE), polyethylene terephthalate (PET), and polypropylene (PP) were identified as the predominant types, each representing 18.8% of the polymers analyzed (Figure 8). Other types of polymers, including nylon, polyvinyl acetate (PVA), and polystyrene (PS), were detected at lower percentages.
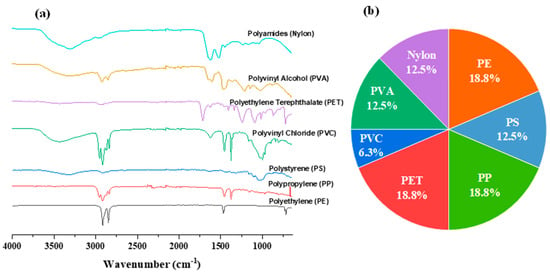
Figure 8.
Microplastic polymer composition. (a) displays the Fourier transform infrared (FTIR) spectra of different microplastic polymers identified in the sediment samples. (b) a pie chart that breaks down the relative proportions of various polymers found in the samples.
4. Discussion
Identifying the distribution and characteristics of microplastics at the water–sediment interface is crucial in understanding their fate and transport in the environment. These factors are influenced by the variability in sediment composition, flow dynamics, river morphology, the land use of the contributing area, and other environmental factors. The current study explored the relationships between the sediment grain size, flow velocity, and microplastic deposition, thereby enhancing our understanding of microplastic pollution in freshwater environments.
4.1. Grain Size Distribution and Sediment Composition among Sites
The sediment size distribution serves as an indicator of the flow energy, which determines the deposition and transportation of microplastics. Locations with elevated silt and clay suggest low-energy environments or regions with slower-moving water, such as river bends, where microplastics may be trapped more effectively [,,]. River bottoms having high sand content could act as zones of microplastic accumulation, while gravelly areas might allow the greater transport of microplastics.
The study area is located in the headwater regions of the White River watershed and is dominated by coarse sediment material (Figure 3). Specifically, the predominance of coarser sediments at WRW suggests a high-energy environment, which may reduce microplastic retention [,]. The sediments at MM are relatively finer, presenting a lower-energy environment conducive to the accumulation of microplastics [,]. The intermediate texture found at WSP suggests a balance between microplastic transport and retention [].
4.2. Microplastic Distribution
Prevalence and Implications of Microplastic Types in Aquatic Ecosystems
Our results showed that fragments were the most abundant types of microplastics detected at the sampling locations (Figure 5), which is consistent with the results from reported studies that also identified fragments as the most prevalent type [,]. This prevalence is likely a result of larger plastic items breaking down into smaller pieces over time. This process is accelerated by environmental factors like UV exposure, mechanical wear, and water flow []. At the WRW site, agricultural activities likely contribute to the presence of fragments through the degradation of plastic materials used in farming [], such as plastic sheeting or containers. The midstream WSP site, which receives urban runoff, likely introduces fragments from litter, consumer packaging, and household waste [,]. Downstream at MM, located near the Muncie Wastewater Treatment Plant, fragments may originate from wastewater effluents, where microplastics from consumer products evade filtration systems, as well as from nearby urban and recreational areas. The widespread presence of fragments underscores the need for stricter waste management practices across agricultural, urban, and industrial settings to minimize the breakdown of plastic materials into the environment.
Fibers, which ranked as the second most abundant microplastic type in the samples, likely originate from various anthropogenic sources. Common contributors include synthetic textiles, which release fibers during washing [], cigarette filters that degrade into microfibers [], and personal care products containing synthetic materials [,,]. The high prevalence of fibers suggests significant inputs from non-point sources, such as urban and agricultural runoff, and recreational sources. Additionally, the atmospheric deposition of fibers from urban areas could also contribute to their presence in river sediments []. Understanding these sources is critical in designing effective mitigation strategies to reduce fiber pollution in aquatic systems.
Films also comprised a significant component, accounting for about 27% of microplastics identified. This points to the persistent issue of single-use plastics and likely agricultural mulch [], highlighting the need for improved waste management and reduction strategies []. The agricultural activities surrounding the WRW site likely contribute to the abundant presence of plastic films in the upstream area through the use of agricultural products like plastic mulch or bags. At WSP, urban runoff may carry plastic bags and wrappers discarded by nearby residents and visitors to the recreational park, further contributing to film pollution. At the MM site, runoff from the nearby high school and surrounding recreational areas, along with the wastewater treatment plant, may be a significant contributor. The lightweight nature of films allows them to be easily transported by the river’s hydrodynamics over long distances, increasing their prevalence throughout the river system [].
The implications of the findings on microplastics are significant, especially for aquatic ecosystems. Numerous studies have revealed that the ingestion of fragments and fibers can harm wildlife, causing damage to the digestive organs and the choking of marine organisms, reducing their growth and reproductive output, and leading to the ingestion of chemical toxins [,]. This underscores the urgent need for improved waste management and plastic use reduction strategies to address the issue at its source [].
4.3. Statistical Analysis of Differential Microplastic Types across River Locations
The one-way ANOVA revealed no significant differences in the microplastic concentrations among the WRW, WSP, and MM sites (p = 0.07), despite the higher levels at WRW. This result does not conclusively support the hypothesis that the river location affects the microplastic concentration.
Overall, the WRW site had a consistently higher mean value for fibers and films compared to the other locations (Table 3). A multivariate ANOVA (MANOVA) test revealed significant differences in the microplastic particle concentrations between the sampling locations (p = 0.04). The analysis of the microplastic types—fragments, fibers, and films—showed a significant difference across the locations for fibers (p = 0.04), but not fragments (p = 0.86), indicating location-specific variations in microplastic accumulation and pollution.
4.4. Flow Velocity and MP Variability in River Samples
A non-significant relationship was noted between the flow velocity and microplastic concentration at WRW and WSP (r = 0.35, p = 0.1; r = 0.27, p = 0.10, respectively), suggesting that the flow velocity is not the predominant factor affecting the microplastic distribution at these high-gradient sites []. This observation challenges the initial hypothesis that higher peak flow velocities, such as those at WRW (Figure 9), reduce microplastic deposition by facilitating particle movement. Instead, it implies that other environmental or anthropogenic factors play a more significant role in influencing the microplastic distribution []. For example, the presence of biofilms, sediment characteristics, and human activities may contribute to the observed patterns of microplastic accumulation and distribution, highlighting the need to consider a broader range of factors beyond hydrodynamics [].
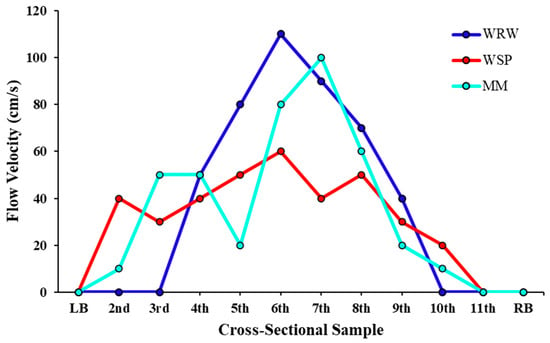
Figure 9.
Cross-sectional flow velocity profile of the White River. This line graph illustrates the flow velocity (y-axis) across a cross-sectional sample from the left bank (LB) to the right bank (RB) at each river sample location (x-axis). Each line represents one of the three sampling locations along the White River, with distinct markers denoting the specific site.
Conversely, the significant relationship observed at MM (r = 0.94, p < 0.001) suggests that the flow velocity significantly influences the microplastic concentration at this site. This finding aligns with the initial hypothesis that moderate to high flow velocities, as observed at MM, facilitate microplastic conveyance in the primary channel, potentially leading to different deposition patterns at locations where the flow decreases. Although a high peak flow velocity occurs at MM, the overall flow is generally slower than at the other two sites. Additionally, there is an in-channel bar near the MM cross-section, which reduces the flow velocity near one side of the bar and results in the deposition of fine sediments in the channel (Figure 9). The strong correlation at MM underscores the role of hydrodynamic forces in microplastic transport and deposition in riverine environments with a slower velocity and finer sediment deposition [].
The overall relationship between the flow velocity and microplastic concentration in the White River sediment illustrates the nuanced and site-specific nature of microplastic pollution in riverine environments. Factors such as the river morphology, water level fluctuations, and the physical properties of microplastics can significantly impact their transport and deposition [,,]. In sites with lower hydrodynamic energy and therefore the greater accumulation of finer sediment fractions, there tends to be a stronger correlation among the sediment size, flow velocity, and microplastic concentration. In contrast, sites dominated by gravel and coarse sand show a weak correlation. These findings highlight the need for site-specific investigations and the multidisciplinary consideration of environmental and anthropogenic factors to understand the dynamics of microplastic pollution. This approach is crucial in developing effective strategies for the monitoring and mitigation of microplastic pollution in rivers and other aquatic ecosystems.
4.5. Dynamics of Microplastics with Downstream Flow
A discernible pattern in the microplastics concentration across the three sampling locations was observed (Figure 7). The concentration of microplastics was greatest at the upstream location (WRW). Here, most of the microplastics appeared as films, followed by fibers and then fragments. At the midstream location, WSP, there was a notable reduction in the concentration of microplastics. The composition of microplastic types remained roughly consistent, although the total count for each type decreased. Lastly, at MM, the microplastic concentration was akin to that at the midstream but considerably lower than that upstream.
Several reasons might elucidate this trend. Firstly, the upstream location may be closer to the microplastic sources, possibly due to increased runoff from agricultural areas, resulting in higher concentrations of films and fibers, which are frequently applied in agricultural practices [,]. As microplastics travel downstream, sedimentation and urban runoff may play a role, causing certain particles to settle in the riverbed []. The dynamics of the river itself, such as the merging of tributaries, might dilute the microplastic concentration, while features like meanders or floodplains could trap these particles []. Over time, larger microplastic particles undergo physical degradation and fragmentation into smaller ones [,]. This fragmentation may lead to an increase in the total amount but can make detection challenging if the monitoring methods are less capable of identifying smaller particles [,]. Biological interactions must also be considered as river organisms may ingest some microplastics, leading to a reduction in free-floating concentrations [,]. Another possible contributor is the Muncie Wastewater Treatment Plant, which releases microplastics into the river. However, the wastewater effluent releases mostly small and lightweight particles, which do not readily settle in sediments in high-gradient headwater streams []. As such, it may not significantly impact the microplastic concentration in sediment collected downstream at the MM site. Hence, although the concentration of microplastics decreases downstream, the reasons for this pattern are multifaceted and warrant further investigation [].
4.6. Polymer Prevalence and Environmental Impact
The prevalence of PE, PET, and PP in the environment is likely due to their widespread use in consumer products and packaging. Industrial discharge and wastewater treatment plant effluents are known to emit microplastic particles into river networks, contributing to their presence, especially in lower-gradient downstream areas []. These common polymer types in microplastics can exert damaging effects on ecosystems—they interact with other potentially harmful elements and organic contaminants, posing risks to soil systems and aquatic life []. Terrestrial microplastic pollution has been shown to decrease the populations of species living below the surface, such as mites and larvae, which are crucial in maintaining soil fertility [,]. Moreover, microplastics persist in the environment; some take hundreds of years to degrade and some can linger within riverbeds for as long as seven years before washing into the ocean [,]. Understanding the distribution and prevalence of these polymers is crucial in understanding the sources of pollution and the potential impacts on ecosystems and in guiding mitigation strategies [].
4.7. Limitations and Future Directions
This research provides insights into the intricacies associated with microplastic contamination and migration in river ecosystems, utilizing the most recent methods in the detection, measurement, and understanding of the effects caused by microplastics. Nevertheless, such research faces constraints due to inconsistencies in sampling and analysis methods and technologies. For instance, the use of FTIR for polymer identification may not fully capture the entire range of microplastic contamination due to its limited detection capabilities. Moreover, visualization using light microscopy may introduce errors due to the similarities of plastic particles and other materials. Although commonly adopted protocols were employed, and FTIR was used for particle identification, there is still the possibility that certain variables will influence the accuracy of the chemical identification of the particles []. Moreover, the findings may be limited in their generalizability due to geographical bias and the sample size, which might not reflect crucial areas of microplastic deposition and its effects. In addition, this research suggests possible detrimental effects on aquatic organisms and broader consequences for the food web, although there is a paucity of studies that provide detailed information on the long-term impacts. Finally, this research acknowledges the necessity of a comprehensive approach that incorporates legislative decisions, technology solutions, and community engagement to effectively reduce microplastic contamination. The efficacy of these efforts in real-life situations has yet to be thoroughly assessed. By acknowledging the limitations of the methodologies used and the associated uncertainties, the findings must be interpreted within the appropriate context.
Comprehensive and innovative research directions are imperative to surmount the above challenges. There is a critical need for the standardization of sampling and analytical protocols to ensure comparability across studies, the expansion of research to underrepresented regions for a global perspective on microplastic distribution, and longitudinal studies focusing on the ecological and health implications of persistent microplastic pollution. Additionally, exploring innovative mitigation technologies and adopting interdisciplinary approaches that merge environmental science, policy, technology, and community engagement will be key in devising effective strategies to tackle microplastic pollution in river ecosystems and beyond. These efforts will not only refine our current understanding but also pave the way for sustainable solutions to this pressing environmental issue.
5. Conclusions
Th reported study investigated the distribution and characteristics of microplastics at the water–sediment interface of samples from various locations along the White River near Muncie, Indiana. This approach underscored the variability in the sediment composition and microplastic dynamics due to the differing environmental energies of the collection sites. The results provide critical insights into the relationships between the sediment grain size, flow velocity, and microplastic deposition, thereby enhancing our understanding of microplastic pollution in freshwater environments.
The findings from studies on microplastics in river sediments and water bodies provide crucial insights into the distribution, abundance, and characteristics of these pollutants in freshwater environments. A significant variation in the microplastic concentrations and types was observed across the sampled locations, with the highest concentration found upstream, suggesting proximity to microplastic sources such as agricultural runoff. The fragment concentrations were consistent across sites; however, the concentrations of films and fibers differed greatly, suggesting that other environmental, geological, or anthropogenic variables may influence microplastic distributions. The polymer analysis identified PE, PP, and PET as the predominant polymers, reflecting common consumer and industrial plastic usage. The results illuminate the variability in sediment composition and microplastic dynamics and the influence of environmental factors like the flow velocity on microplastic deposition.
This research underscores the complexity of microplastic pollution and highlights the need for a multidisciplinary approach to studying these contaminants. While certain findings indicated factors influencing the sediment grain size and flow velocity in the microplastic distribution, other observations revealed the need to consider a broader range of variables, such as biofilms, the sources of pollutants, the sediment characteristics, and human activities. The prevalence of different types of microplastics, such as fragments, fibers, and films, underscores the urgent need for improved waste management strategies to mitigate the environmental impacts of these pollutants on aquatic ecosystems. Additionally, the identification of prevalent polymers like PE, PET, and PP highlights the importance of understanding the pollution sources and potential ecosystem impacts and guiding effective mitigation measures to address microplastic contamination in rivers and their associated environments.
Lastly, this study emphasizes the need for better waste management, pollution control, and targeted strategies to reduce microplastic contamination in rivers and sediments. Recommendations include reducing single-use plastics and agriculture mulch, improving recycling, adopting sustainable agricultural practices, and upgrading wastewater treatment facilities to filter out microplastics. The importance of site-specific monitoring, as microplastic deposition varies with factors like the flow velocity and sediment type, is also highlighted. Sustainable engineering solutions, such as restoring the natural river flow and using sediment traps, can help to manage microplastic transport downstream. Public awareness and stronger regulations targeting plastic pollution are crucial for long-term solutions, requiring collaboration between policymakers, scientists, and local communities.
Author Contributions
B.Y.A.: conceptualization, field work and sample collection, methodology and analysis, and original draft. B.H.: conceptualization, field work and sample collection, methodology and analysis, and resources and funding support. J.P.: manuscript review and editing. E.M.Z.: lab support, manuscript review and editing. R.W.: statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the EPA P3, grant number 84040601, and Ball State Aspire Graduate Research Grant A23-0361-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Acknowledgments
This publication was developed under Assistance Agreement No. 84040601 awarded by the U.S. Environmental Protection Agency to Ball State University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors, and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Acknowledgments. This change does not affect the scientific content of the article.
References
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Fuschi, C.; Pu, H.; Macdonell, M.; Picel, K.; Negri, M.; Chen, J. Microplastics in the Great Lakes: Environmental, Health, and Socioeconomic Implications and Future Directions. ACS Sustain. Chem. Eng. 2022, 10, 14074–14091. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Liu, X.; Singh, V.P. Dispersal and transport of microplastic particles under different flow conditions in riverine ecosystem. J. Hazard. Mater. 2023, 442, 130033. [Google Scholar] [CrossRef]
- Galloway, T.S.; Lewis, C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA 2016, 113, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Claveau-Mallet, D.; Kurusu, R.S.; Lapointe, M.; Bayen, S.; Tufenkji, N. Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: What are we missing? J. Hazard. Mater. 2022, 423, 126955. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. [Google Scholar] [CrossRef]
- Darabi, M.; Majeed, H.; Diehl, A.; Norton, J.; Zhang, Y. A review of microplastics in aquatic sediments: Occurrence, fate, transport, and ecological impact. Curr. Pollut. Rep. 2021, 7, 40–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Zhang, Z. A Critical Review on Artificial Intelligence—Based Microplastics Imaging Technology: Recent Advances, Hot-Spots and Challenges. Int. J. Environ. Res. Public Health 2023, 20, 1150. [Google Scholar] [CrossRef]
- He, B.; Wijesiri, B.; Ayoko, G.A.; Egodawatta, P.; Rintoul, L.; Goonetilleke, A. Influential factors on microplastics occurrence in river sediments. Sci. Total Environ. 2020, 738, 139901. [Google Scholar] [CrossRef]
- Koutnik, V.S.; Leonard, J.; Alkidim, S.; DePrima, F.J.; Ravi, S.; Hoek, E.M.; Mohanty, S.K. Distribution of microplastics in soil and freshwater environments: Global analysis and framework for transport modeling. Environ. Pollut. 2021, 274, 116552. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Smith, M.; Egodawatta, P.; Ayoko, G.A.; Rintoul, L.; Goonetilleke, A. Dispersal and transport of microplastics in river sediments. Environ. Pollut. 2021, 279, 116884. [Google Scholar] [CrossRef]
- Kukkola, A.; Runkel, R.L.; Schneidewind, U.; Murphy, S.F.; Kelleher, L.; Smith, G.H.S.; Nel, H.A.; Lynch, I.; Krause, S. Prevailing impacts of river management on microplastic transport in contrasting US streams: Rethinking global microplastic flux estimations. Water Res. 2023, 240, 120112. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Vermeiren, P.; Muñoz, C.C.; Ikejima, K. Sources and sinks of plastic debris in estuaries: A conceptual model integrating biological, physical and chemical distribution mechanisms. Mar. Pollut. Bull. 2016, 113, 7–16. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Kim, J.-S.; Lee, H.; Lee, H.-J. Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. J. Hazard. Mater. 2021, 403, 123997. [Google Scholar] [CrossRef]
- Alam, M.S.; Han, B.; Pichtel, J. Irrigation suitability of White River in Indiana, Midwestern USA. Environ. Geochem. Health 2021, 43, 4179–4200. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Rane, N.R.; Bankole, P.O.; Krishnaiah, P.; Ahn, Y.; Park, Y.-K.; Yadav, K.K.; Amin, M.A.; Jeon, B.-H. An assessment of micro-and nanoplastics in the biosphere: A review of detection, monitoring, and remediation technology. Chem. Eng. J. 2022, 430, 132913. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.H.; Luo, G.; Kang, Y.; Zhang, L.; Pan, Y.; Zhou, X.; Fan, L.; Liang, B.; Wang, A. The contamination of microplastics in China’s aquatic environment: Occurrence, detection and implications for ecological risk. Environ. Pollut. 2022, 296, 118737. [Google Scholar] [CrossRef]
- Chauret, C.; Volk, C.; Creason, R.; Jarosh, J.; Robinson, J.; Warnes, C. Detection of Aeromonas hydrophila in a drinking-water distribution system: A field and pilot study. Can. J. Microbiol. 2001, 47, 782–786. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Belontz, S.L.; Ryan, K.; Walzak, M.J. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 2019, 54, 818–825. [Google Scholar] [CrossRef]
- Alam, M.J.; Shammi, M.; Tareq, S.M. Distribution of microplastics in shoreline water and sediment of the Ganges River Basin to Meghna Estuary in Bangladesh. Ecotoxicol. Environ. Saf. 2023, 266, 115537. [Google Scholar] [CrossRef]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, L.; Reboa, A.; Geneselli, I.; Capello, M. Considerations on salts used for density separation in the extraction of microplastics from sediments. Mar. Pollut. Bull. 2021, 166, 112216. [Google Scholar] [CrossRef]
- Tian, P.; Muhmood, A.; Xie, M.; Cui, X.; Su, Y.; Gong, B.; Yu, H.; Li, Y.; Fan, W.; Wang, X. New insights into the distribution and interaction mechanism of microplastics with humic acid in river sediments. Chemosphere 2022, 307, 135943. [Google Scholar] [CrossRef] [PubMed]
- Adomat, Y.; Grischek, T. Sampling and processing methods of microplastics in river sediments-a review. Sci. Total Environ. 2021, 758, 143691. [Google Scholar] [CrossRef]
- Ta, A.T.; Babel, S. Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals. Chemosphere 2020, 257, 127234. [Google Scholar] [CrossRef]
- Shamskhany, A.; Li, Z.; Patel, P.; Karimpour, S. Evidence of Microplastic Size Impact on Mobility and Transport in the Marine Environment: A Review and Synthesis of Recent Research. Front. Mar. Sci. 2021, 8, 760649. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhao, Y.; Colin, C.; Lin, A.T.S. Distribution and controlling factors of microplastics in surface sediments of typical deep-sea geomorphological units in the northern South China Sea. Front. Mar. Sci. 2022, 9, 1047078. [Google Scholar] [CrossRef]
- Enders, K.; Käppler, A.; Biniasch, O.; Feldens, P.; Stollberg, N.; Lange, X.; Fischer, D.; Eichhorn, K.-J.; Pollehne, F.; Oberbeckmann, S.; et al. Tracing microplastics in aquatic environments based on sediment analogies. Sci. Rep. 2019, 9, 15207. [Google Scholar] [CrossRef] [PubMed]
- Waldschläger, K.; Brückner, M.Z.; Almroth, B.C.; Hackney, C.R.; Adyel, T.M.; Alimi, O.S.; Belontz, S.L.; Cowger, W.; Doyle, D.; Gray, A.; et al. Learning from natural sediments to tackle microplastics challenges: A multidisciplinary perspective. Earth Sci. Rev. 2022, 228, 104021. [Google Scholar] [CrossRef]
- Amin, B.; Galib, M.; Setiawan, F. Preliminary Investigation on the Type and Ditribution of Microplastics in the West Coast of Karimun Besar Island. IOP Conf. Ser. Earth Environ. Sci. 2020, 430, 012011. [Google Scholar] [CrossRef]
- Fan, J.; Zou, L.; Zhao, G. Microplastic abundance, distribution, and composition in the surface water and sediments of the Yangtze River along Chongqing City, China. J. Soils Sediments 2021, 21, 1840–1851. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; International Maritime Organisation: London, UK, 2015. [Google Scholar]
- Kunz, A.; Schneider, F.; Anthony, N.; Lin, H.T. Microplastics in rivers along an urban-rural gradient in an urban agglomeration: Correlation with land use, potential sources and pathways. Environ. Pollut. 2023, 321, 121096. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef]
- Semcesen, P.O.; Wells, M.G. Biofilm growth on buoyant microplastics leads to changes in settling rates: Implications for microplastic retention in the Great Lakes. Mar. Pollut. Bull. 2021, 170, 112573. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Lu, H.C.; Ziajahromi, S.; Neale, A.; Leusch, F.D.L. A systematic review of freshwater microplastics in water and sediments: Recommendations for harmonisation to enhance future study comparisons. Sci. Total Environ. 2021, 781, 146693. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, W.; Liu, X. Impact of Water Level Fluctuation on Microplastic Transportation and Redistribution in a Floodplain Lake System. Water 2023, 15, 3658. [Google Scholar] [CrossRef]
- Ren, X.; Sun, Y.; Wang, Z.; Barceló, D.; Wang, Q.; Zhang, Z.; Zhang, Y. Abundance and characteristics of microplastic in sewage sludge: A case study of Yangling, Shaanxi province, China. Case Stud. Chem. Environ. Eng. 2020, 2, 100050. [Google Scholar] [CrossRef]
- Syakti, A.D.; Hidayati, N.V.; Jaya, Y.V.; Siregar, S.H.; Yude, R.; Suhendy; Asia, L.; Wong-Wah-Chung, P.; Doumenq, P. Simultaneous grading of microplastic size sampling in the Small Islands of Bintan water, Indonesia. Mar. Pollut. Bull. 2018, 137, 593–600. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar] [CrossRef]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef]
- Malli, A.; Corella-Puertas, E.; Hajjar, C.; Boulay, A.M. Transport mechanisms and fate of microplastics in estuarine compartments: A review. Mar. Pollut. Bull. 2022, 177, 113553. [Google Scholar] [CrossRef]
- Mendrik, F.; Fernández, R.; Hackney, C.R.; Waller, C.; Parsons, D.R. Non-buoyant microplastic settling velocity varies with biofilm growth and ambient water salinity. Commun. Earth Environ. 2023, 4, 30. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Verma, A.; Jha, P.K.; Singh, P.; Gupta, P.K.; Chandra, R.; Prasad, P.V.V. Effect of physical characteristics and hydrodynamic conditions on transport and deposition of microplastics in riverine ecosystem. Water 2021, 13, 2710. [Google Scholar] [CrossRef]
- Atugoda, T.; Piyumali, H.; Liyanage, S.; Mahatantila, K.; Vithanage, M. Fate and Behavior of Microplastics in Freshwater Systems. In Handbook of Microplastics in the Environment; Springer: Cham, Switzerland, 2022; pp. 781–811. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Li, B.; Li, B.; Jia, Q.; Cai, Y.; Xie, Y.; Yuan, X.; Yang, Z. Dynamic characteristics of microplastics under tidal influence and potential indirect monitoring methods. Sci. Total Environ. 2023, 869, 161869. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, H.; Gu, W.; Yang, G.; Liu, Y.; He, Q. Distribution and characteristics of microplastics in the Yulin River, China: Role of environmental and spatial factors. Environ. Pollut. 2020, 265, 115033. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Foo, Y.H.; Ratnam, S.; Lim, E.V.; Abdullah, M.; Molenaar, V.J.; Hwai, A.T.S.; Zhang, S.; Li, H.; Zanuri, N.B.M. Microplastic ingestion by commercial marine fish from the seawater of Northwest Peninsular Malaysia. PeerJ 2022, 10, e13181. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Holsen, T.M.; Baki, A.B.M. Distribution and risk assessment of microplastic pollution in a rural river system near a wastewater treatment plant, hydro-dam, and river confluence. Sci. Rep. 2024, 14, 6006. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 768297. [Google Scholar] [CrossRef]
- Lin, D.; Yang, G.; Dou, P.; Qian, S.; Zhao, L.; Yang, Y.; Fanin, N. Microplastics negatively affect soil fauna but stimulate microbial activity: Insights from a field-based microplastic addition experiment. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201268. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Vermeiren, P.; Lercari, D.; Muñoz, C.C.; Ikejima, K.; Celentano, E.; Jorge-Romero, G.; Defeo, O. Sediment grain size determines microplastic exposure landscapes for sandy beach macroinfauna. Environ. Pollut. 2021, 286, 117308. [Google Scholar] [CrossRef] [PubMed]
- De Frond, H.; Cowger, W.; Renick, V.; Brander, S.; Primpke, S.; Sukumaran, S.; Elkhatib, D.; Barnett, S.; Navas-Moreno, M.; Rickabaugh, K.; et al. What determines accuracy of chemical identification when using microspectroscopy for the analysis of microplastics? Chemosphere 2023, 313, 137300. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).