Expanding Karst Groundwater Tracing Techniques: Incorporating Population Genetic and Isotopic Data to Enhance Flow-Path Characterization
Abstract
1. Introduction
2. Materials and Methods
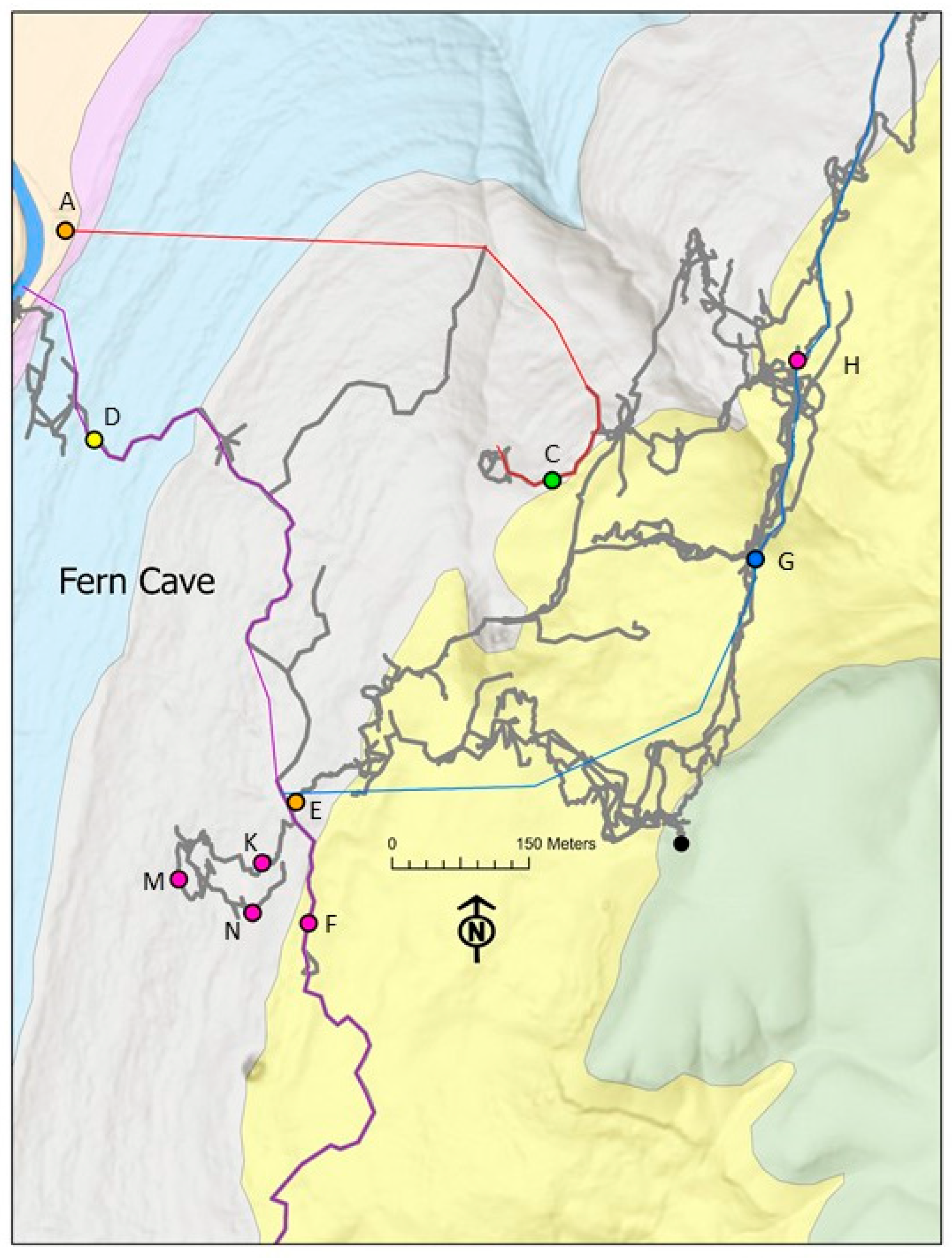
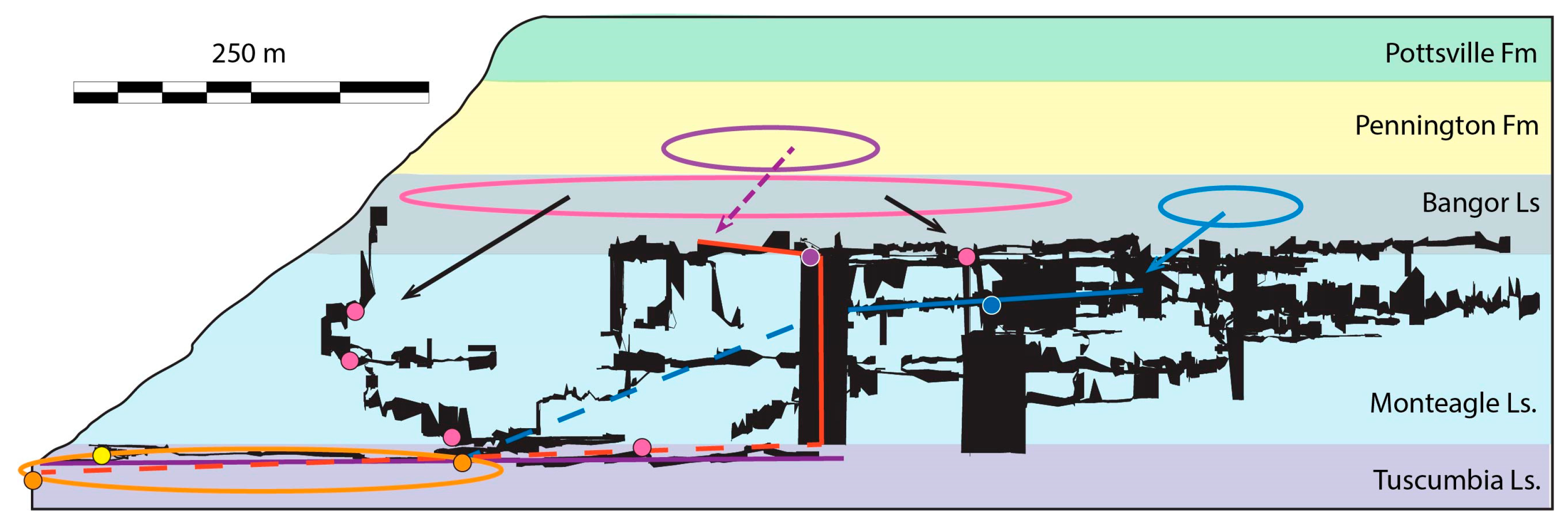
2.1. Study Site
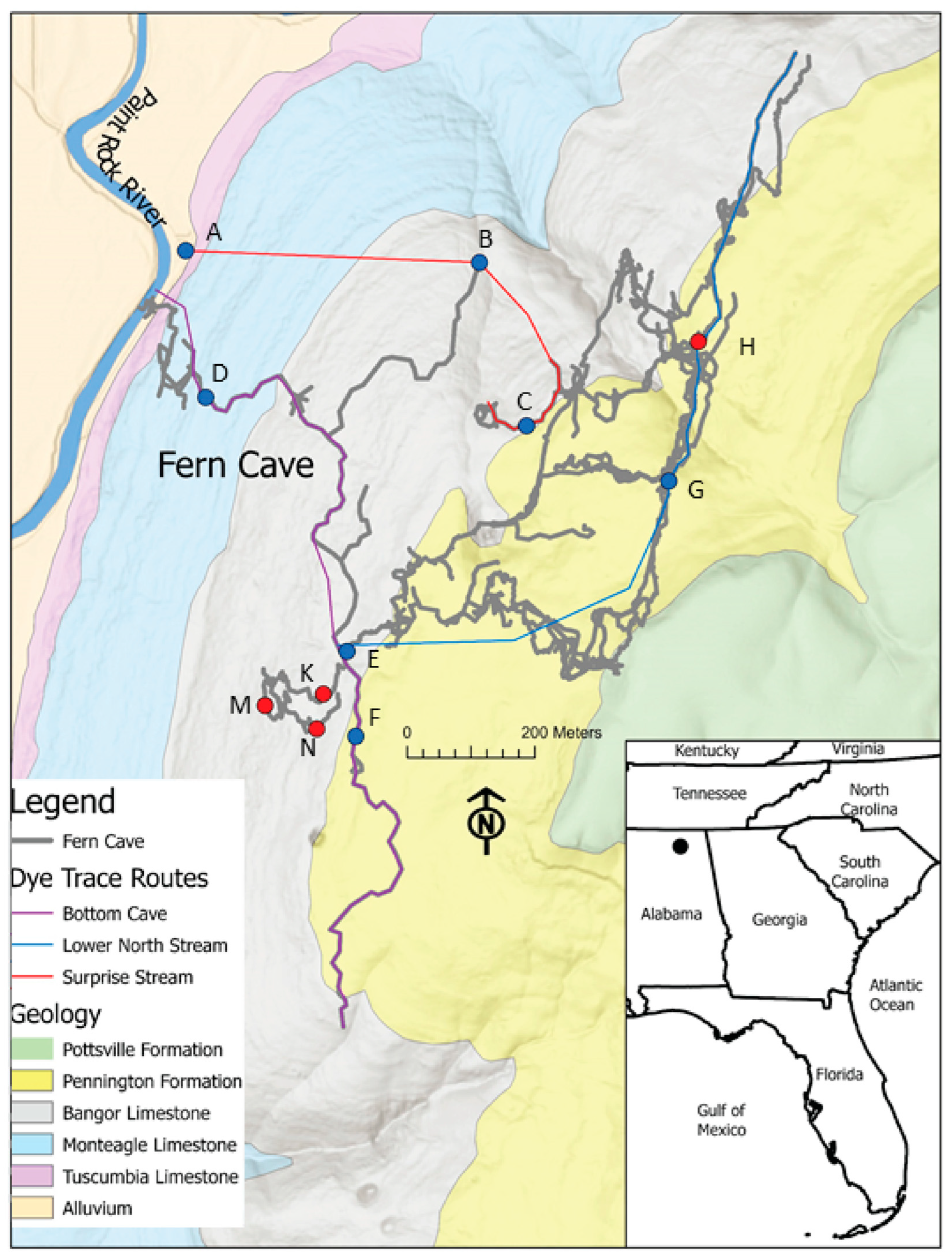
| Site ID | Site Name | Water Isotopes | Water Chemistry | Sediment Isotopes | Isopod Isotopes | Isopod Genetics |
|---|---|---|---|---|---|---|
| A | Haley Spring | x | x | x | x | x |
| B | Earthquake Room | x | x | x | x | x |
| C | Surprise Stream | x | x | |||
| D | Bottom Cave—downstream (DS) | x | x | x | x | x |
| E | Bottom Cave—waterfall | x | x | x | x | x |
| F | Bottom Cave—upstream (US) | x | x | x | x | x |
| G | Lower North—cascade | x | x | x | x | |
| H | Lower North—upstream (US) | x | x | x | ||
| K | Morgue Dome | x | ||||
| M | Refrigerator Passage | x | x | |||
| N | Morgue Entrance | x |
2.2. Field Data Collection and Analysis
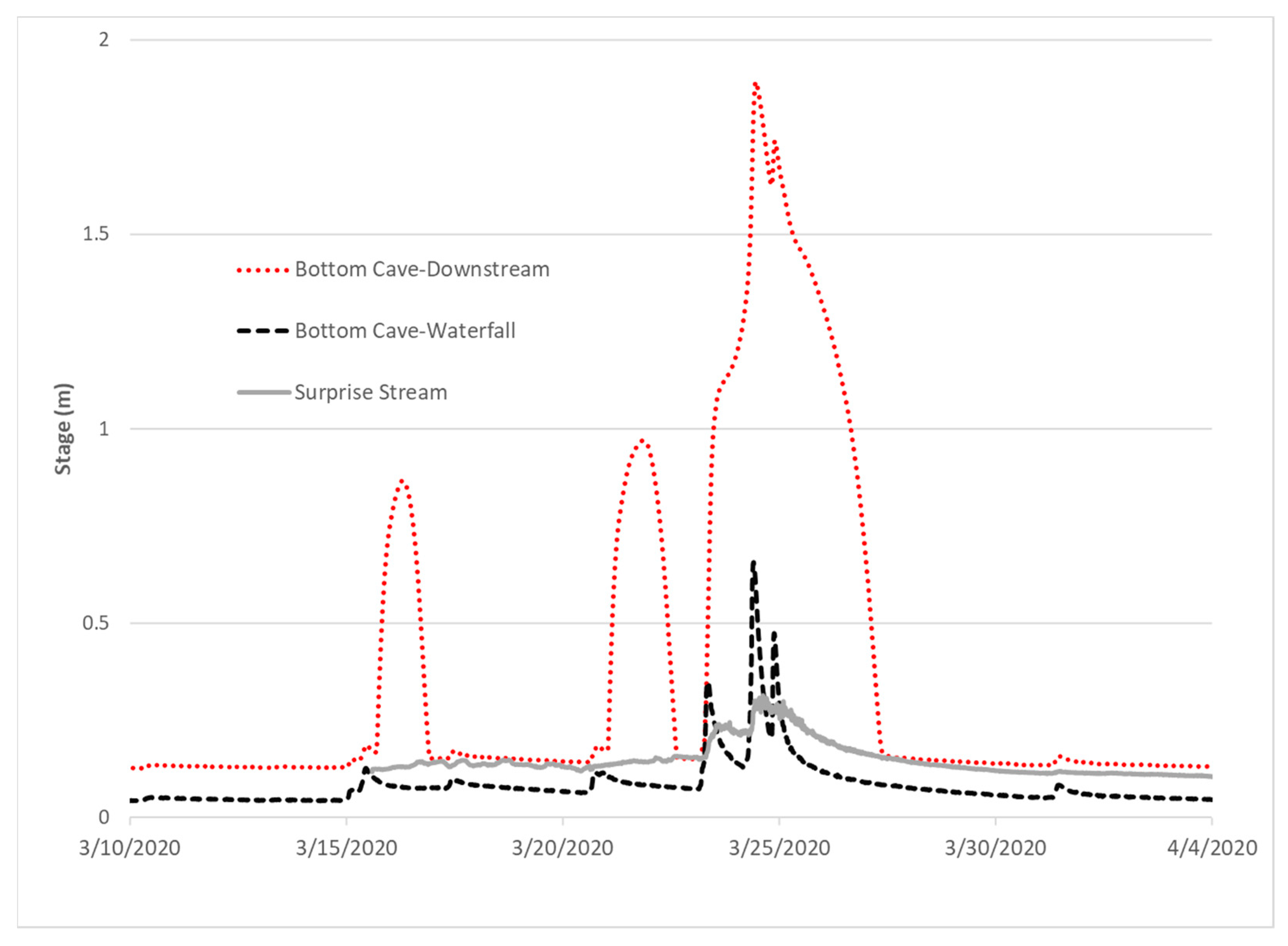
2.2.1. Hydrologic Monitoring
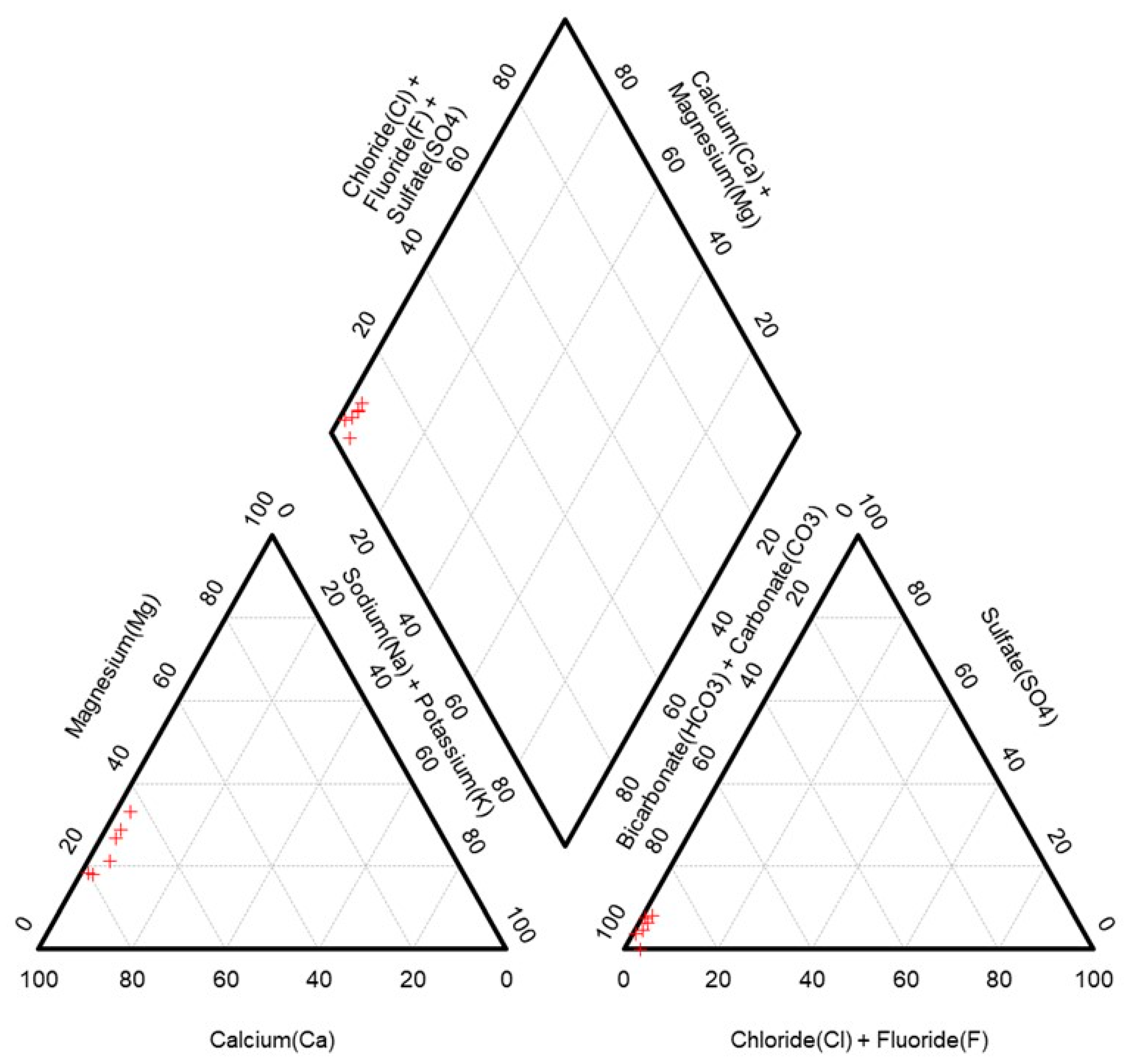
2.2.2. Geochemical Analysis
2.2.3. Genetic Analysis
3. Results
3.1. Ion Geochemistry Analysis
3.2. Hydrograph Analysis
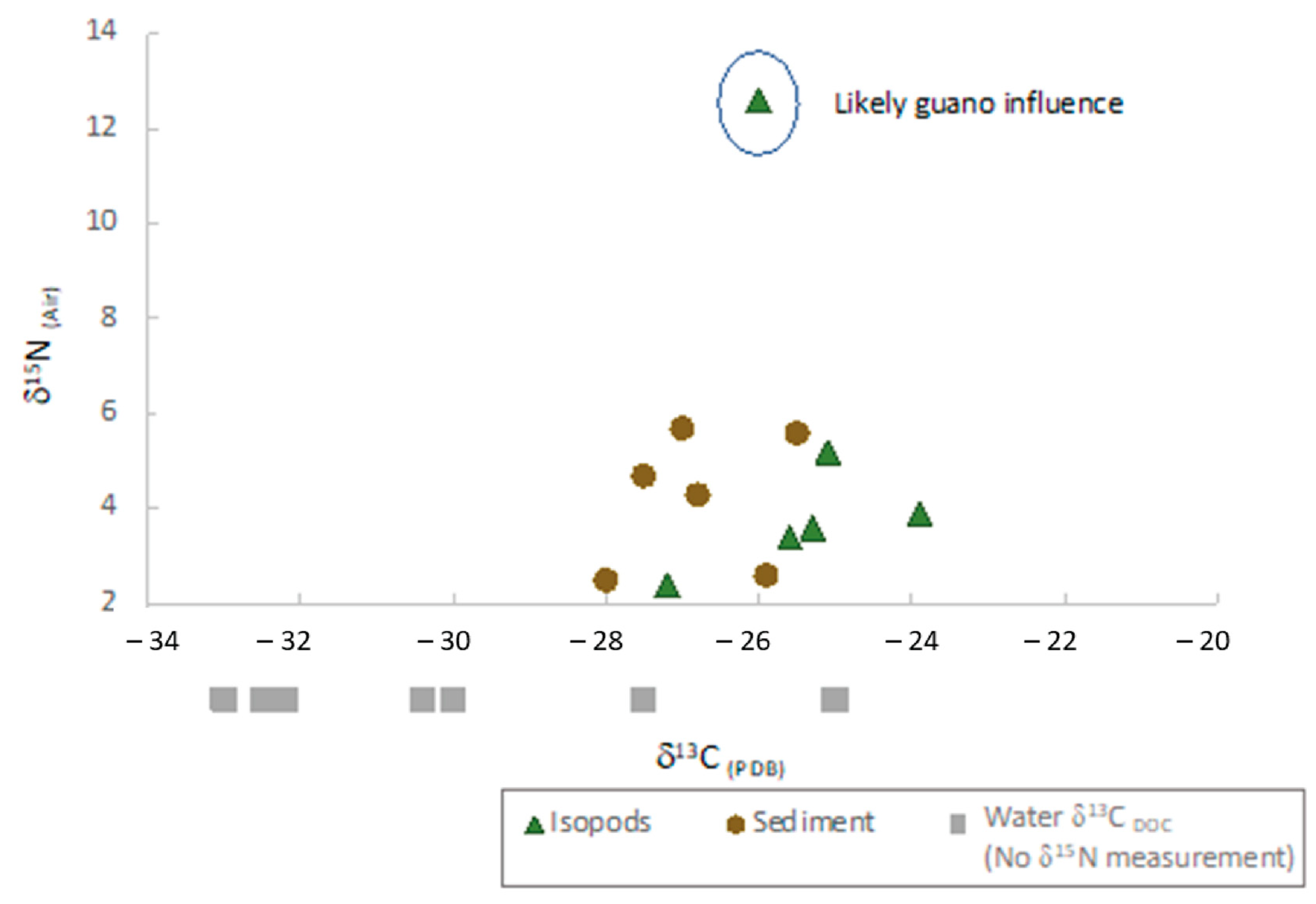
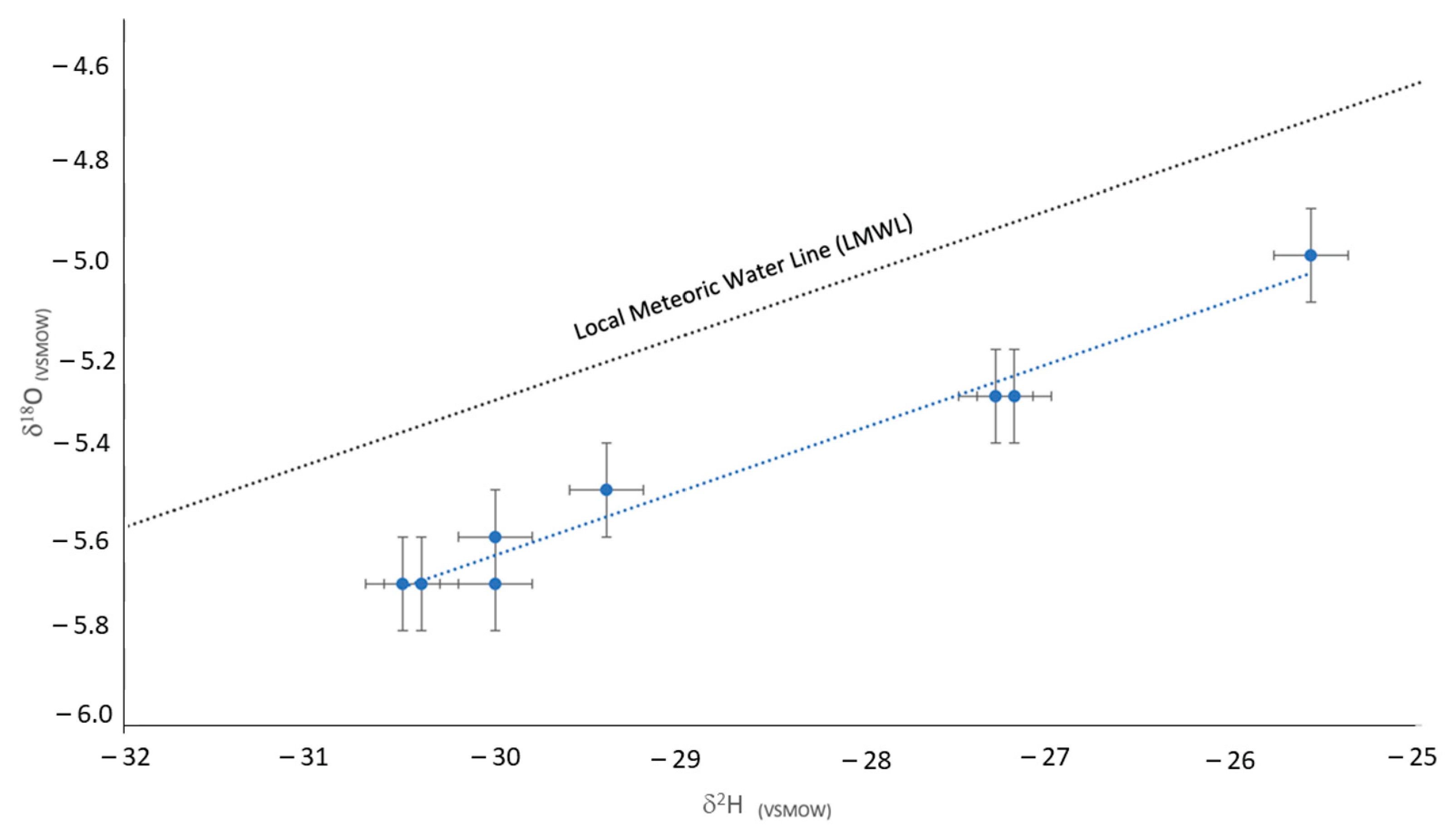
3.3. Isotope Geochemistry
3.4. Genetic Analysis
4. Discussion
4.1. Genetic Tracers
4.2. Isotopic Tracers
4.3. Updated Groundwater Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldscheider, N.; Chen, Z.; Auler, A.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Gutierrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth-Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- Guo, F.; Yuan, D.; Qin, Z. Groundwater contamination in karst areas of southwestern China and recommended countermeasures. Acta Carsologica 2010, 39, 389–399. [Google Scholar] [CrossRef]
- Green, R.T.; Painter, S.L.; Sun, A.; Worthington, S.R.H. Groundwater Contamination in Karst Terranes. Water Air Soil Pollut. Focus 2006, 6, 157–170. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, H.; Lu, H.; Mao, R.; Zheng, H.; Wang, J. Analytical methods and application of stable isotopes in dissolved organic carbon and inorganic carbon in groundwater: Analytical methods for carbon isotopes in DIC and DOC. Rapid Commun. Mass Spectrom. 2015, 29, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.J.; McCormack, T.; Naughton, O.; Johnston, P.M.; Gill, L.W. Modelling groundwater flooding in a lowland karst catchment. J. Hydrol. 2020, 580, 124361. [Google Scholar] [CrossRef]
- Basu, B.; Morrissey, P.; Gill, L.W. Application of nonlinear time series and machine learning algorithms for forecasting groundwater flooding in a lowland karst area. Water Resour. Res. 2022, 58, e2021WR029576. [Google Scholar] [CrossRef]
- Goldscheider, N. Overview of methods applied in karst hydrogeology. In Karst Aquifers—Characterization and Engineering, Professional Practice in Earth Sciences; Springer International Publishing: Cham, Switzerland, 2015; pp. 127–145. [Google Scholar] [CrossRef]
- Benischke, R. Review: Advances in the methodology and application of tracing in karst aquifers. Hydrogeol. J. 2021, 29, 67–88. [Google Scholar] [CrossRef]
- Kambesis, P. The importance of cave exploration to scientific research. J. Cave Karst Stud. 2007, 69, 46–58. [Google Scholar]
- Sovie, A.R.; Tobin, B.W.; Farmer, B. Understanding Karst Landscape Evolution Through Ecosystems: Cave Connectivity and Isolation. Carbonates Evaporites 2022, 37, 8. [Google Scholar] [CrossRef]
- Saccò, M.; Blyth, A.; Bateman, P.W.; Hua, Q.; Mazumder, D.; White, N.; Humphreys, W.F.; Laini, A.; Griebler, C.; Grice, K. New light in the dark—A proposed multidisciplinary framework for studying functional ecology of groundwater fauna. Sci. Total Environ. 2019, 662, 963–977. [Google Scholar] [CrossRef]
- Culver, D.C.; Christman, M.C.; Elliott, W.R.; Hobbs, H.H., III; Reddell, J.R. The North American obligate cave fauna: Regional patterns. Biodivers. Conserv. 2003, 12, 441–468. [Google Scholar] [CrossRef]
- Barr, T.C. Observations on the Ecology of Caves. Am. Nat. 1967, 101, 475–491. [Google Scholar] [CrossRef]
- Goldscheider, N.; Meiman, J.; Pronk, M.; Smart, C. Tracer tests in karst hydrogeology and speleology. Int. J. Speleol. 2008, 37, 27–40. [Google Scholar] [CrossRef]
- Jordan, S.; Hand, B.K.; Hotaling, S.; Delvecchia, A.G.; Malison, R.; Nissley, C.; Luikart, G.; Stanford, J.A. Genomic data reveal similar genetic differentiation in aquifer species with different dispersal capabilities and life histories. Biol. J. Linn. Soc. 2020, 129, 315–322. [Google Scholar] [CrossRef]
- Krejca, J.K.; Weckerley, B. Detection probabilities of karst invertebrates. In Proceedings of the Eighteenth National Cave and Karst Management Symposium, St. Louis, MI, USA, 8–12 October 2008; Elliott, W.R., Ed.; NCKMS Steering Committee: Albuquerque, NM, USA, 2008; pp. 283–289. [Google Scholar]
- Culver, D.C.; Pipan, T. Shifting paradigms of the evolution of cave life. Acta Carsologica 2015, 44, 415–425. [Google Scholar] [CrossRef]
- Johns, T.; Jones, J.I.; Knight, L.; Maurice, L.; Wood, P.; Robertson, A. Regional-scale drivers of groundwater faunal distributions. Freshw. Sci. 2015, 34, 316–328. [Google Scholar] [CrossRef][Green Version]
- Krejca, J.K. Stygobite Phylogenetics as a Tool for Determining Aquifer Evolution. Ph.D. Dissertation, The University of Texas at Austin, Austin, TX, USA, 2005. [Google Scholar]
- Niemiller, M.L.; Near, T.J.; Fitzpatrick, B.M. Delimiting species using multilocus data: Diagnosing cryptic diversity in the southern cavefish, Typhlichthys subterraneus (Teleostei: Amblyopsidae). Evolution 2012, 66, 846–866. [Google Scholar] [CrossRef]
- Devitt, T.J.; Wright, A.M.; Cannatella, D.C.; Hillis, D.M. Species delimitation in endangered groundwater salamanders: Implications for aquifer management and biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; Bloomfield, J. Stygobitic invertebrates in groundwater—A review from a hydrogeological perspective. Freshw. Rev. 2012, 5, 51–71. [Google Scholar] [CrossRef]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Biondi, M.; Di Cicco, M.; Galassi, D.M.P. Spatial Distribution of stygobitic crustacean harpacticoids at the boundaries of groundwater habitat types in Europe. Sci. Rep. 2020, 10, 19043. [Google Scholar] [CrossRef]
- Datry, T.; Malard, F.; Gibert, J. Response of invertebrate assemblages to increased groundwater recharge rates in a phreatic aquifer. J. N. Am. Benth. Soc. 2015, 24, 461–477. [Google Scholar] [CrossRef]
- Wilson, G.D.F. Global diversity of Isopod crustaceans (Crustacea; Isopoda) in freshwater. Hydrobiologia 2008, 595, 231–240. [Google Scholar] [CrossRef]
- Steeves, H.R. The troglobitic Asellids of the United States: The stygius group. Am. Midl. Nat. 1963, 69, 470–481. [Google Scholar] [CrossRef]
- Lewis, J.J. Systematics of the troglobitic Caecidotea (Crustacea: Isopoda: Asellidae) of the southern interior low plateaus. Brimleyana 1982, 8, 65–74. [Google Scholar]
- Hess, A.; Bonett, R. Utilizing native isopods to assess the connectivity and quality of Oklahoma groundwater. In Oklahoma Water Resources Center 2018 Annual Research Report; Oklahoma Water Resources Center: Stillwater, OK, USA, 2019. [Google Scholar]
- Osborne, W.E.; Ward, W.E., II; Irvin, G.D. Geologic Map and Cross Sections of the Paint Rock 7.5-Minute Quadrangle, Jackson and Madison Counties, Alabama. In Geological Survey of Alabama Quadrangle Series Map QS59, Scale 1, 24,000; Geological Survey of Alabama: Tuscaloosa, AL, USA, 2013. [Google Scholar]
- Miller, B.V.; Tobin, B.W. Mapping karst groundwater flow paths and delineating recharge areas for Fern Cave, Alabama through the use of dye tracing. In United States Geological Survey Scientific Investigation Maps; USGS: Reston, VA, USA, 2023. [Google Scholar] [CrossRef]
- Miller, B.V.; Tobin, B.W.; Hourigan, A.M. Mapping Karst Groundwater Flow Paths and Delineating Recharge Areas for Fern Cave, Alabama through the Use of Dye Tracing; U.S. Geological Survey Data Release; USGS: Reston, VA, USA, 2023. [Google Scholar] [CrossRef]
- Shelton, L.R. Field Guide for Collecting and Processing Stream-Water Samples for the National Water-Quality Assessment Program; U.S. Geological Survey Open-File Report 94-455; USGS: Reston, VA, USA, 1994. [Google Scholar]
- Francois, C.M.; Mermillod-Blondin, F.; Malard, F.; Fourel, F.; Lécuyer, C.; Douady, C.J.; Simon, L. Trophic ecology of groundwater species reveals specialization in a low-productivity environment. Funct. Ecol. 2016, 30, 262–273. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Araguas-Araguas, L.; Groning, M.; Kulkarni, K.M.; Kurttas, T.; Newman, B.D.; Tanweer, A. Laser Spectroscopic Analysis of Liquid Water Samples for Stable Hydrogen and Oxygen Isotopes; IAEA: Wien, Austria, 2009; p. 49. [Google Scholar]
- Coplen, T.B.; Wassenaar, L.I. LIMS for lasers 2015 for achieving long-term accuracy and precision of δ2H, δ17O, and δ18O of waters using laser absorption spectrometry: LIMS for lasers 2015. Rapid Commun. Mass Spectrom. 2015, 29, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Rivé, K.; Ader, M.; Jézéquel, D.; Agrinier, P. Improved method for isotopic and quantitative analysis of dissolved inorganic carbon in natural water samples. Rapid Commun. Mass Spectrom. 2006, 20, 2243–2251. [Google Scholar] [CrossRef]
- Wilson, J.W.; Munizzi, J.; Erhardt, A.M. Preservation methods for the isotopic composition of dissolved carbon species in non-ideal conditions. Rapid Commun. Mass Spectrom. 2020, 34, e8903. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.Q.; Bernasconi, S.M.; Früh-Green, G.L. Stable isotope analysis of organic carbon in small (μg C) samples and dissolved organic matter using a GasBench preparation device: δ13C of organic compounds by GasBench. Rapid Commun. Mass Spectrom. 2012, 26, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W. Drainage and flooding in karst terranes. Environ. Geol. 2007, 51, 963–973. [Google Scholar] [CrossRef]
- Midwood, A.J.; Boutton, T.W. Soil carbonate decomposition by acid has little effect on δ13C of organic matter. Soil Biol. Biochem. 1998, 30, 1301–1307. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, W.-S.; Kumar, K.S.; Shin, K.-H.; Robarge, W.; Kim, M.; Lee, S.R. Effects of HCl pretreatment, drying, and storage on the stable isotope ratios of soil and sediment samples. Rapid Commun. Mass Spectrom. 2016, 30, 1567–1575. [Google Scholar] [CrossRef]
- Tobin, B.; Miller, B.V.; Niemiller, M.; Erhardt, A. Hydrology Data for Fern Cave, Alabama (2020–2022); Kentucky Geological Survey: Lexington, KY, USA, 2023. [Google Scholar] [CrossRef]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 2008, 3, e3376. [Google Scholar] [CrossRef] [PubMed]
- Bayona-Vásquez, N.J.; Glenn, T.C.; Kieran, T.J.; Pierson, T.W.; Hoffberg, S.L.; Scott, P.A.; Bentley, K.E.; Finger, J.W.; Louha, S.; Troendle, N.; et al. Adapterama III: Quadruple-indexed, double/triple-enzyme RADseq libraries (2RAD/3RAD). PeerJ 2019, 7, e7724. [Google Scholar] [CrossRef]
- Rohland, N.; Reich, D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2005, 22, 939–946. [Google Scholar] [CrossRef]
- Eaton, D.A.R.; Overcast, I. Ipyrad: Interactive Assembly and Analysis of RADseq Data Sets. 2016. Available online: https://ipyrad.readthedocs.io/ (accessed on 1 May 2022).
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Jombart, T.; Kamvar, Z.N.; Collins, C.; Lustrik, R.; Beugin, M.P.; Knaus, B.J.; Jombart, M.T. Package ‘Adegenet’. Github Repository. 2018. Available online: https://github.com/thibautjombart/adegenet (accessed on 1 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 May 2022).
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; François, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 2014, 196, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Carrière, S.D.; Chalikakis, K.; Danquigny, C.; Davi, H.; Mazzilli, N.; Ollivier, C.; Emblanch, C. The role of porous matrix in water flow regulation within a karst unsaturated zone: An integrated hydrogeophysical approach. Hydrogeol. J. 2016, 24, 1905–1918. [Google Scholar] [CrossRef]
- Wilson, J.W.; Erhardt, A.M.; Tobin, B.W. Isotopic and geochemical tracers of groundwater flow in the Shivwits Plateau, Grand Canyon National Park, USA. Hydrogeol. J. 2022, 30, 495–510. [Google Scholar] [CrossRef]
- Campbell, J.W.; Waters, M.N.; Rich, F. Guano core evidence of palaeoenvironmental change and Woodland Indian inhabitance in Fern Cave, Alabama, USA, from the mid-Holocene to present. Boreas 2017, 46, 462–469. [Google Scholar] [CrossRef]
- Le Mesnil, M.; Charlier, J.-B.; Moussa, R.; Caballero, Y. Interbasin groundwater flow: Characterization, role of karst areas, impact on annual water balance and flood processes. J. Hydrol. 2020, 585, 1245583. [Google Scholar] [CrossRef]


| Site ID | Site Name | Installation | Removal |
|---|---|---|---|
| D | Bottom Cave—downstream | 17 February 2020 | 11 August 2021 |
| E | Bottom Cave—waterfall | 17 February 2020 | 11 August 2021 |
| C | Surprise Stream | 15 March 2020 | 7 February 2021 |
| G | Lower North—cascade | 27 July 2020 | 10 August 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobin, B.W.; Miller, B.V.; Niemiller, M.L.; Erhardt, A.M. Expanding Karst Groundwater Tracing Techniques: Incorporating Population Genetic and Isotopic Data to Enhance Flow-Path Characterization. Hydrology 2024, 11, 23. https://doi.org/10.3390/hydrology11020023
Tobin BW, Miller BV, Niemiller ML, Erhardt AM. Expanding Karst Groundwater Tracing Techniques: Incorporating Population Genetic and Isotopic Data to Enhance Flow-Path Characterization. Hydrology. 2024; 11(2):23. https://doi.org/10.3390/hydrology11020023
Chicago/Turabian StyleTobin, Benjamin W., Benjamin V. Miller, Matthew L. Niemiller, and Andrea M. Erhardt. 2024. "Expanding Karst Groundwater Tracing Techniques: Incorporating Population Genetic and Isotopic Data to Enhance Flow-Path Characterization" Hydrology 11, no. 2: 23. https://doi.org/10.3390/hydrology11020023
APA StyleTobin, B. W., Miller, B. V., Niemiller, M. L., & Erhardt, A. M. (2024). Expanding Karst Groundwater Tracing Techniques: Incorporating Population Genetic and Isotopic Data to Enhance Flow-Path Characterization. Hydrology, 11(2), 23. https://doi.org/10.3390/hydrology11020023






