Detection of Strobilurin Fungicides in Trout Streams within an Agricultural Watershed
Abstract
1. Introduction
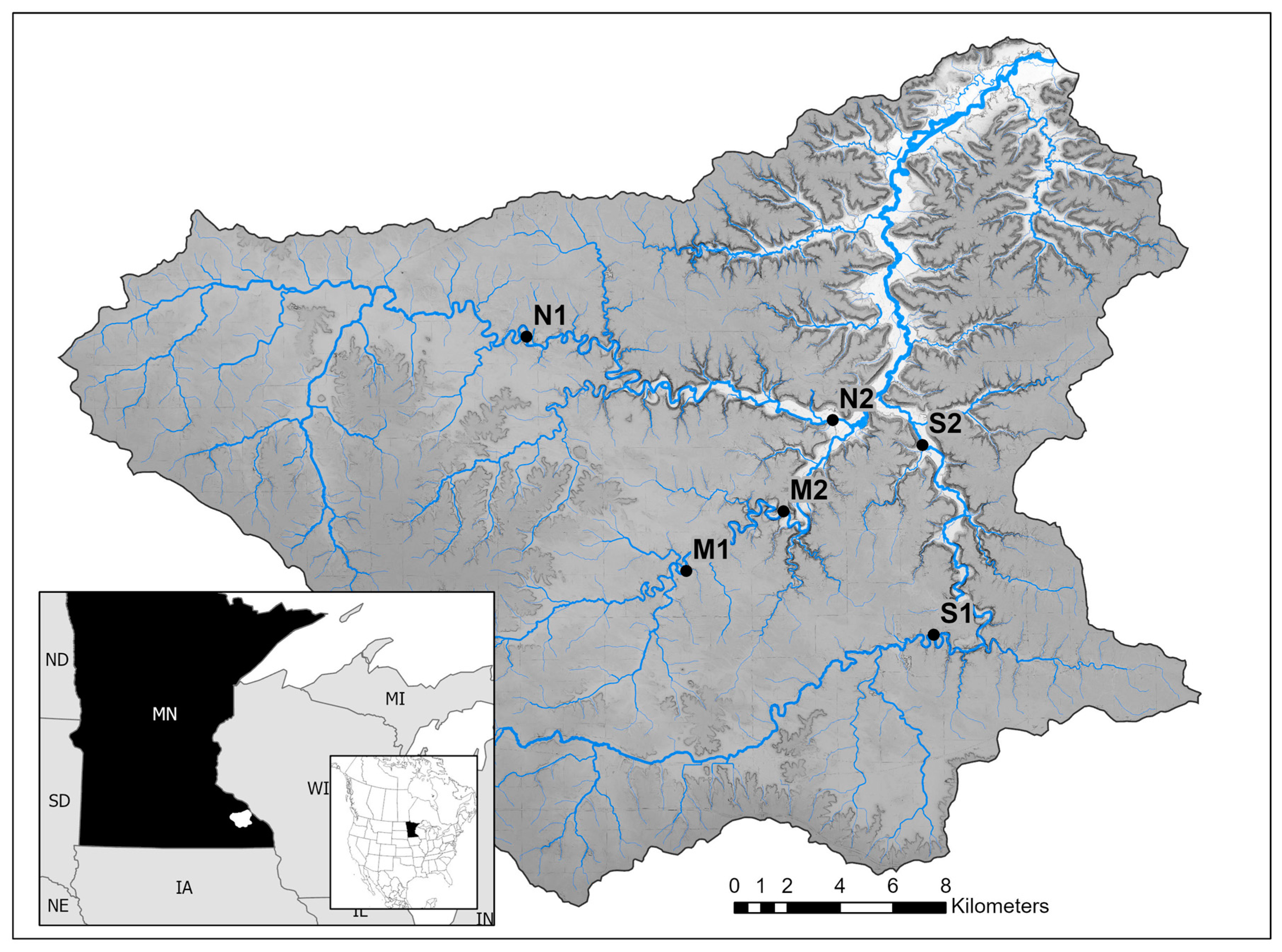
2. Study Area
3. Methods and Materials
3.1. Fungicide Applications
3.2. Sample Collections
3.3. Sample Preparation and Extraction for Fungicide Analysis
3.4. Chemical Sources and Purity
3.5. Sample Concentration Calculations
3.6. Data Analyses
4. Results
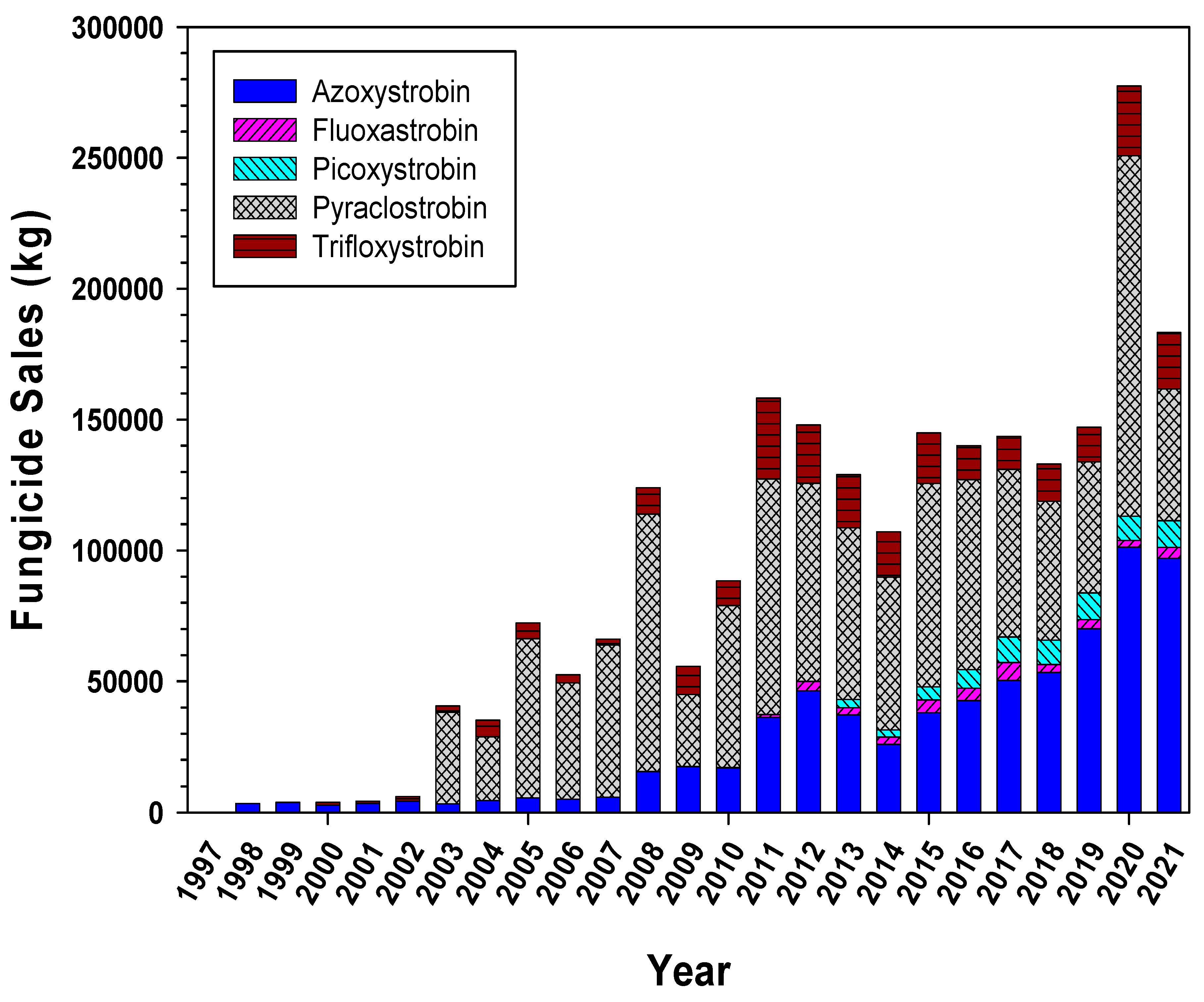
4.1. Fungicide Applications within the Watershed
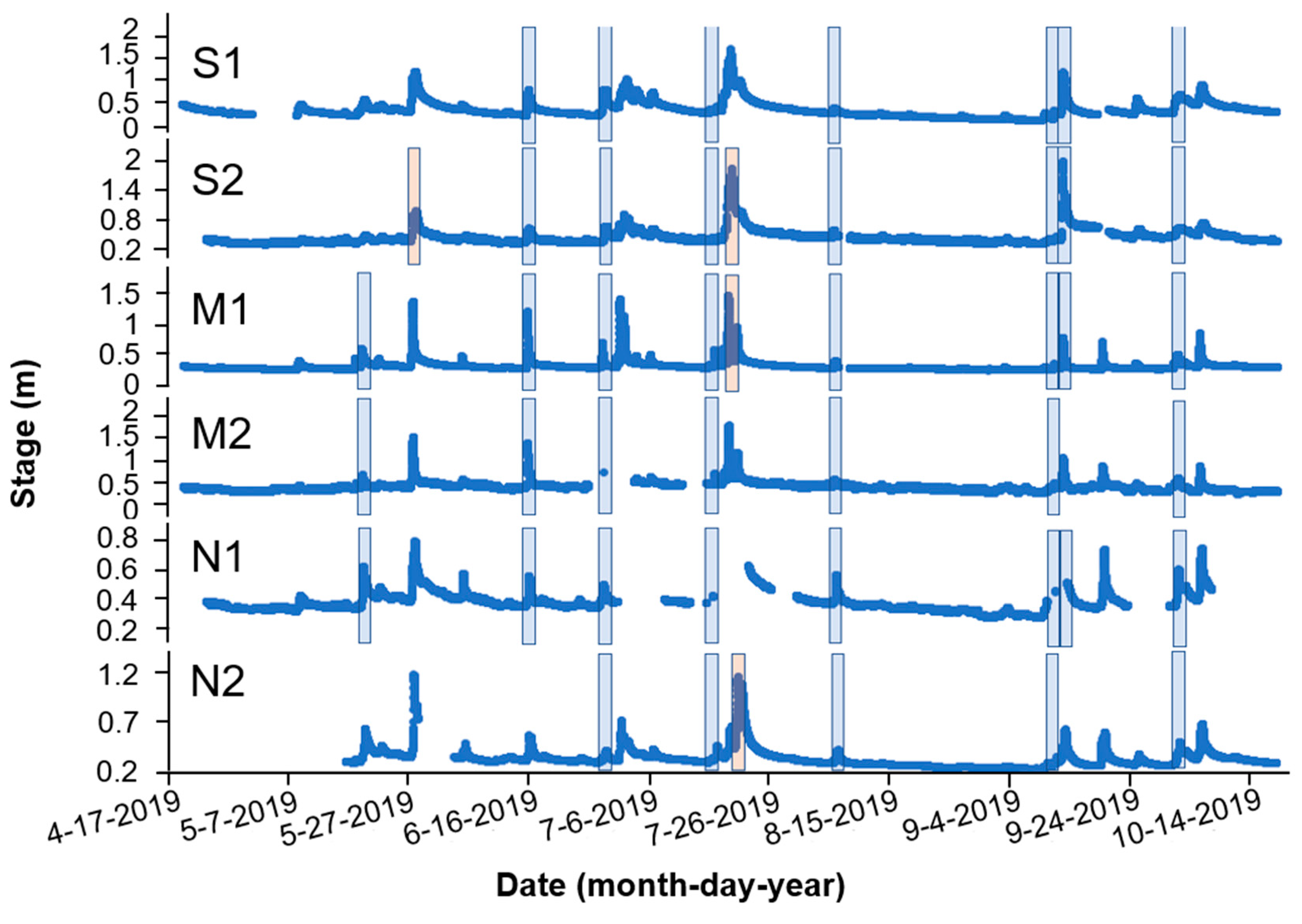
4.2. Routine Water Quality Measurements
4.3. Water Sampling for Strobilurin Fungicides
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Pesticide Fact Sheet: Azoxystrobin; United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances: Washington, DC, USA, 1997. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-128810_07-Feb-97.pdf (accessed on 3 January 2024).
- Dorrance, A.E.; Berry, S.A.; Anderson, T.R.; Meharg, C. Isolation, storage, pathotype characterization, and evaluation of resistance for Phytophthora sojae in soybean. Plant Health Prog. 2008, 9, 35. Available online: https://apsjournals.apsnet.org/doi/pdf/10.1094/PHP-2008-0118-01-DG (accessed on 4 January 2024). [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. Review: The strobilurin fungicides. Pestic. Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Brown-Rytlewski, D.; Vincelli, P.; Allen, T.; Hershman, D.; Berstrom, G.C.; Hollingsworth, C.; Bradley, C.; Hunger, B. Letter from Universities Regarding the Strobilurin, Pyraclostrobin (Headline), Supplemental Label; United States Environmental Protection Agency: Washington, DC, USA, 2009; Available online: https://fliphtml5.com/ytyf/sucq/basic/ (accessed on 3 January 2024).
- Weaver, C. Fungicides in the Whitewater River: An Emerging Concern? Master’s Thesis, Winona State University, Winona, MN, USA, 2020. [Google Scholar]
- Bradley, K.W.; Sweets, L.E. Influence of glyphosate and fungicide coapplications on weed control, spray penetration, soybean response, and yield in glyphosate-resistant soybean. Agron. J. 2008, 100, 1360–1365. [Google Scholar] [CrossRef]
- Yang, X.B.; Navi, S.S.; Pecinovsky, K.; Shriver, J. Use of fungicides to control soybean foliar diseases in Iowa: A 6-year study. In Proceedings of the Integrated Crop Management Conference, Iowa State University, Ames, IA, USA, 10–11 December 2008; pp. 161–175. Available online: https://dr.lib.iastate.edu/server/api/core/bitstreams/6478e29b-fab4-493b-9327-1486a2d7ac81/content (accessed on 3 January 2024).
- Reddy, S.N.; Gupta, S.; Gajbhiye, V.T. Adsorption-desorption and leaching of pyraclostrobin in Indian soils. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 948–959. [Google Scholar] [CrossRef]
- BASF Corporation. Headline Fungicide Supplemental Label; BASF Corporation: Research Triangle Park, NC, USA, 2008; Available online: https://www.cdms.net/LDat/ld62L016.pdf (accessed on 3 January 2024).
- Edwards, P.G.; Murphy, T.M.; Lydy, M.J. Fate and transport of agriculturally applied fungicide compounds, azoxystrobin and propiconazole. Chemosphere 2015, 146, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.J.; Smalling, K.L.; Orlando, J.L.; Kuivila, K.M. Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere 2012, 89, 228–234. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Sandstrom, M.W.; Kuivila, K.M.; Kolpin, D.W.; Meyer, M.T. Occurrence of azoxystrobin, propiconozole, and selected other fungicides in US streams. Water Air Soil Pollut. 2011, 218, 307–322. [Google Scholar] [CrossRef]
- Wightwick, A.M.; Bui, A.D.; Zhang, P.; Rose, G.; Allinson, M.; Myers, J.H.; Reichman, S.M.; Menzies, N.W.; Pettigrove, V.; Allinson, G. Environmental fate of fungicides in surface waters of a horticultural-production catchment in southeastern Australia. Arch. Environ. Contam. Toxicol. 2012, 62, 380–390. [Google Scholar] [CrossRef]
- Smalling, K.L.; Orlando, J.L. Occurrence of Pesticides in Surface Water and Sediments from Three Central California Coastal Watersheds, 2008–2009; United States Geological Survey Data Series 600; US Geological Survey: Washington, DC, USA, 2011. [Google Scholar]
- Carpenter, K.D.; Kuivila, K.M.; Hladik, M.L.; Haluska, T.; Cole, M.B. Storm-event-transport of urban-use pesticides to streams likely impairs invertebrate assemblages. Environ. Monit. Assess. 2016, 188, 345. [Google Scholar] [CrossRef]
- Bereswill, R.; Golla, B.; Streloke, M.; Schulz, R. Entry and toxicity of organic pesticides and copper in vineyard streams; erosion rills jeopardise the efficiency of riparian buffer strips. Agric. Ecosyst. Environ. 2012, 146, 81–92. [Google Scholar] [CrossRef]
- Berenzen, N.; Lentzen-Godding, A.; Probst, M.; Schulz, R.; Liess, M. A comparison of predicted and measured levels of runoff-related pesticides concentrations in small lowland streams on a landscape level. Chemosphere 2005, 58, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Mischke, C.; Avery, J.; Toxicities of Agricultural Pesticides to Selected Aquatic Organisms. Southern Regional Aquaculture Center Publication No. 4600. 2013. Available online: https://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.-4600-Toxicities-of-Agricultural-Pesticides-to-Selected-Aquatic-Organisms1.pdf (accessed on 3 January 2024).
- Morrison, S.A.; McMurry, S.T.; Smith, L.M.; Belden, J.B. Acute toxicity of pyraclostrobin and trifloxystrobin to Hyalella azteca. Environ. Toxicol. Chem. 2013, 32, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Bringolf, R.B.; Cope, W.G.; Eads, C.B.; Lazaro, P.R.; Barnhart, M.C.; Shea, D. Acute and chronic toxicity of technical-grade pesticides to glochidia and juveniles of freshwater mussels (Unionidae). Environ. Toxicol. Chem. 2007, 26, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, M.; Yang, Y.; Shi, H.; Zhou, J.; He, D. Lethality and teratogenicity of strobilurins on Xenopus tropicalis embryos: Basing on ten agricultural fungicides. Environ. Pollut. 2016, 208, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Dijksterhuis, J.; Van Doorn, T.; Samson, R.; Postma, J. Effects of seven fungicides on non-target aquatic fungi. Water Air Soil Pollut. 2011, 222, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Acuña, H.G.; Bialkowski, W.; Yale, G.; Hahn, L. Toxicity of soybean rust fungicides to freshwater algae and Daphnia magna. Ecotoxicology 2009, 18, 440–446. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Minnesota—State Climate Summaries 2022. 2022. Available online: https://statesummaries.ncics.org/chapter/mn/ (accessed on 3 January 2024).
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Hunt, L. South Branch Whitewater River Unified Fish Kill Response 2015; Minnesota Department of Agriculture: Saint Paul, MN, USA, 2015; Available online: https://files.dnr.state.mn.us/areas/fisheries/lanesboro/unified-fish-kill-response12-22-15.pdf (accessed on 3 January 2024).
- Williams, M.A.; Vondracek, B. Spring distributions and relationships with land cover and hydrogeologic strata in a karst landscape in Winona County, Minnesota, USA. Carbonates Evaporites 2010, 25, 333–347. [Google Scholar] [CrossRef]
- Wickert, A.D.; Anderson, R.S.; Mitrovica, J.X.; Carson, E.C. The Mississippi River records glacial-isostatic deformation of North America. Sci. Adv. 2019, 5, eaav2366. [Google Scholar] [CrossRef]
- Whitewater River Watershed Project. A History of the Whitewater Watershed in Minnesota. Whitewater River Watershed Project 2015. Available online: http://www.whitewaterwatershed.org/wp-content/uploads/2017/01/Whitewater-Watershed-Conservation-History_Minnesota.pdf (accessed on 3 January 2024).
- Minnesota Legislature. 2023 Minnesota Statutes: Chapter 18B. Pesticide Control; Office of the Revisor of Statutes, Minnesota Legislature: Saint Paul, MN, USA, 2023. Available online: https://www.revisor.mn.gov/statutes/cite/18B (accessed on 3 January 2024).
- United States Environmental Protection Agency. EPA-NERL: 350.3: Ammonia by Potentiometry; US EPA National Exposure Research Laboratory: Cincinnati, OH, USA, 1974. Available online: https://www.nemi.gov/methods_summary/4871/ (accessed on 3 January 2024).
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- O’Connor, M. Photolysis and Detection of Contaminants of Emerging Concern in Minnesota Waterways. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2020. Available online: https://hdl.handle.net/11299/216125 (accessed on 3 January 2024).
- Smalling, K.L.; Hladik, M.L.; Sanders, C.J.; Kuivila, K.M. Leaching and sorption of neonicotinoid insecticides and fungicides from seed coatings. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 176–183. [Google Scholar] [CrossRef]
- Dux, K.; (Agronomy Sales, Ag-Partners, Lewiston, MN, USA). Personal communication, September 2019.
- Theede, C.; (Crop Consultant, Nutrien Ag Solutions, Rochester, MN, USA). Personal communication, September 2019.
- Klavetter, D.; (Agronomy Sales, All-American Cooperative, Plainview, MN, USA). Personal communication, September 2019.
- Welti, A.; (Agriculture Department Manager, Lakeside Foods, Plainview, MN, USA). Personal communication, September 2019.
- Minnesota Department of Agriculture. Minnesota Pesticide Sales Database. 2023. Available online: https://www.mda.state.mn.us/minnesota-pesticide-sales-information (accessed on 30 November 2023).
- Lofthus, D. Minnesota Ag News—Crop Production; United States Department of Agriculture, National Agricultural Statistics Service, Minnesota Field Office: St. Paul, MN, USA, 2023. Available online: https://www.nass.usda.gov (accessed on 7 December 2023).
- United States Geological Survey NAWQA. The Pesticide National Synthesis Project. 2018. Available online: https://www.usgs.gov/mission-areas/water-resources/science/national-water-quality-assessment-nawqa (accessed on 7 December 2023).
- ChemSafetyPro. Mobility Classification of Chemicals in Soil. 2019. Available online: https://chemsafetypro.com/Topics/CRA/Mobility_Classification_of_Chemicals_in_Soil.html (accessed on 30 November 2023).
- United States Environmental Protection Agency. Pesticide Fact Sheet: Trifloxystrobin; United States Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Health Canada Pest Management Regulatory Agency. Regulatory Note: Pyraclostrobin, Headline EC, Cabri EG. 2003. Available online: https://publications.gc.ca/collections/Collection/H113-7-2003-6E.pdf (accessed on 3 January 2024).
- Menrath, S. Registration of Evito SC Fungicide and Disarm 480 SC Fungicide Containing the New Active Ingredient Fluoxastrobin (Chemical Code 028869), 2014.
- Menrath, S. Registration of DuPont Approach Fungicide (EPA Reg. No. 352-840) Which Contains the New Active Ingredient Picoxystrobin (Chemical Code 129200), 2014.
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Wu, P.; Wu, W.Z.; Han, Z.H.; Yang, H. Desorption and mobilization of three strobilurin fungicides in three types of soil. Environ. Monit. Assess. 2016, 188, 363. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Hu, T.; Chen, X.; Ding, X. Adsorption and leaching of novel fungicide pyraoxystrobin on soils by 14C tracing method. Environ. Monit. Assess. 2018, 190, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, T.; Xu, Y.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Zhu, L. Ecotoxicology of strobilurin fungicides. Sci. Total Environ. 2020, 742, 140611. [Google Scholar] [CrossRef] [PubMed]
- National Water Quality Monitoring Council. Water Quality Portal. 2023. Available online: https://www.waterqualitydata.us (accessed on 4 December 2023).
- Liu, J.; Xia, W.; Wan, Y.; Xu, S. Azole and strobilurin fungicides in source, treated, and tap water from Wuhan, central China: Assessment of human exposure potential. Sci. Total Environ. 2021, 801, 149733. [Google Scholar] [CrossRef] [PubMed]
- Warming, T.P.; Mulderij, G.; Christoffersen, K.S. Clonal variation in physical responses of Daphnia magna to the strobilurin fungicide azoxystrobin. Environ. Toxicol. Chem. 2009, 28, 374–380. [Google Scholar] [CrossRef]
- Andresen, J.; Hilberg, S.; Kunkel, K. Historical climate and climate trends in the midwestern USA. In U.S. Global Change Research Program, National Climate Assessment, Midwest Technical Input Report; Winkler, J., Andresen, J., Hatfield, J., Bidwell, D., Brown, D., Eds.; Great Lake Integrated Sciences and Assessments Center: Ann Arbor, MI, USA, 2012. [Google Scholar]
- Mallakpour, I.; Villarini, G. The changing nature of flooding across the central United States. Nat. Clim. Chang. 2015, 5, 250–254. [Google Scholar] [CrossRef]
- Saint Ores, J.; Alexander, E.C., Jr.; Halsey, C.F. Groundwater Pollution Prevention in Southeast Minnesota’s Karst Region; University of Minnesota Agricultural Extension Service Bulletin 465; University of Minnesota: Saint Paul, MN, USA, 1982. [Google Scholar]
- Goyal, S.M.; Amundson, D.; Robinson, R.A.; Gerba, C.P. Viruses and drug resistant bacteria in groundwater of southeastern Minnesota. J. Minn. Acad. Sci. 1989, 55, 58–62. [Google Scholar]
- Legg, T.D.; Fletcher, J.J.; Easter, K.W. Nitrogen budgets and economic efficiency: A case study of southeastern Minnesota. J. Prod. Agric. 1989, 2, 110–116. [Google Scholar] [CrossRef]
- Troelstrup, N.H., Jr.; Perry, J.A. Water quality in southeastern Minnesota streams: Observations along a gradient of land use and geology. J. Minn. Acad. Sci. 1989, 55, 6–13. [Google Scholar]
- Hallberg, G.R. Agricultural chemicals in ground water: Extent and implications. Am. J. Altern. Agric. 1987, 2, 3–15. [Google Scholar] [CrossRef]
- Keeler, B.L.; Polasky, S. Land-use change and costs to rural households: A case study in groundwater nitrate contamination. Environ. Res. Lett. 2014, 9, 074002. [Google Scholar] [CrossRef]
- Waters, T.F. The Streams and Rivers of Minnesota; University of Minnesota Press: Minneapolis, MN, USA, 1977. [Google Scholar]
- Husk, B.; Sanchez, J.S.; Leduc, R.; Takser, L.; Savary, O.; Cabana, H. Pharmaceuticals and pesticides in rural community drinking waters of Quebec, Canada—A regional study on the susceptibility to source contamination. Water Qual. Res. J. 2019, 54, 88–103. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Mundahl, N.D.; Varela, W.L.; Weaver, C.; Mundahl, E.D.; Cochran-Biederman, J.L. Stream habitats and aquatic communities in an agricultural watershed: Changes related to a mandatory riparian buffer law. Environ. Manag. 2023, 72, 945–958. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Baattrup-Pedersen, A.; Wiberg-Larsen, P.; McKnight, U.S.; Kronvang, B. Buffer strip width and agricultural pesticide contamination in Danish lowland streams: Implications for stream and riparian management. Ecol. Eng. 2011, 37, 1990–1997. [Google Scholar] [CrossRef]
- Vymazal, J.; Brezinová, T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ. Int. 2015, 75, 11–20. [Google Scholar] [CrossRef]
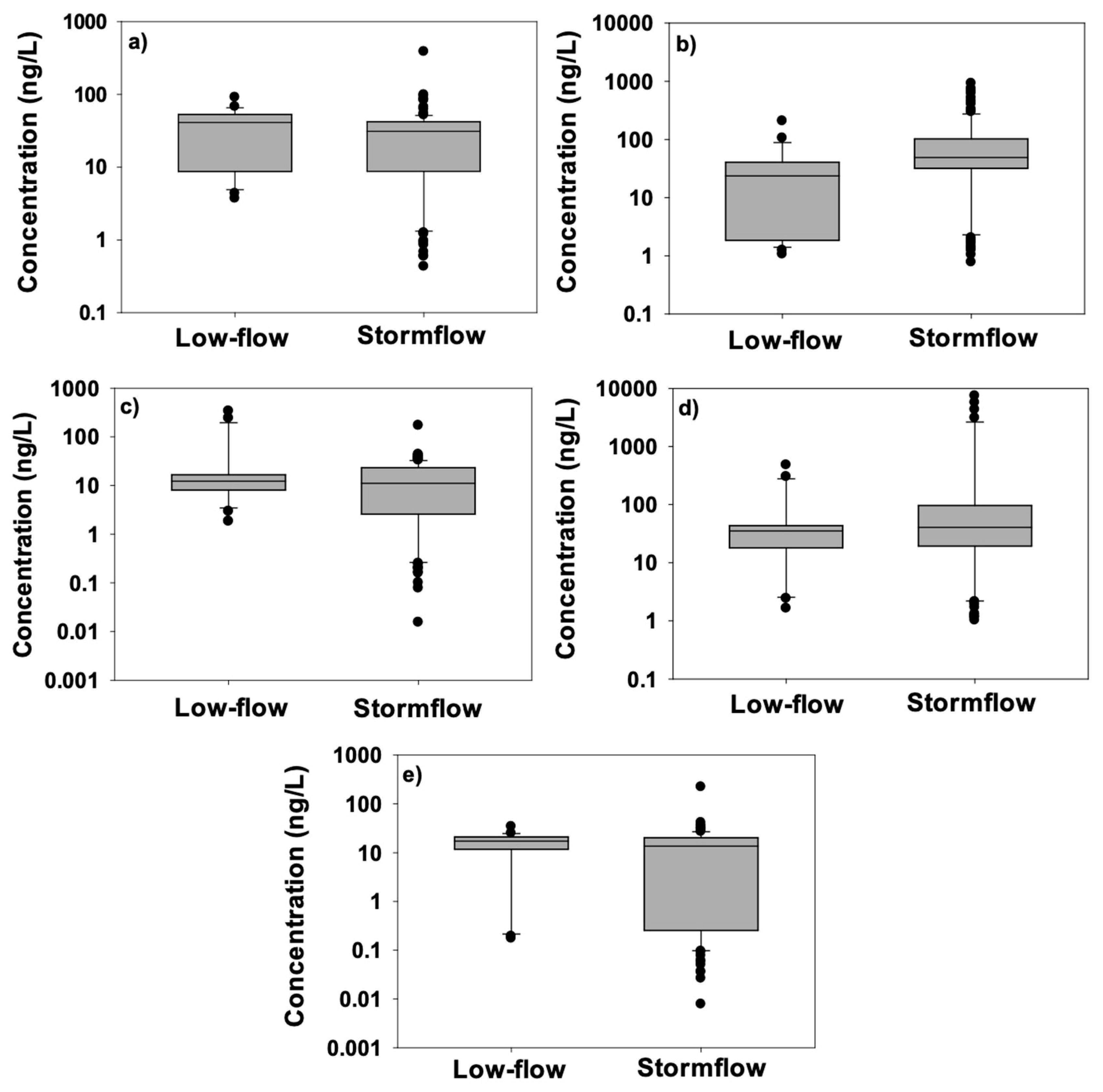
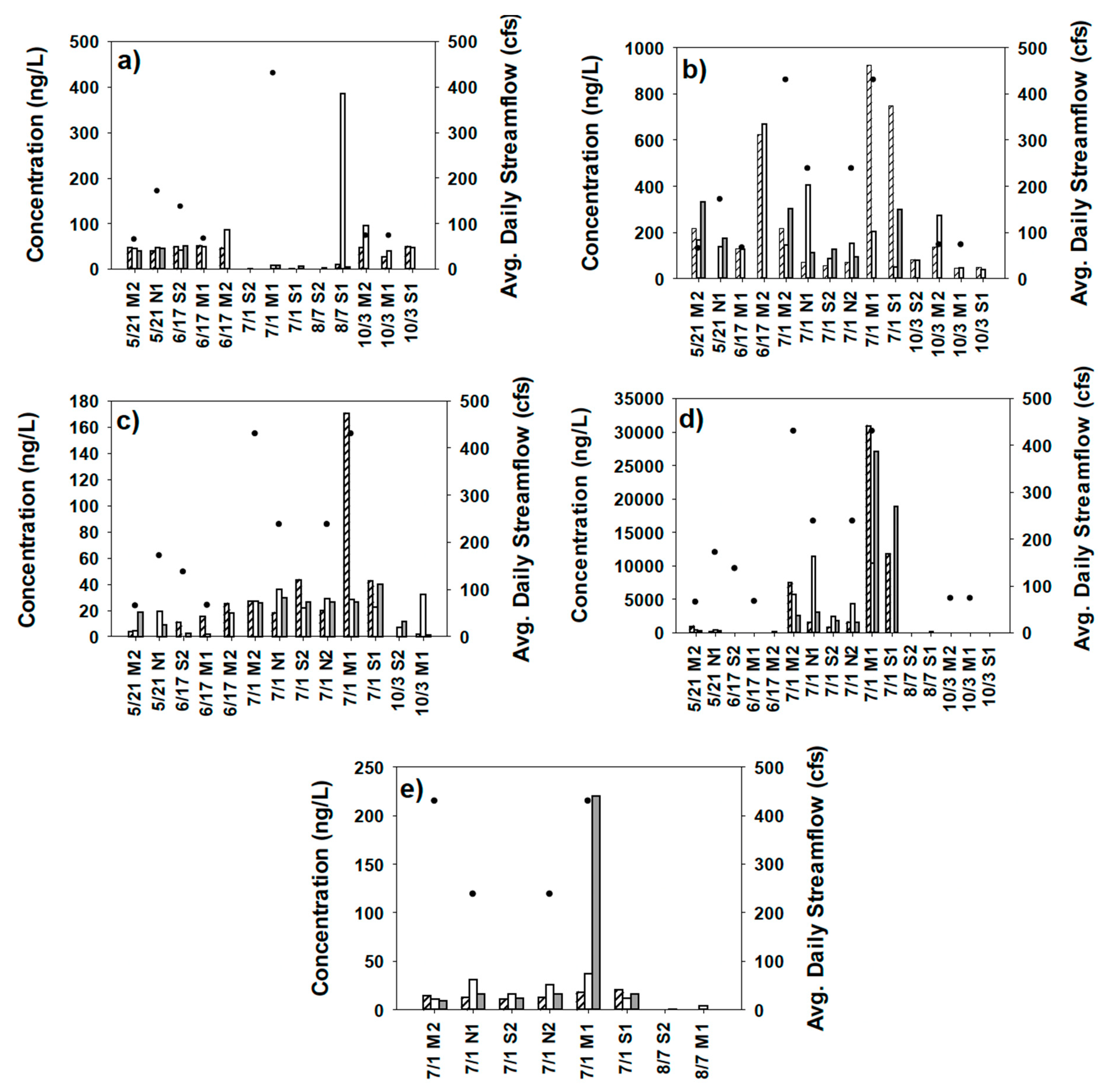
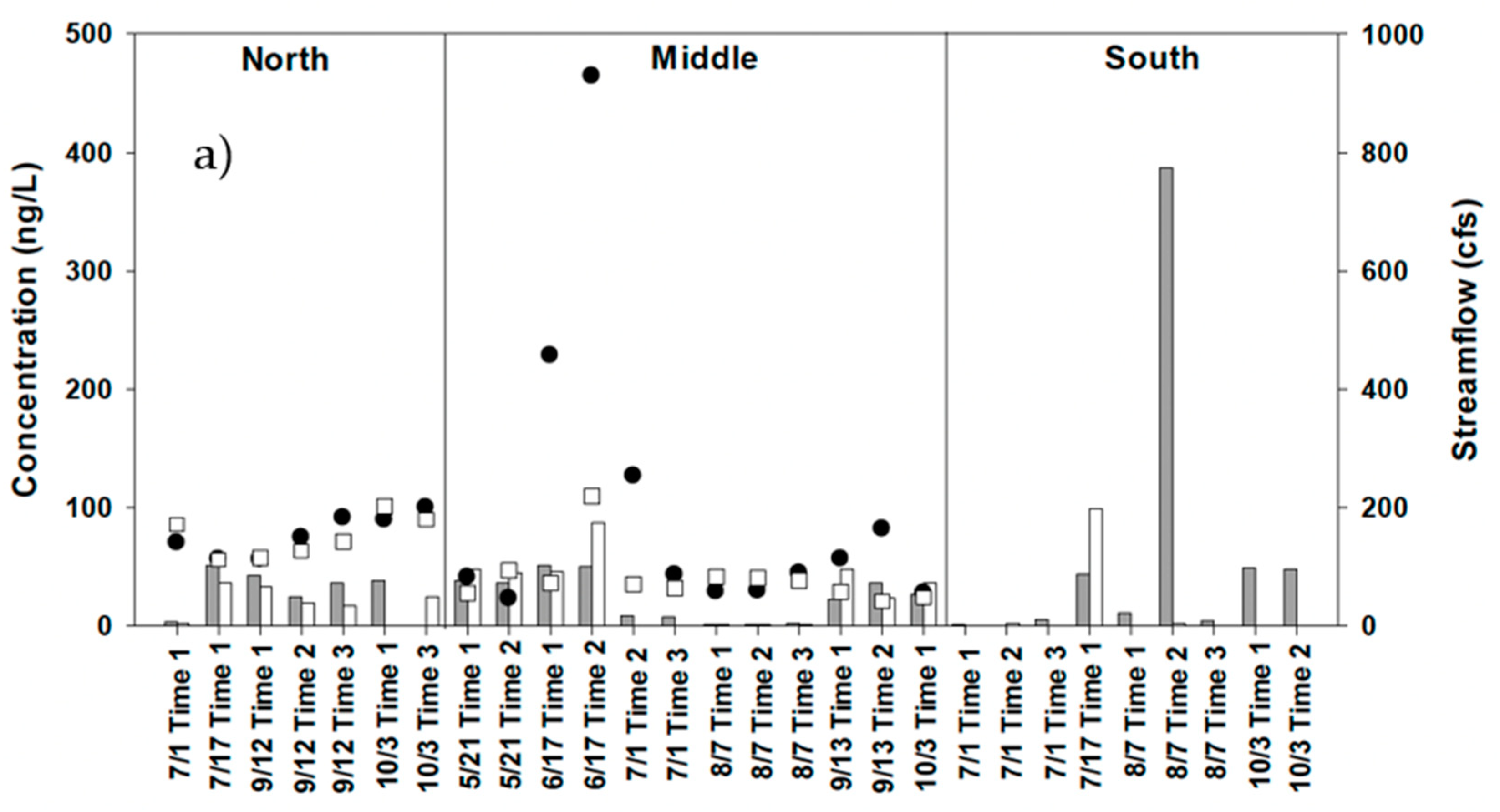
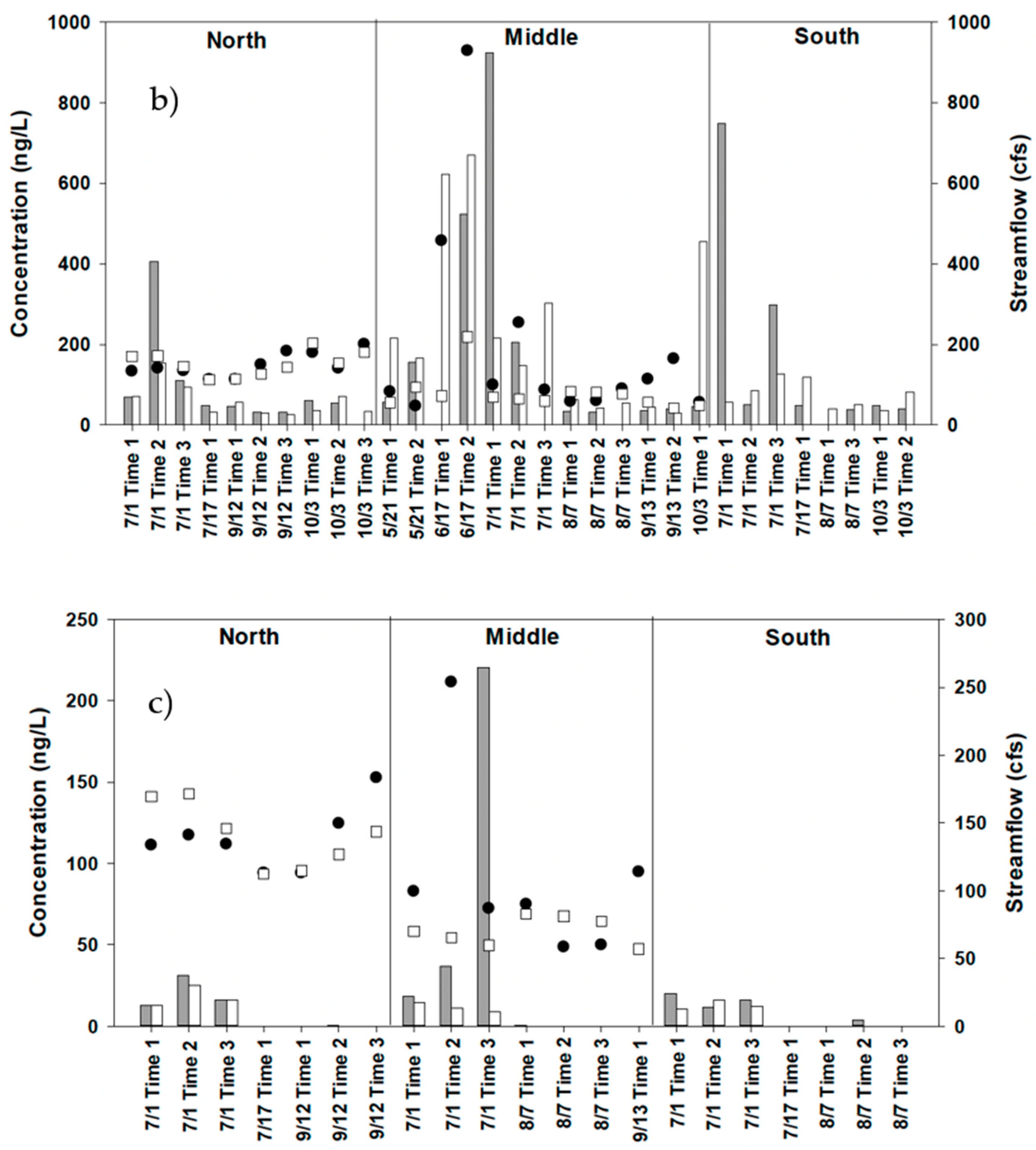
| Baseflow | Stormflow | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Med | Max | n | Mean | Med | Max | n | p Value |
| Ammonia (mg/L–N) | 0.51 | 0.78 * | 7.65 | 31 | 1.14 | 0 | 9.05 | 76 | <0.001 |
| Nitrate (mg/L–N) | 5.87 | 5.66 | 14.3 | 64 | 5.90 | 5.24 | 18.06 | 108 | 0.484 |
| Total Phosphorus (mg/L–P) | 0.51 | 0.56 | 2.03 | 58 | 0.74 | 0.49 | 7.81 | 107 | 0.757 |
| TSS (mg/L) | 19 | 160 | 1000 | 41 | 1781 | 600 * | 18,080 | 107 | 0.044 |
| E. coli (CFU/100 mL) | 9 | 4 * | 78 | 68 | 8 | 0 | 90 | 89 | <0.001 |
| Detections | ||||
|---|---|---|---|---|
| Strobilurin | (Overall, Baseflow, Stormflow) | Percent Detections (Overall, Baseflow, Stormflow) | Median Concentration (ng/L) | Maximum Concentration (ng/L) |
| Azoxystrobin | 106, 18, 88 | 82, 82, 82 | 30 (33) | 386 |
| Fluoxastrobin | 103, 15, 88 | 79, 68, 80 | 45 | 924 |
| Picoxystrobin | 82, 15, 67 | 63, 68, 61 | 1.2 (11) | 338 |
| Pyraclostrobin | 107, 20, 87 | 82, 90, 80 | 37 | 3.1 × 104 |
| Trifloxystrobin | 58, 9, 49 | 44, 41, 45 | 0 (15) | 221 |
| Strobilurin | df | t Value | p Value |
|---|---|---|---|
| Azoxystrobin | 49 | ||
| Without BMRL | 0.88 | 0.08 | |
| With BMRL | 0.68 | 0.50 | |
| Fluoxastrobin | 120 | ||
| Without BMRL | 3.94 | <0.001 | |
| With BMRL | 3.92 | <0.001 | |
| Picoxystrobin | 21 | ||
| Without BMRL | 1.33 | 0.20 | |
| With BMRL | 1.40 | 0.18 | |
| Pyraclostrobin | 120 | ||
| Without BMRL | 2.89 | <0.005 | |
| With BMRL | 2.89 | <0.005 | |
| Trifloxystrobin | |||
| Without BMRL | 120 | 1.79 | 0.08 |
| With BMRL | 80 | 0.53 | 0.60 |
| p Values | ||
|---|---|---|
| Strobilurin | Without BMRL | With BMRL |
| Azoxystrobin | 0.40 | 0.68 |
| Fluoxastrobin | 0.69 | 0.87 |
| Picoxystrobin | 0.93 | 1.00 |
| Pyraclostrobin | 0.75 | |
| Trifloxystrobin | 0.94 | |
| p Values | |||
|---|---|---|---|
| Strobilurin | North Fork | Middle Fork | South Fork |
| Azoxystrobin | |||
| Without BMRL | <0.05 | >0.50 | >0.05 |
| With BMRL | <0.05 | >0.50 | >0.10 |
| Fluoxastrobin | |||
| Without BMRL | <0.001 | >0.05 | >0.20 |
| With BMRL | <0.001 | >0.05 | >0.20 |
| Picoxystrobin | |||
| Without BMRL | 0.20 | >0.50 | >0.50 |
| With BMRL | 0.50 | >0.50 | >0.20 |
| Pyraclostrobin | |||
| Without BMRL | >0.20 | >0.50 | 0.50 |
| With BMRL | >0.20 | >0.50 | 0.50 |
| Trifloxystrobin | |||
| Without BMRL | >0.50 | >0.20 | >0.10 |
| With BMRL | >0.50 | 0.02 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver, C.R.; Brockman, M.; Mundahl, N.D.; Arnold, W.A.; Blumentritt, D.; Varela, W.L.; Franz, J.L. Detection of Strobilurin Fungicides in Trout Streams within an Agricultural Watershed. Hydrology 2024, 11, 13. https://doi.org/10.3390/hydrology11020013
Weaver CR, Brockman M, Mundahl ND, Arnold WA, Blumentritt D, Varela WL, Franz JL. Detection of Strobilurin Fungicides in Trout Streams within an Agricultural Watershed. Hydrology. 2024; 11(2):13. https://doi.org/10.3390/hydrology11020013
Chicago/Turabian StyleWeaver, Cole R., Meghan Brockman, Neal D. Mundahl, William A. Arnold, Dylan Blumentritt, Will L. Varela, and Jeanne L. Franz. 2024. "Detection of Strobilurin Fungicides in Trout Streams within an Agricultural Watershed" Hydrology 11, no. 2: 13. https://doi.org/10.3390/hydrology11020013
APA StyleWeaver, C. R., Brockman, M., Mundahl, N. D., Arnold, W. A., Blumentritt, D., Varela, W. L., & Franz, J. L. (2024). Detection of Strobilurin Fungicides in Trout Streams within an Agricultural Watershed. Hydrology, 11(2), 13. https://doi.org/10.3390/hydrology11020013






