1. Introduction
The scientific and technological development has led to an improvement in human living and working conditions, but it has also brought a series of problems and new challenges, such as waste generation and environmental damage. Aluminum is nowadays a very widely used metal; it can be obtained from the mineral bauxite (Primary Aluminum Production, PAP) by means of purification of bauxite and further electrolytic reduction (Bayer and Hall–Héroult Processes), an expensive and polluting procedure [
1,
2,
3]. Fortunately, aluminum can be recycled indefinitely (Secondary Aluminum Production, SAP), and this recycling is well established in several countries. Unfortunately, this process also generates some waste, which is strongly polluting but contains an important amount of valuable aluminum in the form of metal and several compounds. These wastes must be correctly managed to avoid further contamination and retrieve valuable components [
1,
2,
3]. A general overview of aluminum recycling is included in the
Supplementary Material, which can be provided as introductory information to the students this proposal is aimed at.
Chemistry of aluminum has been addressed in several laboratory demonstrations, both in experiments reported in classical laboratory textbooks and, recently, in short video demonstrations published on social networks. However, these demonstrations usually cover only a part of aluminum chemistry. Classical experiments based on this element are focused on the preparation of anhydrous salts, such as chloride and bromide, some complexes, such as Al(acac)
3 or K
3[Al(oxalate)
3], or alums (double sulfates with potassium or ammonium), or on the use of metallic aluminum for preparing other metals by means of aluminothermic reactions [
4,
5]. Some of these demonstrations are based on redox properties of aluminum and others in their acid–base behavior, sometimes considering amphoterism [
6], but they do not systematically address all the properties of the element but are mainly focused on the preparation of the final solid product. In some cases, commercial aluminum compounds are used as reagents, although other aluminum sources have also been considered, such as metallic aluminum (scrap, cans, foil kitchen paper, etc.) or clays, including the well-known preparation method based on kaolin or other clay minerals [
7,
8,
9,
10,
11]. In some cases, once Al
3+ is extracted, it is used for the preparation of other solids, such as zeolites [
9]. As these experiments are visual, redox reactions, such as the dissolution of the metal in acidic or alkaline solutions or its reaction with a Cu(II) solution, are usually considered in social network demonstrations [
12,
13,
14].
As indicated, these experiments usually report only on a certain part of aluminum behavior, acid–base or redox, and they only scarcely emphasize other properties as solubility. When natural products are used as source of aluminum, very strong and even dangerous conditions are employed; for example, kaolin, which is very inert, is submitted to acid digestion, using concentrated sulfuric acid and a sand bath at high temperature, or to leaching in very strong acid or alkaline aqueous solutions [
7,
8,
9,
10]. In addition, when aluminum is extracted from a natural source, the insoluble residues (mixtures of undissolved clay mineral, feldspars, quartz, etc.) are usually ignored.
The present paper presents an integrated laboratory work studying all aspects of aluminum chemistry, mainly acid–base and redox behavior, but also considering the speciation Pourbaix diagram of this element, and reviewing/incorporating some other important theoretical concepts. On the other hand, the source of aluminum is an industrial waste, which allows us to know the industrial production of this metal and to emphasize the necessity of valorizing its industrial residues, not only by recovering their valuable components, but also by analyzing the composition of the inert final residue (after recovering) and looking for new applications; all this is absolutely mandatory nowadays under the auspices of the Circular Economy and the Sustainable Development Goals.
The “learning pyramid” theory establishes that learning is much more effective if the students practice by doing than if they receive a lecture, they read some documents, or even if they see demonstrations made by others. Also the discussions between students occupy a high position in this pyramid. Thus, the learning advantages of laboratory practice as a pedagogical resource are evident [
15,
16,
17,
18,
19,
20]. Thus, this work proposes an experimental laboratory practice based on a procedure for the management, handling and valorization of aluminum saline slags generated during SAP based on a hydrometallurgical extraction process with acid and basic routes. This laboratory practice could be carried out by students in the third and/or fourth year of Chemistry, Chemical Engineering, Environmental Engineering, Materials Engineering, or related University Degrees. By carrying out this laboratory practice, the students consolidate the contents explained during the theory lectures related to general concepts of Metallurgy, Chemical Engineering, Analytical, Inorganic and Physical Chemistry, as industrial process of aluminum production and chemical behavior of
p- and
d-block metallic elements, solubility, pH, acid–base properties, redox reactions or speciation Pourbaix diagrams of the elements, promoting teamwork in the discussion at the laboratory about the experimental results obtained and elaborating at the end of the practice a comprehensive and rigorous laboratory report. Moreover, this laboratory practice not only allows the students to consolidate these concepts, but also to be introduced to the Chemistry of Materials and come into contact with characterization techniques such as PXRD, as well as to begin to familiarize themselves with research tasks [
9,
20,
21,
22]. Likewise, it also allows the students to learn and reinforce important concepts such as the Circular Economy and the Sustainable Development Goals set by the 2030 Agenda, which aims to decrease the pressure on the environment derived from industrial and social development [
23,
24,
25,
26].
2. Materials and Methods
- A.
Raw aluminum saline slag
The saline slag used in this practice can be kindly provided by companies in the sector (in our case, IDALSA, Pradilla de Ebro, Zaragoza, Spain, a Spanish company working in recycling of aluminum (
https://idalsa.com/ accessed on 5 February 2025). Recycling of aluminum is very well established worldwide, and the companies involved in this process usually provide the slags free of charge. The chemical and phase compositions should be very similar for slags from different companies, since for recycling to be economically profitable, the waste used as raw material must have a high Al content.
- B.
Material and equipment
We used a laboratory mill, but if this equipment is not easily available, its use should be omitted or substituted by mortar and an X–ray diffractometer. In our case, the chemical analyses of the slag and of the washing liquors were carried out by XRF and ICP–MS at the technical research platform of our University. Normal laboratory material is required, including a heating magnetic stirrer, for extraction under reflux conditions.
- C.
Grinding of the raw saline slag
Weigh 400 g of the raw slag and put it together with the alumina balls into the alumina jar. Close the jar and place it in the mill. Grind for 20 min. Open the jar and sieve the solid using a 1 mm light sieve. Return the solid particles larger than 1 mm back into the jar (add more raw slag if necessary, but do not exceed 400 g). Repeat the process until you have enough particles smaller than 1 mm. This process can be completed before by the teacher, who will then provide the required fraction to the students; however it is very useful for illustrating the difference in reactivity depending on the particle size.
- D.
Washing with water
Weigh 20 g of the fraction smaller than 1 mm and place it in a beaker. Add distilled water maintaining the ratio of 1 g of solid/20 mL of distilled water. Shake and heat to boiling point. Allow complete decantation. Remove most of the water and repeat the process until the wash water gives negative in the chloride test with AgNO3 10−2 M. Finally filter under vacuum and allow drying. Sieve through a 0.4 mm light sieve. This process removes the soluble NaCl and KCl (eventually also other soluble salts used as flux salts in the recycling of aluminum), but it may also produce partial hydrolysis of certain aluminum phases, so the process may be performed in a laboratory hood.
- E.
Extraction process
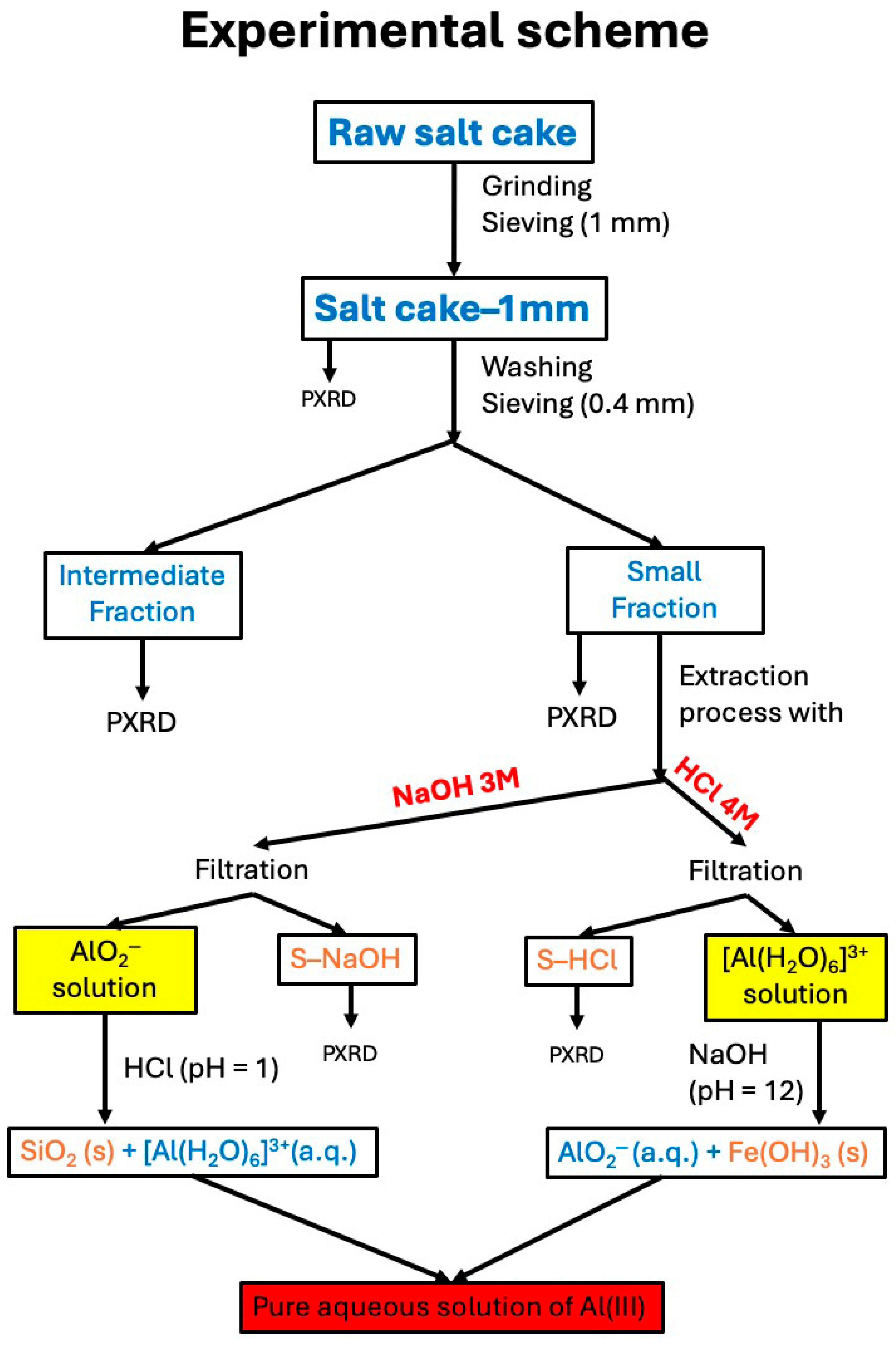
Set up the laboratory equipment as indicated in
Figure 1. It must be set up in a fume hood cabinet. Prepare 25 mL of 3 M NaOH and 25 mL of 4 M HCl solutions. Weigh in two round flasks 7.5 g of the small fraction, add 25 mL of acidic or base solution, place the refrigerant and reflux for 2 h. After this time filter the residue under vacuum, wash it and let it dry. For simplicity of the calculations, make up the extraction liquor to a final volume of 50 or 100 mL. Although HCl and NaOH are the cheapest, easily handled and most used strong acid and base, they can be substituted by other acids or bases, mainly if the extracted Al
3+ solution should be considered for the synthesis of other compounds not considered in this report. Thus, the use of HNO
3 as acid and KOH and CsOH as bases has been reported [
27]; the solution derived from CsOH being used for preparing zeolites of the analcime–pollucite family [
28]. If other extraction agents are used, specific safety precautions may be adopted (see
Supplementary File).
- F.
Elemental chemical analysis, powder X-ray diffraction (PXRD) and X-ray microfluorescence (XRF)
The different fractions and the extraction of solid residues will be analyzed by PRXD and XRF to determine their mineralogical and chemical composition, respectively. The extraction liquors will be analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP–MS) to determine the content of aluminum and other extracted metals such as Fe, Cu, Ti, etc. In our case, both ICP–MS and XRF were performed by NUCLEUS, the research platform of the University of Salamanca (Agilent 7800 (Agilent Technologies Spain, Las Rozas, Madrid, Spain) and Bruker M4 (Bruker Española S.A., Madrid, Spain) models, respectively). In both cases, the technicians of the platform and the own teachers can explain in situ to the students the experimental details of the techniques. In the case of PXRD, available in our own laboratories, the analyses will be performed by the students, with the assistance of the teachers (we used a Siemens D–5000 instrument (Siemens España, Madrid, Spain) using Cu–Kα radiation, λ = 0.154 nm, with fixed divergence, from 5° to 80° (2θ) at a scanning rate of 2° (2θ)/min with steps of 0.05° and time per step of 1.5 s). The assignment of crystalline phases was carried out with the help of the X’Pert HighScore software from Malvern Panalytical (Malvern Panalytical B.V. Madrid, Spain) and using the International Centre for Diffraction Data database (JCPDS–ICDD®, Newtown Square, PA, USA). If these techniques are not available, simpler techniques could be used for elemental analyses, for example Flame Atomic Absorption Spectroscopy (FAAS). In this case, a small amount of insoluble phases in the slag should not be digested, but the technique should be used for determining the amount of aluminum extracted. Although the precise composition of a given slag depends on its origin, for this laboratory practice a standard composition should be taken as example; the data given in this work should be such a standard.
2.1. Time Sequence and Resources Needed
This laboratory experience is designed for a group of 12 to 16 students, organized in duos or trios, and allows the application of knowledge and skills from previous courses in General Chemistry, Analytical and Inorganic Chemistry, Chemical Engineering, while also promoting teamwork. The duration of this laboratory experience is 4 sessions of 4 h each. All sessions take place in the laboratory (a chart summarizing all the steps of the laboratory work is shown in
Scheme 1).
First session. The first session is divided into two parts: The first part consists of a brief literature search on saline slags and the solubility of some elements, in particular aluminum, as a function of pH (additional information is given in the
Supplementary Material, that should be used by the students as a starting point for a bibliographic search on this subject). The second part of the first session is devoted to carrying out the process of grinding and sieving the raw slag until the particle size is decreased to 1 mm or less. At this point, each group of students would have the so-called fraction smaller than 1 mm, which they will work with during the second session.
Second session. This session starts with washing this fraction with hot distilled water to remove the fluxing salts, until the washing water test for chloride is negative (using AgNO3 10−2 M). To conclude this session, the students will perform a sieving process using a 0.4 mm light sieve, thus obtaining the intermediate fraction (particle size between 1 and 0.4 mm) and the small fraction (particle size below 0.4 mm). Each group should reserve a small amount (~1 g) from each fraction to perform PXRD in the fourth session.
Third session. The extraction of aluminum is carried out in this session. The small fraction is treated with NaOH and HCl under reflux conditions for 2 h. Finally, a filtration step is performed, and the extraction residue is left to dry. The students are asked to carefully observe the extraction process, mainly the liberation of hydrogen. It is underlined that, contrary to most metals, the oxidation of metallic aluminum occurs both in acidic and in alkaline solutions. Then, the acid base properties of the extraction liquor are investigated. If it has been obtained under acidic conditions, pH is increased using NaOH until the precipitation of the hydroxide and further dissolution as aluminate (iron hydroxide remaining precipitate) are observed. If the extraction has been performed under alkaline conditions, the pH is decreased with HC, causing the precipitation of the hydroxide, followed by its dissolution as hexaaquacation; at the same time silica precipitates (see details below). The students are encouraged to notice all the physical changes observed, and to discuss these changes among them.
Fourth session. The students will record the PXRD of the raw slag and the different fractions, and also of the final residue (after extraction), identifying the main crystalline phases of each solid. The students will also receive the results of chemical analysis ICP–MS and X–ray microfluorescence, carried out in external laboratories. They should discuss all these results among them. Finally, they will elaborate and discuss the results in a laboratory report, which reflects all the steps followed in the process and the results obtained. The training in the elaboration of laboratory reports is very important nowadays, as a significant part of the students have serious difficulties with reading comprehension and writing correctly in scientific style [
29,
30]. Thus, the teachers should encourage the elaboration of a comprehensive laboratory report, written in a rigorous scientific style.
These sessions should be restructured according to the infrastructure and requirements of each laboratory. If the teachers do not consider it important to illustrate the obtaining of different particle size fractions by grinding, only a previously grinded sample should be given to the students. If an X–ray diffractometer is not available at the laboratory, the diffractograms of the solids should be registered before and given to the students.
The resources needed for this experiment are the following: laboratory hood, magnetic stirrer, laboratory mill (if not available, the aluminum slag may be grinded by the students using an agate mortar), 1 mm and 0.4 mm laboratory mill sieves (if not available, they can be substituted by kitchen strainers of approximately the same size), 3 M NaOH and 4 M HCl solutions, and AgNO3 10−2 M solution (only for the analytical evaluation of the presence of chloride anions). As indicated before, the companies involved in aluminum recycling are usually happy to provide samples of the slag free of charge. Thus, all necessary reagents are routinely used in teaching University laboratories. Usual precautions (laboratory glasses and gloves) should be taken for handling the solutions. Laboratory mask is recommended when handling the slag, mainly if it is grinded by the students.
2.2. Learning Objectives and Concepts
The experiment proposed in this laboratory practice can be carried out in any general chemistry laboratory due to its simplicity, since it uses common laboratory reagents and one of the most abundant wastes generated during SAP. The material required to carry out the practice is also basic laboratory material. The characterization techniques required are the basic ones used in the characterization of materials, such as chemical analysis and PXRD, available in several laboratories of University Departments or in the general research services of most universities.
This laboratory practice allows the student to put into practice the contents studied in previous courses on General, Analytical and Inorganic Chemistry, and Chemical Engineering, mainly the following:
Handling of basic laboratory material.
Preparation and handling of solutions.
Concepts of pH and solubility–precipitation, as well as the relationship between them.
Chemical properties of aluminum and other metals, with special emphasis on redox reactions, solubility and acid–base properties.
Knowledge and handling of common characterization techniques.
Waste management and valorization.
Awareness of correct management of chemical residues.
Discussion of the results (team working) and elaboration of a well-structured laboratory report. If time is available, different groups could present their results in short oral presentations.
As indicated, besides of the content related to Chemistry and Chemical Engineering, the practice illustrates concepts of Circular Economy, by means of valorization of industrial residues, and its relationship with the Sustainable Development Goals set by the 2030 Agenda, such as SDG 11 (Sustainable Cities and Communities), SDG 12 (Responsible Production and Consumption) and SDG 13 (Climate Action), besides of the SDG 4 (Quality Education), which condition all the practices. In this way, the students are made aware of the importance of reducing mankind’s pressure on the environment [
23,
26].
During the development of the laboratory work, teachers have the opportunity to review key concepts from previous courses related to the work being conducted, such as chemical formulation, reaction adjustment, stoichiometry, pH calculations, etc. Thus, general and specific competencies may be acquired, such as the following:
Understanding different types of chemical reactions and how to balance them.
Understanding concentration, solubility, and properties of solutions.
Performing experiments safely and responsibly.
Operating common chemical instruments.
Working effectively in teams and collaborating on scientific projects.
Reading and understanding scientific literature.
Recording, interpreting, and analyzing experimental data.
Integrating knowledge across different areas of chemistry.
Understanding and practicing ethical conduct in scientific research.
Communicating scientific information clearly and effectively in written reports and oral presentations.
Specific competencies, such as the skill in working with ICP, DRX and XRF, interpretation of diffractograms, handling of speciation diagrams, etc.
3. Results
Here, we summarize the main results derived from our work. Although the results should be slightly different depending on the origin and composition of the raw residue used, these results should help with the interpretation of the results and also give ideas to the educators on the different subjects to be considered at each step.
The fractions of different particle size were analyzed by XRF and PXRD; the results obtained are shown below [
27].
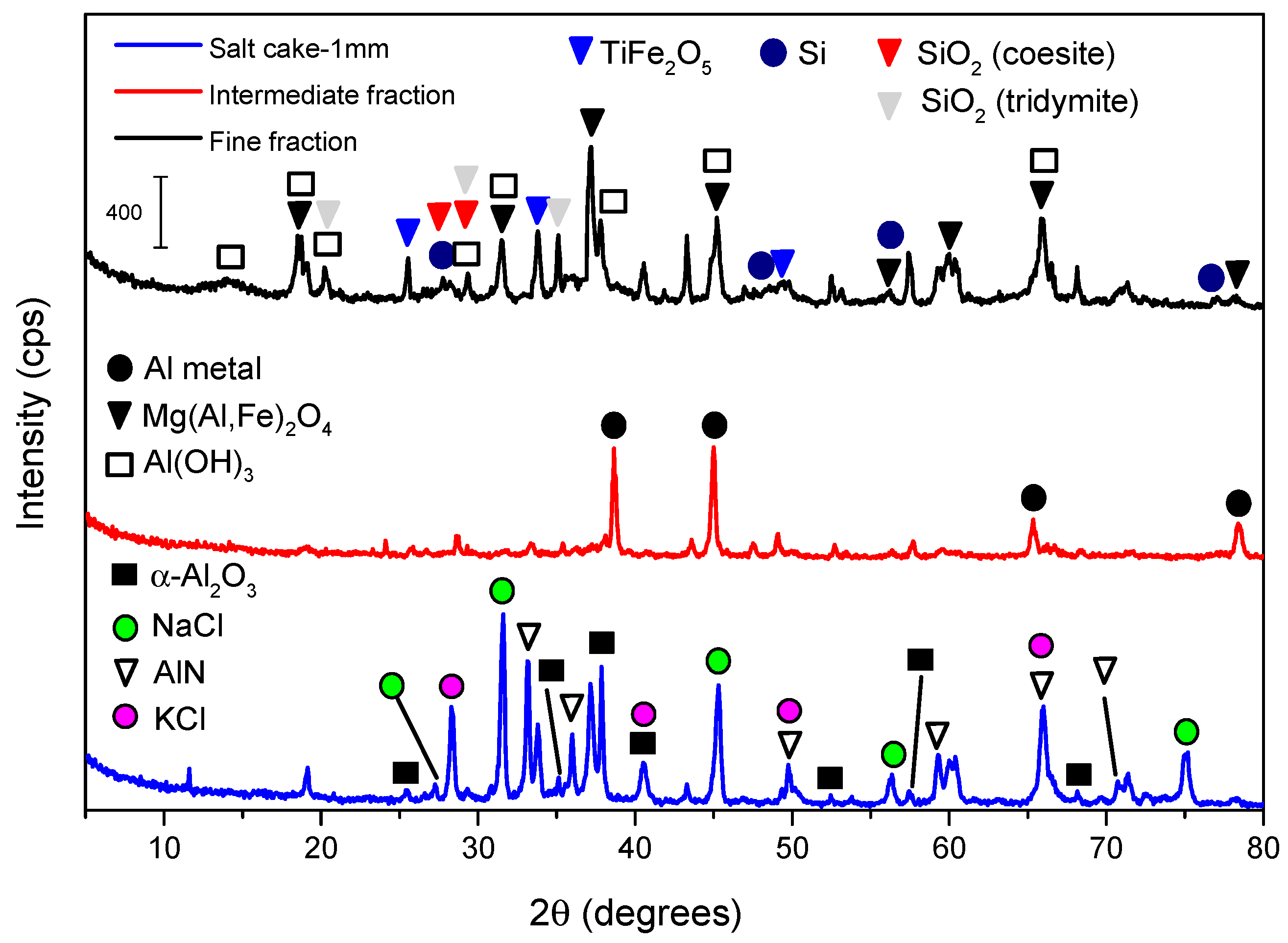
Table 1 shows the chemical composition of salt cake–1 mm. The high content of Al, Na, and K (expressed in the form of its oxide) stands out. The presence of aluminum is logical, as the residue used comes from the recycling of this element, and those of Na and K derives from the uses of their chlorides as flux salts (following the general procedures, the content of these elements are given in oxide from, although PXRD demonstrate their presence as chlorides, which was also confirmed by the high amount of Cl in the sample). On the other hand,
Figure 2 shows the mineralogical composition of salt cake–1 mm. As indicated, both sodium and potassium were mainly in the form of chlorides, while aluminum was present in various phases, mainly Al(OH)
3, α–Al
2O
3, AlN, MgAl
2O
4 and metallic Al. Concerning iron and silicon, two important elements which can be extracted together with aluminum, they were found in small amounts in the forms of TiFe
2O
5, metallic Si, and coesite and tridimyte phases of SiO
2 in acidic and alkaline medium, respectively. Students should conduct a short literature search on the properties and chemical characteristics of each of these phases, their solubility in water, acidic solutions, and alkaline solutions, employing the speciation diagrams of each element. The amphoteric properties of aluminum should be underlined here, emphasizing that they allows us to extract this element both by treatment with an acidic solution or an alkaline solution, considering the differences that the extraction medium should cause in the composition of the extraction liquor. Al(OH)
3, also formulated as Al
2O
3.3H
2O or Al
2O
3.nH
2O, precipitates from pH ≈ 5–11 (depending on the concentration), with K
s = 10
−33.5, which can also be taken into account for the comprehension of the solution behavior of this element.
After subjecting salt cake–1 mm to the washing process, a mass loss of about 33% was observed. This mass loss can be attributed to the removal of the fluxing salts, as shown in
Table 1 [
27]. The Na and K content were drastically decreased, which showed that the washing process was adequate to remove the fluxing salts from the residue. This was confirmed by PXRD (
Figure 2), where no diffraction peaks corresponding to NaCl and KCl were observed in the diffractogram of either the intermediate or small fraction. In addition, the AlN phase disappeared. This is one of the most dangerous phases present in the slag, since in the presence of water it hydrolyses, giving rise to Al(OH)
3, and evolving into highly toxic gaseous NH
3. This showed that the washing process was effective in eliminating toxic phases present in the waste. Regarding the mineralogical composition, significant differences can be observed between the intermediate fraction, in which diffraction peaks corresponding to metallic Al were mainly observed, and the fine fraction, where the phases Al(OH)
3, α–Al
2O
3 and MgAl
2O
4 were observed.
Several environmental concerns should be addressed here. First, a washing liquid containing almost exclusively NaCl and KCl was obtained (depending on the origin of the slag, it may contain fluoride, carbonate, etc.). The students should be asked about a possible use of this solution in order to avoid creating a new residue (i.e., crystallization and use as anti-icing during winter). The emission of NH
3 should be noted, as AlN is relatively abundant in the slags, especially considering the common disposal of this residue in legal dumps (and sometimes even on illegal ones), where its reaction with water may cause emission of this gas. Additionally, in exceptional cases, methane and very toxic gases such as H
2S or PH
3, may be emitted if the slag contains small amounts of compounds such as Al
4C
3, Al
2S
3, or AlP. The students should be asked about ways to recover ammonia when valorizing the slag, or at least to avoid its emission into the atmosphere, such as reaction with water to produce concentrated aqueous ammonia solutions, or reaction with acidic solutions for neutralization. If time and material allow, ammonia can be recovered and quantified by titration with boric acid or collected over a standardized HCl solution, which can be later titrated with standardized NaOH [
27], allowing the incorporation of acid–base titrations to the practice.
At this point, it should be underlined that several aluminum compounds react with water through an acid–base hydrolysis reaction, while metallic aluminum reacts through a redox reaction; in the first case, the oxidation state of aluminum does not change, whereas in the second, the metal is oxidized. Thus, the hydrolysis reaction for several compounds in water should be given, such as
In the case of the most abundant aluminum compound, Al
2O
3, also produced in the hydrolysis reactions of other compounds, it should be underlined that it is stable in water, but its amphoteric character allows it to dissolve under both acidic and alkaline conditions. Under acidic conditions, where Al
2O
3 acts as a base, it dissolves forming [Al(H
2O)
6]
3+, usually abbreviated as Al
3+ (although other species can be found as a function of pH), by means of the reaction
The dissolution of iron under acidic conditions should be noted here. Although underlying that this element is usually present in the saline slags forming double oxides as Mg(Al,Fe)
2O
4 spinel or TiFe
2O
5 pseudobrookite, Fe
2O
3 can be used as model compound to illustrate its solubility:
While in alkaline medium, where Al
2O
3 acts as an acid, it dissolves forming the aluminate anion [Al(OH)
4]
−, frequently abbreviated as AlO
2−:
Again, a comparison with iron can be performed here; the reaction does not occur:
The analysis of the acid–base behavior should be completed, on the basis of the speciation diagram, commenting on the existence of the species [Al(H2O)5(OH)]2+ (usually abbreviated [AlOH]2+) and [Al(H2O)4(OH)2]+ (usually abbreviated [Al(OH)2]+), and the respective regions of stability, and even that of the tridecameric Keggin–type polycation [Al13O4(OH)24(H2O)12]7+, underlying that Al3+ is one of the cations that polymerizes forming polycationic species.
In contrast, metallic aluminum reacts by means of a redox reaction, and for the reasons mentioned before, the reaction can be carried out both in acidic and in base medium:
The strong reducing properties of Al can be noted here, also remembering the difficulties in obtaining this element by reduction in its salts, the impossibility of achieving this in aqueous medium (ε°(Al3+/Al) = −1.66 V, much lower than ε°(H+/H2) = 0.00 V) and the subsequent necessity of carrying out the process in molten medium (Hall–Héroult process in PAP). The interest in storing the H2 produced, if the process is carried out at an industrial scale, can also be commented on.
The extraction residues were also analyzed by XRF and PXRD.
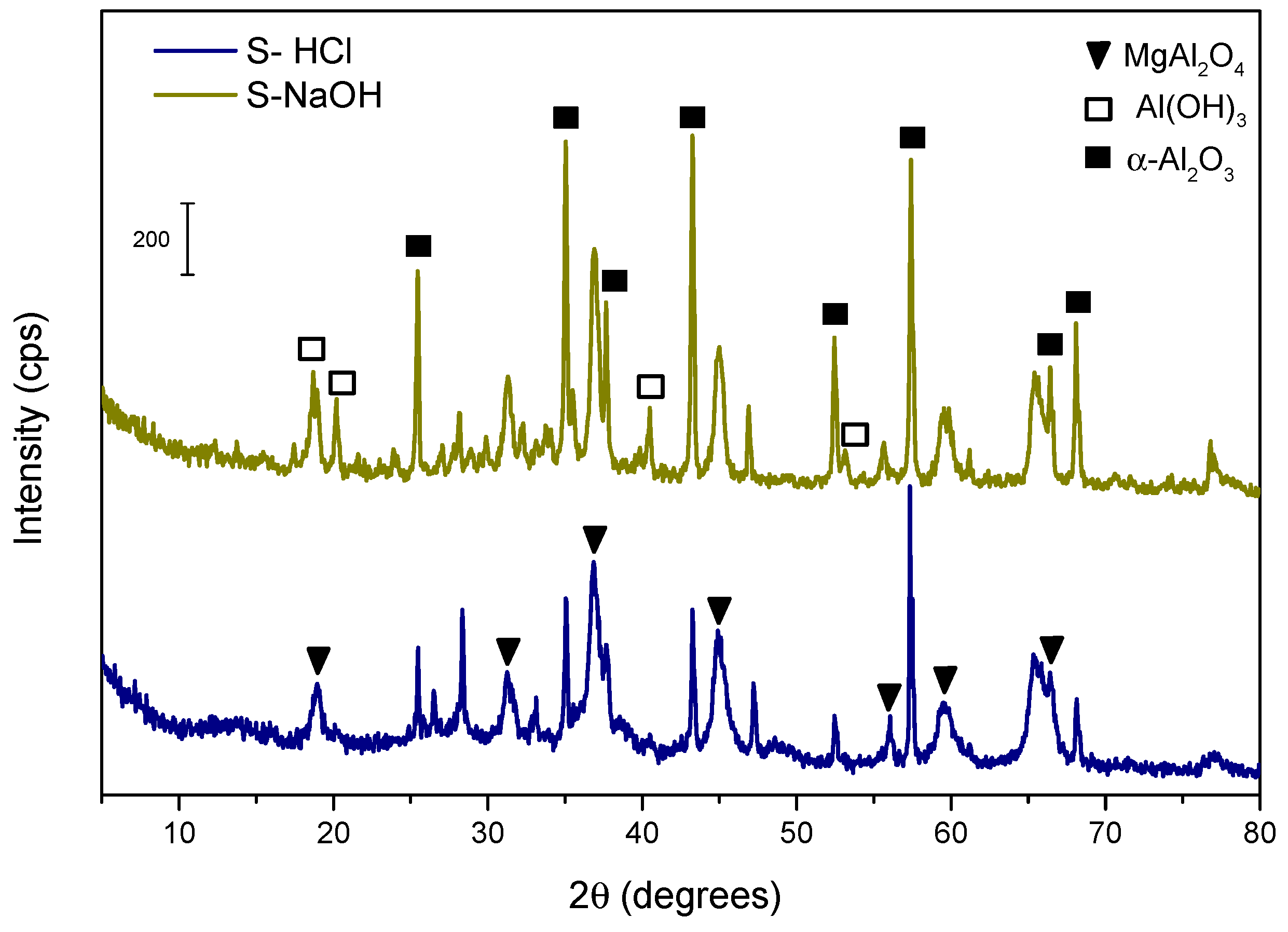
Table 2 shows the chemical composition of the basic (S–NaOH) and acidic (S–HCl) extraction residues, and
Table 3 that of the extraction liquors in each medium [
27]. A decrease in aluminum content was observed in both residues compared to the composition of the small fraction, which showed that aluminum solubilizes in both media. Some important differences in chemical composition can be observed between the acidic and basic residues, and these differences were related to the different solubility of the species as a function of pH. Thus, the Fe content in the basic residue is higher than the one in the acidic residue due to the low solubility of iron at neutral and basic pH. As for Si, the opposite trend is observed, higher in the acidic residue than in the basic residue; again this is related to the low solubility of Si at acidic pH, since it is soluble at very basic pH as silicate anion. On the other hand,
Figure 3 shows the mineralogical composition of the acidic and basic extraction residues. In the case of the acid route, the only crystalline phases observed are α–Al
2O
3 and MgAl
2O
4, while in the case of the basic route, in addition to these two phases, the presence of Al(OH)
3 was also identified. Both α–Al
2O
3 and MgAl
2O
4 are thermodynamically very stable phases, very unreactive and highly insoluble, while Al(OH)
3 is very poorly soluble under natural pH conditions. This final residue, after extraction, is not considered toxic, and it has been used, for example, as additive for road pavement.
Table 3 shows the composition of the extraction liquors in basic (L–NaOH) and acidic (L–HCl) media. The aluminum content was slightly higher in the case of the acidic medium. However, the purity of L–HCl was lower than that of L–NaOH, since more elements are soluble in an acidic medium, and L–HCl is impurified with Fe, Mg, Cu or Zn. However, only Si was observed in the case of the basic medium. Thus, these results are strongly illustrative of the behavior of the elements. Most of them form soluble species in an acidic medium (where the elements are present as hydrated cationic complexes), so the corresponding phases in the slag solubilize in this medium, except the very insoluble phases such as α–Al
2O
3 and MgAl
2O
4. In contrast, only SiO
2 dissolves in an alkaline medium, as silicate anion, while it remains insoluble in an acidic medium (depending on the year in which the experiment is performed, a nice explanation or a reminder of the Lux–Flood definition of acids and bases should be given here). Finally, the amphoteric character of aluminum should be highlighted again, reviewing the species soluble at each pH according to the speciation diagram (
Figure 4).
The % of extracted aluminum can be calculated by the expression:
which is converted to:
Finally, an approximate mass balance of all the process can be given at this point.
The behavior of aluminum in solution can be nicely compared to that of iron and silicon, which can be related to the Bayer process of purification of bauxite, helping to understand this very important process. Both aluminum and iron are dissolved in acidic media, and according to their redox potentials, the dissolution of aluminum is easier; this can be related here, for example, with the use of aluminum as sacrificial anode for avoiding corrosion of iron in acidic medium. Thus, as observed in
Table 3, iron is dissolved in acidic medium, where it should be as [Fe(H
2O)
6]
3+, but not in alkaline medium because, contrary to aluminum, it is not amphoteric (its speciation diagram is given in
Figure S6). Thus, if the liquor obtained under acidic conditions is alkalinized, the hydroxides of both elements precipitate together, but while further alkalization dissolves the amphoteric Al(OH)
3, (Fe(OH)
3 remains a brown gelatinous solid. The observation of this effect should depend on the content of iron in the slag used, and on the solubility of the phases it is forming, but, in general, it is very soluble under acidic conditions. On the other hand,
Table 3 shows that in addition to amphoteric aluminum, only silicon is dissolved in alkaline media, which allows for a comment on the acidic character of SiO
2, which forms silicate anions under alkaline conditions (usually formulated as SiO
32−, although it is more correctly formulated as SiO
44−, with existence of protonated forms; its speciation diagram is given in
Figure S7). Thus, if the liquor obtained in the alkaline medium is acidified, silica gel precipitates, although again the observation of this effect depends on the amount of silicon in the slag and in the solubility of the phases it is forming.
3.1. Evaluation of the Level of Attainment of the Goals
Several complementary questions can be proposed to evaluate the level of goal attainment and reinforce the contents worked with during this laboratory experience (the students should answer these in their laboratory reports). This may also strongly depend on the previous knowledge of the students; we list here four examples that should be included in the lab report.
Question 1. Look for information about other methods of recovery of aluminum from saline slags. As mentioned, other acids or bases should be used for hydrometallurgical recovery. Propose other compounds and discuss the advantages or disadvantages of each one.
Question 2. Determine the percentage of aluminum extracted and suggest possible methods to optimize this percentage.
Question 3. How could Fe, Mg, Cu, and Zn be eliminated from the acid extraction liquor? Look for information about the origin of these metals in recyclable aluminum materials.
Question 4. Could the amount of NH3 generated during the AlN hydrolysis washing process be determined? Propose a method for such determination. Propose methods for the recovery of other gases that can be evolved during the hydrometallurgical treatment of the saline slag.
3.2. Evaluation of the Methodology
This methodology has not been fully implemented, and since the proposed practice is rather long, it should be easier to implement part of it. In fact, we have already explained the acid–base properties of aluminum in Basic Practical Inorganic Chemistry using this methodology but within the framework of laboratory practices devoted to the preparation of potassium tris(oxalato)aluminate(III) and aluminum tris(acetylacetonato) complexes. The results were excellent (>95% of students passed the courses), although we did not conduct surveys or other monitoring mechanisms. It should be emphasized again that the viability of the experiments is guaranteed, given that they were developed within our research, and the detailed results have been published elsewhere. Therefore, the practice, in whole or in part, can help teachers with similar subjects around the world. The method’s strengths lie primarily in the simplicity of Al extraction, given its amphoteric characteristics, which allows it to be extended to other acids and bases and to use the extracted solutions to prepare advanced materials. Its weaknesses relate to the process’s efficiency, since not all the Al present in the waste can be extracted, and therefore, its efficiency is not 100% from a recycling and recovery perspective.