Bitumen Extraction from Bituminous Sands by Ultrasonic Irradiation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Influence of Ultrasound Irradiation Power and Frequency on Bitumen Extraction
3.2. The Influence of the Concentration of Alkaline Solutions on Bitumen Extraction
3.3. The Influence of the Bituminous Sand/Solution Ratio on the Bitumen Extraction
3.4. The Influence of the Temperature on the Bitumen Extraction
3.5. The Influence of Ultrasonic Irradiation Time
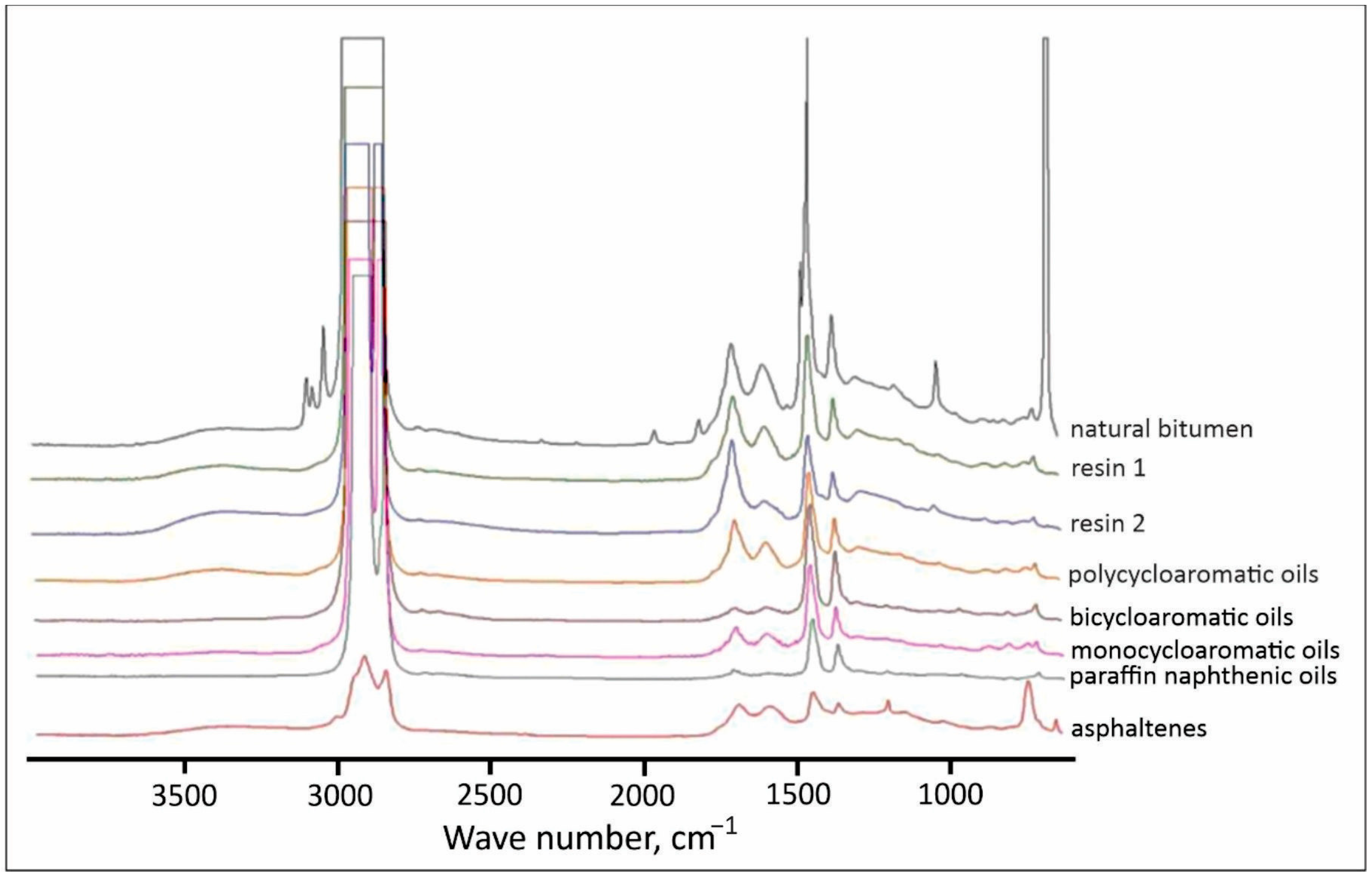
3.6. Composition and Properties of Bitumen Extracted Using Ultrasound
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soroush, M.; Hosseini, S.A.; Roostaei, M.; Pourafshary, P.; Mahmoudi, M.; Ghalambor, A.; Fattahpour, V. Challenges and potentials for sand control design and management in oil reservoirs of Kazakhstan. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, H.; Chen, J. Water and environmental management in oil sands regions. J. Environ. Manag. 2022, 315, 115264. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-oil.pdf (accessed on 11 July 2021).
- Ehsani, M.A.; Akbari, M.; Khalili, Y. A Comprehensive Review of Ultrasonic-Assisted Oil Recovery: Principles, Applications, and Future Prospects. J. Chem. Pet. Eng. 2025, 59, 81–113. [Google Scholar] [CrossRef]
- Myltykbayeva, Z.; Mussabayeva, B.; Ongarbayev, Y.; Imanbayev, Y.; Muktaly, D. Ultrasonic Technology for Hydrocarbon Raw Recovery and Processing. Processes 2024, 12, 2162. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Thring, R.W.; Hu, X.; Song, X. Oil recovery from refinery oily sludge via ultrasound and freeze/thaw. J. Hazardous Mater. 2012, 203–204, 195–203. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.; Zou, C.; Gu, T. Study On Ultrasonic Extraction Of Kerogen From Huadian Oil Shale By Solvents. Oil Shale 2013, 30, 491–500. [Google Scholar] [CrossRef]
- Kasongo, T.; Zhou, Z.; Xu, Z.; Masliyah, J. Effect of clays and calcium ions on bitumen extraction from Athabasca oil sands using flotation. Can. J. Chem. Eng. 2000, 78, 674–681. [Google Scholar] [CrossRef]
- Mierez, J.; AlTammar, M.J.; Alruwaili, K.M.; Alfaraj, R.T. Recent advances of ultrasound applications in the oil and gas industry. Ultrason. Sonochem. 2024, 91, 106256. [Google Scholar] [CrossRef] [PubMed]
- Hanxuan, S.; Yan, Y.; Weiru, Z.; Bibiche, E.E.A.F.; Qingwen, Z.; Jixiang, G. Advances in ultrasonic treatment of oily sludge: Mechanisms, industrial applications, and integration with combined treatment technologies. Environ. Sci. Pollut. Res. 2023, 30, 12345–12358. [Google Scholar] [CrossRef]
- Luo, X.; Gong, H.; He, Z.; Zhang, P.; He, L. Research on mechanism and characteristics of oil recovery from oily sludge in ultrasonic fields. J. Hazard. Mater. 2020, 399, 123137. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, R.; Wu, S.; Wu, Y.; Zhang, Y.; Yang, M. Influence of ultra-sonic waves on the removal of different oil components from oily sludge. Environ. Technol. 2015, 36, 1771–1775. [Google Scholar] [CrossRef]
- Okawa, H.; Saito, T.; Yasuda, S.; Kawamura, Y.; Kato, T.; Sugawara, K.; Babadagli, T. Enhancement of bitumen recovery from the oil sand in an alkaline solution using ultra-sound irradiation and carbon dioxide. Jpn. J. Appl. Phys. 2020, 59, SKKD02. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Patel, N. Influence of pH and surfactant addition on bitumen extraction efficiency from oil sands. Fuel 2021, 287, 119371. [Google Scholar]
- Li, Y.; Wang, T.; Chen, Q.; Zhang, L. CO2-assisted ultrasonic extraction of bitumen from oil sands: Process optimization and recovery efficiency. J. Clean. Prod. 2022, 338, 130618. [Google Scholar] [CrossRef]
- Ahmed, H.; Zhao, X.; Li, S.; Wu, D. Enhancement of bitumen recovery from Athabasca oil sands by ultrasound combined with CO2 injection. Energy Fuels 2021, 35, 12345–12356. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Xu, Y.; Zhou, H. Optimization of ultrasonic parameters for bitumen extraction from oil sands. Ultrason. Sonochem. 2021, 70, 105318. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Sun, F.; Huang, Y. Effects of surfactant type and concentration on ultrasonic-assisted bitumen extraction. Fuel 2022, 310, 122219. [Google Scholar] [CrossRef]
- Zhao, P.; Li, M.; Zhang, Y.; Gu, T. Integrated ultrasonic and green solvent technologies for sustainable bitumen recovery. J. Hazard. Mater. 2023, 451, 131131. [Google Scholar] [CrossRef]
- Ahmed, K.; Wang, Z.; Li, X.; Zhang, P. Sustainable industrial-scale bitumen extraction using combined ultrasound, surfactants, and eco-friendly solvents. Chem. Eng. J. 2022, 446, 137179. [Google Scholar] [CrossRef]
- Ongarbayev, Y.K.; Golovko, A.K.; Krivtsov, E.B.; Imanbayev, Y.I.; Tileuberdi, E.; Tuleutaev, B.; Mansurov, Z.A. Thermocatalytic cracking of the natural bitumens of Kazakhstan. Solid Fuel Chem. 2016, 50, 81–87. [Google Scholar] [CrossRef]
- Madeira, N.C.; de Souza, L.M.; Pereira, A.R.; Chinelatto, L.S.; Cravo, M.C.; Nascimento, L.A.H.D.; Lacerda, V.; Romão, W. Study of thermal and photochemical aging of saturates, naphtenic-aromatics, resins, and asphaltene fractions of asphalt cement by FTIR and FT-ICR MS. Fuel 2024, 367, 131371. [Google Scholar] [CrossRef]
- ISO 3838-2004; Grude Petroleum and Liquid or Solid Petroleum Products-Determination of Density or Relative Density-Capillary-Stoppered Pyknometer and Graduated Bicapillary Pyknometer Methods, MOD. Available online: https://online.zakon.kz/Document/?doc_id=31655032 (accessed on 1 July 2025).
- DIN E No. 1426:1999; Bitumen and Bitumen-Based Binders. Determination of Needle Penetration, MOD. Available online: https://online.zakon.kz/Document/?doc_id=30025370&pos=2;-117#pos=2;-117 (accessed on 1 July 2025).
- DIN E No. 1427:1999; Bitumen and Bitumen-Based Binders. Determination of the Softening Point by the Ring and Ball Method, MOD. Available online: https://online.zakon.kz/Document/?doc_id=30023663 (accessed on 1 July 2025).
- DI No. 52013:1985; Bitumen and Bitumen-Based Binders. Method for Determining Ductility. Available online: https://online.zakon.kz/Document/?doc_id=30141521&pos=4;-106#pos=4;-106 (accessed on 1 July 2025).
- GOST 1461-75; Petroleum and Petroleum Products. Method of Ash Test. Available online: https://online.zakon.kz/Document/?doc_id=30007736 (accessed on 1 July 2025).
- DIN E No. 12593:2000; Bitumen and Bitumen-Based Binders. Fraass Brittleness Temperature Test, MOD. Available online: https://online.zakon.kz/Document/?doc_id=30007865 (accessed on 1 July 2025).
- GOST 6370-2018; Petroleum, Petroleum Products and Additives. Method for Determination of Mechanical Admixtures. Available online: https://online.zakon.kz/Document/?doc_id=37268623 (accessed on 1 July 2025).
- ST RK 1224-2003; Bitumen and Bitumen-Based Binders. Methods for Determining Resistance to Aging Under the Influence of Heat and Air Environment. Available online: https://online.zakon.kz/Document/?doc_id=30023530&pos=2;-118#pos=2;-118 (accessed on 1 July 2025).
- Primerano, K.; Mirwald, J.; Hofko, B. Asphaltenes and maltenes in crude oil and bitumen: A comprehensive review of properties, separation methods, and insights into structure, reactivity and aging. Fuel 2024, 368, 131616. [Google Scholar] [CrossRef]
- Ma, L.; Varveri, A.; Jing, R.; Erkens, S. Chemical characterization of bitumen type and ageing state based on FTIR spectroscopy and discriminant analysis integrated with variable selection methods. Road Mater. Pavement Des. 2023, 24, 1181–1198. [Google Scholar] [CrossRef]
- Mirwald, J.; Nura, D.; Hofko, B. Recommendations for handling bitumen prior to FTIR spectroscopy. Mater. Struct. 2022, 55, 26. [Google Scholar] [CrossRef]
- Almomtan, M.; Al Ibrahim, E.; Farooq, A. Fuelprop: Fuel property prediction from ATR-FTIR spectroscopic data. arXiv 2025, arXiv:2506.01601. [Google Scholar] [CrossRef]
- Comesana, A.E.; Chen, S.S.; Niemeyer, K.E.; Rapp, V.H. A Structured Framework for Predicting Sustainable Aviation Fuel Properties Using Liquid-Phase FTIR and Machine Learning. Available online: https://arxiv.org/pdf/2408.01530 (accessed on 1 July 2025).



| Particle Size of Bituminous Sands, mm | Concentration of NaOH Solution, wt.% | ||||
|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | 5 | |
| Bitumen yield, wt.% | |||||
| 1.0 | 85 | 75 | 75 | 75 | 75 |
| 2.5 | 96 | 98 | 98 | 98 | 98 |
| 5.0 | 35 | 75 | 78 | 78 | 81 |
| 10.0 | 8 | 10 | 15 | 17 | 20 |
| Temperature (°C) | Extraction Rate of Bitumen from the Beke Deposit (%) | |
|---|---|---|
| NaOH Solution | KOH Solution | |
| 5 | 80 | 80 |
| 15 | 82 | 88 |
| 25 | 84 | 90 |
| 50 | 86 | 90 |
| 75 | 98 | 98 |
| 90 | 87 | 94 |
| Extraction Time (min) | Extraction Rate of Bitumen from the Beke Deposit (%) | ||
|---|---|---|---|
| Pure Water | NaOH Solution | KOH Solution | |
| 2 | 0 | 5 | 6 |
| 4 | 5 | 20 | 20 |
| 6 | 10 | 75 | 80 |
| 8 | 40 | 98 | 98 |
| 20 | 80 | - | - |
| Indicators | Regulatory Documents on Test Methods | Bituminous Sand Bitumen | Bitumen 100/130 |
|---|---|---|---|
| Density, kg/m3 | [23] | 940.1 | 1030.0 |
| Penetration at 25 °C, 0.1 mm | [24] | 133 | 110 |
| Softening point, °C | [25] | 29 | 44 |
| Ductility at 25 °C, cm | [26] | 95 | 150 |
| Ash content, wt.% | [27] | 0.4 | 3.1 |
| Brittleness temperature, °C | [28] | −13 | −24 |
| Mechanical impurities, wt.% | [29] | 6.5 | 2.5 |
| Mass change after heating, % | [30] | 2.5 | 0.5 |
| Bitumen content, wt.% | 8–10 | – | |
| Asphaltenes, wt.% | 11.1 | 19.8 | |
| Oils, wt.% | 46.7 | 48.9 | |
| Resins, wt.% | 42.2 | 31.3 |
| Hydrocarbons | Contents (wt.%) | Hydrocarbons | Contents (wt.%) |
|---|---|---|---|
| n-Alkanes | 68.7 | n-Alkyltoluenes | 1.9 |
| Cyclohexanes | 1.4 | Alkylnaphthalenes | 0.4 |
| Terpans | 20.1 | Alkylphenanthrenes | 1.1 |
| Sterans | 2.4 | Naphthenophenanthrenes | 0.5 |
| Naphthenomonoarenes | 2.3 | Dibenzothiophenes | 0.2 |
| n-Alkylbenzenes | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanbayev, Y.; Ongarbayev, Y.; Abylaikhan, A.; Mussabayeva, B.; Muktaly, D.; Myltykbayeva, Z. Bitumen Extraction from Bituminous Sands by Ultrasonic Irradiation. ChemEngineering 2025, 9, 109. https://doi.org/10.3390/chemengineering9050109
Imanbayev Y, Ongarbayev Y, Abylaikhan A, Mussabayeva B, Muktaly D, Myltykbayeva Z. Bitumen Extraction from Bituminous Sands by Ultrasonic Irradiation. ChemEngineering. 2025; 9(5):109. https://doi.org/10.3390/chemengineering9050109
Chicago/Turabian StyleImanbayev, Yerzhan, Yerdos Ongarbayev, Akerke Abylaikhan, Binur Mussabayeva, Dinara Muktaly, and Zhannur Myltykbayeva. 2025. "Bitumen Extraction from Bituminous Sands by Ultrasonic Irradiation" ChemEngineering 9, no. 5: 109. https://doi.org/10.3390/chemengineering9050109
APA StyleImanbayev, Y., Ongarbayev, Y., Abylaikhan, A., Mussabayeva, B., Muktaly, D., & Myltykbayeva, Z. (2025). Bitumen Extraction from Bituminous Sands by Ultrasonic Irradiation. ChemEngineering, 9(5), 109. https://doi.org/10.3390/chemengineering9050109








