Decoloration of Waste Cooking Oil by Maghnia Algerian Clays via Ion Exchange and Surface Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Adsorption Protocol
2.3. UV-VIS Color Analysis
2.4. FT-IR Analysis
2.5. X-Ray Diffraction (XRD) Analysis
2.6. X-Ray Fluorescence Analysis
2.7. pH at Point of Zero Charge (pHPZC) Determination
3. Results
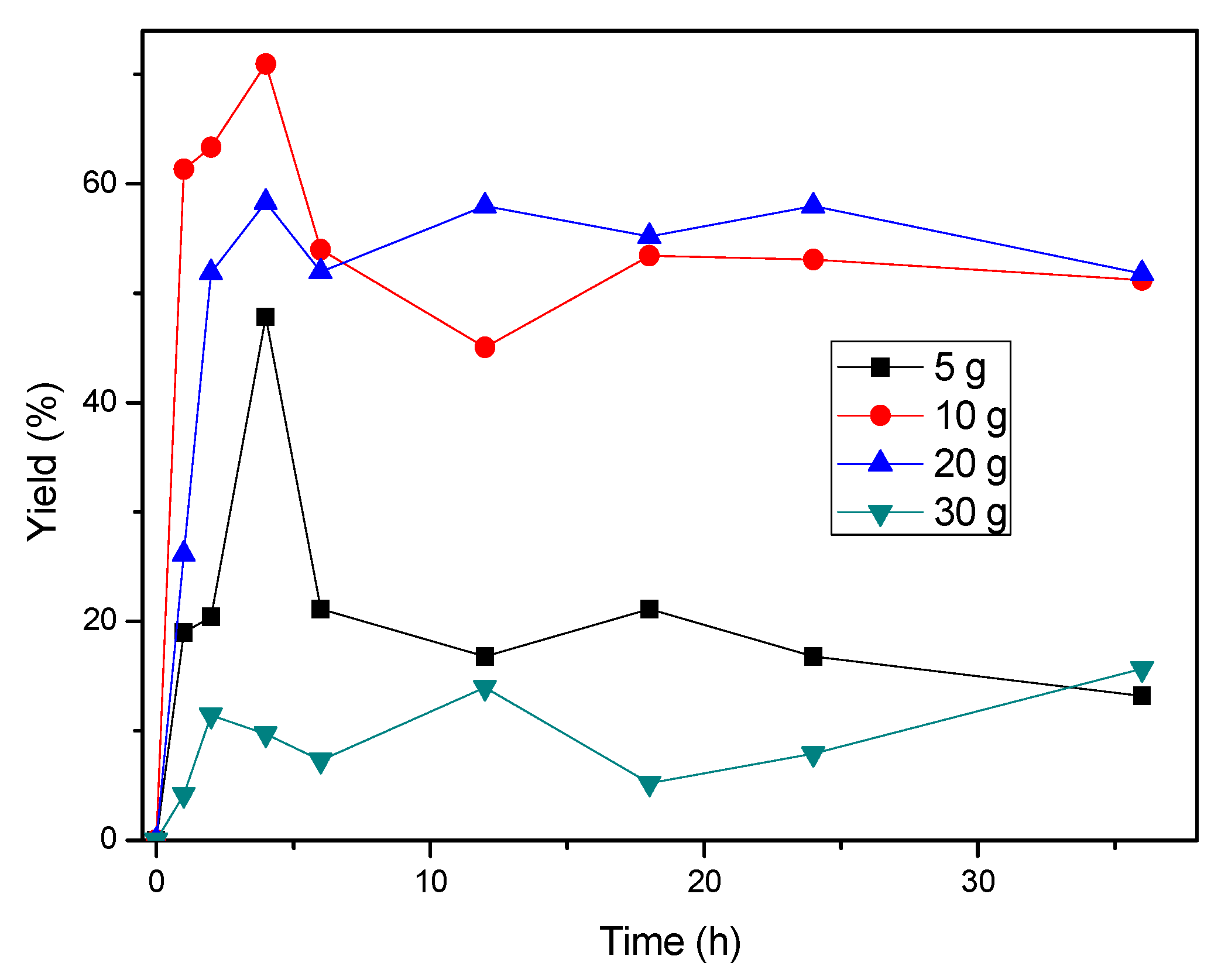
3.1. Adsorption Kinetics Assessment
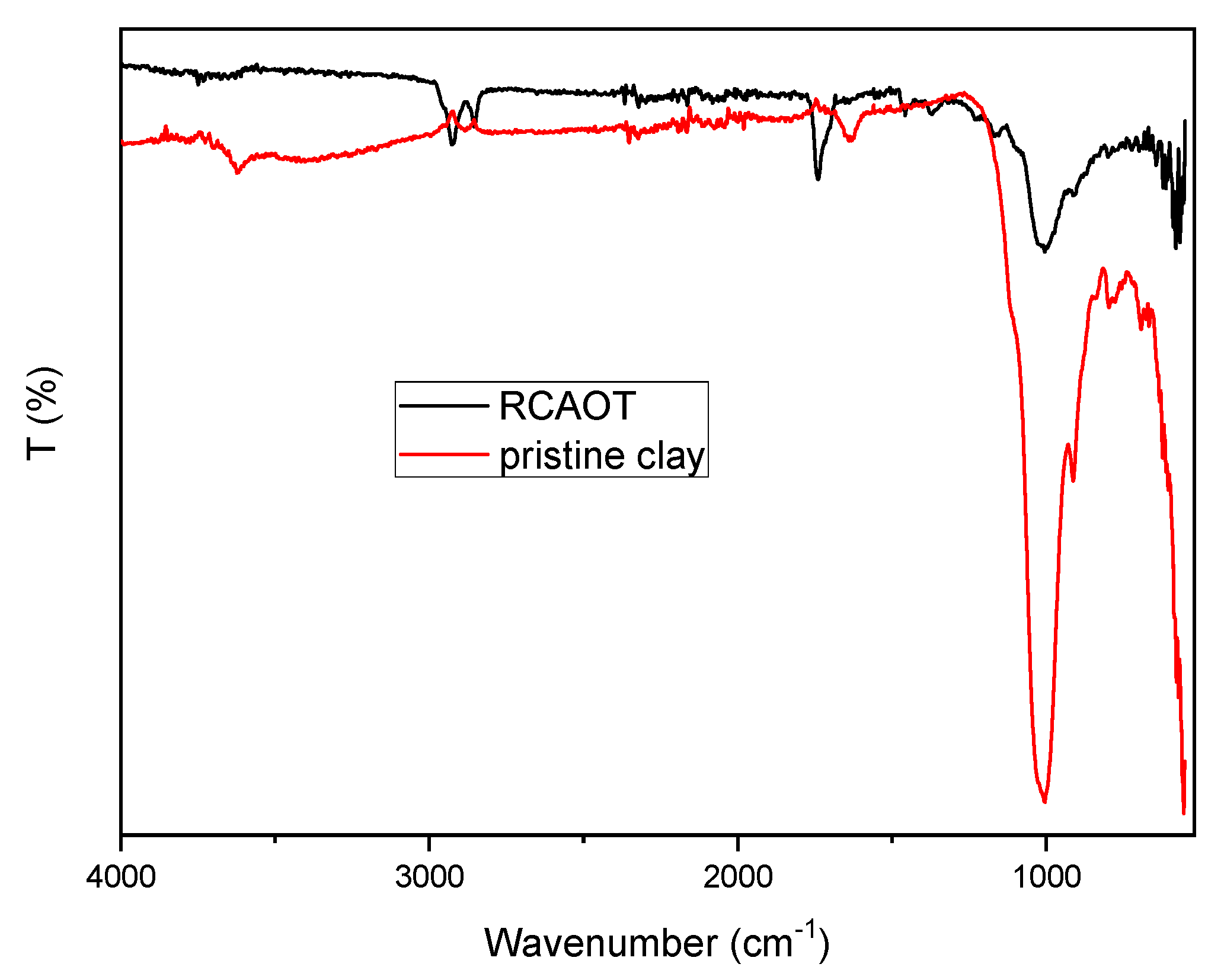
3.2. Chemical Modification Investigation Through FTIR
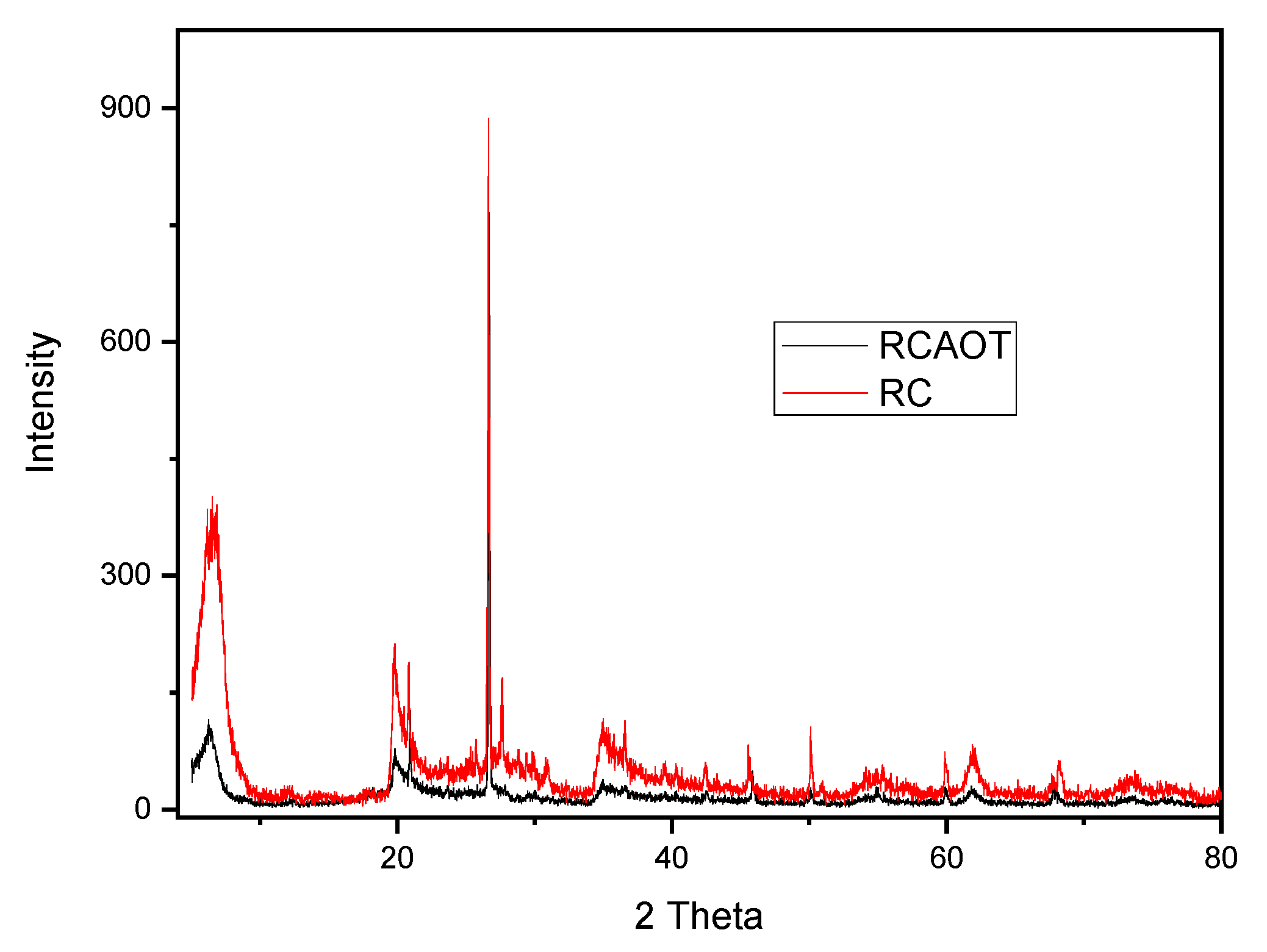
3.3. Structural Investigation by Powder X-Ray Analysis
3.4. Structural Investigation Through XRF Analysis
- (I)
- The loss of Na and Mg, coupled with an increase in Ca and K, suggests that interlayer cations in the clay were replaced by metal-containing organic species from WCO. This mechanism is common in bentonites used for oil purification.
- (II)
- The increase in Fe, Rh, and Pd content implies that clay surface sites interacted with oil impurities, leading to the retention of transition metals and possibly oxidation processes.
- (III)
- The reduction in Si and Al suggests that some oil components penetrated the clay’s structure, covering active sites and altering the elemental composition.
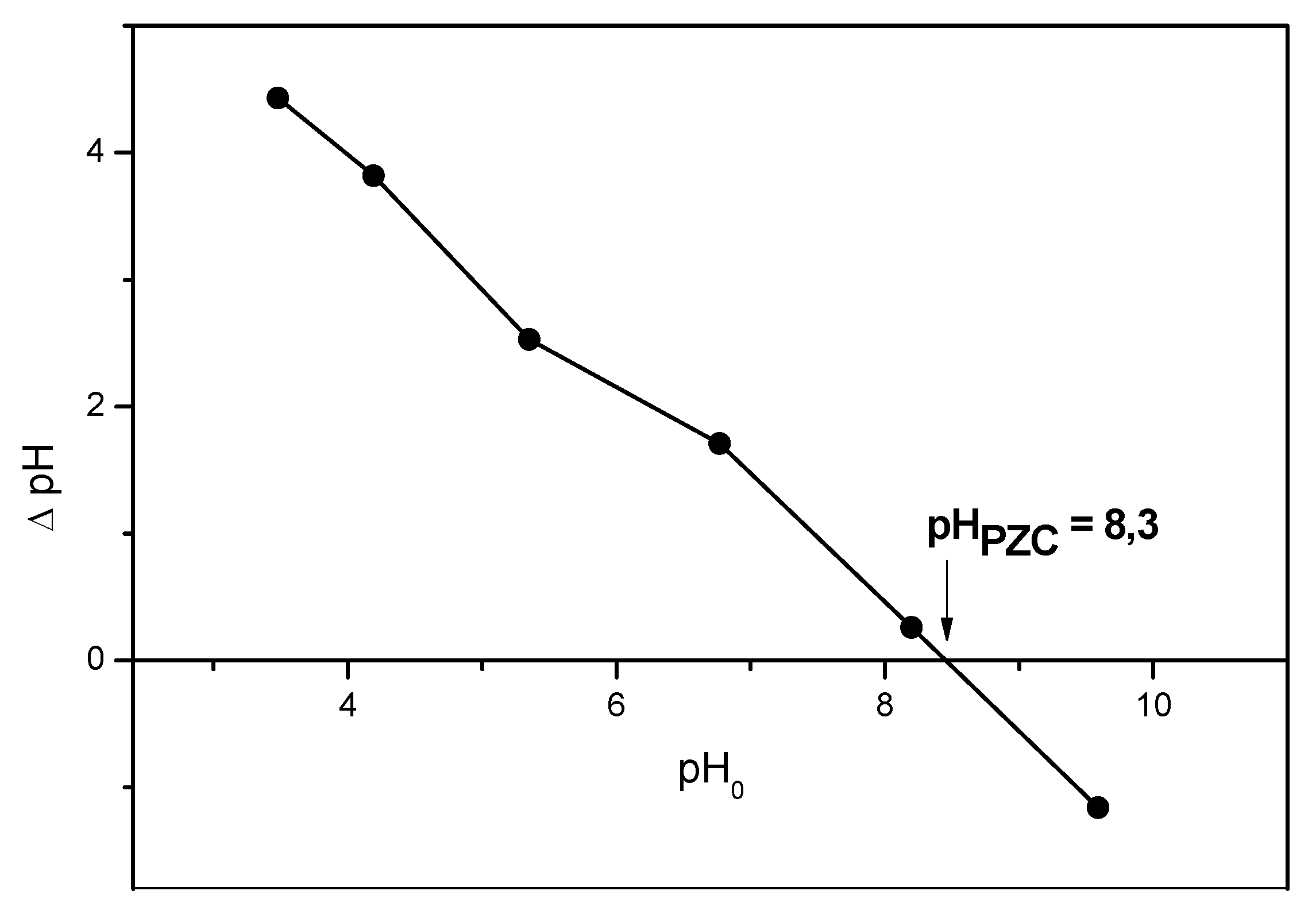
3.5. Adsorption Investigation: pH at Point of Zero Charge (pHPZC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrikopoulos, N.K.; Boskou, G.; Dedoussis, G.V.Z.; Chiou, A.; Tzamtzis, V.A.; Papathanasiou, A. Quality assessment of frying oils and fats from 63 restaurants in Athens, Greece. Food Serv. Technol. 2003, 3, 49–59. [Google Scholar] [CrossRef]
- Ziaiifar, A.M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 2008, 43, 1410–1423. [Google Scholar] [CrossRef]
- Mannu, A.; Almendras Flores, P.; Briatico Vangosa, F.; Di Pietro, M.E.; Mele, A. Sustainable production of raw materials from waste cooking oils. RSC Sustain. 2025, 3, 300–310. [Google Scholar] [CrossRef]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y. Saifuddin Nomanbhay, Kit Wayne Chew, Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Dangal, A.; Tahergorabi, R.; Acharya, D.R.; Timsina, P.; Rai, K.; Dahal, S.; Acharya, P.; Giuffrè, A.M. Review on deep-fat fried foods: Physical and chemical attributes, and consequences of high consumption. Eur. Food Res. Technol. 2024, 250, 1537–1550. [Google Scholar] [CrossRef]
- Mannu, A.; Ferro, M.; Di Pietro, M.E.; Mele, A. Innovative applications of waste cooking oil as raw material. Sci. Prog. 2019, 102, 153–160. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Kaushik, S.; Sati, V.; Kanojia, N.; Mehra, K.S.; Malkani, H.; Pant, H.; Gupta, H.; Singh, A.P.; Kumar, A.; Paul, A.R.; et al. Biodiesel a Substitution for Conventional Diesel Fuel: A Comprehensive Review. In Advances in Mechanical Engineering; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Applications of Waste Cooking Oil Other Than Biodiesel. Iran. J. Chem. Eng. 2015, 12, 55. [Google Scholar]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available Technologies and Materials for Waste Cooking Oil Recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Hymore, F.K. Effects of some additives on the performance of acidactivated clays in the bleaching of palm oil. Appl. Clay Sci. 1996, 10, 379–385. [Google Scholar] [CrossRef]
- Mannu, A.; Poddighe, M.; Mureddu, M.; Castia, S.; Mulas, G.; Murgia, F.; Di Pietro, M.E.; Mele, A.; Garroni, S. Impact of morphology of hydrophilic and hydrophobic bentonites on improving the pour point in the recycling of waste cooking oils. Appl. Clay Sci. 2024, 262, 107607. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Urgeghe, P.; Ferro, M.; Del Caro, A.; Taras, A.; Garroni, S.; Rourke, J.P.; Cabizza, R.; Petretto, G.L. Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration. Resources 2019, 8, 108. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Didi, M.A.; Villemin, D.; Azzouz, A. Acid activation of Bentonite for use as a vegetable oil bleaching agent. Grasas Aceites 2009, 60, 343–349. [Google Scholar] [CrossRef]
- Avelino Ratkievicius, L.; Vieira Da Cunha Filho, F.J.; De Barros Neto, E.L.; Santanna, V.C. Modification of bentonite clay by a cationic surfactant to be used as a viscosity enhancer in vegetable-oil-based drilling fluid. Appl. Clay Sci. 2017, 135, 307–312. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Maximum Bleaching of Vegetable Oils by Acid-Activated Bentonite: Influence of Nanopore Radius. Adsorpt. Sci. Technol. 2012, 30, 97–104. [Google Scholar] [CrossRef]
- Egbuna, S.O. Development of kinetic model for adsorption of carotenoids on activated clay in the bleaching of PalmOil. Int. J. Res. Eng. Technol. 2014, 3, 371–380. [Google Scholar]
- Ifa, L.; Wiyania, L.; Nurdjannaha, N.; Muhammad, A.; Ghaliba, T.; Ramadhaniara, S.; Kusuma, H.S. Analysis of bentonite performance on the quality of refined crude palm oil’s color, free fatty acid and carotene: The effect of bentonite concentration and contact time. Heliyon 2021, 7, e07230. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Radha Krishnan, K.; Mishra, B.K.; Rayaguru, K. Process optimization for minimizing residual free fatty acid levels in fried mustard oil: Isotherm and kinetics studies. J. Food Process Eng. 2017, 40, e12426. [Google Scholar] [CrossRef]
- Foletto, E.L.; Volzone, C.; Porto, L.M. Clarification of cottonseed oil: How structural properties of treated bentonites by acid affect bleaching efficiency. Lat. Am. Appl. Res. 2006, 36, 37–40. [Google Scholar]
- Yan, N.; Masliyah, J.H. Adsorption and desorption of clay particles at the oil-water interface. J. Colloid Interface Sci. 1994, 168, 386–392. [Google Scholar] [CrossRef]
- Vogt, E.; Pacura, W. Hydrophobization of bleaching clay used for purification of waste frying oils. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012009. [Google Scholar] [CrossRef]
- Luo, J.; Liu, M.; Xing, Y.; Gui, X.; Li, J. Investigating agglomeration of kaolinite particles in the presence of dodecylamine by force testing and molecular dynamics simulation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128930. [Google Scholar] [CrossRef]
- Serouri, A.; Taleb, Z.; Mannu, A.; Garroni, S.; Senes, N.; Taleb, S.; Brini, S.; Abdoun, S.K. Variation of Used Vegetable Oils’ Composition upon Treatment with Algerian Clays. Recycling 2021, 6, 68. [Google Scholar] [CrossRef]
- Tlemsani, S.; Taleb, Z.; Piraúlt-Roy, L.; Taleb, S. Temperature and pH influence on diuron adsorption by Algerian Mont-Na clay. Int. J. Environ. Anal. Chem. 2024, 104, 2316–2333. [Google Scholar] [CrossRef]
- 2- Taleb, Z.; Ramdani, A.; Berenguer, R.; Ramdani, N.; Adjir, M.; Taleb, S.; Morallón, E.; Nemmich, S.; Tilmatine, A. Combined ozonation process and adsorption onto bentonite natural adsorbent for the o-cresol elimination. Int. J. Environ. Anal. Chem. 2023, 103, 977–994. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Sireus, V.; Petretto, G.L.; Mulas, G.; Garroni, S. Bentonite as refining agent in waste cooking oils recycling: Flash point, density and color evaluation. Nat. Prod. Commun. 2018, 12, 1–2. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.J. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: New York, NY, USA, 1989; p. 332. [Google Scholar]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.P.; Fernández, L.G. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Maged, A.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Characterization of activated bentonite clay mineral and the mechanisms underlying its sorption for ciprofloxacin from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 32980–32997. [Google Scholar] [CrossRef]
- Angar, Y.; Djelali, N.E.; Kebbouche-Gana, S. Study of the Effect of the Bentonite Modification Treatments on the Adsorption Removal of Ammonium Ions from Aqueous Solution. Fresenius Environ. Bull. 2016, 25, 3646–3653. [Google Scholar]
- Boufatit, M.; Ait-Amar, H.; McWhinnie, W.R. Development of an Algerian material montmorillonite clay. Adsorption of phenol, 2-dichlorophenol and 2, 4, 6-trichlorophenol from aqueous solutions onto montmorillonite exchanged with transition metal complexes. Desalination 2007, 206, 394–406. [Google Scholar] [CrossRef]
- Mohammed-Azizi, F.; Dib, S.; Boufatit, M. Removal of heavy metals from aqueous solutions by Algerian bentonite. Desalin.Water Treat. 2013, 51, 4447–4458. [Google Scholar] [CrossRef]
- Abdullahi, S.L.; Audu, A.A. Comparative analysis on chemical composition of bentonite clays obtained from Ashaka and tango deposits in Gombe State, Nigeria. ChemSearch J. 2017, 8, 35–40. [Google Scholar]
- Aljlil, S.A.; Alsewailem, F.D. Adsorption of cu & ni on bentonite clay from waste water. Athens J. Sci. 2014, 1, 21–30. [Google Scholar]
- Liu, X.; Tournassat, C.; Grangeon, S.; Kalinichev, A.G.; Takahashi, Y.; Marques Fernandes, M. Molecular-level understanding of metal ion retention in clay-rich materials. Nat. Rev. Earth Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Pavlov, D.I.; Yu, X.; Ryadun, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Sun, N.; Sun, Y.; Fedin, V.P.; Potapov, A.S. Multiresponsive luminescent metal–organic framework for cooking oil adulteration detection and gallium(III) sensing. Food Chem. 2024, 445, 138747. [Google Scholar] [CrossRef]
- Apeiranthitis, N.; Greenwell, H.C.; Carteret, C. Far-and mid-infrared examination of nontronite-1 clay mineral–Redox and cation saturation effects. Appl. Clay Sci. 2022, 228, 106628. [Google Scholar] [CrossRef]
- Pavan Korf, E.; Lange Salvia, A.; Larisse Scopel, S.; Marques Prietto, P.D.; Thomé, A. Competitive Sorption of Metallic Species under Different pH in a Residual Clayey Soil. J. Environ. Eng. 2017, 143, 06017009. [Google Scholar] [CrossRef]
- Sposito, G. On Points of Zero Charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Akinyemi, S.A.; Msagati, T.A. Performance of Mn2+-modified bentonite clay for the removal of fluoride from aqueous solution. S. Afr. J. Chem. 2018, 71, 15–23. [Google Scholar] [CrossRef]
- Kallay, N.; Žalac, S. Charged surfaces and interfacial ions. J. Colloid Interface Sci. 2020, 230, 1–11. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.R.P.; Olenka, L.; Peternella, W.S. A study of degradation in vegetable oils by exposure to sunlight using fourier transform infrared spectroscopy. Mater. Sci. Appl. 2020, 11, 678–691. [Google Scholar] [CrossRef]
- Tlemsani, S.; Taleb, Z.; Pirault-Roy, L.; Taleb, S. Heterogeneous Catalytic Degradation of Diuron Using Algerian Sodium Montmorillonite. Clean-Soil Air Water 2022, 50, 2000468. [Google Scholar] [CrossRef]

| Identified Phase | Plane [hkl] | Pristine (2Θ) | Exhausted (2Θ) |
|---|---|---|---|
| Montmorillonite | (001) | 6.67 | 6.35 |
| Illite | (002) | 19.85 | 19.89 |
| Montmorillonite/Illite | (110) | 20.79 | 20.91 |
| Quartz/Illite | (020) | 26.63 | 26.69 |
| Calcite/Illite | (002) | 27.59 | / * |
| Illite | (110) | 34.99 | 34.89 |
| Quartz/Illite | (200) | 36.51 | / * |
| Quartz/Illite | / | 45.55 | 45.89 |
| Quartz/Illite | / | 50.15 | 50.11 |
| Montmorillonite/Illite | (060) | 61.86 | 61.82 |
| Elements | Pristine Clay (wt%) * | Exhausted Clay (wt%) * |
|---|---|---|
| Na | 1.88 | n.d. |
| Mg | 4.57 | 3.03 |
| Al | 20.50 | 17.65 |
| Si | 53.39 | 48.87 |
| S | 0.54 | 0.64 |
| K | 2.95 | 3.68 |
| Ca | 1.63 | 5.42 |
| Sc | 0.00 | 0.00 |
| Ti | 0.85 | 1.09 |
| V | 0.03 | 0.04 |
| Mn | 0.22 | 0.29 |
| Fe | 10.02 | 12.30 |
| Zn | 0.02 | 0.03 |
| Rb | 0.03 | 0.04 |
| Sr | 0.11 | 0.11 |
| Y | 0.01 | -- |
| Zr | 0.05 | 0.04 |
| Ru | 0.73 | 1.60 |
| Rh | 1.92 | 3.20 |
| Pd | 0.56 | 1.96 |
| Ga | -- | 0.01 |
| Total | 100 | 100 |
| Category | Parameter/Observation | Key Findings |
|---|---|---|
| Adsorption Performance | Optimal bentonite dosage | 10 wt% |
| Optimal contact time | 4 h | |
| Maximum decolorization yield | 70–71% | |
| Adsorption kinetics model | Pseudo-second-order | |
| High clay loading effect | Agglomeration → reduced efficiency; oscillatory kinetics at 30 wt% clay | |
| Structural Changes | FT-IR: Functional group interactions | Disappearance of OH bands, appearance of -CH2 and C=O bands in exhausted clay |
| FT-IR implication | Adsorption of hydrocarbon impurities confirmed | |
| PXRD: Montmorillonite (001) peak shift | From 6.67° to 6.35° → suggests interlayer incorporation of organics | |
| PXRD: General structural stability | No major phase changes; slight reorganization | |
| Elemental Composition | XRF: Decrease in Si, Al, Mg | Indicates surface coverage and leaching |
| XRF: Disappearance of Na | Suggests ion exchange with oil-borne cations | |
| XRF: Increase in Ca, K, Fe, transition metals (Rh, Pd) | Confirms ion exchange, surface adsorption, and possible oxidation | |
| Adsorption mechanisms (from XRF + PXRD + FTIR) | (I) Ion exchange, (II) surface adsorption, (III) physical entrapment | |
| Surface Properties | pHPZC | 8.3—favors adsorption under acidic/neutral conditions |
| pHPZC shift post-adsorption | Negligible—implies predominantly physical adsorption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serouri, A.; Taleb, Z.; Mannu, A.; Kedir, C.N.; Memou, C.H.; Garroni, S.; Mele, A.; Zinai, O.; Taleb, S. Decoloration of Waste Cooking Oil by Maghnia Algerian Clays via Ion Exchange and Surface Adsorption. ChemEngineering 2025, 9, 50. https://doi.org/10.3390/chemengineering9030050
Serouri A, Taleb Z, Mannu A, Kedir CN, Memou CH, Garroni S, Mele A, Zinai O, Taleb S. Decoloration of Waste Cooking Oil by Maghnia Algerian Clays via Ion Exchange and Surface Adsorption. ChemEngineering. 2025; 9(3):50. https://doi.org/10.3390/chemengineering9030050
Chicago/Turabian StyleSerouri, Abdelhak, Zoubida Taleb, Alberto Mannu, Chahineze Nawel Kedir, Cherifa Hakima Memou, Sebastiano Garroni, Andrea Mele, Oussama Zinai, and Safia Taleb. 2025. "Decoloration of Waste Cooking Oil by Maghnia Algerian Clays via Ion Exchange and Surface Adsorption" ChemEngineering 9, no. 3: 50. https://doi.org/10.3390/chemengineering9030050
APA StyleSerouri, A., Taleb, Z., Mannu, A., Kedir, C. N., Memou, C. H., Garroni, S., Mele, A., Zinai, O., & Taleb, S. (2025). Decoloration of Waste Cooking Oil by Maghnia Algerian Clays via Ion Exchange and Surface Adsorption. ChemEngineering, 9(3), 50. https://doi.org/10.3390/chemengineering9030050











