Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignocellulosic Composition of Cocoa Pod Powder (CCP)
2.1.1. Extractables
2.1.2. Hemicellulose
2.1.3. Lignin
2.1.4. Cellulose
2.1.5. Proximate Analysis
2.1.6. Thermogravimetric Analysis
2.2. Pectin Extraction
2.3. Fourier Transform Infrared Spectroscopy
2.3.1. Degree of Esterification
2.3.2. Methoxylation Percentage
2.3.3. Pectin Yield
3. Result and Discussion
3.1. Cocoa Pod Powder Characterization
3.1.1. Proximate and Lignocellulosic Analysis
3.1.2. Thermogravimetric Analysis
3.1.3. Infrared Spectroscopy Analysis
3.2. Pectin Extraction
3.2.1. Extracted Pectin Characterization by FT-IR
3.2.2. FT-IR Characterization of Residual Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in the circular economy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Nieto-Figuero, K.H.B.; Oomah, D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Amboni, R.D.M.C.; de Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot water-soluble pectins. Ind. Crops Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Abdul Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern Med. 2014, 14, 381. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, T.D.; Vu, L.B.; Nguyen, V.H.; Tran, N.L. Microwave-assisted Extraction for Maximizing the Yield of Phenolic Compounds and Antioxidant Capacity from Cacao Pod Husk (Theobroma cacao L.). Curr. Nutr. Food Sci. 2020, 17, 225–237. [Google Scholar] [CrossRef]
- Oduro-Mensah, D.; Ocloo, A.; Lowor, S.T.; Mingle, C.; Okine, L.K.N.A.; Adamafio, N.A. Bio-detheobromination of cocoa pod husks: Reduction of ochratoxin A content without change in nutrient profile. Microb. Cell Fact. 2018, 17, 79. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.H. Proximate Composition, Extraction, and Purification of Theobromine from Cacao Pod Husk (Theobroma cacao L.). Technologies 2017, 5, 14. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, Y.F.; Salas-Calderón, K.T.; Orozco-Blanco, D.A.; Gentile, P.; Girón-Hernández, J. Cocoa Pod Husk: A High-Pectin Source with Applications in the Food and Biomedical Fields. CheBioEng Rev. 2022, 9, 462–474. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Tran, P.T.T. Characterization of microencapsulated powders rich in saponins from cocoa pod husk (Thebroma cacao L.) and medicinal plant an xoa (Helicteres hirsuta Lour.). Heliyon 2024, 10, e32703. [Google Scholar] [CrossRef]
- Díaz-González, A.; Luna, M.Y.P.; Morales, E.R.; Saldaña-Trinidad, S.; Blanco, L.R.; de la Cruz-Arreola, S.; Pérez-Sariñana, B.Y.; Robles-Ocampo, J.B. Assessment of the Pretreatments and Bioconversion of Lignocellulosic Biomass Recovered from the Husk of the Cocoa Pod. Energies 2022, 15, 3544. [Google Scholar] [CrossRef]
- Adiletta, G.; Brachi, P.; Riianova, E.; Crescitelli, A.; Miccio, M.; Kostryukova, N. A Simplified Biorefinery Concept for the Valorization of Sugar Beet Pulp: Ecofriendly Isolation of Pectin as a Step Preceding Torrefaction. Waste Biomass Valorization 2019, 11, 2721–2733. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, C.; Hu, H.; Li, Y.; Sun, D.; Wang, Y.; Peng, L. Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol. Adv. 2016, 34, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/pectin-market (accessed on 17 February 2025).
- Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M.; Encalada, A.M.I.; Zukowski, E.F.; Coelho, R.A.H. Pectins obtained by ultrasound from agroindustrial by-products. Food Hydrocoll. 2021, 118, 106–799. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–376. [Google Scholar] [CrossRef]
- Kumar, K.; Shivmurti, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 175–181. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Boyaci, I.H.; Eksi-Kocak, H. Determination of chemical changes in heat-treated wood using ATR-FTIR and FT Raman spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 395–400. [Google Scholar] [CrossRef]
- Parhizkar, E.; Ghazali, M.; Ahmadi, F.; Sakhteman, A. PLS-LS-SVM based modeling of ATR-IR as a robust method in detection and qualification of alprazolam. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 87–92. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Oliveira TÍ, S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Posthasrvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Zhang, J. Rheological properties of natural low-methoxyl pectin extracted from sunflower head. Food Hydrocoll. 2015, 44, 122–128. [Google Scholar] [CrossRef]
- Löfgren, C.; Guillotin, S.; Hermansson, A.M. Microstructure and kinetic rheological behavior of amidated and nonamidated LM pectin gels. Biomacromolecules 2006, 7, 114–121. [Google Scholar] [CrossRef]
- Racape, E.; Thibault, J. Properties of amidated pectin II. Polyelectrolyte behavior and calcium binding of amidated pectins and amidated pectic acids. Biopolymers 1989, 28, 1435–1448. [Google Scholar] [CrossRef]
- Selvaraju, G.; Bakar, N.K.A. Production of a new industrially viable green-activated carbon from Artocarpus integer fruit processing waste and evaluation of its chemical, morphological and adsorption properties. J. Clean. Prod. 2017, 141, 989–999. [Google Scholar] [CrossRef]
- Li, S.; Su, S.; Liu, S.; Yang, C.; Lu, Q. Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2014, 10, 480–487. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto, L.; Soccol, C.R. A biorefinery approach for pectin extraction and second-generation bioethanol production from cocoa pod husk. Bioresour. Technol. 2021, 346, 126635. [Google Scholar] [CrossRef]
- Sobamiwa, O.; Longe, O.G. Utilization of cocoa-pod pericarp fractions in broiler chick diets. Anim. Feed. Sci. Technol. 1994, 47, 237–244. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of cacao pod husks by pyrolysis and catalytic reaction to produce useful chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. The potential of using cocoa pod husks as green solid base catalysts for the transesterification of soybean oil into biodiesel: Effects of biodiesel on engine performance. Chem. Eng. J. 2013, 220, 395–401. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112–122. [Google Scholar] [CrossRef]
- Gavahian, M.; Mathad, G.N.; Pandiselvam, R.; Lin, J.; Sun, D.-W. Emerging technologies to obtain pectin from food processing by-products: A strategy for enhancing resource efficiency. Trends Food Sci. Technol. 2021, 115, 42–54. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Shahvandi, A.; Okoro, O.V.; Denayer, J.F.M.; Karimi, K. Biorefining of corn stover for efficient production of bioethanol, biodiesel, biomethane, and value-added byproducts. Energy Convers. Manag. 2023, 283, 116877. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.; Tsang, D.C.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef]
- Rath, S.; Pradhan, D.; Du, H.; Mohapatra, S.; Thatoi, H. Transforming lignin into value-added products: Perspectives on lignin chemistry, lignin-based biocomposites, and pathways for augmenting ligninolytic enzyme production. Adv. Compos. Hybrid Mater. 2024, 7, 27. [Google Scholar] [CrossRef]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the role of lignin for preparing the lignin-based wood adhesive: A review. Ind. Crops Prod. 2022, 187, 115388. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Reszczyńska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Mund, N.K.; Dash, D.; Mishra, P.; Nayak, N.R. Cellulose solvent–based pretreatment and enzymatic hydrolysis of pineapple leaf waste biomass for efficient release of glucose towards biofuel production. Biomass Convers. Biorefin. 2021, 12, 4117–4126. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Nair, D. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ala’a, H.; García-Peñas, A.; Mola, G.T.; Si, C.; Stadler, F.J. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review. Chem. Eng. J. 2020, 382, 122937. [Google Scholar] [CrossRef]
- Saffe, A.; Fernández, A.; Mazza, G.; Rodriguez, R. Prediction of regional agro-industrial wastes characteristicas by thermogravimetic analysis to obtain bioenergy using thermal process. Energy Explor. Exploit. 2019, 37, 544–557. [Google Scholar] [CrossRef]
- Milian-Luperón, L.; Hernández-Rodríguez, M.; Falcón-Hernández, J.; Otero-Calvis, A. Obtainin bioproducts slow pyrolysis of coffee and cocoa husks as suitable candidates for beins used as soil amendment and source of energy. Rev. Colomb. Quim. 2020, 49, 23–29. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Parthasarathy, R.; Pramanik, B.; Paz-Ferreiro, J.; Shah, K. TGA-FTIR study on the slow pyrolysis of lignin and cellulose-rich fractions derived from imidazolium-based ionic liquid pre-treatment of sugarcane straw. Energy Convers. Manag. 2019, 200, 112067. [Google Scholar] [CrossRef]
- Sohni, S.; Norulaini, N.A.N.; Hashim, R.; Khan, S.B.; Fadhullah, W.; Omar, A.K.M. Physicochemical characterization of Malaysian crop and agro-industrial biomass residues as renewable energy resources. Ind. Crops Prod. 2017, 111, 642–650. [Google Scholar] [CrossRef]
- Fernandez, A.; Saffe, A.; Pereyra, R.; Mazza, G.; Rodriguez, R. Kinetic study of regional agro-industrial wastes pyrolysis using non-isothermal TGA analysis. Appl. Therm. Eng. 2016, 106, 1157–1164. [Google Scholar] [CrossRef]
- Fernandez, A.; Palacios, C.; Echegaray, M.; Mazza, G.; Rodriguez, R. Pyrolysis and Combustion of Regional Agro-Industrial Wastes: Thermal Behavior and Kinetic Parameters Comparison. Combust. Sci. Technol. 2017, 190, 114–135. [Google Scholar] [CrossRef]
- Sangaré, D.; Bostyn, S.; Moscosa-Santillan, M.; Belandria, V.; García-Alamilla, P.; González-Chávez, M.M.; Gökalp, I. Hydrothermal carbonization of cocoa shell: Hydrochar characterization, kinetic triplets, and thermodynamic aspects of the process. Biomass Conv. Bioref. 2024, 14, 93–108. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Guo, F.; Salmén, L.; Yu, Y. Cell wall polymer distribution in bamboo visualized with in situ imaging FTIR. Carbohydr. Polym. 2021, 274, 118653. [Google Scholar] [CrossRef] [PubMed]
- Canteri, M.H.; Renard, C.M.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2016, 52, 456–506. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2013, 100, 9–16. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier Transform Infrared Microspectroscopy Is a New Way to Look at Plant Cell Walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apple development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2012, 8, 29–42. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainabiliy Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A Novel Pectin Material: Extraction, Characterization and Gelling Properties. Int. J. Mol. Sci. 2010, 11, 3686–3695. [Google Scholar] [CrossRef]
- Baum, A.; Dominiak, M.; Vidal-Melgosa, S.; Willats, W.G.; Søndergaard, K.M.; Hansen, P.W.; Meyer, A.S.; Mikkelsen, J.D. Prediction of Pectin Yield and Quality by FTIR and Carbohydrate Microarray Analysis. Food Bioprocess Technol. 2017, 10, 143–154. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Rodi Kandri, Y.; Ouazzani Chahdi, F.; Touzani, H.; Errachidi, F. Yield, Esterification Degree and Molecular Weight Evaluation of Pectins Isolated from Orange and Grapefruit Peels under Different Conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Ni, Y.; Yates, K.M.; Zarzycki, R. High Molecular Weight, Low Methodyl Pectins, and Their Production and Uses. U.S. Patent No. 2003/0220485, 27 November 2023. [Google Scholar]
- Ghoshal, G.; Negi, P. Isolation of pectin from kinnow peels and its characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.; Oduro, I.N.; Morris, G.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Fellah, A.; Anjukandi, P.; Waterland, M.R.; Williams, M.A. Determining the degree of methylesterification of pectin by ATR/FT-IR: Methodology optimisation and comparison with theoretical calculations. Carbohydr. Polym. 2009, 78, 847–853. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Wei, M.-C.; Hong, S.-J.; Huang, T.-C.; Lee, S.-Z. Development/optimization of a green procedure with ultrasound-assisted improved supercritical carbon dioxide to produce extracts enriched in oleanolic acid and ursolic acid from Scutellaria barbata D. Don. Ind. Crops Prod. 2013, 49, 542–553. [Google Scholar] [CrossRef]
- Olsson, A.-M.; Salmén, L. The association of water to cellulose and hemicellulose in paper examined by FTIR spectroscopy. Carbohydr. Res. 2004, 339, 813–818. [Google Scholar] [CrossRef]

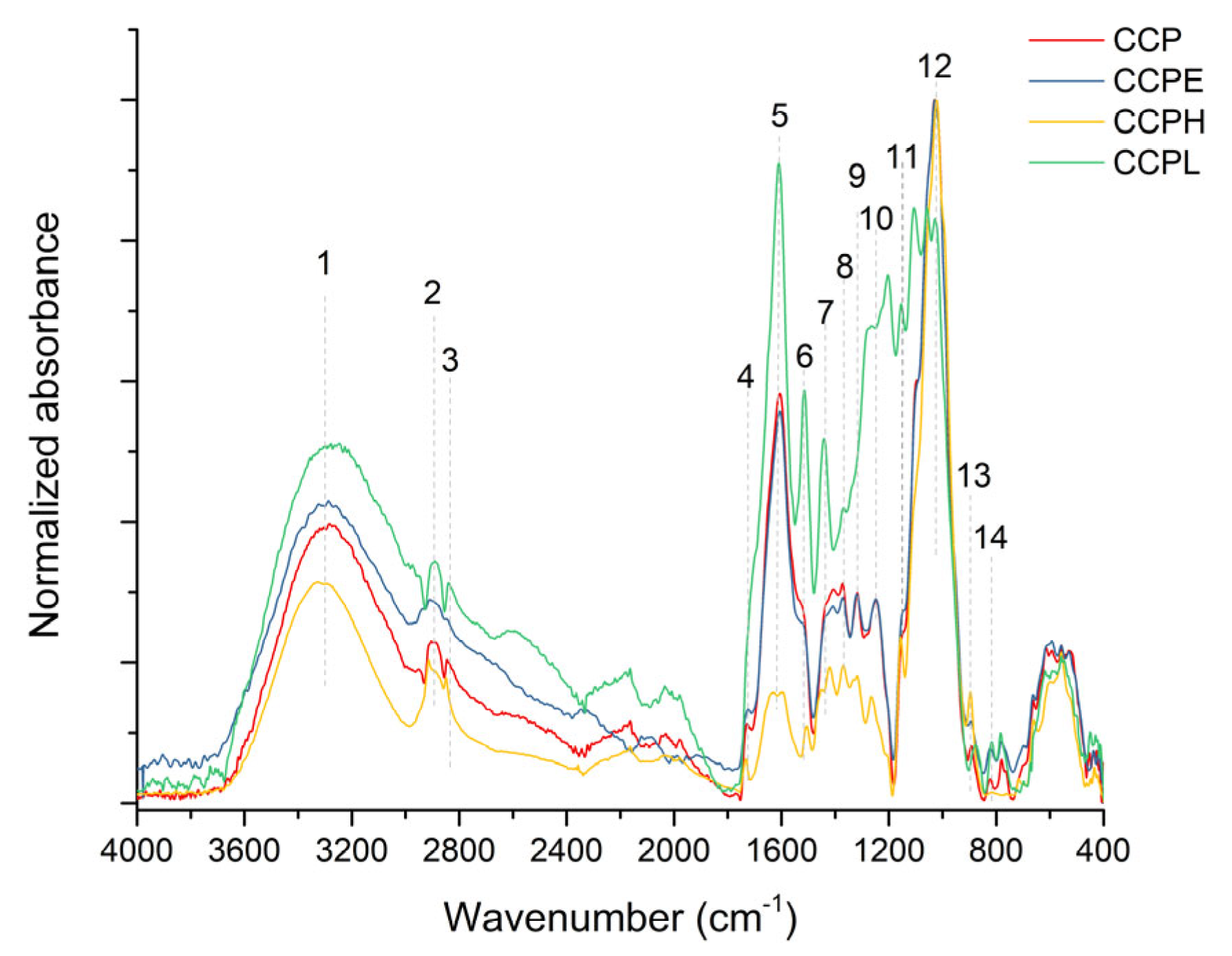
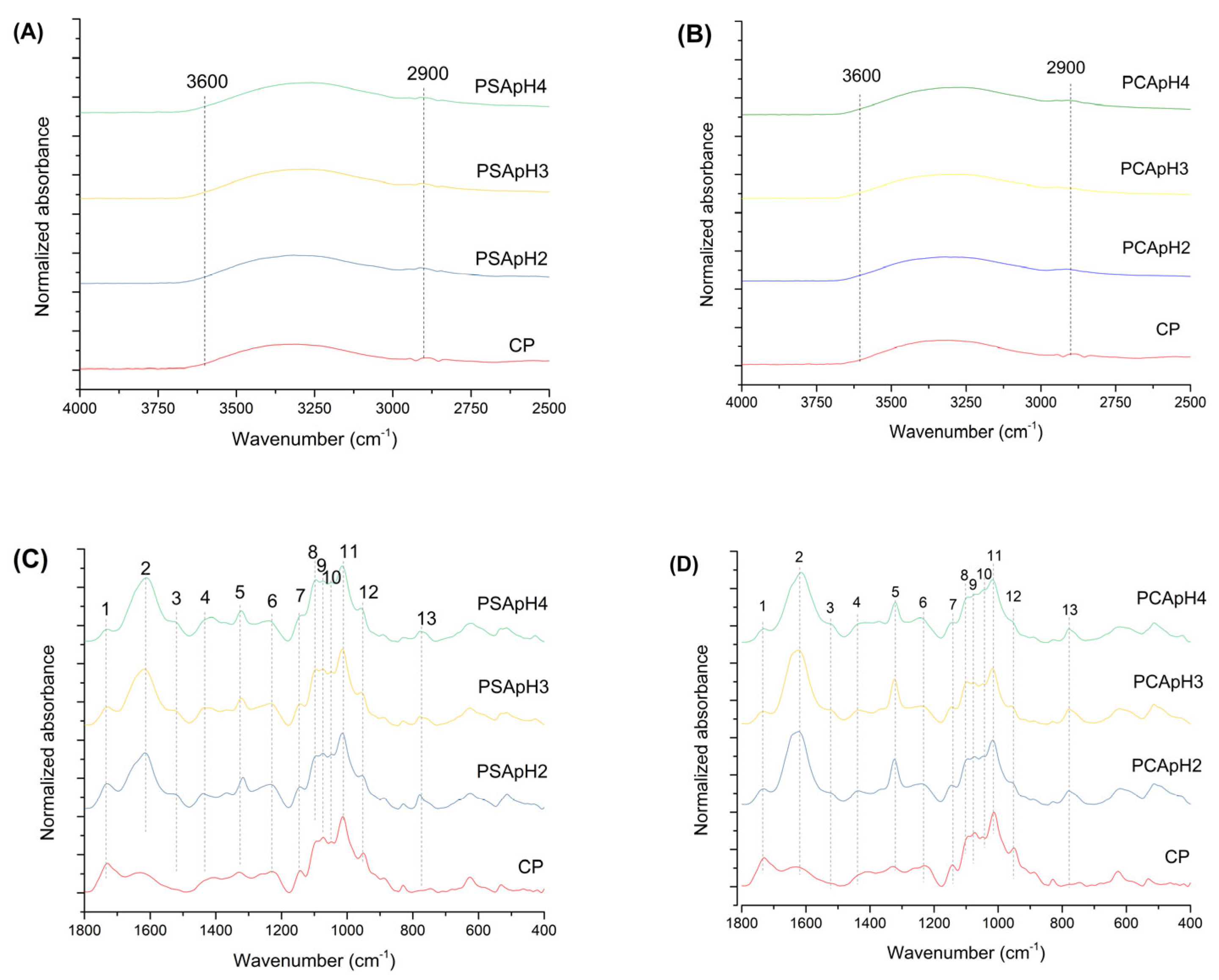

| Property | Content (wt.%) |
|---|---|
| Extractable | 13.4 ± 0.5 |
| Hemicellulose | 48 ± 8 |
| Lignin | 19 ± 10 |
| Cellulose | 20 ± 1 |
| Protein | 5.3 ± 0.2 |
| Fat | 7.3 ± 0.1 |
| Fiber | 23 ± 1 |
| Moisture | 9.2 ± 0.0 |
| Ash (%) | 8.0 ± 0.1 |
| Carbohydrate | 46.9 ± 0.0 |
| Sulfuric Acid | Citric Acid | |||||
|---|---|---|---|---|---|---|
| pH | Pectin (%) * | DE (%) | MeO (%) | Pectin (%) * | DE (%) | MeO (%) |
| 2 | 2.6 ± 0.2 | 18 ± 4.27 a | 3.0 ± 0.7 a | 2.5 ± 0.2 | 7.4 ± 0.3 b | 1.12 ± 0.04 b |
| 3 | 2.4 ± 0.2 | 12.8 ± 0.6 ab | 2.1 ± 0.1 ab | 2.3 ± 0.3 | 12.5 ± 0.6 ab | 1.9 ± 0.1 a |
| 4 | 2.5 ± 0.7 | 9 ± 3 b | 1.5 ± 0.5 a | 2.3 ± 0.2 | 6.9 ± 0.5 b | 1.13 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Gómez, I.; Carrera-Lanestosa, A.; González-Alejo, F.A.; Guerra-Que, Z.; García-Alamilla, R.; Rivera-Armenta, J.L.; García-Alamilla, P. Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy. ChemEngineering 2025, 9, 25. https://doi.org/10.3390/chemengineering9020025
Santiago-Gómez I, Carrera-Lanestosa A, González-Alejo FA, Guerra-Que Z, García-Alamilla R, Rivera-Armenta JL, García-Alamilla P. Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy. ChemEngineering. 2025; 9(2):25. https://doi.org/10.3390/chemengineering9020025
Chicago/Turabian StyleSantiago-Gómez, Ismael, Areli Carrera-Lanestosa, Fanny Adabel González-Alejo, Zenaida Guerra-Que, Ricardo García-Alamilla, José Luis Rivera-Armenta, and Pedro García-Alamilla. 2025. "Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy" ChemEngineering 9, no. 2: 25. https://doi.org/10.3390/chemengineering9020025
APA StyleSantiago-Gómez, I., Carrera-Lanestosa, A., González-Alejo, F. A., Guerra-Que, Z., García-Alamilla, R., Rivera-Armenta, J. L., & García-Alamilla, P. (2025). Pectin Extraction Process from Cocoa Pod Husk (Theobroma cacao L.) and Characterization by Fourier Transform Infrared Spectroscopy. ChemEngineering, 9(2), 25. https://doi.org/10.3390/chemengineering9020025








