Operative Improvement in the Naphtha Catalytic Reforming Process to Reduce the Environmental Impact of Benzene Fugitive Emissions from Gasoline
Abstract
1. Introduction
1.1. NCR and Low-Benzene Gasoline Production
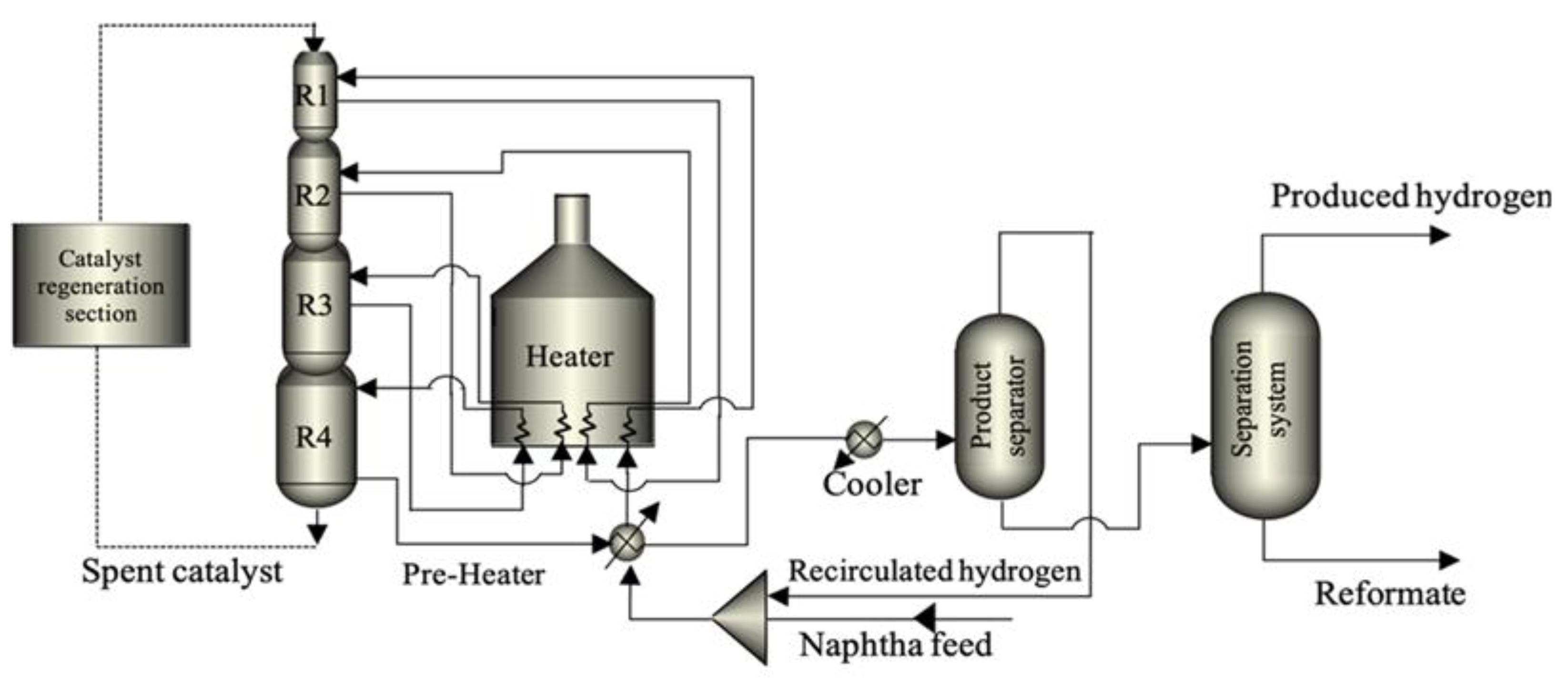
1.2. Description of the NCR Process
1.3. Advancements in Sustainable Fuel Production
1.4. Strategies for Benzene Reduction in Gasoline
1.5. Environmental and Public Health Impacts from Benzene Exposure
2. Materials and Methods
2.1. Mass Balance of Benzene Emissions and Scenario Analysis
2.2. Simulation Design
2.3. Surface Response Method (SRM)
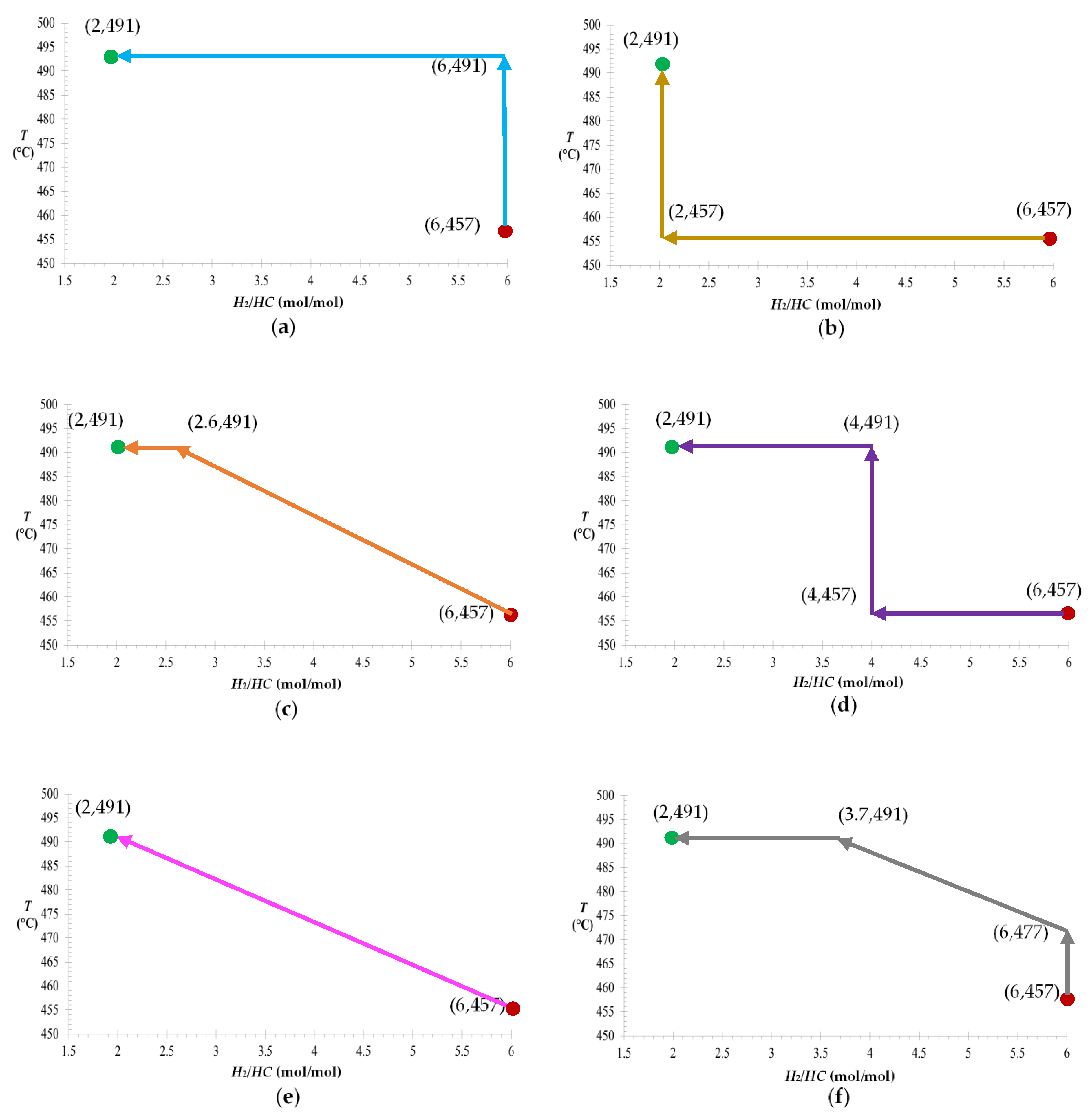
2.4. Procedure for Identifying an Optimal TOR
2.5. Multi-Objective Optimization
2.6. Pilot Plant Experiments
- Preparing the pilot plant and conditioning, including filling the reactors with the catalyst, cleaning the filters of the separation system, and filling the feed tank with non-reformed naphtha.
- Checking the pipelines by performing leaking tests using nitrogen and then using hydrogen to detect leaks even with smaller molecules.
- Catalyst heating up and setting under inert conditions using nitrogen.
- Pilot plant stabilization under the previously settled experimental conditions, before starting a test.
- Feeding the system with hydro-desulfurized naphtha.
- Confirming the mass balance.
- Starting the heating up of the reactor section.
- Initiating operation, monitoring, and on/off-line sampling of reformation products.
2.7. Analytical Methods for the NCR Products and Byproducts
2.7.1. GC for Detailed Hydrocarbons Analysis (DHA)
2.7.2. Multidimensional GC
2.8. GC Measurement and Estimation of the RON
3. Results
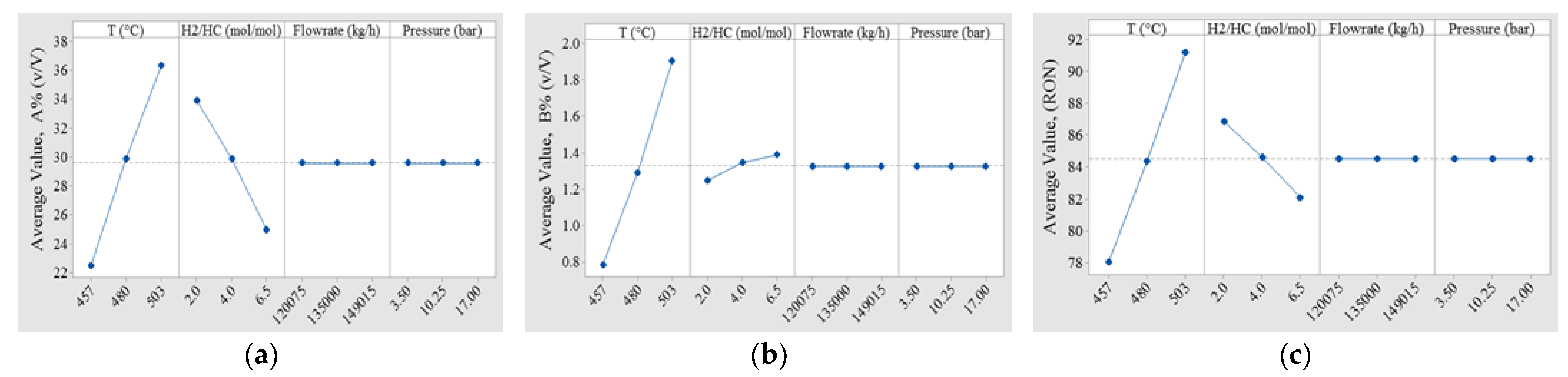
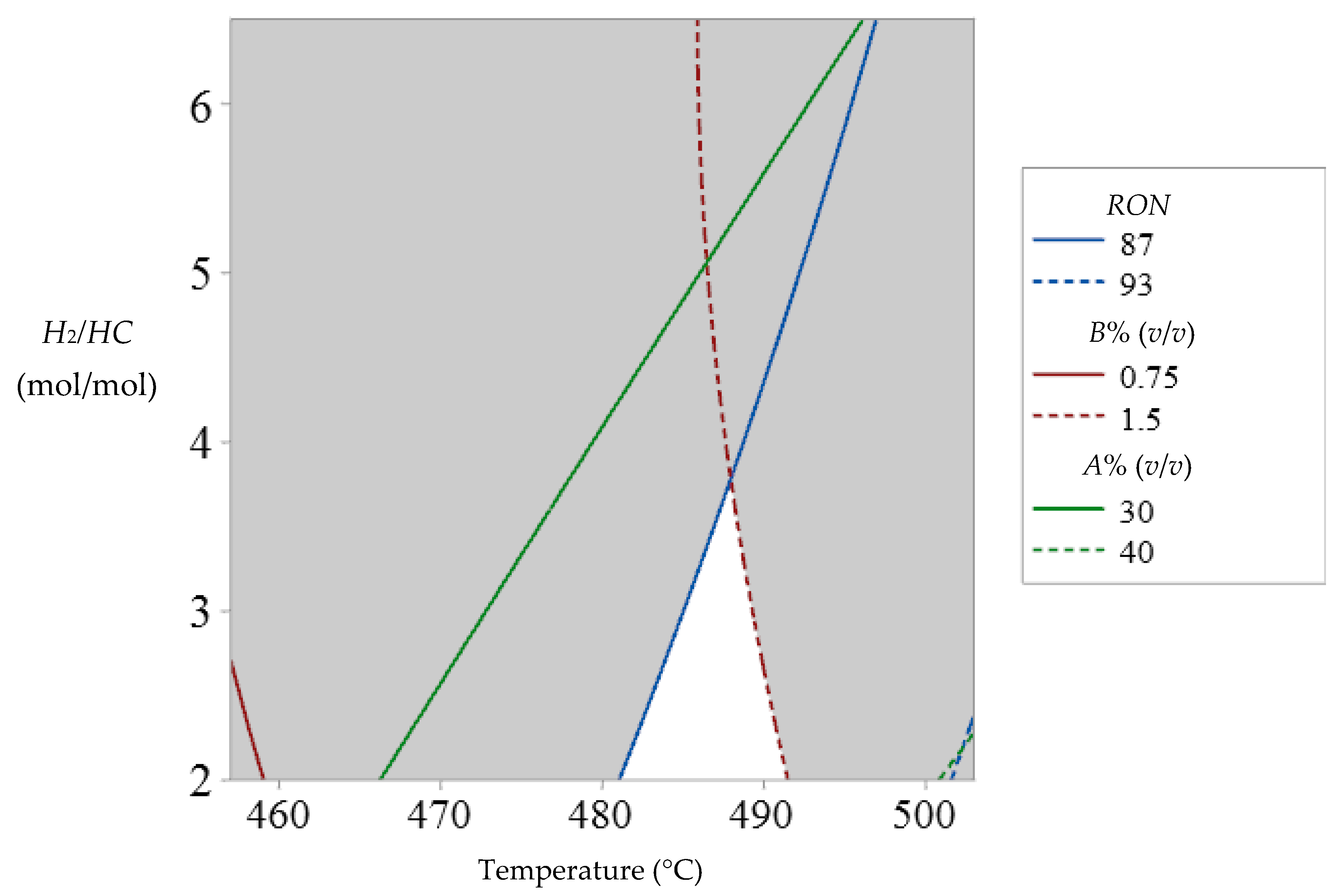
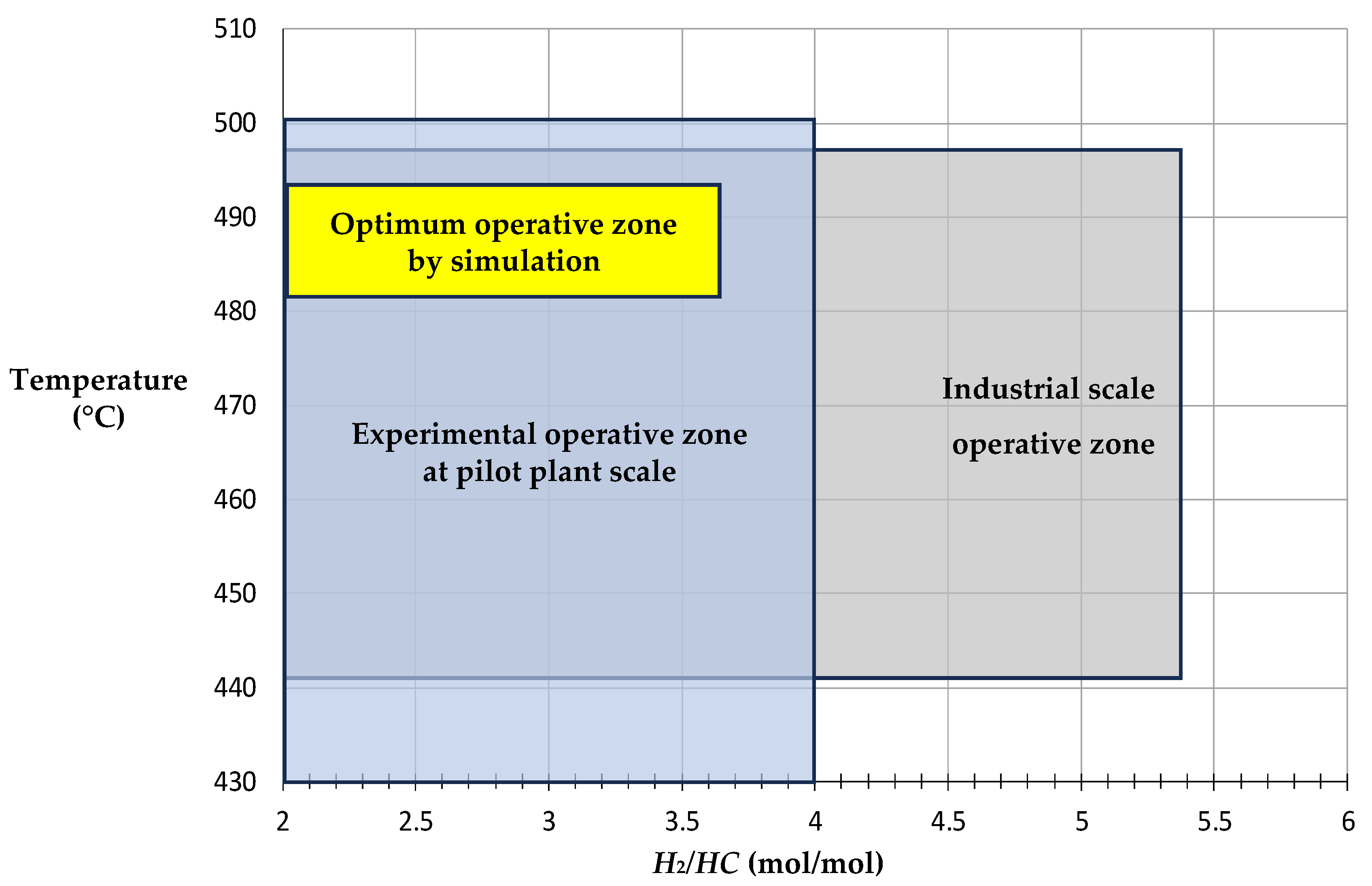
3.1. Operative Zones and Transitional Operative Routes (TOR)
- RON values between 87 and 93.
- Aromatic compound content between 30 and 40% (v/v).
- Benzene content between 0.75 and 1.5% (v/v).
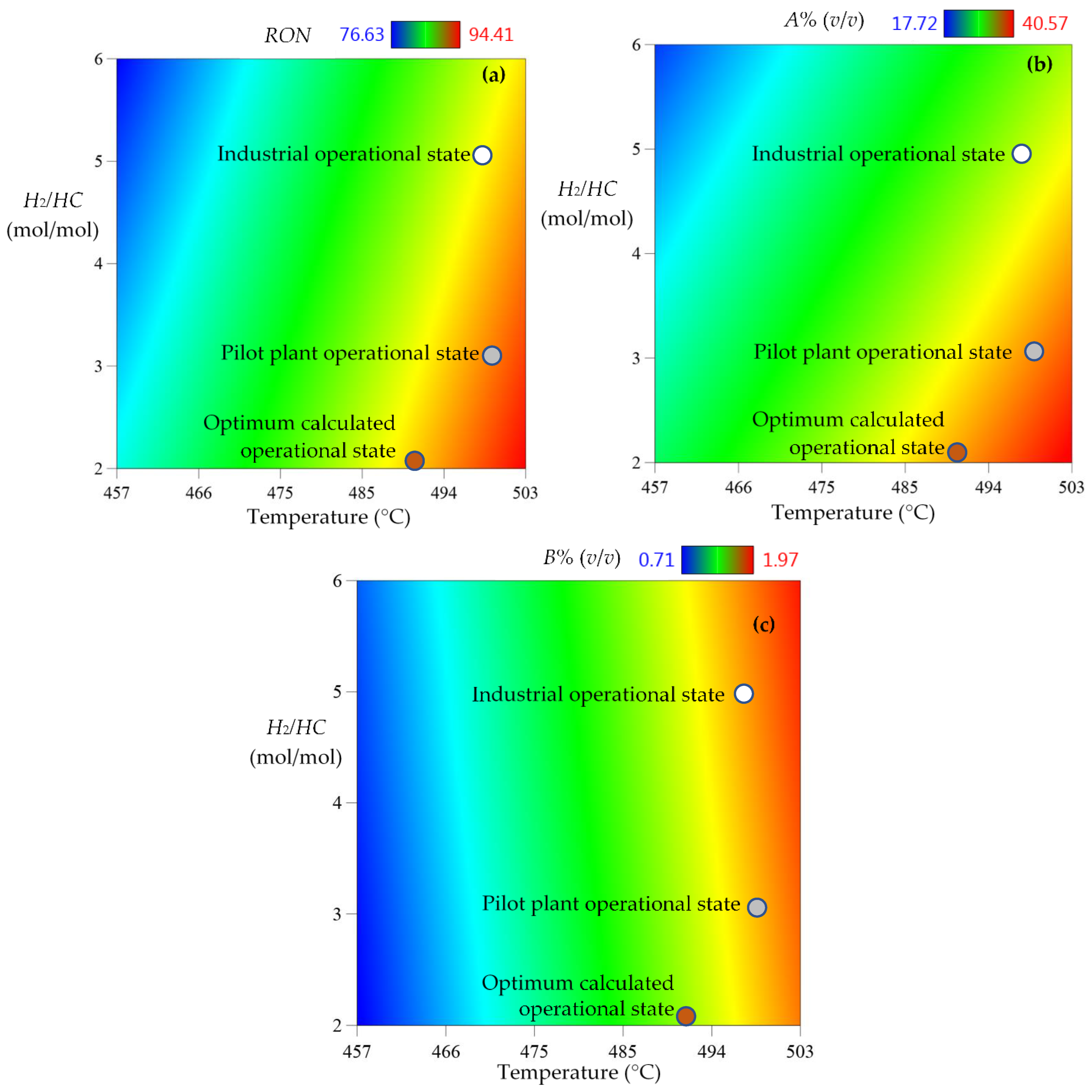
3.2. NCR Operative Improvement
3.3. Experimental Results from the Pilot Plant
Analytical Results from Non-Reformed and Reformed Naphtha
3.4. Comparisons Between Industrial Data, Pilot Plant and Simulation Results
4. Discussion
4.1. Production of High-Quality Fuels
4.2. NCR Optimization for Fuels Benzene Reduction
4.3. Potential Challenges
4.4. Comparative Analysis
4.5. Environmental Benefits
4.6. Further Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable Symbol | Description | Benzene Content Scenarios %(v/v) | |||
|---|---|---|---|---|---|
| 2.0 | 1.5 | 1.0 | 0.75 | ||
| F | Total gasoline production from refineries (installed capacity in Mexico) | 252.4 | 252.4 | 252.4 | 252.4 |
| XB,F | Total benzene content of gasoline produced | 5.048 | 3.786 | 2.524 | 1.893 |
| E1 | Fugitive benzene emissions from the NCR process (2%) | 0.101 | 0.076 | 0.050 | 0.038 |
| S1 | Gasoline production internally sent to storage terminals | 252.29 | 252.32 | 252.35 | 252.36 |
| XB,S1 | Benzene content of produced gasoline | 5.046 | 3.785 | 2.524 | 1.893 |
| I | Imported gasoline | 419.50 | 419.50 | 419.50 | 419.50 |
| XB,I | Benzene content of imported gasoline (0.75% v/v) | 3.146 | 3.146 | 3.146 | 3.146 |
| E2 | Fugitive benzene emissions from storage of imported and nationally produced gasoline (6.7%) | 0.548 | 0.464 | 0.379 | 0.337 |
| S2 | Total gasoline stock for distribution | 671.24 | 671.35 | 671.47 | 671.52 |
| XB,S2 | Total benzene content in gasoline for distribution | 7.644 | 6.467 | 5.291 | 4.702 |
| TD | Gasoline transported by duct (inlet) (65%) | 436.31 | 436.37 | 436.46 | 436.49 |
| XB,TD | Benzene content of gasoline transported by duct | 4.969 | 4.204 | 3.439 | 3.056 |
| TC | Gasoline transported by tank car (inlet) (26%) | 174.52 | 174.55 | 174.58 | 174.59 |
| XB,TC | Benzene content of gasoline transported by tank car | 1.987 | 1.681 | 1.375 | 1.222 |
| TB | Gasoline transported by tanker (inlet) (6%) | 40.274 | 40.281 | 40.288 | 40.291 |
| XB,TB | Benzene content of gasoline transported by tanker | 0.459 | 0.388 | 0.317 | 0.282 |
| TT | Gasoline transported by train (inlet) (3%) | 20.137 | 20.141 | 20.144 | 20.145 |
| XB,TT | Benzene content of gasoline transported by train | 0.229 | 0.197 | 0.158 | 0.141 |
| E3 | Fugitive benzene emissions from duct transport (2.5%) | 0.124 | 0.105 | 0.086 | 0.076 |
| E4 | Fugitive benzene emissions from tank car transport (0.4%) | 0.0079 | 0.0067 | 0.0055 | 0.0049 |
| E5 | Fugitive benzene emissions from tanker transport (0.4%) | 0.0018 | 0.0015 | 0.0012 | 0.0011 |
| E6 | Fugitive benzene emissions from train transport (0.4%) | 0.00092 | 0.00078 | 0.00063 | 0.00056 |
| TDs | Gasoline transported by duct (outlet) | 436.19 | 436.26 | 436.37 | 436.41 |
| TCs | Gasoline transported by tank car (outlet) | 174.51 | 174.54 | 174.58 | 174.59 |
| TBs | Gasoline transported by tanker (outlet) | 40.272 | 40.279 | 40.286 | 40.289 |
| TTs | Gasoline transported by train (outlet) | 20.136 | 20.140 | 20.143 | 20.144 |
| XB,TDs | Benzene content of gasoline transported by duct (outlet) | 4.845 | 4.099 | 3.353 | 2.980 |
| XB,TCs | Benzene content of gasoline transported by tank car (outlet) | 1.979 | 1.674 | 1.369 | 1.217 |
| XB,TBs | Benzene content of gasoline transported by tanker (outlet) | 0.457 | 0.386 | 0.315 | 0.280 |
| XB,TTs | Benzene content of gasoline transported by train (outlet) | 0.228 | 0.196 | 0.157 | 0.140 |
| S3 | Total gasoline to final storage terminals | 671.10 | 671.22 | 671.37 | 671.43 |
| XB,S3 | Benzene content of gasoline to final storage terminals | 7.509 | 6.356 | 5.196 | 4.618 |
| E7 | Fugitive benzene emissions from final storage terminals (6.7%) | 0.503 | 0.426 | 0.348 | 0.309 |
| S4 | Total gasoline to be distributed at gasoline stations | 670.60 | 670.80 | 671.03 | 671.12 |
| XB,S4 | Benzene content of gasoline to be distributed at gasoline stations | 7.006 | 5.930 | 4.847 | 4.309 |
| E8 | Fugitive benzene emissions from gasoline transported by tank car to gasoline stations (0.4%) | 0.028 | 0.023 | 0.019 | 0.017 |
| S5 | Total gasoline dispensed at gasoline stations | 670.57 | 670.77 | 671.01 | 671.11 |
| XB,S5 | Benzene content of gasoline stored at gasoline stations | 6.980 | 5.906 | 4.828 | 4.292 |
| E9 | Benzene fugitive emissions from gasoline stored and dispensed at gasoline stations (0.5%) | 0.035 | 0.029 | 0.024 | 0.021 |
| S6 | Total gasoline dispensed from gasoline stations | 670.54 | 670.74 | 670.98 | 671.08 |
| XB,S6 | Benzene content of gasoline dispensed to vehicles | 6.943 | 5.877 | 4.804 | 4.270 |
| E10 | Fugitive benzene emissions from vehicles (2%) | 0.139 | 0.117 | 0.096 | 0.085 |
References
- Liu, Y.; Lu, S.; Yan, X.; Gao, S.; Cui, X.; Cui, Z. Life cycle assessment of petroleum refining process: A case study in China. J. Clean. Prod. 2020, 256, 120422. [Google Scholar] [CrossRef]
- Barton, C.C. Benzene. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Elsevier: Washington, DC, USA, 2024; Volume 1, pp. 961–966. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Y.; Li, Z.; Lv, Z.; Zhang, J.; Song, A.; Wang, T.; Wu, L.; Mao, H.; Peng, J. Tailpipe volatile organic compounds (VOCs) emissions from Chinese gasoline vehicles under different vehicles standards, fuel types, and driving conditions. Atmos. Environ. 2024, 323, 120348. [Google Scholar] [CrossRef]
- Li, Q.; Padilla, L.; Thompson, T.; Xiao, S.; Mohr, E.J.; Zhou, X.; Kacharava, N.; Cui, Y.; Wang, C. A modeling framework to assess fenceline monitoring and self-reported upset emissions of benzene from multiple oil refineries in Texas. Atmos. Environ. X 2024, 23, 100281. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chang, C.-F.; Lai, C.-H.; Peng, Y.-P.; Su, Y.-J.; Chen, G.-F. Multivariate analysis of carcinogenic equivalence (CEQ) to characterize carcinogenic VOC emissions in a typical petrochemical industrial park in Taiwan. Environ. Int. 2024, 186, 108548. [Google Scholar] [CrossRef] [PubMed]
- Hienuki, S. Environmental and socio-economic analysis of naphtha reforming hydrogen energy using input-output tables: A case study from Japan. Sustainability 2017, 9, 1376. [Google Scholar] [CrossRef]
- Zahed, M.A.; Salehi, S.; Khoei, M.A.; Esmaeili, P.; Mohajeri, L. Risk assessment of Benzene, Toluene, Ethyl benzene, and Xylene (BTEX) in the atmospheric air around the world: A review. Toxic. Vitr. 2024, 98, 105825. [Google Scholar] [CrossRef] [PubMed]
- Alcántar-González, F.S.; Elizalde-Segovia, R.; Olvera-Santos, M.G.; López-Herrera, D.; Cruz-Gómez, M.J. Análisis del contenido de benceno en las gasolinas y estimación de emisiones de este compuesto al ambiente. Rev. Int. Contam. Ambient. 2020, 36, 321–331. [Google Scholar] [CrossRef]
- Romo, D. Refinación del petróleo en México y perspectiva de la reforma energética. Probl. Des. 2016, 47, 139–164. [Google Scholar] [CrossRef]
- Li, Z.; Ying, Y.; Yang, M.; Zhao, L.; Zhao, L.; Du, W. Monitoring and path optimization of catalytic reformer in a refinery: Principal component analysis and A* algorithm application. Expert Syst. Appl. 2022, 209, 118358. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Aitani, A.M.; Ummer, A.C.; Alasiri, H.S. Review of the catalytic conversion of naphtha to aromatics: Advances and outlook. Energy Fuels 2023, 37, 2586–2607. [Google Scholar] [CrossRef]
- Aznárez, A.; Korilli, S.A.; Gil, A. Progress and recent novelties in naphtha reforming catalysts. J. Environ. Chem. Eng. 2024, 12, 113066. [Google Scholar] [CrossRef]
- Atarianshandiz, M.; McAuley, K.B.; Shahsavand, A. Modeling and parameter tuning for continuous catalytic reforming of naphtha in an industrial reactor system. Process 2023, 11, 2838. [Google Scholar] [CrossRef]
- Zapf, F.; Wallek, T. Case-study of a flowsheet simulation using deep-learning process models for multi-objective optimization of petrochemical production plants. Comp. Chem. Eng. 2022, 162, 107823. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Nakhjiri, A.T.; Rezakazemi, M.; Marjani, A.; Shirazian, S. Mechanistic model and numerical simulation of axial flow catalytic reactor for naphtha reforming unit. PLoS ONE 2020, 15, e0242343. [Google Scholar] [CrossRef] [PubMed]
- Boukezoula, T.F.; Bencheikh, L.; Belkhiat, D.E.C. A heterogeneous model for a cylindrical fixed bed axial flow reactors applied to a naphtha reforming process with a non-uniform catalyst distribution n in the pellet. React. Kinet. Mech. Catal. 2020, 131, 335–351. [Google Scholar] [CrossRef]
- Yusuf, A.Z.; John, Y.M.; Aderemi, B.O.; Patel, R.; Mujtaba, I.M. Modelling simulation and sensitivity analysis of naphtha catalytic reforming reactions. Comp. Chem. Eng. 2019, 130, 106531. [Google Scholar] [CrossRef]
- Issa, H.M. Streamlining aromatic content detection in automotive gasoline for environmental protection: Utilizing a rapand simplified prediction model based on some physical characteristics and regression analysis. Results Eng. 2024, 21, 101771. [Google Scholar] [CrossRef]
- Martínez, J.; Zúñiga-Hinojosa, M.A.; Ruiz- Martínez, R.S. A thermodynamic analysis of naphtha catalytic reforming reactions to produce high octane gasoline. Process 2022, 10, 313. [Google Scholar] [CrossRef]
- Petrova, D.A.; Gushchin, P.A.; Ivanov, E.V.; Lyubimenko, V.A.; Kolesnikov, I.M. Modelling industrial catalytic reforming of low octane gasoline. Chem. Technol. Fuels Oils 2021, 57, 143–159. [Google Scholar] [CrossRef]
- Ivanchina, E.; Chernyakova, E.; Pchelintseva, I.; Poluboyartsev, D. Mathematical modelling and optimization of semi-regenerative catalytic reforming of naphtha. Oil Gas Sci. Technol.—Rev. IFP Energies Nouv. 2021, 76, 64–75. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Jafari, M.; Rafiei, R.; Amiri, S.; Karimi, M.; Iranshahi, D.; Rahimpour, M.R.; Mahdiyar, H. Combining continuous catalytic regenerative naphtha reformer with thermally coupled concept for improving the process yield. Int. J. Hydrogen Energy 2013, 38, 10327–10344. [Google Scholar] [CrossRef]
- Hou, W.F.; Su, H.Y.; Mu, S.J.; Chu, J. Multiobjective optimization of the industrial catalytic naphtha reforming process. Chin. J. Chem. Eng. 2007, 15, 75–80. [Google Scholar] [CrossRef]
- Belgamwar, R.; Santosh, K.; Valavarasu, G.; Sarawade, P.; Polshettivar, V. Tuning the interfacial interactions between alumina support and pseudo single atom platinum-tin catalytic sites for heavy naphtha reforming. Catal. Today 2024, 432, 114606. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Shakeel, M.R.; Harale, A.; Paglieri, S.; Mansour, R.B. Fuel reforming processes for hydrogen production. Fuel 2024, 359, 130427. [Google Scholar] [CrossRef]
- Velázquez- Alonso, F.; González-Ramírez, C.A.; Villagómez-Ibarra, J.R.; Otazo-Sánchez, E.M.; Hernández-Juárez, M.; Pérez-Villaseñor, F.; Castro-Agüero, A.; Alemán-Vázquez, L.O. Transitional analysis for multi-objective operative improvement of reformate quality and hydrogen production from a naphtha catalytic reforming process. Heliyon 2025, 11, e41428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Li, Y.; Wu, J.; Zhang, L. Explicit molecule-based reaction network simplification: Theory and application on catalytic reforming. Chem. Eng. Sci. 2023, 277, 118833. [Google Scholar] [CrossRef]
- Mohebi, A.R.; Najafi, F.; Mozzafarian, M.; Dabir, B.; Amrabadi, N.E. Fludynamics and erosion analysis in industrial naphtha reforming: A CFD-DPM simulation approach. Powder Technol. 2024, 436, 119417. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; Tian, J.; Zhang, K.; Ma, H. Feasibility analysis of replacing benzene in gasoline with cyclohexane: Engine performance and emissions. J. Energy Inst. 2025, 119, 101996. [Google Scholar] [CrossRef]
- Laredo, G.C.; Quintana-Solórzano, R.; Castillo, J.J.; Armendáriz-Herrera, H.; García-Gutiérrez, J.L. Benzene reduction in gasoline by alkylation with propylene over MCM-22 zeolite with a different Brønsted-Lewis acidity ratios. Appl. Catal. A Gen. 2013, 454, 37–45. [Google Scholar] [CrossRef]
- Rostamikia, T.; Parsafard, N.; Peyrovi, M.H. Benzene reduction in reformate gasoline by competitive hydrogenation: Effect of the preparation method. ChemistrySelect 2019, 4, 4861–4866. [Google Scholar] [CrossRef]
- Verma, D.K.; des Tombe, K. Benzene in gasoline and crude oil: Occupational and environmental implications. Am. Ind. Hyg. Assoc. J. 2002, 63, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Soni, D.; Aggarwal, S.G. Benzene: A critical review on measurement methodology, certified reference material, exposure limits with its impact on human health and mitigation strategies. Environ. Anal. Health Toxicol. 2024, 39, e2024012. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Asuquo, A.E. Recent advances in occupational and environmental health hazards of workers exposed to gasoline compounds. Int. J. Occup. Med. Environ. Health 2017, 30, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Weisel, C.P. Benzene exposure: An overview of monitoring methods and their findings. Chem.-Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fetisov, V.; Gonopolsky, A.M.; Davardoost, H.; Ghanbari, A.R.; Mohammadi, A.H. Regulation and impact of VOC and CO2 emissions in low carbon energy systems resilient to climate change: A case study on an environmental issue in the oil and gas industry. Energy Sci. Eng. 2022, 11, 1516–1535. [Google Scholar] [CrossRef]
- Secretaría de Energía, México. Estadísticas de Petrolíferos (Gasolina). Available online: https://estadisticashidrocarburos.energia.gob.mx/gas.aspx (accessed on 1 October 2024).
- Velázquez-Alonso, F.; (TECNM campus Pachuca, Pachuca, Hidalgo, México). Personal communication, 2024.
- González-Ramírez, C.A.; (Universidad Autónoma del Estado de Hidalgo, Mineral de la Reforma, Hidalgo, México). Personal communication, 2024.
- EPA. Emission Factor Documentation for AP-42 Section 7.1 Organic Liqustorage Tanks Final Report, September 2006. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100NVV2.txt (accessed on 22 October 2024).
- Mata, T.M.; Smith, R.L.; Young, D.M.; Costa, C.A.V. Life cycle assessment of gasoline blending options. Environ. Sci. Technol. 2003, 37, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Sheartson, J.A.; Hilpert, M. Gasoline vapor emissions during vehicle refueling events in a vehicle fleet saturated with onboard refueling vapor recovery systems: Need for an exposure assessment. Front. Public Health 2020, 8, 18. [Google Scholar] [CrossRef]
- Hilpert, M.; Mora, B.A.; Ni, J.; Rule, A.M.; Nachman, K.E. Hydrocarbon release during fuel storage and transfer at gas stations: Environmental and health effects. Curr. Environ. Health Rep. 2015, 2, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Diario Oficial de la Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5450011&fecha=29/08/2016#gsc.tab=0 (accessed on 22 October 2024).
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Gutiérrez Pulido, H.; De la Vara Salazar, R. Análisis y Diseño de Experimentos, 2nd ed.; McGraw-Hill/ Interamericana Eds.: Mexico City, Mexico, 2008; pp. 384–420. [Google Scholar]
- Yusup, S.; Khan, Z.; Ahmad, M.M.; Rashidi, N.A. Optimization of hydrogen production in in-situ catalytic adsorption (ICA) steam gasification based on Response Surface Methodology. Biomass-Bioenergy 2014, 60, 98–107. [Google Scholar] [CrossRef]
- Al-Jamimi, H.A.; BinMakhashen, G.M.; Deb, K.; Saleh, T.A. Multiobjective optimization and analysis of petroleum catalytic processes: A review. Fuel 2021, 288, 119678. [Google Scholar] [CrossRef]
- ASTM D6729-20; Standard Test Method for Determination of Individual Components in Spark Ignition Engine Fuels by 100 Meter Capillary High Resolution Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2020; p. 51.
- ASTM D6839-21a; Standard Test Method for Hydrocarbon Types, Oxygenated Compounds, Benzene, and Toluene in Spark Ignition Engine Fuels by Multidimensional Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2021; p. 17.
- Cherepitsa, S.V.; Bychkov, S.M.; Gatsikha, S.V.; Kovalenko, A.N.; Mazanik, A.L.; Kuzmenkov, D.E.; Luchinina, Y.L.; Gremyako, N.N. Gas chromatographic analysis of automobile gasolines. Chem. Technol. Fuels Oils 2001, 37, 283–290. [Google Scholar] [CrossRef]
- He, Z.; Zao, W.; Liu, G.; Qian, Y.; Lu, X. Effects of short chain aromatics in gasoline on GDI engine combustion and emissions. Fuel 2021, 297, 120725. [Google Scholar] [CrossRef]
- Health Canada. Benzene Releases from Gasoline Stations: Implications for Human Health, 1st ed.; Government of Canada: Ottawa, ON, Canada, 2023; pp. 1–40. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/benzene-releases-gasoline-stations-implications-human-health.html (accessed on 13 January 2025).
- El-Malky, E.; Umansky, B.; Moy, E.; Donahoe, G.; Thom, T. Gasoline benzene reduction. Dig. Refin. 2013, 4, 1000839. [Google Scholar]
- Ma, X.C.; He, C.; Chen, Q.L.; Zhang, B.J. Modeling and optimization for the continuous catalytic reforming process based on the hybrsurrogate optimization model. Comp. Chem. Eng. 2024, 191, 108841. [Google Scholar] [CrossRef]
- Elfghi, F.M. Catalytic naphtha reforming; challenges for selective gasoline; an overview and optimization case study. J. Adv. Catal. Sci. Technol. 2016, 3, 27–42. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Ali-Abdelkareem, M.; Kamil, M.; Olabi, A.G. Recent trends for introducing promising fuel components to enhance the anti-knock quality of gasoline: A systematic review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Zainullin, R.Z.; Zagoruiko, A.N.; Koledina, K.F.; Gubaidullin, I.M.; Faskhutdinova, R.I. Multi-criterion optimization of a catalytic reforming unit using a genetic algorithm. Catal. Ind. 2020, 12, 133–140. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Z.; Sun, Y.; Jiang, S.; Tian, J. Optimization of the countercurrent continuous reforming process based on equation oriented modeling and the SQP algorithm. ACS Omega 2020, 7, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Abdoulkarimi, V.; Sari, A.; Shokri, S. Robust prediction and optimization of gasoline quality using data-driven adaptive modeling for a light naphtha isomerization reactor. Fuel 2022, 328, 125304. [Google Scholar] [CrossRef]
- Otaraku, I.J.; Egun, I.L. Optimization of hydrogen production from Nigerian crude oil samples through continuous catalyst regeneration (CCR) reforming process using Aspen Hysys. React. Kinet. Catal. Lett. 2017, 5, 69–72. [Google Scholar] [CrossRef][Green Version]
- Laredo, G.C.; Castillo, J.; Cano, J.L. Benzene reduction in gasoline range streams by adsorption process using a PVDC-PVC carbon molecular sieve. Fuel 2014, 135, 459–467. [Google Scholar] [CrossRef]
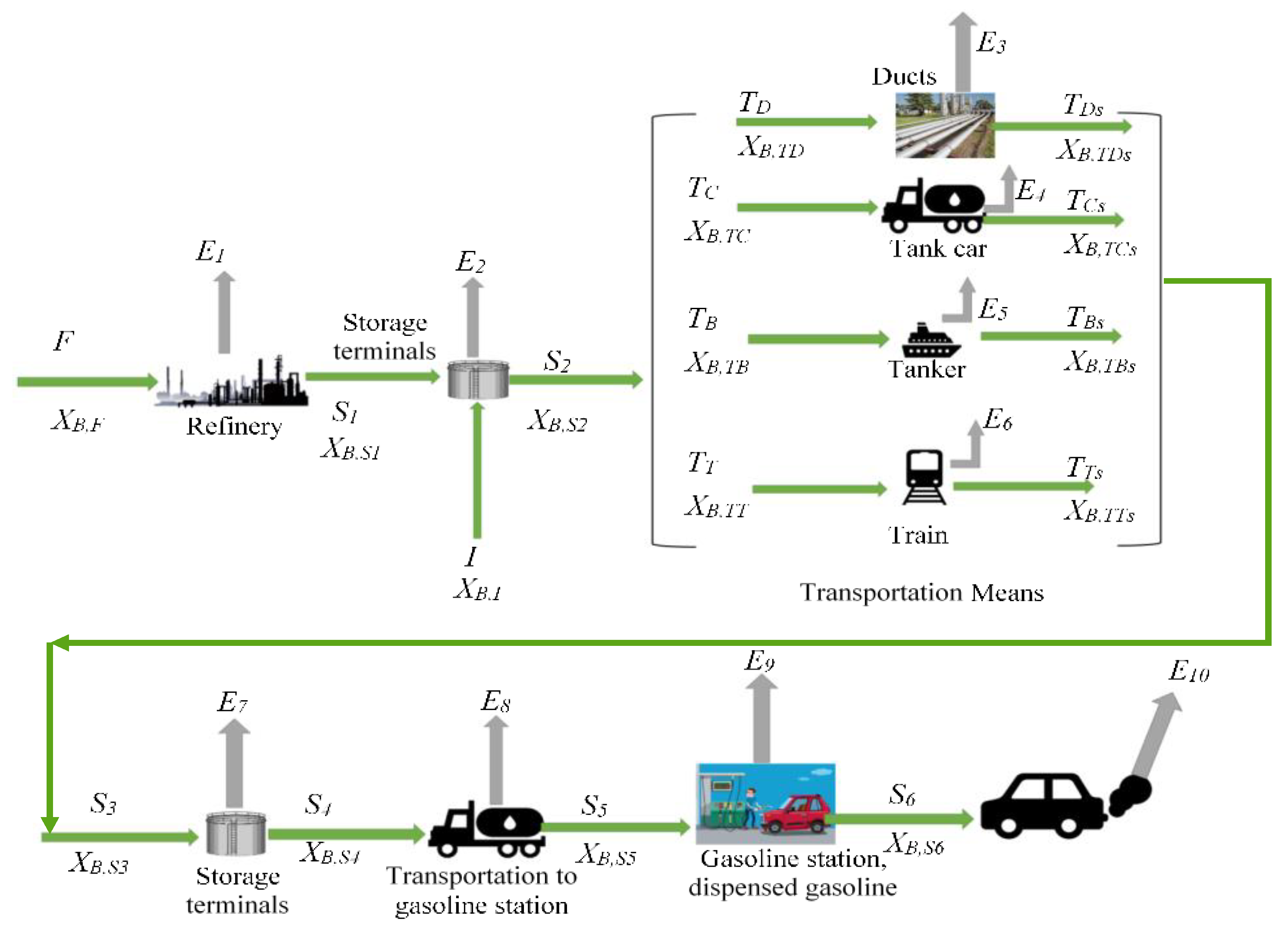


| Evaporation During | Evaporative Loss (% v/v) | Reference |
|---|---|---|
| Tank storage | 6.7 | [41] |
| Ducts transportation | 2.5 | [42] |
| Other transportation means | 0.4 | [43] |
| Dispensing at gasoline stations | 0.5 | [44] |
| Gasoline Benzene Content % (v/v) | Total Gasoline Production to be Sold (KBPD) | Total Benzene Emissions (KBPD) | Total Benzene Content of Dispensed Gasoline (KBPD) |
|---|---|---|---|
| 0.75 | 671.08 | 0.89 | 4.27 |
| 1.00 | 670.98 | 1.01 | 4.80 |
| 1.50 | 670.74 | 1.25 | 5.87 |
| 2.00 | 670.54 | 1.48 | 6.94 |
| Operative Variable | Units | Values | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Temperature (T) | °C | 457.0 | 480.0 | 503.0 |
| H2/HC molar ratio | mol/mol | 2.0 | 4.0 | 6.0 |
| Dependent Variables | Symbol | Units |
|---|---|---|
| Octane index | RON | Dimensionless |
| Volumetric fraction of aromatic compounds | A% | % (v/v) |
| Volumetric fraction of benzene | B% | % (v/v) |
| yi | c0 | c1 | c2 | c3 | c4 | c5 | r2 |
| RON | 59.906 | −0.1559 | −2.2783 | 4.52 ×10−4 | 0.0234 | 0.0021 | 0.9758 |
| A% (v/v) | −315.4 | 1.184 | −3.422 | −9.30 ×10−4 | 0.0154 | 0.0028 | 0.9614 |
| B% (v/v) | 13.10 | −0.0741 | 0.0423 | 1.02 ×10−4 | −0.0071 | 0.0001 | 0.9948 |
| TOR No. | Average Estimated Values Across Each TOR | |||
|---|---|---|---|---|
| RON | A% (v/v) | B% (v/v) | Average Position | |
| 1 | 84.491 (1) | 29.211 (1) | 1.410 (6) | 2.666 |
| 2 | 81.365 (6) | 27.198 (6) | 0.929 (1) | 4.333 |
| 3 | 83.540 (3) | 28.997 (3) | 1.227 (4) | 3.333 |
| 4 | 83.526 (4) | 28.981 (4) | 1.226 (3) | 3.666 |
| 5 | 82.871 (5) | 28.297 (5) | 1.170 (2) | 4.000 |
| 6 | 83.941 (2) | 29.077 (2) | 1.306 (5) | 3.000 |
| Maximum values of the response variables | 89.871 | 37.395 | 1.488 | |
| Day | Data Origin | RON | Volumetric Fraction [% (v/v)] | |||
|---|---|---|---|---|---|---|
| n-Paraffins | Aromatics | Naphthenes | Benzene | |||
| 1 | Simulation | 77.54 | 65.31 | 13.54 | 19.61 | 0.28 |
| Experiments | 79.50 | 68.95 | 12.90 | 18.12 | 0.31 | |
| 2 | Simulation | 84.03 | 56.20 | 24.30 | 15.92 | 0.83 |
| Experiments | 85.93 | 60.48 | 21.03 | 17.54 | 0.77 | |
| 3 | Simulation | 92.25 | 50.91 | 36.54 | 7.49 | 1.98 |
| Experiments | 90.33 | 54.56 | 33.01 | 8.38 | 1.87 | |
| Correlation coefficient (r2) | 0.9844 | 0.9987 | 0.9996 | 0.9693 | 0.9995 | |
| Variables | Data from the Industry | Experimental Data from the Pilot Plant | Results from Simulation |
|---|---|---|---|
| Independent | |||
| Temperature (°C) | 442.9–498.7 | 430.0–500.0 | 482.0–491.0 |
| H2/HC ratio (mol/mol) | 2.0–5.2 | 2.0–4.0 | 2.0–3.5 |
| Objective | |||
| Aromatics [A% (v/v)] | 38.47–58.4 | 12–36 | 30–40 |
| Benzene [B% (v/v)] | 2.58 | 0.28–1.98 | 0.75–1.5 |
| RON | 83.2–92.8 | 77.0–92.0 | 87.0–94.0 |
| Variables | Operative Points | ||
|---|---|---|---|
| Industrial | Pilot Plant | Optimum Simulated | |
| Temperature (°C) | 498.7 | 500 | 491 |
| H2/HC (mol/mol) | 5.00 | 3.00 | 2.00 |
| Aromatics [A% (v/v)] | 34.56 | 36 | 37.39 |
| Benzene [B% (v/v)] | 1.8 | 1.5 | 1.48 |
| RON | 91 | 92 | 89.87 |
| Variable | Units | Reported Data | Reference | This Work | ||
|---|---|---|---|---|---|---|
| Operative State | Optimum Result | Operative State | Optimum Result | |||
| Temperature | °C | 510 | 514.67 | [60] | 482–491 | 491 |
| 400 | 479.6 (R1) 478.5 (R2) 500.0 (R3) | [59] | ||||
| H2/HC ratio | mol /mol | 2.12 | 2.05 | [60] | 2.00–3.50 | 2.00 |
| NR | NR | [59] | ||||
| A | %(v/v) | 53.02 | 53.86 | [60] | 30–40 %(v/v) | 37.39 %(v/v) |
| %(wt) | 56.00 | 45.00 | [59] | |||
| B | --- | NR | NR | [60] | 0.75–1.50 %(v/v) | 1.48 %(v/v) |
| %(wt) | 4.00 | 3.08 | [59] | |||
| RON | --- | 99.30 | 99.70 | [60] | 87–94 | 89.87 |
| 92.70 | 91.80 | [59] | ||||
| Decision Issue | Decision Making | |
|---|---|---|
| Traditional Industrial Approach | Computational/ Experimental Approach | |
| Temperature | To maintain stable values under controlled conditions, avoiding catalyst severity. | To analyze the optimum transition pathways for reaching fuel quality goals. |
| H2/HC ratio | To adjust only when the feedstock flowrates change. | To analyze the optimum TORs for promoting selected reactions. |
| Benzene content | To keep it within environmental regulations. | To minimize the benzene content, even under regulated limits, whilst assuring the reformate quality. |
| Aromatics content | To maintain concentration levels, favoring the final RON of the reformate. | To achieve an adequate concentration by increasing alkylated aromatics without increasing the benzene content. |
| RON | To reach the highest possible value. | To obtain a reasonably high value that can be complemented at the final gasoline blend, without increasing the benzene content. |
| Catalyst lifetime | To extend it as much as possible. | To avoid polymerization reactions and coke formation from the analysis of operative conditions. |
| Coke formation | To solve it in the regeneration unit. | To minimize coke formation by controlling the operative conditions in the reactor. |
| Selectivity | To be assessed only if the RON of gasoline is not achieved. | To promote selectivity from a kinetic analysis by assessing the operative conditions with process simulation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Alonso, F.; González-Ramírez, C.A.; Villagómez-Ibarra, J.R.; Otazo-Sánchez, E.M.; Hernández-Juárez, M.; Pérez-Villaseñor, F.; Castro-Agüero, Á.; Alemán-Vázquez, L.O.; Camacho-López, C.; Romo-Gómez, C. Operative Improvement in the Naphtha Catalytic Reforming Process to Reduce the Environmental Impact of Benzene Fugitive Emissions from Gasoline. ChemEngineering 2025, 9, 21. https://doi.org/10.3390/chemengineering9020021
Velázquez-Alonso F, González-Ramírez CA, Villagómez-Ibarra JR, Otazo-Sánchez EM, Hernández-Juárez M, Pérez-Villaseñor F, Castro-Agüero Á, Alemán-Vázquez LO, Camacho-López C, Romo-Gómez C. Operative Improvement in the Naphtha Catalytic Reforming Process to Reduce the Environmental Impact of Benzene Fugitive Emissions from Gasoline. ChemEngineering. 2025; 9(2):21. https://doi.org/10.3390/chemengineering9020021
Chicago/Turabian StyleVelázquez-Alonso, Fabiola, César Abelardo González-Ramírez, José Roberto Villagómez-Ibarra, Elena María Otazo-Sánchez, Martín Hernández-Juárez, Fernando Pérez-Villaseñor, Ángel Castro-Agüero, Laura Olivia Alemán-Vázquez, César Camacho-López, and Claudia Romo-Gómez. 2025. "Operative Improvement in the Naphtha Catalytic Reforming Process to Reduce the Environmental Impact of Benzene Fugitive Emissions from Gasoline" ChemEngineering 9, no. 2: 21. https://doi.org/10.3390/chemengineering9020021
APA StyleVelázquez-Alonso, F., González-Ramírez, C. A., Villagómez-Ibarra, J. R., Otazo-Sánchez, E. M., Hernández-Juárez, M., Pérez-Villaseñor, F., Castro-Agüero, Á., Alemán-Vázquez, L. O., Camacho-López, C., & Romo-Gómez, C. (2025). Operative Improvement in the Naphtha Catalytic Reforming Process to Reduce the Environmental Impact of Benzene Fugitive Emissions from Gasoline. ChemEngineering, 9(2), 21. https://doi.org/10.3390/chemengineering9020021







