Experimental Data and Thermodynamics Modeling (eNRTL and mUNIFAC) of the (Cyclohexane + Benzene + N,N-Dimethylformamide + Sodium Thiocyanate) Systems
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Apparatus and Procedures for Equilibrium Liquid–Liquid
2.3. Quality Test of the Experimental Data
2.4. Distribution Coefficient and Selectivity
3. Thermodynamic Modeling
3.1. eNRTL Model
3.2. mUNIFAC Model
4. Results and Discussion
4.1. Experimental Data
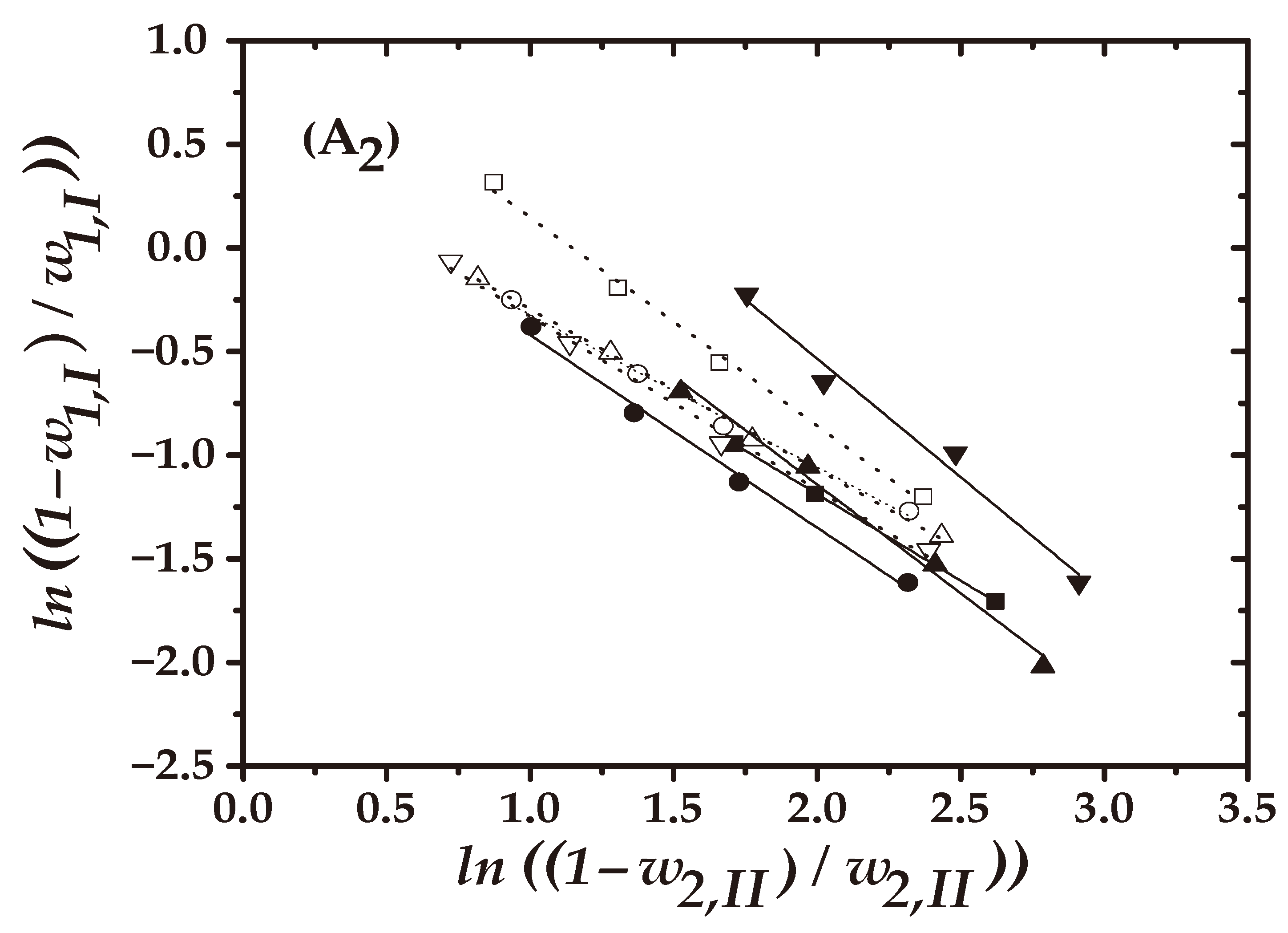
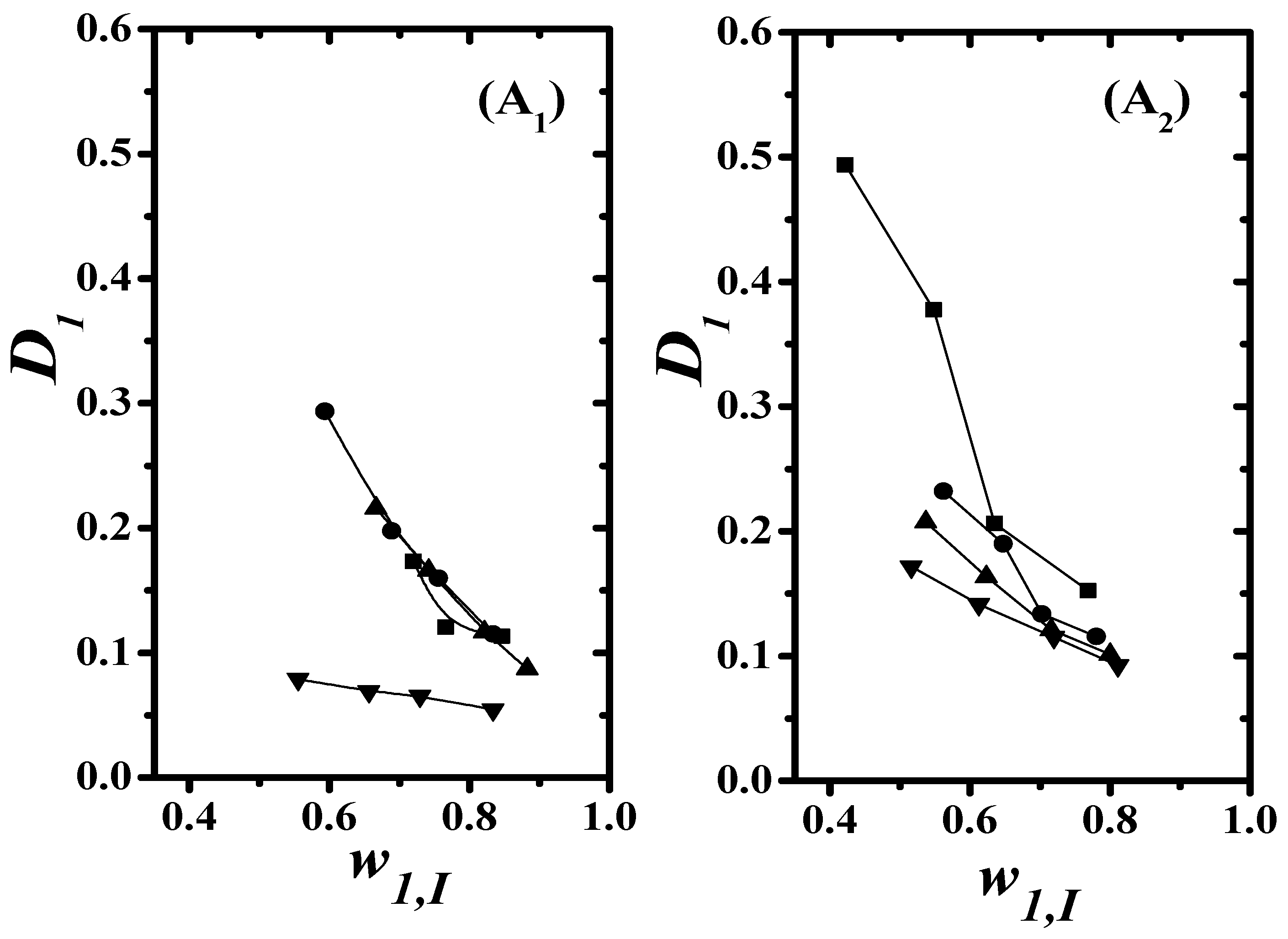
4.2. Quality Test of the LLE Data
 ) 8 wt%; (
) 8 wt%; ( ) 16 wt%]: (A1): Hand and (A2) Othmer–Tobias.
) 16 wt%]: (A1): Hand and (A2) Othmer–Tobias.
 ) 8 wt%; (
) 8 wt%; ( ) 16 wt%]: (A1): Hand and (A2) Othmer–Tobias.
) 16 wt%]: (A1): Hand and (A2) Othmer–Tobias.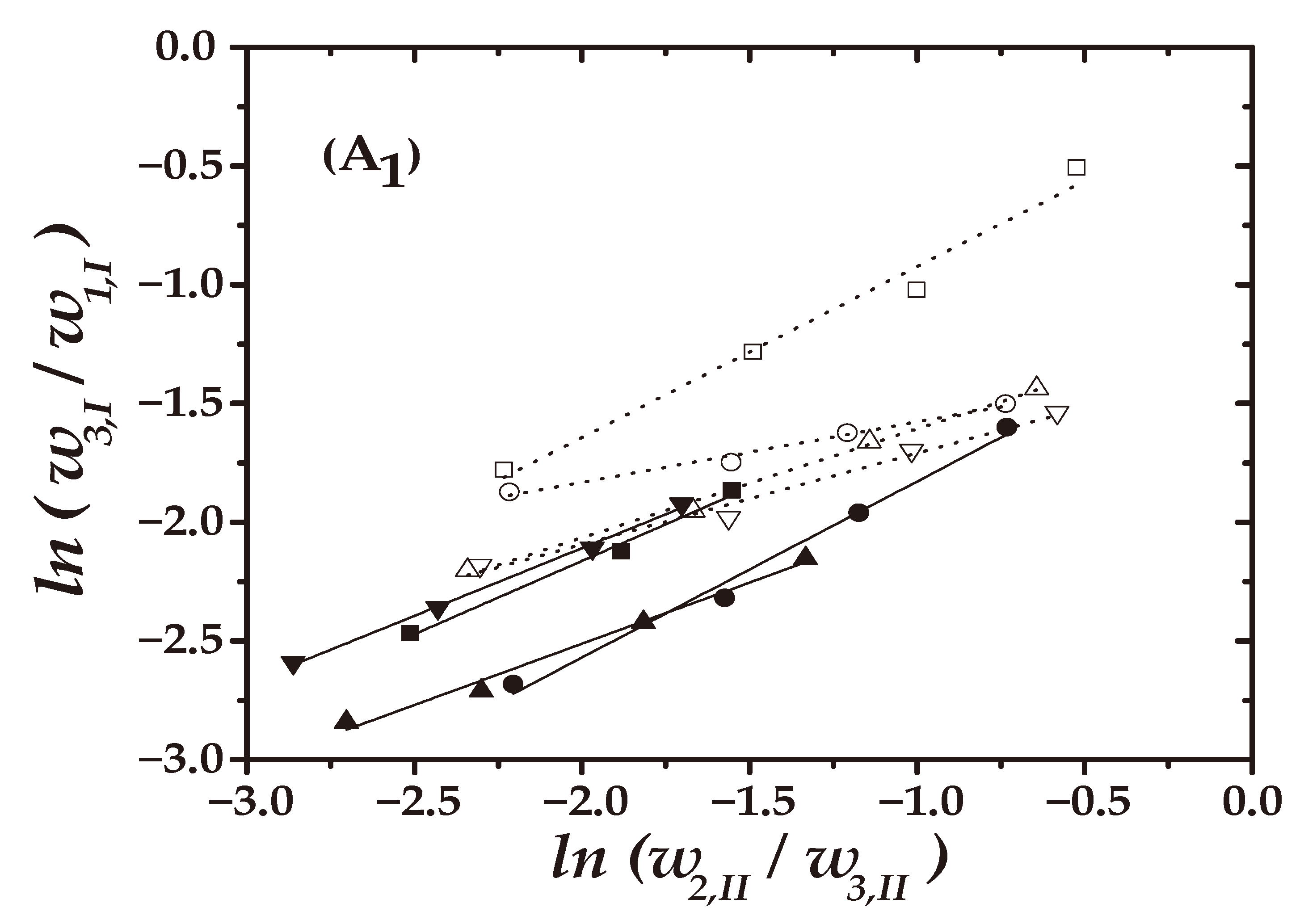
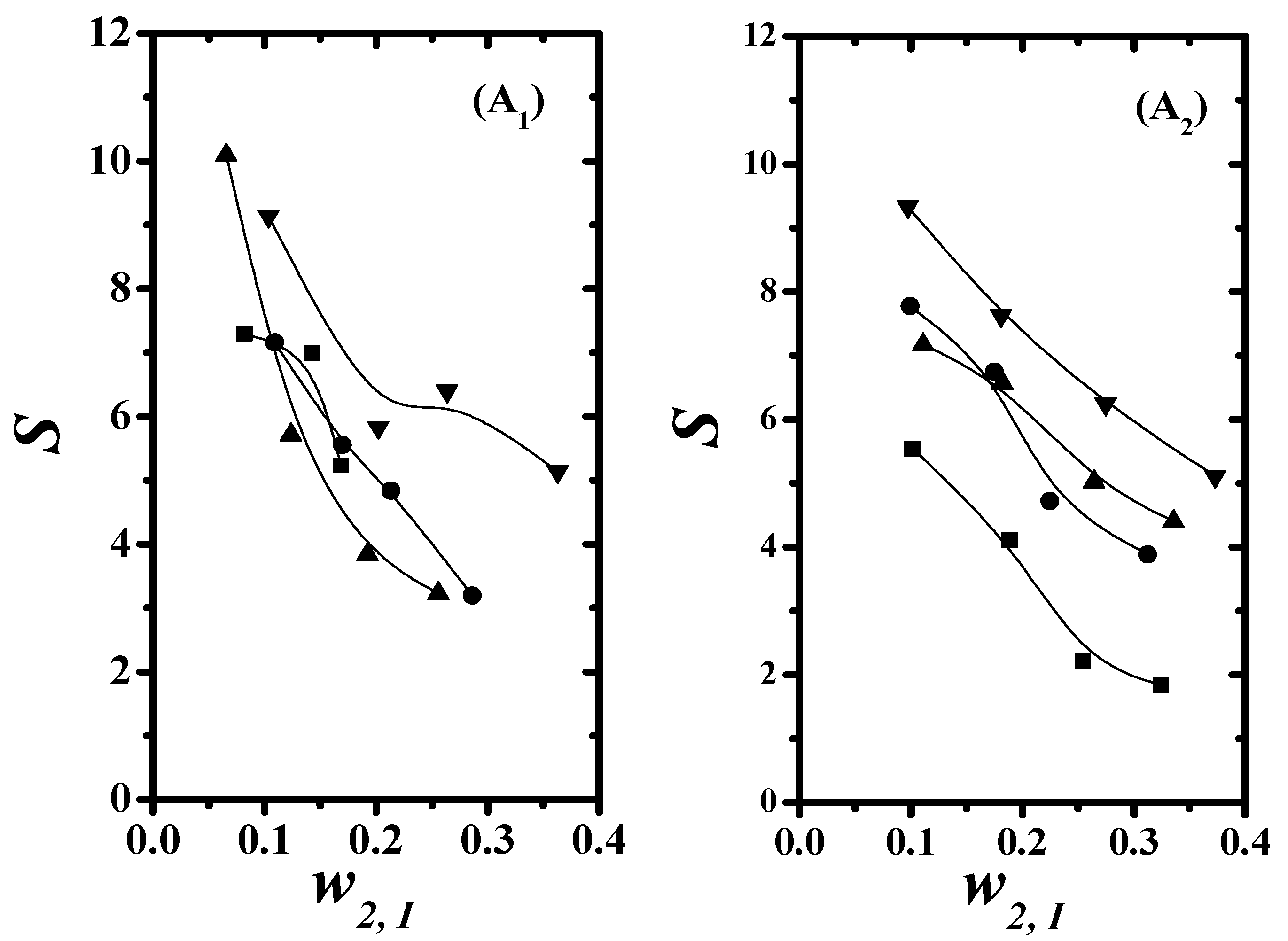
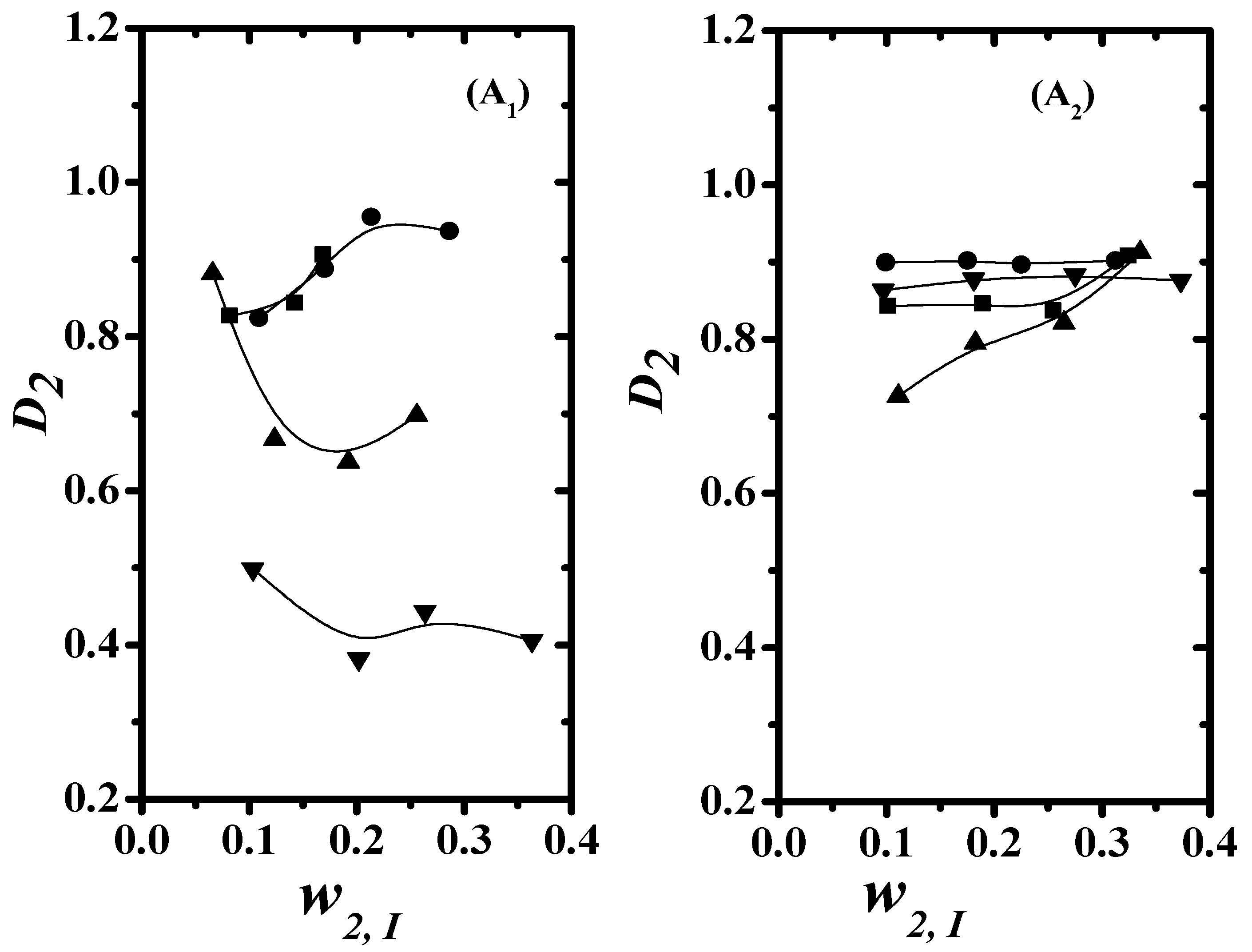
4.3. Separation Factor and Distribution Coefficient
4.4. Thermodynamic Modeling Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Jia, B.; Wang, L.; Yan, M.; Liu, H.; Yu, Y. Liquid-Liquid Equilibrium for Ternary Systems of Water + 2,2,3,3-Tetrafluoro-1-Propanol + Isopropyl Ether/Tert-Butyl Methyl Ether at 298.2, 308.2 K. J. Chem. Thermodyn. 2018, 124, 32–37. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Zhang, J. Liquid−Liquid Equilibria for Benzene + Cyclohexane + N, N -Dimethylformamide + Potassium Thiocyanate. J. Chem. Eng. Data 2010, 55, 3972–3975. [Google Scholar] [CrossRef]
- LUE, S. Pervaporation of Benzene/Cyclohexane Mixtures Using Ion-Exchange Membrane Containing Copper Ions. J. Membr. Sci. 2004, 240, 149–158. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Hu, C.; Wu, H.; Jiang, Z. Significant Increase of Permeation Flux and Selectivity of Poly(Vinyl Alcohol) Membranes by Incorporation of Crystalline Flake Graphite. J. Membr. Sci. 2005, 259, 65–73. [Google Scholar] [CrossRef]
- Garcia Villaluenga, J.P.; Tabe-Mohammadi, A. A Review on the Separation of Benzene/Cyclohexane Mixtures by Pervaporation Processes. J. Membr. Sci. 2000, 169, 159–174. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Meng, H.; Li, C.; Wang, Z. Liquid−Liquid Equilibria for Benzene + Cyclohexane + 1-Methyl-3-Methylimidazolium Dimethylphosphate or + 1-Ethyl-3-Methylimidazolium Diethylphosphate. J. Chem. Eng. Data 2008, 53, 1159–1162. [Google Scholar] [CrossRef]
- García, J.; García, S.; Torrecilla, J.S.; Oliet, M.; Rodríguez, F. Separation of Toluene and Heptane by Liquid–Liquid Extraction Using z-Methyl-N-Butylpyridinium Tetrafluoroborate Isomers (Z = 2, 3, or 4) at T = 313.2 K. J. Chem. Thermodyn. 2010, 42, 1004–1008. [Google Scholar] [CrossRef]
- Aspi, K.K.; Surana, N.M.; Ethirajulu, K.; Vennila, V. Liquid−Liquid Equilibria for the Cyclohexane + Benzene + Dimethylformamide + Ethylene Glycol System. J. Chem. Eng. Data 1998, 43, 925–927. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Wang, S.; Gao, J.; Xu, D.; Zhang, L.; Wang, Y. Liquid–Liquid Equilibrium for Ternary Systems (Ethyl Acetate/Isopropyl Acetate + 2,2-Difluoroethanol + Water) at 298.15 and 308.15 K. J. Chem. Eng. Data 2021, 66, 1399–1405. [Google Scholar] [CrossRef]
- Santos, F.S.; d’Ávila, S.G.; Aznar, M. Salt Effect on Liquid–Liquid Equilibrium of Water+1-Butanol+acetone System: Experimental Determination and Thermodynamic Modeling. Fluid Phase Equilib. 2001, 187–188, 265–274. [Google Scholar] [CrossRef]
- Patai, S. (Ed.) Saul Patai Cyanates and Their Thio Derivatives; John Wiley & Sons, Ltd.: Chichester, UK, 1977; Volume 2, ISBN 9780470771532. [Google Scholar]
- McCullough, J.P.; Scott, D.W.; Waddington, G. Thermodynamics of Organic Sulfur Compounds. In Organic Sulfur Compounds; Kharasch, N., Ed.; Elsevier: Amsterdam, The Netherlands, 1961; Volume 1, pp. 20–29. [Google Scholar]
- Fernanda Bonfim de Souza, B.; Lenhare, S.; Cristaldo Heck, S.; Zuber, A.; Beneti, S.C.; Zanette, A.F.; Filho, L.C. COSMO Study on the Heptane–Toluene–DMF/DEG-KSCN Liquid–Liquid Equilibrium System. Ind. Eng. Chem. Res. 2022, 61, 653–659. [Google Scholar] [CrossRef]
- Song, S.; Lin, C.; You, N. Phase Equilibria Study of the (Benzene + Cyclohexane + N,N-Dimethylformamide + Potassium Thiocyanate) Quaternary Systems. Fluid Phase Equilib. 2014, 376, 154–158. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Zhang, X.; Dong, H.; Wang, Y.; Yue, G.; Liu, J. Liquid–Liquid Equilibria of Benzene + Cyclohexane + N, N-Dimethyl Acetamide + Ammonium Thiocyanate at 298.15 K and Atmospheric Pressure. J. Chem. Eng. Data 2015, 60, 971–975. [Google Scholar] [CrossRef]
- Wilding, W.V.; Rowley, R.L.; Oscarson, J.L. DIPPR® Project 801 Evaluated Process Design Data. Fluid. Phase Equilib. 1998, 150–151, 413–420. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems; Linstrom, P.J., Mallard, W.G., Eds.; NIST Standard Reference Database Number 69; NIST Chemistry WebBook: Gaithersburg, MD, USA, 1998.
- Martínez-Reina, M.D.; Amado-González, E. Refraction Indexes and Density for Binary Mixtures of Heptane with Cyclohexane, Benzene and Toluene at 293.15, 298.15, 303.15 K. Orinoquia 2012, 16, 110–120. [Google Scholar] [CrossRef][Green Version]
- Qiu, D.; Tang, J.; Li, S.; Zhai, Q.; Jiang, Y.; Hu, M. Solubility, Density and Refractive Index of Formamide/N-Methylformamide/N,N-Dimethylformamide + Rb2SO4 + H2O Ternary Systems at 283.2, 298.2 and 313.2 K. J. Chem. Thermodyn. 2022, 175, 106907. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Yue, G.; Zhang, W.; Zhang, J. (Liquid + liquid) Equilibria for Benzene + cyclohexane + N,N-Dimethylformamide + sodium Thiocyanate. J. Chem. Thermodyn. 2013, 63, 169–172. [Google Scholar] [CrossRef]
- Othmer, D.; Tobias, P. Liquid-Liquid Extraction Data—The Line Correlation. Ind. Eng. Chem. 1942, 34, 693–696. [Google Scholar] [CrossRef]
- Grob, R.L.; Barry, E.F. Modern Practice of Gas Chromatography, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 0471229830. [Google Scholar]
- Hand, D.B. Dineric Distribution. J. Phys. Chem. 1929, 34, 1961–2000. [Google Scholar] [CrossRef]
- Imahara, H.; Minami, E.; Saka, S. Thermodynamic Study on Cloud Point of Biodiesel with Its Fatty Acid Composition. Fuel 2006, 85, 1666–1670. [Google Scholar] [CrossRef]
- Brandani, V.; Chianese, A.; Rossi, M. Ternary Liquid-Liquid Equilibrium Data for the Water-Ethanol-Benzene System. J. Chem. Eng. Data 1985, 30, 27–29. [Google Scholar] [CrossRef]
- Follegatti-Romero, L.A.; Oliveira, M.B.; Batista, F.R.M.; Batista, E.A.C.; Coutinho, J.A.P.; Meirelles, A.J.A. Liquid–Liquid Equilibria for Ternary Systems Containing Ethyl Esters, Ethanol and Glycerol at 323.15 and 353.15K. Fuel 2012, 94, 386–394. [Google Scholar] [CrossRef]
- Mesquita, F.M.R.; Evangelista, N.S.; de Sant’Ana, H.B.; de Santiago-Aguiar, R.S. Liquid–Liquid Equilibrium for the Glycerol + Alcohol + Coconut Biodiesel System at Different Temperatures and Atmospheric Pressure. J. Chem. Eng. Data 2012, 57, 3557–3562. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Barbedo, S.; Soletti, J.I.; Carvalho, S.H.; Queimada, A.J.; Coutinho, J.A. Liquid–Liquid Equilibria for the Canola Oil Biodiesel+ ethanol+ glycerol System. Fuel 2011, 90, 2738–2745. [Google Scholar] [CrossRef]
- Walas, S.M. Phase Equilibria in Chemical Engineering, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1984; ISBN 9781483145082. [Google Scholar]
- Sandler, S.I. Chemical, Biochemical and Engineering Thermodynamics, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J. Chemical Thermodynamics for Process Simulation; Wiley: Hoboken, NJ, USA, 2019; ISBN 9783527343256. [Google Scholar]
- Renon, H.; Prausnitz, J.M. Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Chen, C.-C.; Britt, H.I.; Boston, J.F.; Evans, L.B. Local Composition Model for Excess Gibbs Energy of Electrolyte Systems. Part I: Single Solvent, Single Completely Dissociated Electrolyte Systems. AIChE J. 1982, 28, 588–596. [Google Scholar] [CrossRef]
- Maia, F.M.; Rodríguez, O.; Macedo, E.A. LLE for (Water+ionic Liquid) Binary Systems Using [Cxmim][BF4] (X=6, 8) Ionic Liquids. Fluid Phase Equilib. 2010, 296, 184–191. [Google Scholar] [CrossRef]
- Gmehling, J.; Li, J.; Schiller, M. A Modified UNIFAC Model. 2. Present Parameter Matrix and Results for Different Thermodynamic Properties. Ind. Eng. Chem. Res. 1993, 32, 178–193. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Gaube, J. A. Fredenslund, J. Gmehling, and P. Rasmussen: Vapor-Liquid equilibria using UNIFAC a group-contribution method. Elsevier Scientific Publishing Company, Amsterdam, Oxford, New York 1977. 380 Seiten, Preis: $ 59.75, Dfl 146,-. Berichte Der Bunsenges. Für. Phys. Chem. 1978, 82, 551. [Google Scholar] [CrossRef]
- Aznar, M.; Telles, A.S. Prediction of Electrolyte Vapor-Liquid Equilibrium by UNIFAC-Dortmund. Braz. J. Chem. Eng. 2001, 18, 127–137. [Google Scholar] [CrossRef]
- Vera, J.H. Molecular thermodynamics of fluid-phase equilibria by John M. Prausnitz, Rüdiger N. Lichtenthaler and Edmundo Comes de Azevedo, Third Edition, 1999; Prentice Hall PTR, Upper-Saddle River, New Jersey 07458, xxiii + 860 pages; price $131.95 CND; ISBN 0-13-977745-8. Can. J. Chem. Eng. 2000, 78, 429–430. [Google Scholar] [CrossRef]
- Michelsen, M.L.; Mollerup, J.M. Thermodynamic Models: Fundamentals & Computational Aspects, 2nd ed.; Tie- Line Publications: Holte, Denmark, 2007; ISBN 87-989961-3-4. [Google Scholar]
- Taylor, B.N.; Kuyatt, C.E. Guidelines for the Evaluation and Expression of Uncertainty in NIST Measurement Results Technical Note 1297 for NIST; NIST: Gaithersburg, MD, USA, 1994.
- Macedo, E.A.; Skovborg, P.; Rasmussen, P. Calculation of Phase Equilibria for Solutions of Strong Electrolytes in Solvent—Water Mixtures. Chem. Eng. Sci. 1990, 45, 875–882. [Google Scholar] [CrossRef]
- Roughton, B.C.; Christian, B.; White, J.; Camarda, K.V.; Gani, R. Simultaneous Design of Ionic Liquid Entrainers and Energy Efficient Azeotropic Separation Processes. Comput. Chem. Eng. 2012, 42, 248–262. [Google Scholar] [CrossRef]
- Arce, P.F.; Guimarães, D.H.P.; de Aguirre, L.R. Experimental Data and Prediction of the Physical and Chemical Properties of Biodiesel. Chem. Eng. Commun. 2019, 206, 1273–1285. [Google Scholar] [CrossRef]

 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.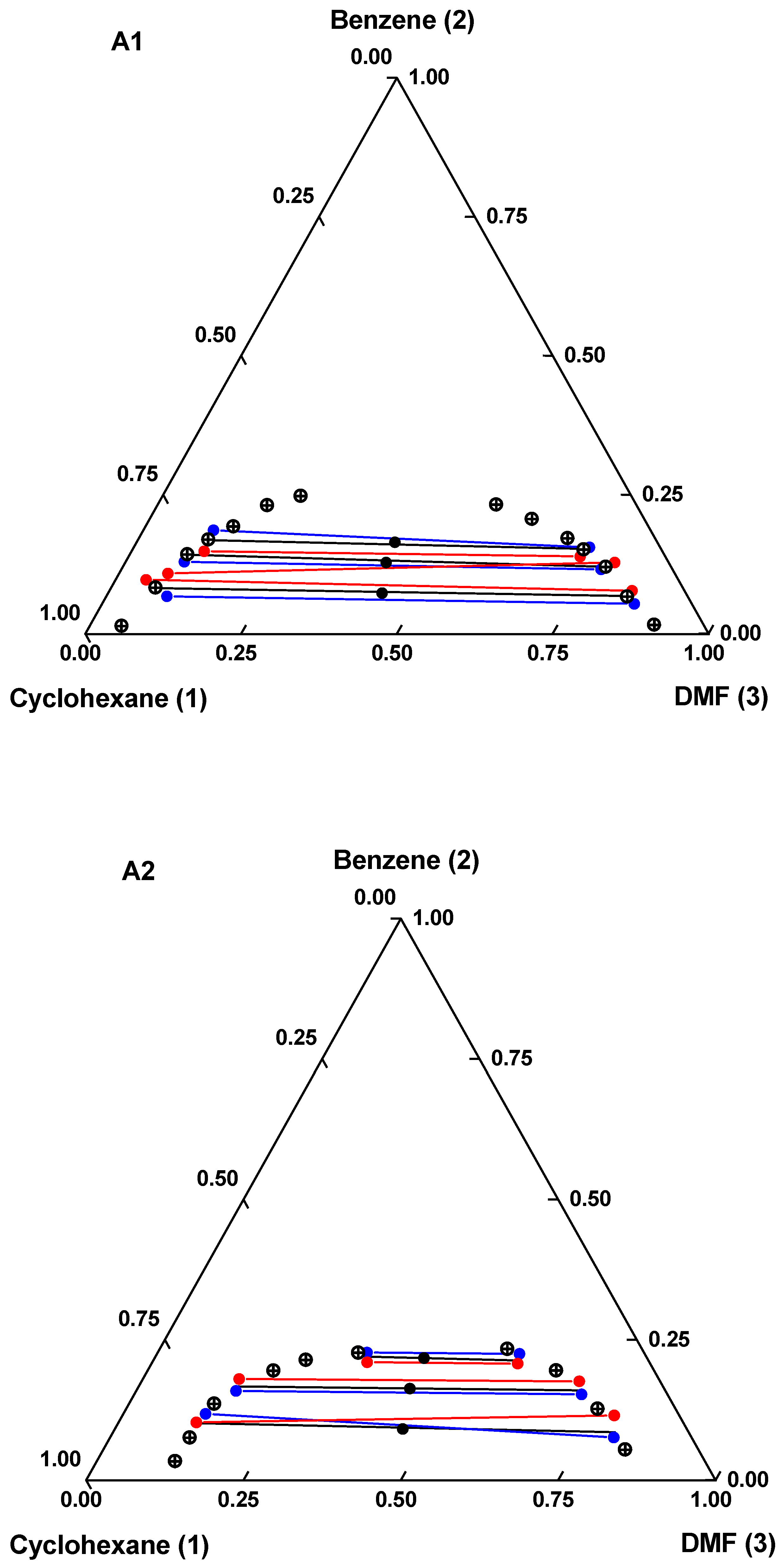
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.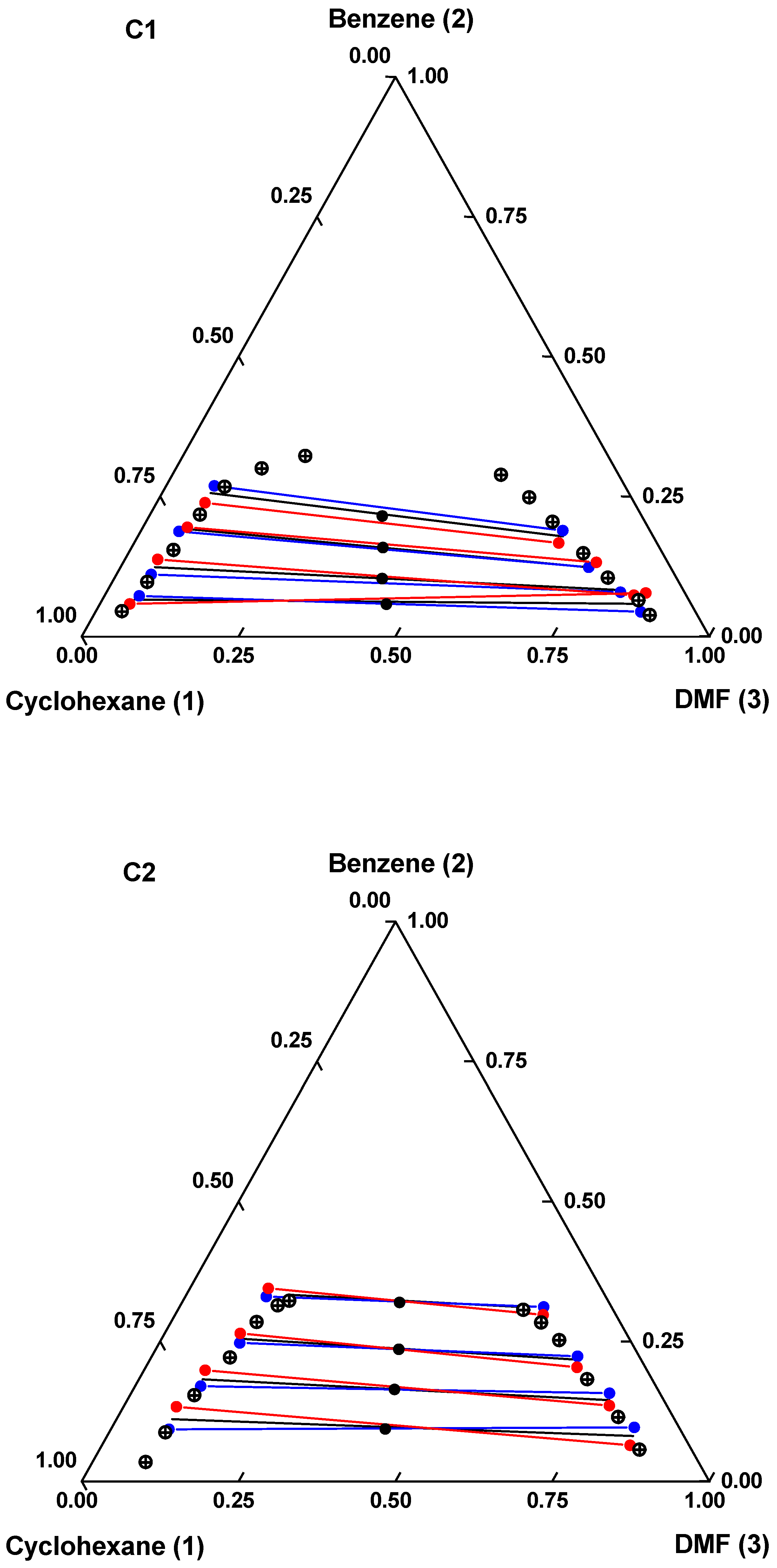
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.
 ) binodal curve; (
) binodal curve; ( ) global composition; (
) global composition; ( ) experimental tie lines; (
) experimental tie lines; ( ) calculated (mUNIFAC) tie lines; (
) calculated (mUNIFAC) tie lines; ( ) calculated (eNRTL) tie lines.
) calculated (eNRTL) tie lines.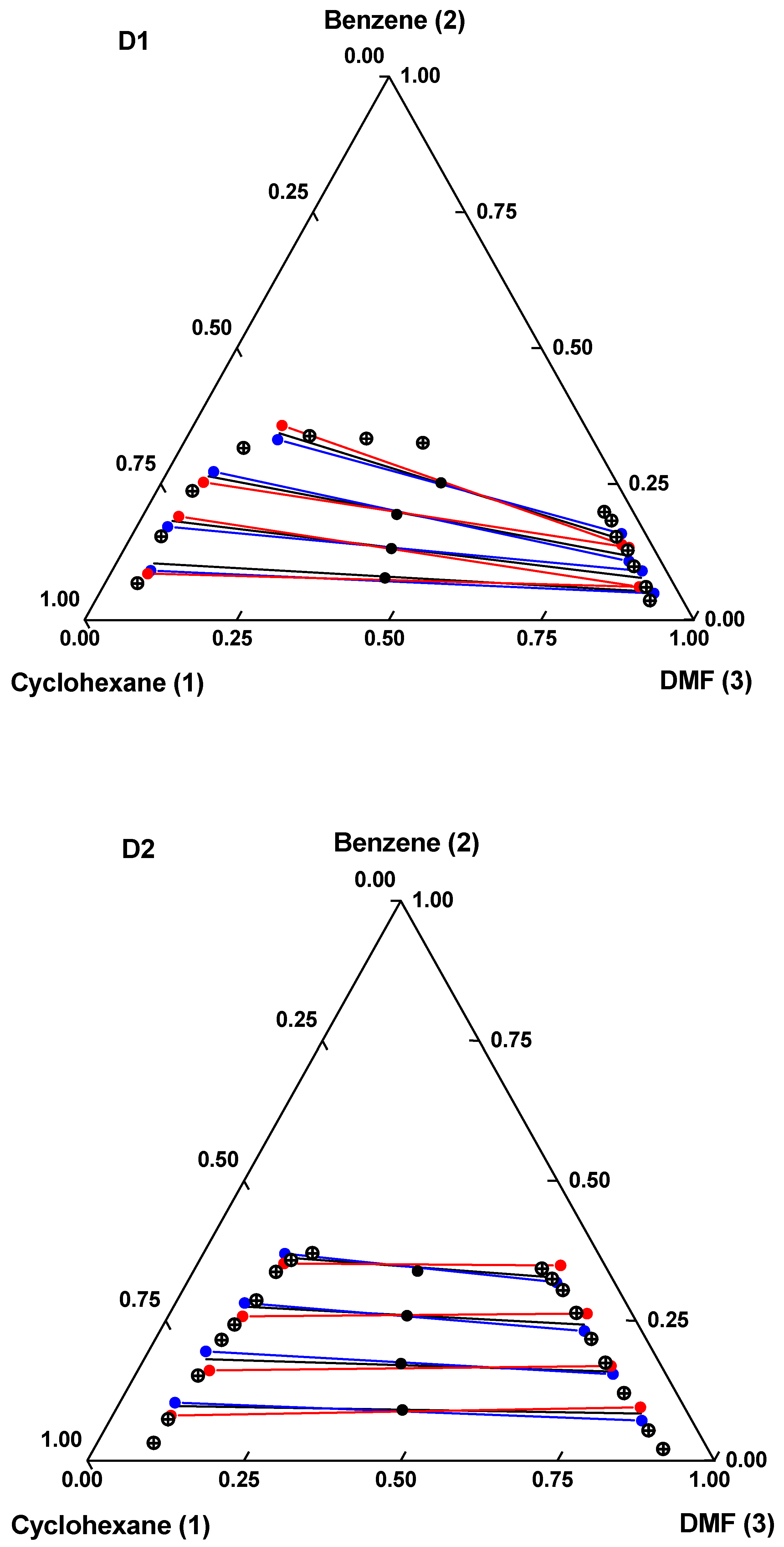
| Compounds | CAS | MW (g·moL−1) | Refractive Index | Density (g·cm−3) | ||
|---|---|---|---|---|---|---|
| This Work | Literature | This Work | Literature | |||
| Cyclohexane | 110-82-7 | 84.161 | 1.4232 | 1.4235 a | 0.7732 | 0.7738 a |
| Benzene | 71-43-2 | 74.114 | 1.4972 | 1.4974 a | 0.8733 | 0.8736 a |
| N,N-dimethylformamide | 68-12-2 | 73.095 | 1.4273 | 1.4279 b | 0.9484 | 0.9488 b |
| Sodium thiocyanate | 540-72-7 | 81.072 [19] | --- | --- | --- | --- |
| T (K) | Overall Composition | Experimental (Tie Lines) | D2 | S | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclohexane Rich-Phase | DMF Rich-Phase | ||||||||||
| w1 b | w2 b | w3 b | w1 b | w2 b | w3 b | w1 b | w2 b | w3 b | |||
| 298.15 | NaSCN = 3 wt% | ||||||||||
| 0.4581 | 0.4280 | 0.1138 | 0.8463 | 0.0820 | 0.0717 | 0.0959 | 0.0678 | 0.8363 | 0.83 | 7.31 | |
| 0.4209 | 0.4144 | 0.1647 | 0.7660 | 0.1422 | 0.0917 | 0.0924 | 0.1200 | 0.7881 | 0.84 | 7.05 | |
| 0.3682 | 0.4109 | 0.2209 | 0.7199 | 0.1687 | 0.1114 | 0.1248 | 0.1529 | 0.7223 | 0.91 | 5.22 | |
| NaSCN = 5 wt% | |||||||||||
| 0.4906 | 0.4096 | 0.0998 | 0.8340 | 0.1090 | 0.0570 | 0.0960 | 0.0898 | 0.8142 | 0.82 | 7.21 | |
| 0.4677 | 0.3725 | 0.1598 | 0.7558 | 0.1699 | 0.0743 | 0.1209 | 0.1509 | 0.7282 | 0.89 | 5.53 | |
| 0.4240 | 0.3630 | 0.2130 | 0.6893 | 0.2136 | 0.0971 | 0.1362 | 0.2040 | 0.6598 | 0.96 | 4.81 | |
| 0.3952 | 0.3259 | 0.2789 | 0.5937 | 0.2864 | 0.1199 | 0.1742 | 0.2683 | 0.5575 | 0.94 | 3.24 | |
| NaSCN = 8 wt% | |||||||||||
| 0.4858 | 0.4566 | 0.0576 | 0.8827 | 0.0658 | 0.0515 | 0.0771 | 0.0580 | 0.8649 | 0.88 | 10.10 | |
| 0.4695 | 0.4271 | 0.1033 | 0.8217 | 0.1236 | 0.0547 | 0.0959 | 0.0824 | 0.8217 | 0.67 | 5.74 | |
| 0.4407 | 0.4006 | 0.1587 | 0.7419 | 0.1923 | 0.0658 | 0.1233 | 0.1226 | 0.7541 | 0.64 | 3.83 | |
| 0.4134 | 0.3714 | 0.2152 | 0.6664 | 0.2561 | 0.0775 | 0.1440 | 0.1787 | 0.6773 | 0.70 | 3.27 | |
| NaSCN = 16 wt% | |||||||||||
| 0.4683 | 0.4549 | 0.0768 | 0.8341 | 0.1035 | 0.0624 | 0.0455 | 0.0516 | 0.9029 | 0.52 | 9.18 | |
| 0.4307 | 0.4383 | 0.1310 | 0.7293 | 0.2019 | 0.0688 | 0.0478 | 0.0771 | 0.8751 | 0.50 | 5.83 | |
| 0.3904 | 0.4156 | 0.1940 | 0.6566 | 0.2638 | 0.0796 | 0.0455 | 0.1169 | 0.8376 | 0.38 | 6.40 | |
| 0.2918 | 0.4637 | 0.2545 | 0.5556 | 0.3632 | 0.0812 | 0.0439 | 0.1474 | 0.8087 | 0.44 | 5.12 | |
| 313.15 | NaSCN = 3 wt% | ||||||||||
| 0.4041 | 0.4328 | 0.1631 | 0.7689 | 0.1014 | 0.1297 | 0.1172 | 0.0863 | 0.7973 | 0.84 | 5.54 | |
| 0.3547 | 0.4279 | 0.2174 | 0.6350 | 0.1889 | 0.1761 | 0.1315 | 0.1605 | 0.7091 | 0.85 | 4.10 | |
| 0.3093 | 0.3921 | 0.2986 | 0.5480 | 0.2547 | 0.1973 | 0.2077 | 0.2131 | 0.5806 | 0.84 | 2.22 | |
| NaSCN = 5 wt% | |||||||||||
| 0.4906 | 0.4096 | 0.0998 | 0.7814 | 0.0993 | 0.1203 | 0.0902 | 0.0892 | 0.8204 | 0.89 | 7.82 | |
| 0.4677 | 0.3725 | 0.1598 | 0.7022 | 0.1751 | 0.1226 | 0.0944 | 0.1585 | 0.7480 | 0.90 | 6.75 | |
| 0.4240 | 0.3630 | 0.2130 | 0.6476 | 0.2256 | 0.1282 | 0.1237 | 0.2021 | 0.6753 | 0.90 | 4.72 | |
| 0.3952 | 0.3259 | 0.3000 | 0.5620 | 0.3130 | 0.1254 | 0.1313 | 0.2828 | 0.5882 | 0.90 | 3.95 | |
| NaSCN = 8 wt% | |||||||||||
| 0.4697 | 0.4368 | 0.0934 | 0.8003 | 0.1114 | 0.0892 | 0.0819 | 0.0814 | 0.8384 | 0.73 | 7.20 | |
| 0.4194 | 0.4164 | 0.1641 | 0.7161 | 0.1821 | 0.1027 | 0.0875 | 0.1457 | 0.7686 | 0.79 | 6.63 | |
| 0.3767 | 0.3875 | 0.2358 | 0.6247 | 0.2658 | 0.1123 | 0.1027 | 0.2172 | 0.6813 | 0.82 | 5.03 | |
| 0.3335 | 0.3465 | 0.3199 | 0.5373 | 0.3363 | 0.1281 | 0.1112 | 0.3060 | 0.5828 | 0.91 | 4.42 | |
| NaSCN = 16 wt% | |||||||||||
| 0.4523 | 0.4574 | 0.0903 | 0.8112 | 0.0973 | 0.0915 | 0.0757 | 0.0847 | 0.8410 | 0.86 | 9.34 | |
| 0.4131 | 0.4137 | 0.1732 | 0.7197 | 0.1811 | 0.0992 | 0.0835 | 0.1592 | 0.7582 | 0.88 | 7.67 | |
| 0.3605 | 0.3806 | 0.2590 | 0.6128 | 0.2750 | 0.1122 | 0.0878 | 0.2433 | 0.6715 | 0.88 | 6.23 | |
| 0.3038 | 0.3575 | 0.3387 | 0.5161 | 0.3730 | 0.1109 | 0.0892 | 0.3276 | 0.5853 | 0.88 | 5.18 | |
| T (K) | Hand | T (K) | Othmer–Tobias | ||||
|---|---|---|---|---|---|---|---|
| R2 | A | B | R2 | A′ | B′ | ||
| NaSCN 3 wt% | NaSCN 3 wt% | ||||||
| 298.15 | 0.9913 | 0.6172 | −0.9287 | 298.15 | 0.9999 | −0.8363 | 0.4846 |
| 313.15 | 0.9811 | 0.7197 | −0.2038 | 313.15 | 0.9959 | −1.0064 | 1.1536 |
| NaSCN 5 wt% | NaSCN 5 wt% | ||||||
| 298.15 | 0.9898 | 0.7412 | −1.0875 | 298.15 | 0.9932 | −0.9293 | 0.5088 |
| 313.15 | 0.9856 | 0.2543 | −1.3235 | 313.15 | 0.9946 | −0.7353 | 0.4125 |
| NaSCN 8 wt% | NaSCN 8 wt% | ||||||
| 298.15 | 0.9887 | 0.5154 | −1.4809 | 298.15 | 0.9904 | −1.0489 | 0.9555 |
| 313.15 | 0.9935 | 0.4582 | −1.1491 | 313.15 | 0.9985 | −0.7738 | 0.4786 |
| NaSCN 16 wt% | NaSCN 16 wt% | ||||||
| 298.15 | 0.9972 | 0.5701 | −0.9693 | 298.15 | 0.9813 | −1.1416 | 1.7481 |
| 313.15 | 0.9823 | 0.384 | −1.3253 | 313.15 | 0.9948 | −0.8366 | 0.5061 |
| i/j | Cyclohexane | Benzene | DMF | NaSCN |
|---|---|---|---|---|
| Cyclohexane | 0.00 | −2142.55 + 162.17/T | −1740.34 + 89.72/T | −2382.48 + 102.30/T |
| Benzene | −1928.41 + 281.92/T | 0.00 | −1506.45 + 85.07/T | −1805.82 + 113.35/T |
| DMF | −2675.23 + 153.38/T | −1510.28 + 162.20/T | 0.00 | −1865.22 + 236.37/T |
| NaSCN | −2341.74 + 336.75/T | −1845.33 + 160.48/T | −1592.74 + 226.27/T | 0.00 |
| i | j | αij |
|---|---|---|
| Cyclohexane | Benzene | 0.2845 |
| Cyclohexane | DMF | 0.2941 |
| Cyclohexane | NaSCN | 0.3654 |
| Benzene | DMF | 0.2748 |
| Benzene | NaSCN | 0.3241 |
| DMF | NaSCN | 0.3514 |
| Group | Subgroup | Rk | Qk |
|---|---|---|---|
| CH2 | CH | 0.4469 | 0.2280 |
| ACH | ACH | 0.5313 | 0.4000 |
| DMF | DMF | 3.0856 | 2.7360 |
| Na+ (*) | Na | 3.0000 | 3.0000 |
| SCN− (**) | SCN | 1.9446 | 1.1752 |
| m | N | amn (K) | bmn | cmn (K−1) | anm | cnm (K−1) |
|---|---|---|---|---|---|---|
| CH | ACH | 1142.8 | −4.410 | 0.001041 | −1468.3 | 0.000841 |
| CH | DMF | 1840.4 | −3.293 | 0.002021 | −88.6 | 0.000124 |
| CH | Na+ | 2845.1 | −0.465 | 0.00 | −317.3 | 0.00 |
| CH | SCN− | 2664.3 | −0.834 | 0.00 | −181.6 | 0.00 |
| ACH | DMF | −1251.6 | 2.342 | 0.001521 | 158.3 | 0.000074 |
| ACH | Na+ | 2338.7 | −0.335 | 0.00 | −109.8 | 0.00 |
| ACH | SCN− | 2603.5 | −0.636 | 0.00 | −201.5 | 0.00 |
| DMF | Na+ | 2412.0 | −0.841 | 0.00 | −175.6 | 0.00 |
| DMF | SCN− | 2674.3 | −0.556 | 0.00 | −181.1 | 0.00 |
| T (K) | NaSCN (wt%) | ARD (%) | |
|---|---|---|---|
| eNRTL | mUNIFAC | ||
| 298.15 | 3 | 0.38 | 0.75 |
| 5 | 0.51 | 0.81 | |
| 8 | 0.64 | 0.86 | |
| 16 | 0.41 | 0.91 | |
| 313.15 | 3 | 0.36 | 0.81 |
| 5 | 0.34 | 0.76 | |
| 8 | 0.48 | 0.80 | |
| 16 | 0.43 | 0.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenhare, S.; de Souza, B.F.B.; Miyasaki, F.V.; Zuber, A.; Arce, P.; Ferreira-Pinto, L.; Beneti, S.C.; Cardozo-Filho, L.; Zanette, A.F. Experimental Data and Thermodynamics Modeling (eNRTL and mUNIFAC) of the (Cyclohexane + Benzene + N,N-Dimethylformamide + Sodium Thiocyanate) Systems. ChemEngineering 2025, 9, 18. https://doi.org/10.3390/chemengineering9010018
Lenhare S, de Souza BFB, Miyasaki FV, Zuber A, Arce P, Ferreira-Pinto L, Beneti SC, Cardozo-Filho L, Zanette AF. Experimental Data and Thermodynamics Modeling (eNRTL and mUNIFAC) of the (Cyclohexane + Benzene + N,N-Dimethylformamide + Sodium Thiocyanate) Systems. ChemEngineering. 2025; 9(1):18. https://doi.org/10.3390/chemengineering9010018
Chicago/Turabian StyleLenhare, Stephanie, Beatriz Fernanda Bonfim de Souza, Fernanda Viana Miyasaki, André Zuber, Pedro Arce, Leandro Ferreira-Pinto, Stéphani Caroline Beneti, Lúcio Cardozo-Filho, and Andréia Fátima Zanette. 2025. "Experimental Data and Thermodynamics Modeling (eNRTL and mUNIFAC) of the (Cyclohexane + Benzene + N,N-Dimethylformamide + Sodium Thiocyanate) Systems" ChemEngineering 9, no. 1: 18. https://doi.org/10.3390/chemengineering9010018
APA StyleLenhare, S., de Souza, B. F. B., Miyasaki, F. V., Zuber, A., Arce, P., Ferreira-Pinto, L., Beneti, S. C., Cardozo-Filho, L., & Zanette, A. F. (2025). Experimental Data and Thermodynamics Modeling (eNRTL and mUNIFAC) of the (Cyclohexane + Benzene + N,N-Dimethylformamide + Sodium Thiocyanate) Systems. ChemEngineering, 9(1), 18. https://doi.org/10.3390/chemengineering9010018







