Abstract
Ethyl hydrocinnamate is an ester with a sweet, fruity, honey-like scent commonly used as a flavor and fragrance agent. Due to its chemical structure, it can be easily obtained through enzymatic reactions without the need for harsh substances and processes. This study investigated the immobilization of the commercial lipase Sustine® 131 onto spent coffee grounds (SCG) as a low-cost support for the enzymatic synthesis of ethyl hydrocinnamate. Spent coffee grounds underwent pretreatment with water, hexane, and ethanol to serve as a lipase adsorption platform and extract valuable bioactive compounds such as polyphenols. The immobilized lipase displayed both hydrolytic and synthetic activities during 12 weeks of storage at room temperature. The optimal reaction conditions for the synthesis of ethyl hydrocinnamate were determined using a Box–Behnken plan. It was shown that the enzyme concentration and the temperature were crucial for achieving high yields of ethyl hydrocinnamate with a conversion rate above 92%. Specifically, at least 18% enzyme concentration and a temperature of 45 °C were necessary. This eco-friendly approach utilized abundant food waste residue as an inexpensive and renewable immobilization support, enabling efficient biocatalytic production of the high-value flavor ester ethyl hydrocinnamate.
1. Introduction
Ethyl hydrocinnamate (ethyl 3-phenylpropionate) is a chemical compound with the molecular formula C11H14O2 and a molecular mass of 178.23 g/mol. It has a sweet, fruity, honey-like scent and is commonly used as a flavor and fragrance agent [1]. This compound and other 3-phenylpropionic acid and cinnamic acid derivatives are listed as flavoring substances in the Commission Implementing Regulation (EU) No 872/2012 [2]. Ethyl ester of 3-phenylpropionic acid is identified as a flavoring substance by several numbers, including 644 by JECFA (Joint FAO/WHO Expert Committee on Food Additives), 429 by CoE (The Council of Europe), 2455 by FEMA (Flavor Extract Manufacturers Association), or 09.747 by Flavis.
This valuable aroma compound has gained significant attention due to its widespread applications in the food, cosmetic, and pharmaceutical industries. With the odor of honey and beeswax reminiscence, it is widely used as a flavoring and fragrance agent, imparting desirable sensory characteristics to various products [3,4,5]. The traditional production of ethyl hydrocinnamate relies on chemical synthesis methods, which often involve harsh conditions and toxic solvents and generate substantial amounts of waste [6]. This has driven the search for more sustainable and environmentally friendly alternatives, such as enzymatic synthesis and the application of biocatalysis [7].
Enzymatic synthesis offers several advantages over conventional chemical methods, including milder reaction conditions, higher selectivity, and reduced formation of undesired byproducts [8,9]. This approach aligns with the principles of green chemistry and sustainable manufacturing practices [10]. Therefore, the enzymatic synthesis of ethyl hydrocinnamate typically involves using lipases, a class of enzymes that catalyze the esterification or transesterification reactions between carboxylic acids and alcohols. Due to their high catalytic activity, lipases from various microbial sources, such as Candida antarctica, Yarrowia lipolytica, and Pseudomonas (Burkholderia) cepacia, have been explored for this purpose [1,11,12].
One of the critical challenges in enzymatic synthesis is the immobilization of enzymes onto suitable solid supports to maintain enzyme stability in reactions in organic solvents. Moreover, immobilization facilitates enzyme recovery and reuse, improving overall process efficiency and economics [13,14]. The current study focuses on using spent coffee grounds (SCG) as a low-cost and sustainable support material for enzyme immobilization. SCG is a highly abundant residue generated by the coffee industry, and its valorization aligns with the principles of a circular economy and sustainable development goals [15,16]. Spent coffee grounds have been utilized as immobilization carriers so far for cellulases and lipases [17,18,19,20]. Key advantages of using spent coffee grounds include their low cost, abundance as a waste product, high porosity, large surface area, and presence of residual compounds that can aid enzyme binding [19,20].
The primary aim of this work was to develop a sustainable method for the biosynthesis of ethyl hydrocinnamate, a valuable aromatic compound via enzymatic esterification with the Sustine® 131 lipase immobilized onto spent coffee grounds, a readily available and inexpensive waste material. Through the application of the statistical design of the experiment, the current study aimed to create a novel low-cost biocatalytic system that can efficiently catalyze the synthesis of ethyl hydrocinnamate.
2. Materials and Methods
2.1. Materials
Sustine® 131 was kindly donated by Novozymes (Bagsværd, Denmark). All chemicals used during the study were purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland) and Sigma-Aldrich (Poznań, Poland). Spent coffee grounds (SCG) were obtained from a household using a fully automatic coffee machine, Siemens TK56001 (Munich, Germany), which operates at a pump pressure of 15 bar. After their delivery to the laboratory, the SCG were dried at 80 °C for 24 h until reaching a constant weight.
2.2. Support Preparation
Ten grams of spent coffee grounds (SCG) were subjected to extraction using a Soxhlet apparatus. Initially, 150 mL of water was utilized for 4 h, followed by 150 mL of hexane, which was heated in a flask to its boiling point of 68 °C, allowing vapor to rise through the extractor and condense in the condenser for 12 cycles of solvent transfer. Subsequently, 150 mL of ethanol, with a boiling point of 78 °C, was employed under the same conditions as hexane. After each solvent change and the final extraction, the SCG was dried at 80 °C until a constant weight was reached. The resulting defatted material, free of polyphenols, was then used in the immobilization process. The obtained water, hexane, and ethanol extracts were stored for further analysis.
2.3. Extraction Yield and Total Polyphenol Content in the Obtained Extracts
The extraction yield was determined based on the precise masses of the SCG used for extraction and the remaining SCG after the process. The total polyphenolic contents in the water and ethanolic extracts were determined using the Folin–Ciocalteu method according to Rybak et al. [21]. The content of phenolic compounds was calculated as chlorogenic acid equivalents (mg CGA/g SCG).
2.4. Elemental Compositions of Spent Coffee Grounds
Total C, N, and S contents were determined by dry combustion (Vario MacroCube, Elementar, Langenselbold, Germany).
2.5. Lipase Immobilization
Sustine® 131 was immobilized according to the methodology of de S. Lira et al. [22] with slight modification. Briefly, 1 g of SCG was added to 10 mL of hexane and 10 mL of lipase in 0.025 M sodium phosphate buffer solution and stirred for 24 h at room temperature. At the end of the process, Sustine® 131-SCG preparation was filtered with a Büchner funnel, washed with 10 mL of hexane, and dried at room temperature. The obtained filtrate was used to determine the protein content and calculate the immobilization yield (%).
2.6. Protein Content Determination
Protein concentration was determined spectrophotometrically using Lowry’s method following the protocol from Jasińska et al. [23]. The amount of protein adsorbed onto the SCG was calculated based on differences between protein concentration in free lipase and filtrates after immobilization.
2.7. Hydrolytic Activity Determination
The hydrolytic activity was measured using a spectrophotometric method based on the hydrolysis of p-nitrophenyl laurate. A total of 25 mg of immobilized biocatalyst in 100 μL of distilled water was stirred at 37 °C with 25 μL of 0.3 mmol p-nitrophenyl laurate dissolved in 2 mL of heptane. After 15 min of incubation, the absorbance was measured at 410 nm using a RayLeigh UV-1601 (BRAIC, Beijing, China) spectrophotometer. The unit of lipase enzymatic activity was 1 U, which is the amount of enzyme that released one µmol of p-nitrophenol per minute under the assay conditions. Hydrolytic activities were determined after 12 weeks of storage at room temperature, precisely after 1, 2, 4, 8, and 12 weeks.
2.8. Synthetic Activity Determination
The synthetic activity of the immobilized lipase was examined using the colorimetric method developed by Zheng et al. [24], with some modifications [23]. Briefly, the measurement was based on the reaction between vinyl acetate and 1-butanol through transesterification. The reaction was conducted in an Eppendorf tube with 100 mM of vinyl acetate and 100 mM of 1-butanol in 1 mL of hexane, adding 5 mg of immobilized lipase. The assay involved preparing diluted samples, adding MBTH (3-methyl-2-benzothialinone) and H4FeNO4S2·12H2O solutions, and conducting colorimetric measurements at 595 nm. The unit of lipase synthetic activity was 1 U, indicating the amount of enzyme that converted 0.1 mmol of vinyl acetate into acetaldehyde per minute under the assay conditions. Synthetic activities were determined after 12 weeks of storage at room temperature, precisely after 1, 2, 4, 8, and 12 weeks.
2.9. Synthesis of Ethyl Hydrocinnamate, Solvent Selection, and Reusability of Preparation
The reaction involved hydrocinnamic acid and ethyl alcohol (Figure 1) in a 1:2 molar ratio, with the amount of acid being 0.00125 moles. The reactions were carried out for 96 h in an incubator at 40 °C, 200 rpm, and with the addition of 15% enzyme preparation based on the mass of the substrates. The following solvents with a volume of 20 mL were compared: isooctane (log p = 4.6), methyl tert-butyl ether (log p = 0.94), tert-butanol (log p = 0.35), and acetone (log p = −0.24). For the reusability of the immobilized lipase, isooctane was used as the reaction solvent, and five continuous reactions were carried out under the same conditions as mentioned above.
Figure 1.
Lipase-catalyzed esterification of hydrocinnamic acid with ethyl alcohol.
The reaction yield was calculated using the ester mass obtained after column chromatography. Ethyl hydrocinnamate was purified using column chromatography. Approximately 35 g of silica gel 60 (0.040–0.063 mm; 230–400 mesh) and chloroform were used as the stationary and mobile phases, respectively. The purification process utilized a glass chromatographic column with dimensions of 400 mm in length, 20 mm in diameter, and a capacity of 125 mL. The fractions containing ester, whose presence was confirmed by thin-layer chromatography (TLC) using a standard, were subsequently dried with magnesium sulfate (MgSO4), filtered, and the solvent evaporated. Yield: 100 × (actual quantity received/theoretical quantity calculated). Aluminum TLC plates coated with silica gel 60 matrix and a fluorescent indicator (254 nm; Sigma-Aldrich) were used for thin-layer chromatography. Chloroform was used as the eluent, and the plates were checked under a UV lamp at 254 nm.
2.10. Box–Behnken Design for the Optimization of Reaction Conditions
A three-factor, three-level experiment was planned using Box–Behnken designs (Table 1). The following factors were analyzed: enzyme concentration (5, 15, and 25%), temperature (30, 40, 50 °C), and reaction time (48, 96, 144 h).

Table 1.
Experimental matrix for the three-variable Box–Behnken design with the yield of ethyl hydrocinnamate synthesis.
2.11. Statistical Analysis
The obtained results and the Box–Behnken experiment were analyzed statistically using Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). A one-way analysis of variance (ANOVA) and Tukey’s post hoc test were applied.
3. Results and Discussion
3.1. Preparing the Carrier for Lipase Immobilization
The extraction of coffee grounds for subsequent immobilization aimed to purify them from lipids and other substances. This process was crucial to achieve the highest possible degree of enzyme immobilization and eliminate lipids that might impede the adsorption process and encapsulation into pores. According to Budžaki et al. [18], compounds other than cellulose and hemicellulose should be removed through extraction. Due to differences in polarity and extractability, a multistep extraction system should be applied to achieve suitable carriers for enzyme immobilization. Therefore, in the current study, spent coffee grounds were extracted with water for 4 h, then with hexane, and finally with ethanol. The extraction yields are presented in Figure 2a. Approximately 10.5% of extraction yield was observed for water as the solvent. As the saying goes, “like dissolves like”, indicating that polar compounds dissolve in polar solvents. Over these four hours, water, being a polar solvent, may primarily extract sugars and proteins. This step was followed by hexane extraction, and this time, the nonpolar solvent extracted lipids (7.7%), whereas coffee grounds obtained from industrial coffee processing may contain 10 to 15% lipids [18]. The application of the third solvent, i.e., ethanol, allowed for the extraction of other compounds with a yield of 2.5%.
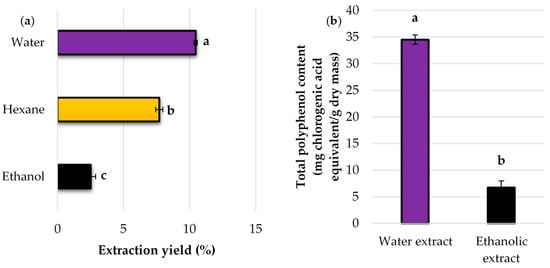
Figure 2.
The effect of the solvent on (a) extraction yield and (b) total polyphenol content in spent coffee grounds. The values with the same lowercase letter (a–c) did not differ significantly (α = 0.05). Error bars represent the standard deviation (SD) of three independent measurements.
Due to the fact that coffee grounds are still a valuable source of bioactive compounds [25,26], aqueous and ethanol extracts were tested for the content of polyphenolic compounds using the Folin–Ciocalteu spectrophotometric method. As can be seen in Figure 2b, there are substantial variations in total polyphenol content between the aqueous and ethanol extracts, with 34.5 mg CGA/g SCG in the aqueous extract compared to 6.8 mg CGA/g SCG in the ethanol extract. Interestingly, the most commonly used solvents for polyphenol extraction are ethanol or methanol, usually in the form of 70–80% aqueous solutions [26]. On the contrary, in the current study, more than five times more polyphenols were detected in the aqueous extract, which indicates that the vast majority of these bioactive compounds could be extracted using water as a solvent, and then other compounds with a greater affinity for ethanol were extracted. The most common compounds found in coffee samples and spent coffee grounds are chlorogenic, gallic, p-coumaric, and trans-ferulic acids, which are examples of polyphenols, and caffeine, a methylxanthine alkaloid [27].
The solid residue after Soxhlet extraction, i.e., the defatted coffee grounds without antioxidants, was analyzed for its carbon, nitrogen, and sulfur content using the dry combustion method (Table 2). The elemental analysis of SCG was quite similar to that reported in the literature [19].

Table 2.
Elemental analysis of the pretreated spent coffee grounds used to immobilize Sustine® 131.
The samples of SCG in the current study showed a relatively high carbon content of 53.28%, a significantly low nitrogen content of 2.48%, and only 0.12% sulfur, which is not as abundant in plant-based materials as other elements. In the study of Girelli et al. [19], the carbon content decreases during the pretreatment processes, likely due to removing some carbon-containing compounds. Still, its levels in different types of spent coffee grounds were in the range of 43.8–50.86%. Similarly, nitrogen and sulfur content is marginal and did not exceed 2.5 and 0.25%, respectively.
3.2. Monitoring the Activity of the Obtained Preparation over Time
After the immobilization of lipase onto the SCG, the immobilization yield was assessed based on the difference in protein concentration at the beginning and the collected filtrate after the process. The yield was 47.82% ± 1.16%. This indicates that only about half of the introduced liquid lipase was immobilized onto the carrier surface. This result is significantly lower than those available in the scientific literature. In the study by Jasińska et al. [23], it was 88.26% when Novozym 51032 and SCG were used as an enzyme and carrier, respectively. When coffee grounds of other origins were used and their purification methods were compared, the content of adsorbed enzymatic proteins for Palatase 20,000 L ranged from 60 to 80% [20]. Properly selecting the material for lipase immobilization is also a significant issue. The carrier should be characterized by good porosity, large surface area, and an appropriate hydrophobic/hydrophilic balance, allowing the enzyme to act more efficiently [28]. Such properties impact the immobilization yield. In the study of Kowalczykiewicz et al. [29], Candida antarctica Lipase B (CALB) was immobilized onto highly porous siliceous pellets, which were chemically modified to improve their adsorption properties. The results indicate that the support grafted with both octyl and amino groups exhibited the highest immobilization yield of 78.4%, while grafting only with amino or octyl groups led to an immobilization yield of 56.0% or 44.3%, respectively. Furthermore, the immobilization yield is also influenced by enzyme concentration and contact time during the immobilization process. As shown by the experiment conducted by Qian et al. [30], CALB was immobilized on silica nanoparticles by physical adsorption, and higher lipase concentrations led to decreased immobilization yield and enzyme activity, likely due to the agglomeration of lipase molecules adsorbed on the surface of SiO2 nanoparticles, which probably covered the active sites. Moreover, the adsorption process reached saturation after 2 h, and prolonging the time further had the opposite effect [30].
It is widely recognized that the surface area is another crucial factor in the immobilization process through physical adsorption, as it enhances the interaction between enzyme and support. Furthermore, a larger surface area allows for greater loading capacity, enabling more active materials to be attached, which boosts performance in biocatalytic applications. Several papers addressed the surface area of spent coffee grounds. Primarily, it is accomplished using the BET (Brunauer, Emmett, and Teller) method by nitrogen adsorption. Ballesteros et al. [31] compared the specific surface area of SCG and coffee silverskin with 4.3 m2/g vs. 2.1 m2/g along with no micropores, respectively, which were generally considered low, especially in the context of materials typically used for adsorption or catalytic applications, while Yang et al. [32] reported higher value of 8.05 m2/g and average pore size of 0.56 nm. Interestingly, hydrothermal carbonization at 210 °C of spent coffee grounds allowed for almost doubling the surface area (15.15 m2/g) and a remarkable increase in pore size to 7.82 nm. Other studies also evaluated the surface area and porosity in spent coffee grounds using scanning electron microscopy (SEM) analyses [19,20].
The next step in evaluating the immobilized enzyme was to assess its hydrolytic and synthetic activity after 12 weeks of storage at room temperature. Figure 3 illustrates a significant decrease in the synthetic activity of the immobilized enzyme after two weeks of storage, with only 50% of the initial activity remaining. In the subsequent weeks of storage, a further decrease in activity was observed, with the enzyme activity dropping to less than 10% of its initial levels. In the case of hydrolytic activity, the activity of the immobilized enzyme also decreased, but not as significantly as in the case of synthetic activity. After four weeks of storage, approximately 70% of the initial activity was retained. After eight weeks, more than 50% of activity was still observed, and about 25% after 12 weeks. The differences in observed patterns of activity may be due to the mechanisms underlying synthetic and hydrolytic activities, resulting in variations in stability and performance over time. Furthermore, the immobilization process itself can affect enzyme stability differently for each type of activity. Ranjbakhsh et al. [33] evaluated the storage stability of lipase from porcine pancreas covalently immobilized onto modified magnetite nanoparticles. The scientists observed stability improvement over time in the immobilized enzyme compared to the free enzyme, but after 21 days (3 weeks), the immobilized enzyme retained 64% of its initial activity. Moreover, the lipase from Penicillium sp. immobilized onto bentonite activated with glutaraldehyde showed enhanced stability and usability [34]. The authors also noted improved performance at high temperatures, reusability, and storage stability at 4 °C. They observed that approximately 70% of the enzyme’s initial activity was retained after 21 days under these conditions [34].
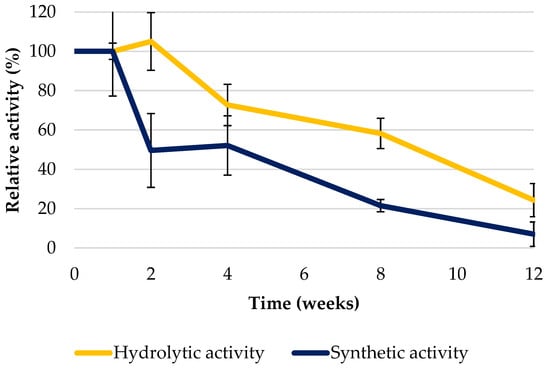
Figure 3.
Relative activity of the immobilized Sustine® 131 onto spent coffee grounds during 12 weeks of storage. Hydrolytic activity was indicated with a yellow line, and synthetic activity was shown with a blue line. Error bars represent the standard deviation (SD) of three independent measurements.
Controlling temperature, pH, and humidity is important to preserve enzyme stability and activity during storage. Enzymes’ specific structural characteristics also significantly impact their stability. Temperature changes, especially temperature increases, can cause molecular movements that result in permanent structural changes and loss of enzyme activity. Additionally, during storage, enzyme activity decreases over time due to a slow aging process after production [35,36]. The aforementioned papers found that immobilizing enzymes is essential, as it leads to improved activity during storage compared to free enzymes [33,34]. In addition, the water content and water activity in dried enzyme formulations, including immobilized lipases, are crucial for ensuring protein stability and extending shelf life, while excessive water can negatively affect lipase-catalyzed reactions in organic solvents [37]. The choice of support material is significant, as its water-absorbing capacity influences the final moisture content and water activity [37,38]. Moreover, maintaining low moisture content and water activity is also essential to prevent microbial growth and product deterioration [38].
3.3. Optimizing the Enzymatic Synthesis of Ethyl Hydrocinnamate
The enzymatic synthesis of ethyl hydrocinnamate was conducted using immobilized lipase in four different solvents: isooctane, MTBE (methyl tert-butyl ether), tert-butanol, and acetone. This stage of the study evaluated the impact of solvent choice on the esterification yield of ethyl hydrocinnamate (Figure 4a). The highest yield of 74% was achieved with isooctane, indicating that this solvent provides an optimal environment for the enzymatic reaction. Isooctane, being a non-polar solvent (log p = 4.6), likely facilitates better solubility of the substrates and minimizes enzyme denaturation, thus enhancing the catalytic efficiency of the immobilized lipase [39]. About 60% yield was achieved with MTBE, demonstrating favorable performance despite being lower than isooctane. MTBE’s moderate polarity (log p = 0.94) may balance substrate solubility and enzyme activity, leading to a reasonable yield. The yield dropped significantly to approximately 8% in tert-butanol. The higher polarity (log p = 0.35) and potential for hydrogen bonding of this solvent may interfere with the enzyme’s active site, leading to reduced catalytic activity [40]. The lowest yield of 0.6% was observed with acetone. Its strong polarity (log p = −0.24) and ability to solvate both polar and non-polar compounds may disrupt the enzyme–substrate interactions necessary for effective catalysis [41]. In solvents with high log p-values, the biocatalyst demonstrates significantly enhanced activity compared to solvents with low log p-values. This is due to the superior ability of hydrophobic solvents to preserve essential water for catalytic activity in enzyme molecules, thereby maximizing the efficiency of the biocatalytic process [40]. Based on these results, the subsequent experiments were carried out using isooctane as a solvent.
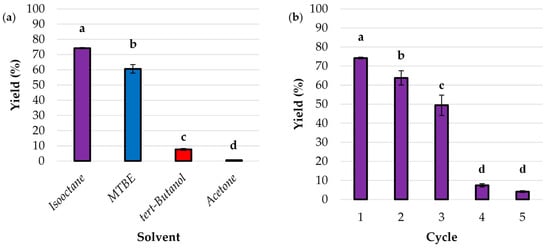
Figure 4.
The impact of (a) the solvent and (b) the ability to reuse the immobilized lipase on the production yield of ethyl hydrocinnamate. The values with the same lowercase letter (a–d) did not differ significantly (α = 0.05). Error bars represent the standard deviation (SD) of three independent measurements. Abbreviation: MTBE—methyl tert-butyl ether.
The immobilized lipase was subjected to five consecutive reaction cycles to determine its efficiency and stability. The yields of ethyl hydrocinnamate were measured after each cycle, providing insights into the enzyme’s reusability and performance over time (Figure 4b). The initial yield was 74%, and after the second cycle, a decrease to 64% was observed, which suggests a reduction in enzyme activity, likely due to minor enzyme denaturation. The yield further declined to 49%, indicating continued loss of activity. This could be attributed to factors such as enzyme leaching or conformational changes in the immobilized lipase [42]. Subsequently, in the fourth cycle, a dramatic drop to 7% and 4% after the fifth cycle reflects the significant loss of catalytic efficiency, suggesting that the immobilized lipase is nearing the end of its functional lifespan, making it ineffective for further reactions.
A response surface methodology was utilized to optimize the enzymatic synthesis of ethyl hydrocinnamate via the direct esterification of hydrocinnamic acid with ethanol using Sustine® 131-SCG as a biocatalyst. A total of 15 runs (Table 1) were conducted to evaluate different enzyme concentrations (5–25%), temperatures (30–50 °C), and times (48–144 h). The results, in the form of a Pareto chart and 3D response surface plots, are presented in Figure 5. The response model evaluated in this study had an R2 of 0.995 and an Adjusted R2 of 0.987, with a 95% confidence level, where a good model fit should have an R2 of at least 0.8 [43].
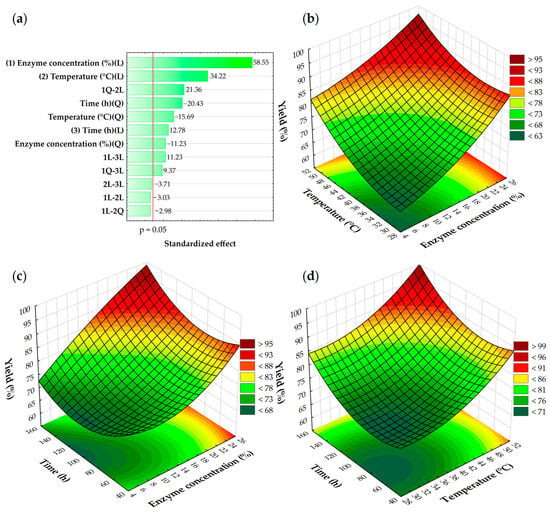
Figure 5.
(a) Pareto chart and 3D response surface plots showing the effects of the interactions of (b) temperature and enzyme concentration, (c) time and enzyme concentration, and (d) time and temperature on the production yield of ethyl hydrocinnamate. Explanations: 1—enzyme concentration, 2—temperature, 3—time, L—linear; Q—quadratic, 1Q-2L—the interaction between a quadratic effect of enzyme concentration and a linear effect of temperature in the statistical model, etc.
As can be seen in 3D plots (Figure 5b–d), the duration of the enzymatic reactions minimally impacted the ester yield. It is noticeable that the reaction does not proceed efficiently at lower temperatures and lower concentrations of lipase. Based on the Pareto chart (Figure 5a), the linear effects (L) of enzyme concentration and temperature had the greatest influence on the final yield of ethyl hydrocinnamate. The experimental findings indicate that the best conditions for the enzymatic synthesis of ethyl hydrocinnamate require a temperature of at least 45 °C and a lipase concentration of approximately 18%. These conditions result in over 80% conversion to the target ester in as little as 40 h.
The response surface methodology was applied several times for efficient enzymatic synthesis. The Box–Behnken design was used to study the effects of temperature, reaction time, and ultrasonic power on the production of octyl cinnamate. The highest conversion of octyl cinnamate was achieved at 74.6 °C, 11.1 h, and 150 W, resulting in a 93.8% conversion rate. Additionally, the authors reduced the reaction time using ultrasound and a vacuum system [9]. Kharrat et al. [43] studied the enzymatic synthesis of 1,3-dihydroxyphenylacetoyl-sn-glycerol with its optimization via Box–Behnken design. Contrary to the current study, Kharrat et al. [43] observed that increasing the enzyme amount led to decreased reaction yield. This was attributed to potential mass transfer limitations, restricted diffusion of the substrate to the enzyme’s active site, and enzyme agglomeration, all of which contributed to reduced catalytic efficiency. Ijaz and Sun [44] successfully synthesized structured lipids from tiger nut oil and methyl behenate using Lipozyme RMIM as a catalyst. They optimized the reaction conditions using response surface methodology and achieved a yield of 78.8%. The best conditions were 85 °C, an enzyme load of 12.5%, a substrate ratio of 1:6, and a reaction time of 1.25 h.
4. Conclusions
The immobilization of the lipase Sustine® 131 onto spent coffee grounds (SCG) combines the use of waste biomass with the sustainable production of a valuable aroma compound through enzymatic synthesis. This approach offers an environmentally friendly alternative to chemical synthesis and contributes to the valorization of an abundant waste stream, potentially enhancing the process’s overall sustainability and economic viability. The SCG underwent pretreatment with water, hexane, and ethanol, which served as a platform for lipase adsorption and allowed for the extraction of valuable bioactive compounds such as polyphenols. Notably, the immobilized lipase maintained both hydrolytic and synthetic activities over a period of 12 weeks at room temperature. Using a Box–Behnken design, the optimal conditions for the synthesis of ethyl hydrocinnamate were established, revealing that an enzyme concentration of at least 18% and a temperature of 45 °C are crucial for achieving conversion rates exceeding 92%. Overall, this research shows the potential for incorporating waste materials into bioprocesses, leading to more sustainable practices in flavor and fragrance production.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Zieniuk, B.; Fabiszewska, A.; Wołoszynowska, M.; Białecka-Florjańczyk, E. Synthesis of Flavor Compound Ethyl Hydrocinnamate by Yarrowia lipolytica Lipases. Biocatal. Biotransform. 2021, 39, 455–464. [Google Scholar] [CrossRef]
- Implementing Regulation—872/2012—EN—EUR-Lex. Available online: http://data.europa.eu/eli/reg_impl/2012/872/oj (accessed on 22 August 2024).
- Nikolaou, A.; Santarmaki, V.; Mitropoulou, G.; Sgouros, G.; Kourkoutas, Y. Novel Low-Alcohol Sangria-Type Wine Products with Immobilized Kefir Cultures and Essential Oils. Microbiol. Res. 2023, 14, 543–558. [Google Scholar] [CrossRef]
- Qian, X.; Ling, M.; Sun, Y.; Han, F.; Shi, Y.; Duan, C.; Lan, Y. Decoding the Aroma Characteristics of Icewine by Partial Least-Squares Regression, Aroma Reconstitution, and Omission Studies. Food Chem. 2024, 440, 138226. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Yuan, Y.; Liang, Z.; Yan, Y.; Chen, Y.; Ni, L.; Lv, X. Metagenomic Insights into the Regulatory Effects of Microbial Community on the Formation of Biogenic Amines and Volatile Flavor Components during the Brewing of Hongqu Rice Wine. Foods 2023, 12, 3075. [Google Scholar] [CrossRef]
- Korneev, S. Hydrocinnamic Acids: Application and Strategy of Synthesis. Synthesis 2013, 45, 1000–1015. [Google Scholar] [CrossRef]
- Buller, R.; Lutz, S.; Kazlauskas, R.J.; Snajdrova, R.; Moore, J.C.; Bornscheuer, U.T. From Nature to Industry: Harnessing Enzymes for Biocatalysis. Science 2023, 382, eadh8615. [Google Scholar] [CrossRef]
- Bandara, R.R.; Louis-Gavet, C.; Bryś, J.; Mańko-Jurkowska, D.; Górska, A.; Brzezińska, R.; Siol, M.; Makouie, S.; Palani, B.K.; Obranović, M.; et al. Enzymatic Interesterification of Coconut and Hemp Oil Mixtures to Obtain Modified Structured Lipids. Foods 2024, 13, 2722. [Google Scholar] [CrossRef]
- Tsai, M.-F.; Huang, S.-M.; Huang, H.-Y.; Tsai, S.-W.; Kuo, C.-H.; Shieh, C.-J. Ultrasound Plus Vacuum-System-Assisted Biocatalytic Synthesis of Octyl Cinnamate and Response Surface Methodology Optimization. Molecules 2022, 27, 7148. [Google Scholar] [CrossRef]
- De Regil, R.; Sandoval, G. Biocatalysis for Biobased Chemicals. Biomolecules 2013, 3, 812–847. [Google Scholar] [CrossRef]
- Priya, K.; Venugopal, T.; Chadha, A. Pseudomonas cepacia Lipase—Mediated Transesterification Reactions of Hydrocinnamates. Indian J. Biochem. Biophys. 2002, 39, 259–263. [Google Scholar]
- Priya, K.; Chadha, A. Synthesis of Hydrocinnamic Esters by Pseudomonas cepacia Lipase. Enzyme Microb. Technol. 2003, 32, 485–490. [Google Scholar] [CrossRef]
- Salgado, C.A.; dos Santos, C.I.A.; Vanetti, M.C.D. Microbial Lipases: Propitious Biocatalysts for the Food Industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of Immobilized Lipases in Enzymatic Reactors: A Review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Hernández-Varela, J.D.; Medina, D.I. Revalorization of Coffee Residues: Advances in the Development of Eco-Friendly Biobased Potential Food Packaging. Polymers 2023, 15, 2823. [Google Scholar] [CrossRef]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A Review on Valorization of Spent Coffee Grounds (SCG) towards Biopolymers and Biocatalysts Production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Brekalo, M.; Rajs, B.B.; Aladić, K.; Jakobek, L.; Šereš, Z.; Krstović, S.; Jokić, S.; Budžaki, S.; Strelec, I. Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier. Sustainability 2023, 15, 13142. [Google Scholar] [CrossRef]
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef]
- Girelli, A.M.; Chiappini, V.; Amadoro, P. Immobilization of Lipase on Spent Coffee Grounds by Physical and Covalent Methods: A Comparison Study. Biochem. Eng. J. 2023, 192, 108827. [Google Scholar] [CrossRef]
- Jasińska, K.; Zieniuk, B.; Piasek, A.M.; Wysocki, Ł.; Sobiepanek, A.; Fabiszewska, A. Obtaining a Biodegradable Biocatalyst—Study on Lipase Immobilization on Spent Coffee Grounds as Potential Carriers. Biocatal. Agric. Biotechnol. 2024, 59, 103255. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- K. de S. Lira, R.; T. Zardini, R.; C. C. de Carvalho, M.; Wojcieszak, R.; G. F. Leite, S.; Itabaiana, I., Jr. Agroindustrial Wastes as a Support for the Immobilization of Lipase from Thermomyces lanuginosus: Synthesis of Hexyl Laurate. Biomolecules 2021, 11, 445. [Google Scholar] [CrossRef]
- Jasińska, K.; Zieniuk, B.; Jankiewicz, U.; Fabiszewska, A. Bio-Based Materials versus Synthetic Polymers as a Support in Lipase Immobilization: Impact on Versatile Enzyme Activity. Catalysts 2023, 13, 395. [Google Scholar] [CrossRef]
- Zheng, J.; Fu, X.; Ying, X.; Zhang, Y.; Wang, Z. A sensitive colorimetric high-throughput screening method for lipase synthetic activity assay. Anal. Biochem. 2014, 452, 13–15. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent Coffee Grounds as a Valuable Source of Phenolic Compounds and Bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.-M.; Mourtzinos, I.; Goula, A.M. Recovery of Phenolic Compounds from Spent Coffee Grounds through Optimized Extraction Processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, Identification and Quantification of Polyphenols from Spent Coffee Grounds by Chromatographic Methods and Chemometric Analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Kowalczykiewicz, D.; Szymańska, K.; Gillner, D.; Jarzębski, A.B. Rotating Bed Reactor Packed with Heterofunctional Structured Silica-Supported Lipase. Developing an Effective System for the Organic Solvent and Aqueous Phase Reactions. Microporous Mesoporous Mater. 2021, 312, 110789. [Google Scholar] [CrossRef]
- Qian, J.; Huang, A.; Zhu, H.; Ding, J.; Zhang, W.; Chen, Y. Immobilization of Lipase on Silica Nanoparticles by Adsorption Followed by Glutaraldehyde Cross-Linking. Bioprocess Biosyst. Eng. 2023, 46, 25–38. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Hu, Y.; Abbey, L.; Cesarino, I.; Goonetilleke, A.; He, Q. Exploring the Properties and Potential Uses of Biocarbon from Spent Coffee Grounds: A Comparative Look at Dry and Wet Processing Methods. Processes 2023, 11, 2099. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of Stability and Catalytic Activity of Immobilized Lipase on Silica-Coated Modified Magnetite Nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Pourkhanali, K.; Khayati, G.; Mizani, F.; Raouf, F. Characterization of Free and Immobilized Lipase from Penicillium sp. onto Three Modified Bentonites: A Comparative Study. J. Biotechnol. 2022, 344, 57–69. [Google Scholar] [CrossRef]
- Drago, G.A.; Gibson, T.D. Enzyme Stability and Stabilisation: Applications and Case Studies. In Focus on Biotechnology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 361–376. ISBN 9780792369271. [Google Scholar]
- Silva, C.; Martins, M.; Jing, S.; Fu, J.; Cavaco-Paulo, A. Practical Insights on Enzyme Stabilization. Crit. Rev. Biotechnol. 2018, 38, 335–350. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Bachmann, L.; Said, S.; Oliveira, W.P. Immobilized Enzyme-Driven Value Enhancement of Lignocellulosic-Based Agricultural Byproducts: Application in Aroma Synthesis. J. Clean. Prod. 2021, 284, 124728. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Bachmann, L.; Said, S.; Oliveira, W.P. Enhancement Lipase Activity via Immobilization onto Chitosan Beads Used as Seed Particles during Fluidized Bed Drying: Application in Butyl Butyrate Production. Appl. Catal. A Gen. 2021, 622, 118217. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A.; Białecka-Florjańczyk, E. Screening of Solvents for Favoring Hydrolytic Activity of Candida antarctica Lipase B. Bioprocess Biosyst. Eng. 2020, 43, 605–613. [Google Scholar] [CrossRef]
- Matte, C.R.; Bordinhão, C.; Poppe, J.K.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Synthesis of Butyl Butyrate in Batch and Continuous Enzymatic Reactors Using Thermomyces Lanuginosus Lipase Immobilized in Immobead 150. J. Mol. Catal. B Enzym. 2016, 127, 67–75. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.-H.; Zhang, J.-Y.; Chen, N.; Zhi, G.-Y. High-Yield Synthesis of Bioactive Ethyl Cinnamate by Enzymatic Esterification of Cinnamic Acid. Food Chem. 2016, 190, 629–633. [Google Scholar] [CrossRef]
- Da S. Pereira, A.; L. Fraga, J.; M. Diniz, M.; C. Fontes-Sant’Ana, G.; F. F. Amaral, P. High Catalytic Activity of Lipase from Yarrowia lipolytica Immobilized by Microencapsulation. Int. J. Mol. Sci. 2018, 19, 3393. [Google Scholar] [CrossRef]
- Kharrat, N.; Aissa, I.; Dgachi, Y.; Aloui, F.; Chabchoub, F.; Bouaziz, M.; Gargouri, Y. Enzymatic Synthesis of 1,3-Dihydroxyphenylacetoyl-sn-Glycerol: Optimization by Response Surface Methodology and Evaluation of Its Antioxidant and Antibacterial Activities. Bioorg. Chem. 2017, 75, 347–356. [Google Scholar] [CrossRef]
- Ijaz, H.; Sun, S. Enzymatic Synthesis of Novel Structured Lipids with Tiger Nut Oil: Optimized by Response Surface Methodology. Biomass Convers. Biorefinery 2024, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).