Microstructure and First Hydrogenation Properties of Individual Phases in TiFe + 12 wt.% ZrV2 Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
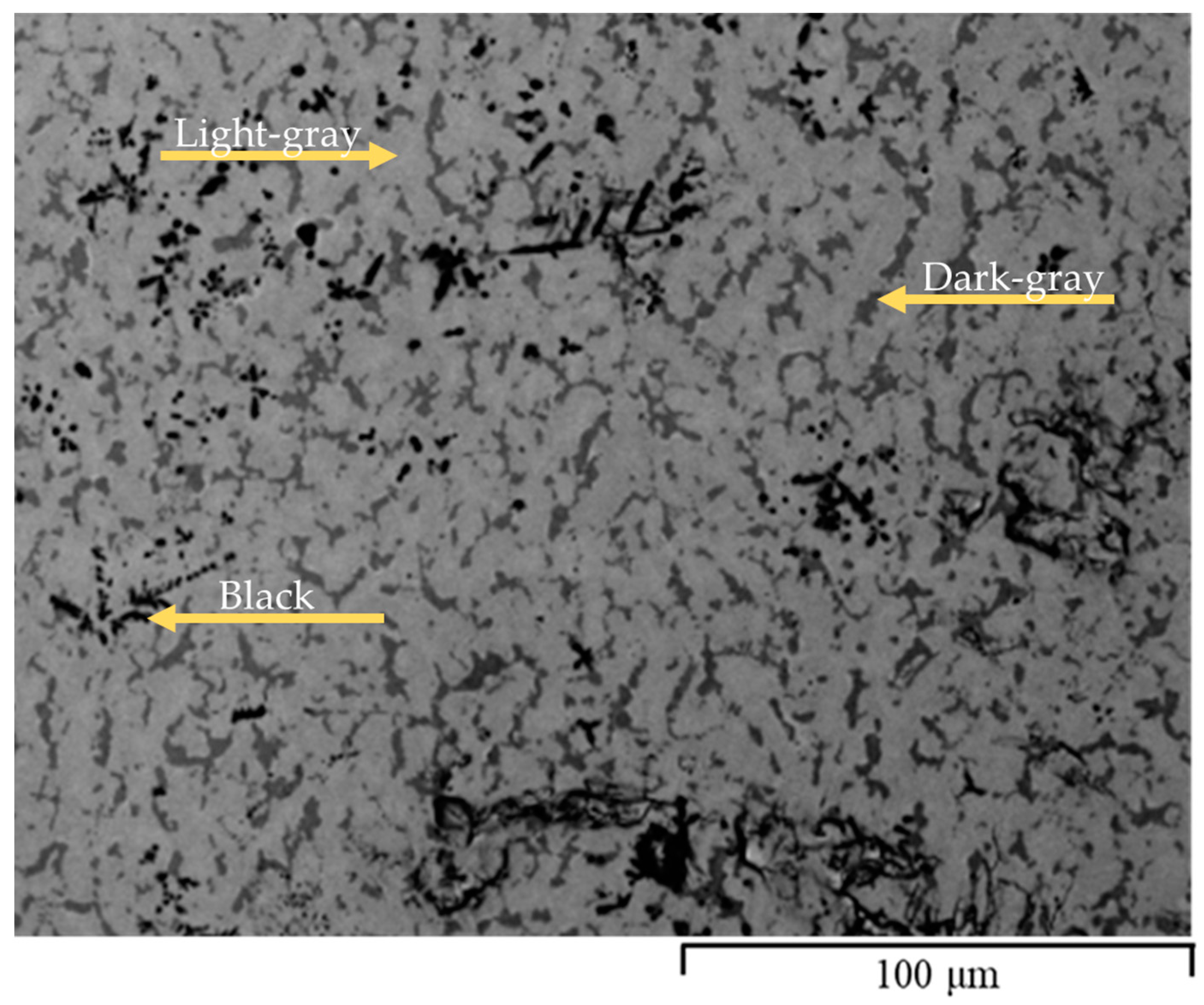
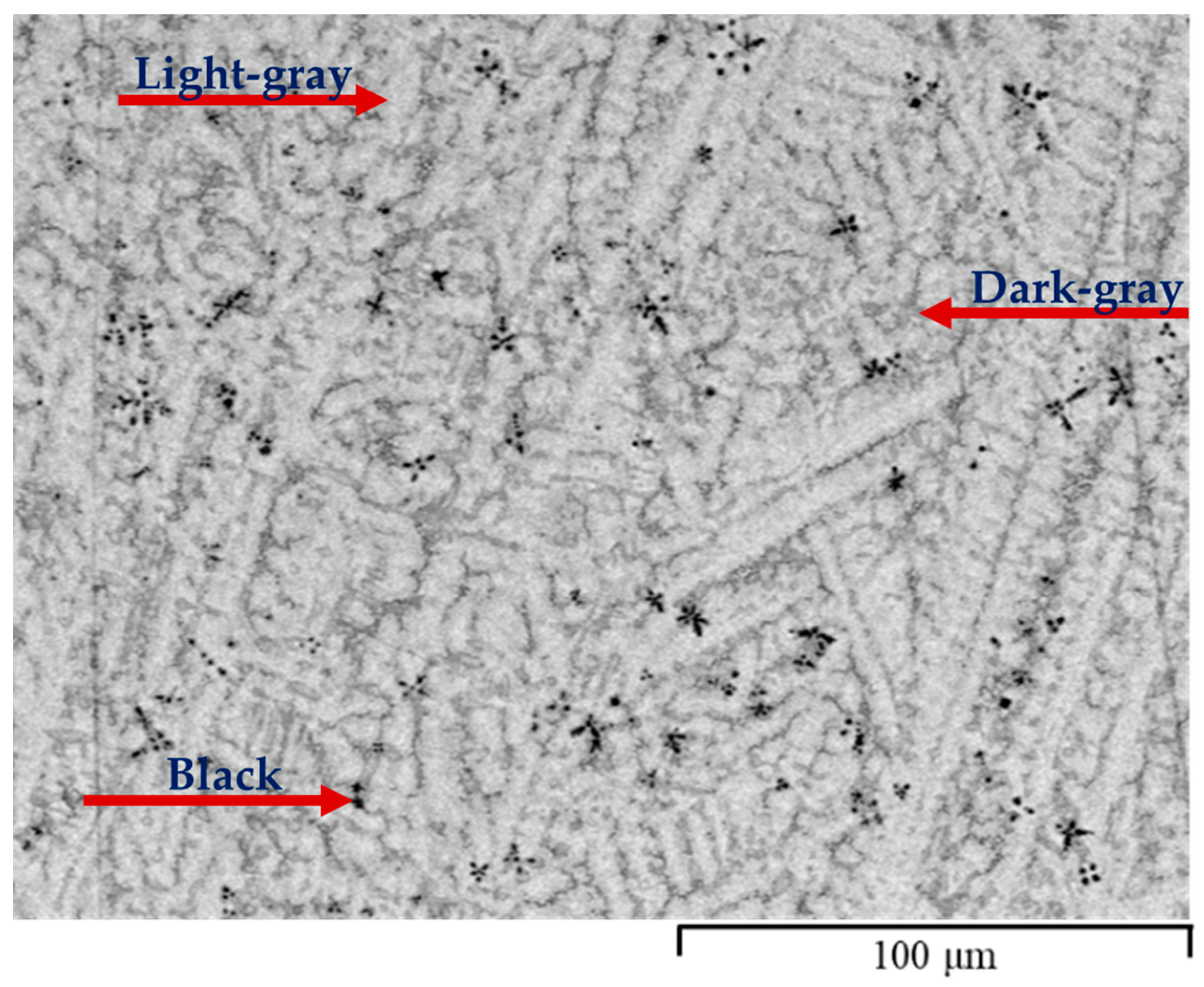
3.1. Morphology
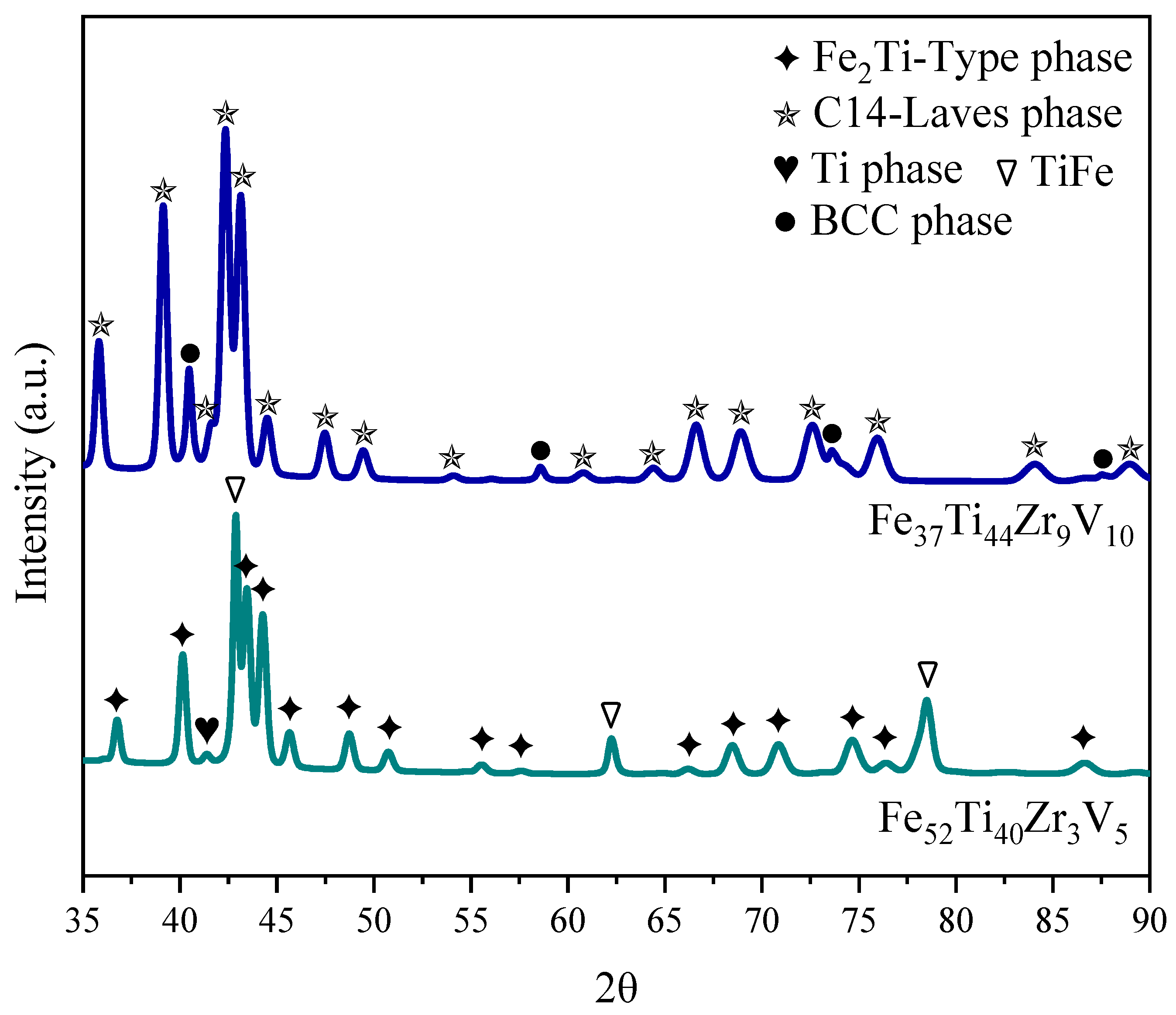
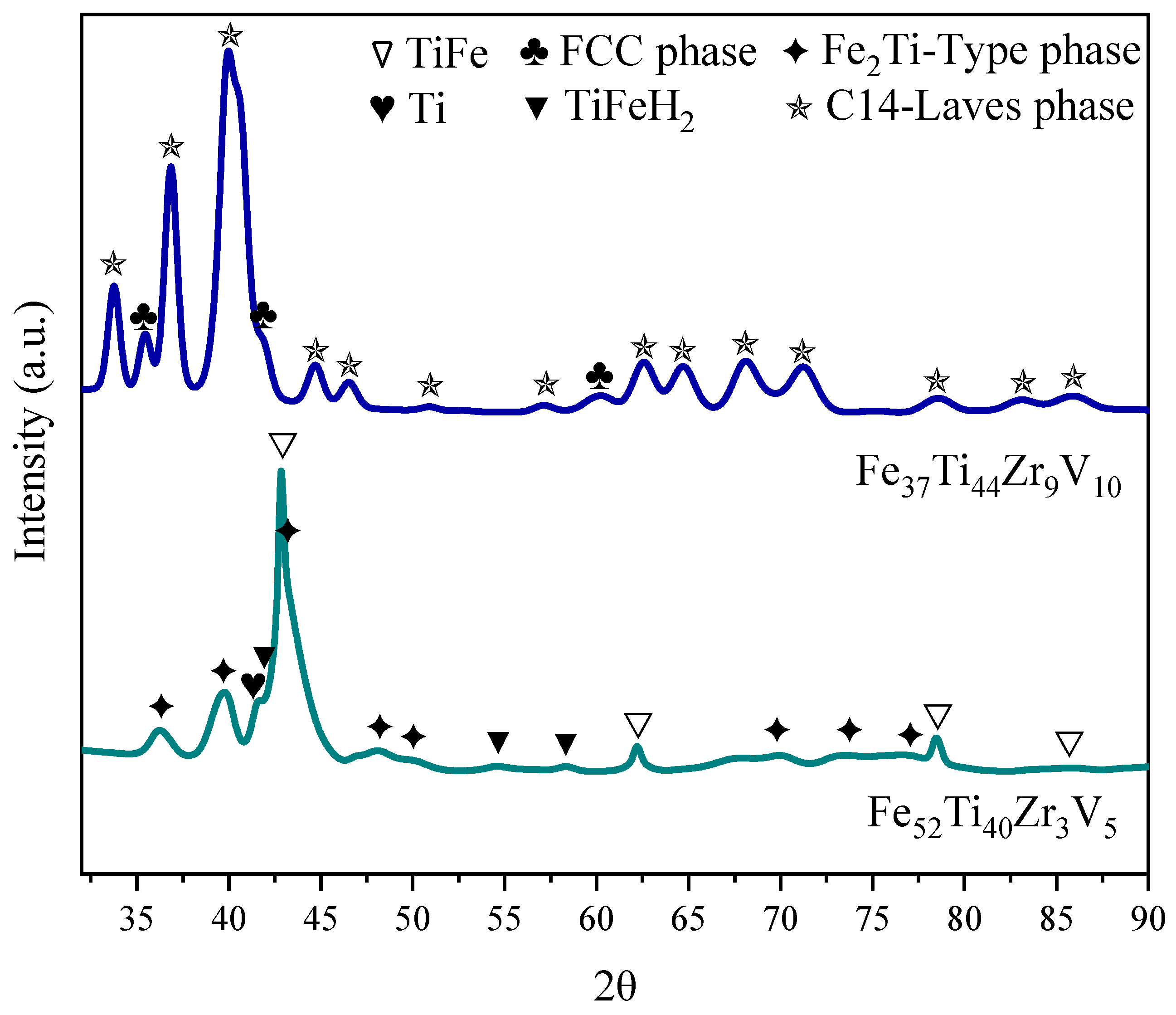
3.2. Crystal Structure of As-Cast Alloys
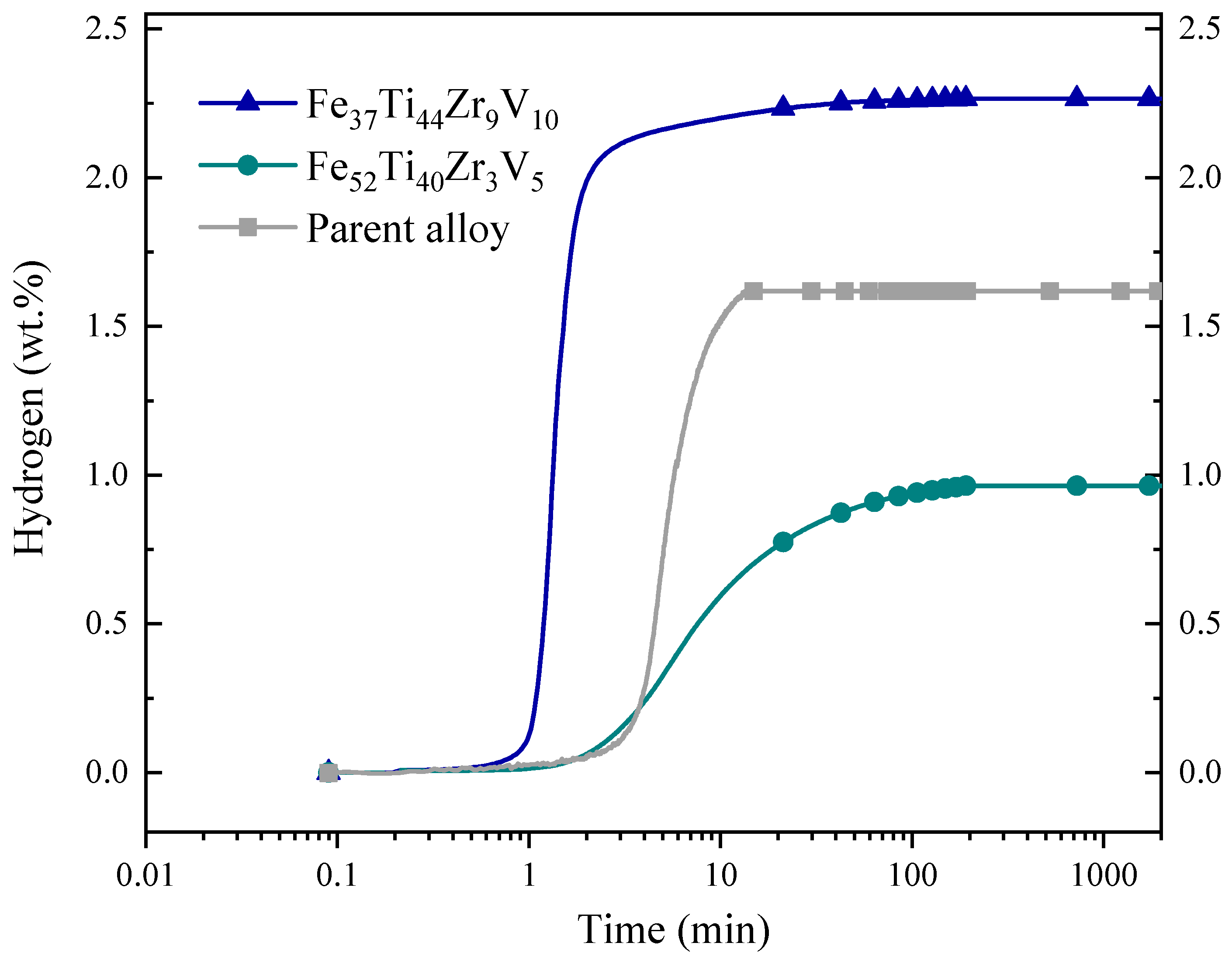
3.3. First Hydrogenation (Activation)
3.4. Crystal Structure of Hydrided Alloys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aranda, V.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Hydrogen storage properties of the TiVFeZr multicomponent alloy with C14-type laves phase structure. Intermetallics 2023, 162, 108020. [Google Scholar] [CrossRef]
- Baquero, J.E.G.; Monsalve, D.B. From fossil fuel energy to hydrogen energy: Transformation of fossil fuel energy economies into hydrogen economies through social entrepreneurship. Int. J. Hydrogen Energy 2024, 54, 574–585. [Google Scholar] [CrossRef]
- Patel, A.K.; Duguay, A.; Tougas, B.; Schade, C.; Sharma, P.; Huot, J. Microstructure and first hydrogenation properties of TiFe alloy with Zr and Mn as additives. Int. J. Hydrogen Energy 2019, 45, 787–797. [Google Scholar] [CrossRef]
- He, X.; Hu, H.; Tang, R.; Zhou, W.; Xiao, H.; Zhang, X.; Ma, C.; Chen, Q. Effect of cobalt substitution for nickel on microstructural evolution and hydrogen storage properties of La0.66Mg0.34Ni3.5–Co alloys. J. Rare Earths 2024, 42, 930–939. [Google Scholar] [CrossRef]
- Hitam, C.; Aziz, M.; Ruhaimi, A.; Taib, M. Magnesium-based alloys for solid-state hydrogen storage applications: A review. Int. J. Hydrogen Energy 2021, 46, 31067–31083. [Google Scholar] [CrossRef]
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and outlook on high-entropy alloys for hydrogen storage. Energy Environ. Sci. 2021, 14, 5191–5227. [Google Scholar] [CrossRef]
- Serrano, L.; Moussa, M.; Yao, J.-Y.; Silva, G.; Bobet, J.-L.; Santos, S.F.; Cardoso, K.R. Development of Ti-V-Nb-Cr-Mn high entropy alloys for hydrogen storage. J. Alloys Compd. 2023, 945, 169289. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H.; Wang, H.; Ouyang, L.; Zhu, M. Amorphous alloys for hydrogen storage. J. Alloys Compd. 2023, 941, 168945. [Google Scholar] [CrossRef]
- Thangarasu, S.; Palanisamy, G.; Im, Y.M.; Oh, T.H. An alternative platform of solid-state hydrides with polymers as composite/encapsulation for hydrogen storage applications: Effects in intermetallic and complex hydrides. Int. J. Hydrogen Energy 2023, 48, 21429–21450. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; de Faria, L.I.L.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2019, 45, 5356–5366. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Zhang, Y. The application of MOFs for hydrogen storage. Inorg. Chim. Acta 2023, 557, 121683. [Google Scholar] [CrossRef]
- Nagar, R.; Srivastava, S.; Hudson, S.L.; Amaya, S.L.; Tanna, A.; Sharma, M.; Achayalingam, R.; Sonkaria, S.; Khare, V.; Srinivasan, S.S. Recent developments in state-of-the-art hydrogen energy technologies—Review of hydrogen storage materials. Sol. Compass 2023, 5, 100033. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Nivedhitha, K.; Beena, T.; Banapurmath, N.; Umarfarooq, M.; Ramasamy, V.; Soudagar, M.E.M.; Ağbulut, Ü. Advances in hydrogen storage with metal hydrides: Mechanisms, materials, and challenges. Int. J. Hydrogen Energy 2024, 61, 1259–1273. [Google Scholar] [CrossRef]
- Bishnoi, A.; Pati, S.; Sharma, P. Architectural design of metal hydrides to improve the hydrogen storage characteristics. J. Power Sources 2024, 608, 234609. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Manna, J.; Huot, J. Effect of KCl Addition on First Hydrogenation Kinetics of TiFe. Compounds 2022, 2, 240–251. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Matsuda, J.; Akiba, E.; Horita, Z. Hydrogen storage performance of TiFe after processing by ball milling. Acta Mater. 2015, 88, 190–195. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Iwaoka, H.; Toh, S.; Akiba, E.; Horita, Z. High-pressure torsion of TiFe intermetallics for activation of hydrogen storage at room temperature with heterogeneous nanostructure. Int. J. Hydrogen Energy 2013, 38, 4622–4627. [Google Scholar] [CrossRef]
- Lang, J.; Huot, J. A new approach to the processing of metal hydrides. J. Alloys Compd. 2011, 509, L18–L22. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloys Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Gosselin, C.; Huot, J. Hydrogenation Properties of TiFe Doped with Zirconium. Materials 2015, 8, 7864–7872. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Huot, J. Hydrogenation improvement of TiFe by adding ZrMn2. Energy 2017, 138, 375–382. [Google Scholar] [CrossRef]
- Lv, P.; Huot, J. Hydrogen storage properties of Ti0.95FeZr0.05, TiFe0.95Zr0.05 and TiFeZr0.05 alloys. Int. J. Hydrogen Energy 2016, 41, 22128–22133. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, G.; Sonak, S.; Jain, U.; Krishnamurthy, N. High performance FeTi—3.1 mass % V alloy for on board hydrogen storage solution. Energy 2014, 75, 520–524. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, Y.; Li, Y.; Qi, Y.; Guo, S.; Zhao, D. Effects of adding over-stoichiometrical Ti and substituting Fe with Mn partly on structure and hydrogen storage performances of TiFe alloy. Renew. Energy 2018, 135, 1481–1498. [Google Scholar] [CrossRef]
- Bratanich, T.; Solonin, S.; Skorokhod, V. Mechanical activation of hydrogen sorption with intermetallic compounds LaNi5 and TiFe in powder systems. Int. J. Hydrogen Energy 1995, 20, 353–355. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.Y.; Klyamkin, S.N.; Zadorozhnyy, M.Y.; Bermesheva, O.V.; Kaloshkin, S.D. Mechanical alloying of nanocrystalline intermetallic compound TiFe doped by aluminum and chromium. J. Alloys Compd. 2014, 586, S56–S60. [Google Scholar] [CrossRef]
- Lyu, P. Effect of Mechanical Deformation on Hydrogen Storage Properties of TiFe-Based Alloys. Doctoral Thesis, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada, 2018. Available online: https://depot-e.uqtr.ca/id/eprint/8472/1/032073258.pdf (accessed on 7 August 2024).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Bououdina, M.; Lambert-Andron, B.; Ouladdiaf, B.; Pairis, S.; Fruchart, D. Structural investigations by neutron diffraction of equi-atomic Zr–Ti(V)–Ni(Co) compounds and their related hydrides. J. Alloys Compd. 2003, 356–357, 54–58. [Google Scholar] [CrossRef]
- Grytsiv, A.; Chen, X.-Q.; Witusiewicz, V.T.; Rogl, P.; Podloucky, R.; Pomjakushin, V.; Maccio, D.; Saccone, A.; Giester, G.; Sommer, F. Atom order and thermodynamic properties of the ternary Laves phase Ti(TiyNixAl1–x–y)2. Z. Fur Krist. Mater. 2006, 221, 334–348. [Google Scholar] [CrossRef]
- Oliynyk, A.; Oryshchyn, S.; Lomnytska, Y. New compounds and phase equilibria in the Zr–Ti–P system. J. Alloys Compd. 2012, 545, 80–84. [Google Scholar] [CrossRef]
| Alloy | Region | Chemical Composition (at%) | |||
|---|---|---|---|---|---|
| Fe | Ti | Zr | V | ||
| Fe52Ti40Zr3V5 | Nominal value | 52.0 | 40.0 | 3.0 | 5.0 |
| Measured Value | 50.5 | 40.3 | 4.9 | 4.3 | |
| Alloy | Region | Phase Abundance (%) | Chemical Composition (at%) | |||
|---|---|---|---|---|---|---|
| Fe | Ti | Zr | V | |||
| Fe52Ti40Zr3V5 | Light-gray | 66 | 59.9 | 32.5 | 2.6 | 5.0 |
| Dark-gray | 28 | 48.8 | 47.1 | 1.1 | 3.1 | |
| Black | 6 | 1.3 | 93.2 | 5.0 | 0.5 | |
| Alloy | Region | Chemical Composition (at%) | |||
|---|---|---|---|---|---|
| Fe | Ti | Zr | V | ||
| Fe37Ti44Zr9V10 | Nominal value | 37.0 | 44.0 | 9.0 | 10.0 |
| Measured Value | 35.2 | 43.3 | 11.9 | 9.6 | |
| Alloy | Region | Phase Abundance (%) | Chemical Composition (at%) | |||
|---|---|---|---|---|---|---|
| Fe | Ti | Zr | V | |||
| Fe37Ti44Zr9V10 | Light grey | 64 | 35.1 | 46.0 | 8.2 | 10.6 |
| Dark grey | 32 | 37.0 | 40.2 | 12.3 | 10.5 | |
| Black | 4 | 5.3 | 82.4 | 10.3 | 2.1 | |
| Alloy | Phase | Phase Abundance (%) | a (Å) | c (Å) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|---|---|---|
| Fe52Ti40Zr3V5 | Fe2Ti | 71.9 (4) | 4.8919 (4) | 7.9528 (8) | 65 (6) | 0.195 (3) |
| TiFe | 26.0 (4) | 2.9835 (2) | -- | 34 (2) | 0.077 (5) | |
| Ti | 2.1 (2) | 2.874 (1) | 4.539 (4) | 25 (5) | -- | |
| Fe37Ti44Zr9V10 | C14 | 92.0 (2) | 5.0109 (4) | 8.1420 (9) | 57 (4) | 0.231 (3) |
| BCC | 8.0 (2) | 3.1502 (4) | -- | 21 (1) | -- |
| Alloy | Phase | Phase Abundance (%) | a (Å) | c (Å) | Crystallite Size (nm) | Microstrain (%) |
|---|---|---|---|---|---|---|
| Fe52Ti40Zr3V5 | Fe2Ti | 69.0 (6) | 4.955 (1) | 8.070 (3) | 45 (10) | 0.75 (1) |
| TiFe | 15.6 (4) | 2.9901 (5) | -- | 16.1 (5) | -- | |
| TiFeH2 | 8.2 (4) | 4.738 (2) | 4.670 (2) | 17 (1) | -- | |
| Ti | 7.2 (4) | 2.921 (2) | 4.391 (5) | 8.7 (6) | -- | |
| Fe37Ti44Zr9V10 | C14 | 92.2 (3) | 5.2886 (8) | 8.600 (1) | 29 (1) | 0.443 (3) |
| FCC | 7.8 (3) | 4.3706 (8) | -- | 8.8 (3) | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellon Monsalve, D.; Ulate-Kolitsky, E.; Cubero-Sesin, J.M.; Martínez-Amariz, A.-D.; Huot, J. Microstructure and First Hydrogenation Properties of Individual Phases in TiFe + 12 wt.% ZrV2 Alloy. ChemEngineering 2024, 8, 81. https://doi.org/10.3390/chemengineering8040081
Bellon Monsalve D, Ulate-Kolitsky E, Cubero-Sesin JM, Martínez-Amariz A-D, Huot J. Microstructure and First Hydrogenation Properties of Individual Phases in TiFe + 12 wt.% ZrV2 Alloy. ChemEngineering. 2024; 8(4):81. https://doi.org/10.3390/chemengineering8040081
Chicago/Turabian StyleBellon Monsalve, Daniela, Elena Ulate-Kolitsky, Jorge M. Cubero-Sesin, Alejandro-David Martínez-Amariz, and Jacques Huot. 2024. "Microstructure and First Hydrogenation Properties of Individual Phases in TiFe + 12 wt.% ZrV2 Alloy" ChemEngineering 8, no. 4: 81. https://doi.org/10.3390/chemengineering8040081
APA StyleBellon Monsalve, D., Ulate-Kolitsky, E., Cubero-Sesin, J. M., Martínez-Amariz, A.-D., & Huot, J. (2024). Microstructure and First Hydrogenation Properties of Individual Phases in TiFe + 12 wt.% ZrV2 Alloy. ChemEngineering, 8(4), 81. https://doi.org/10.3390/chemengineering8040081






