Magnetic Three-Dimensional Graphene: A Superior Adsorbent for Selective and Sensitive Determination of Nitrite in Water Samples by Ion-Pair Based-Surfactant-Assisted Solid-Phase Extraction Combined with Spectrophotometry
Abstract
1. Introduction
2. Experimental
2.1. Procedures and Materials
2.2. Instrumentation
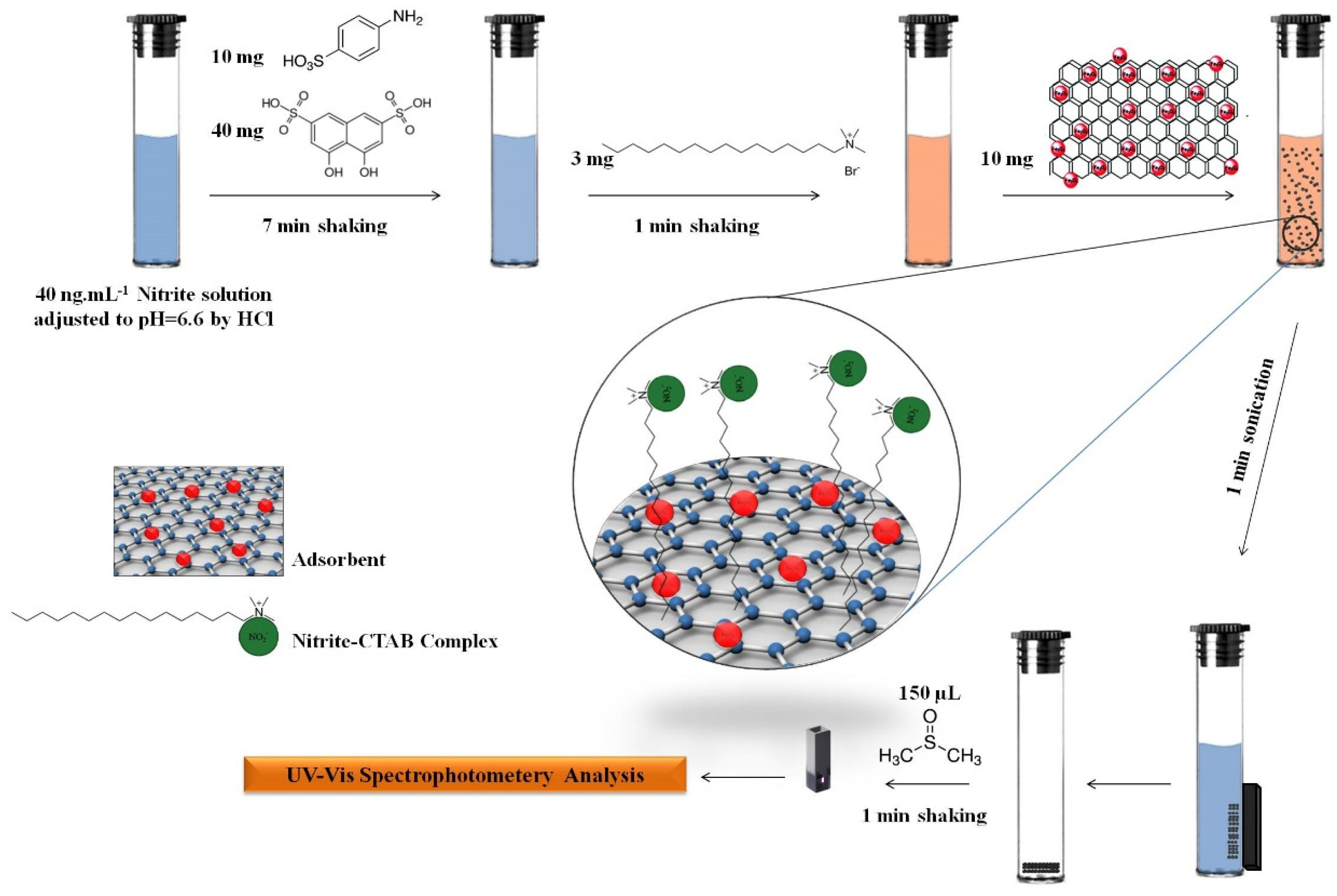
2.3. Preparation of the Adsorbent
2.4. The Procedure
3. Results and Discussion
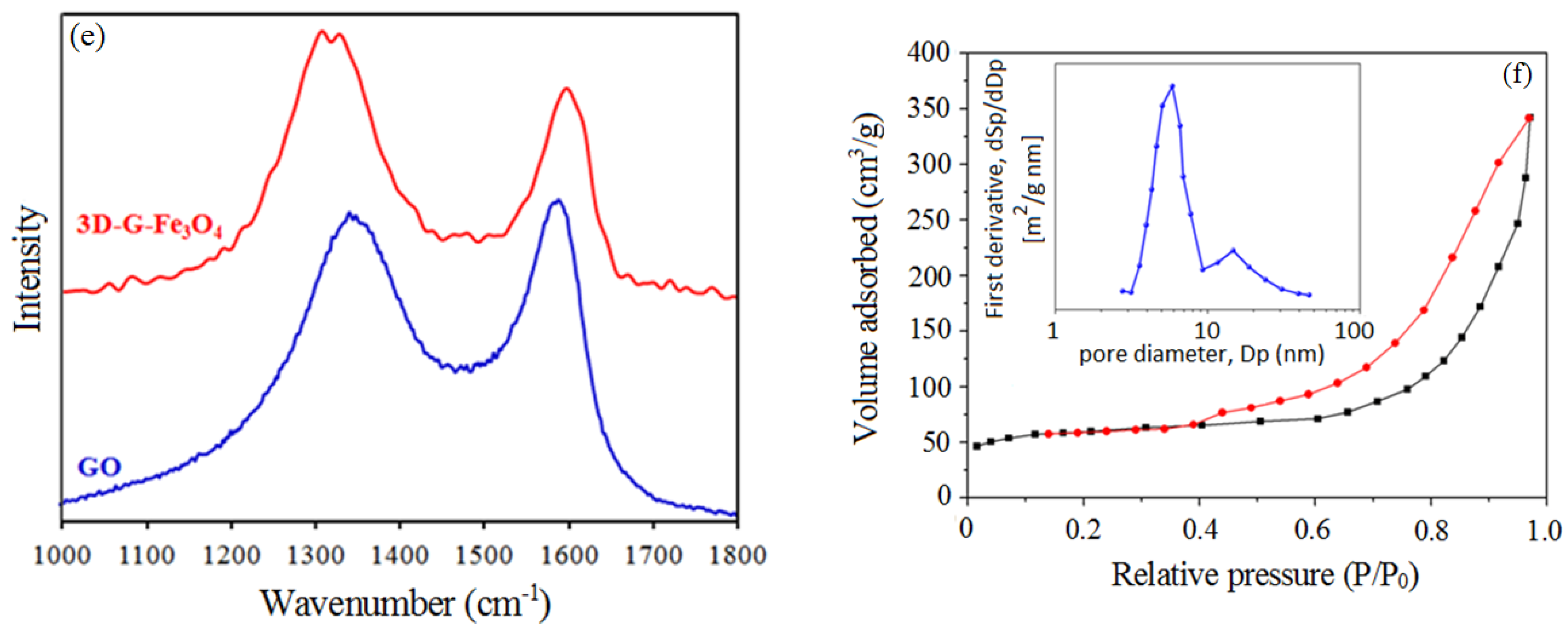
3.1. Characterization
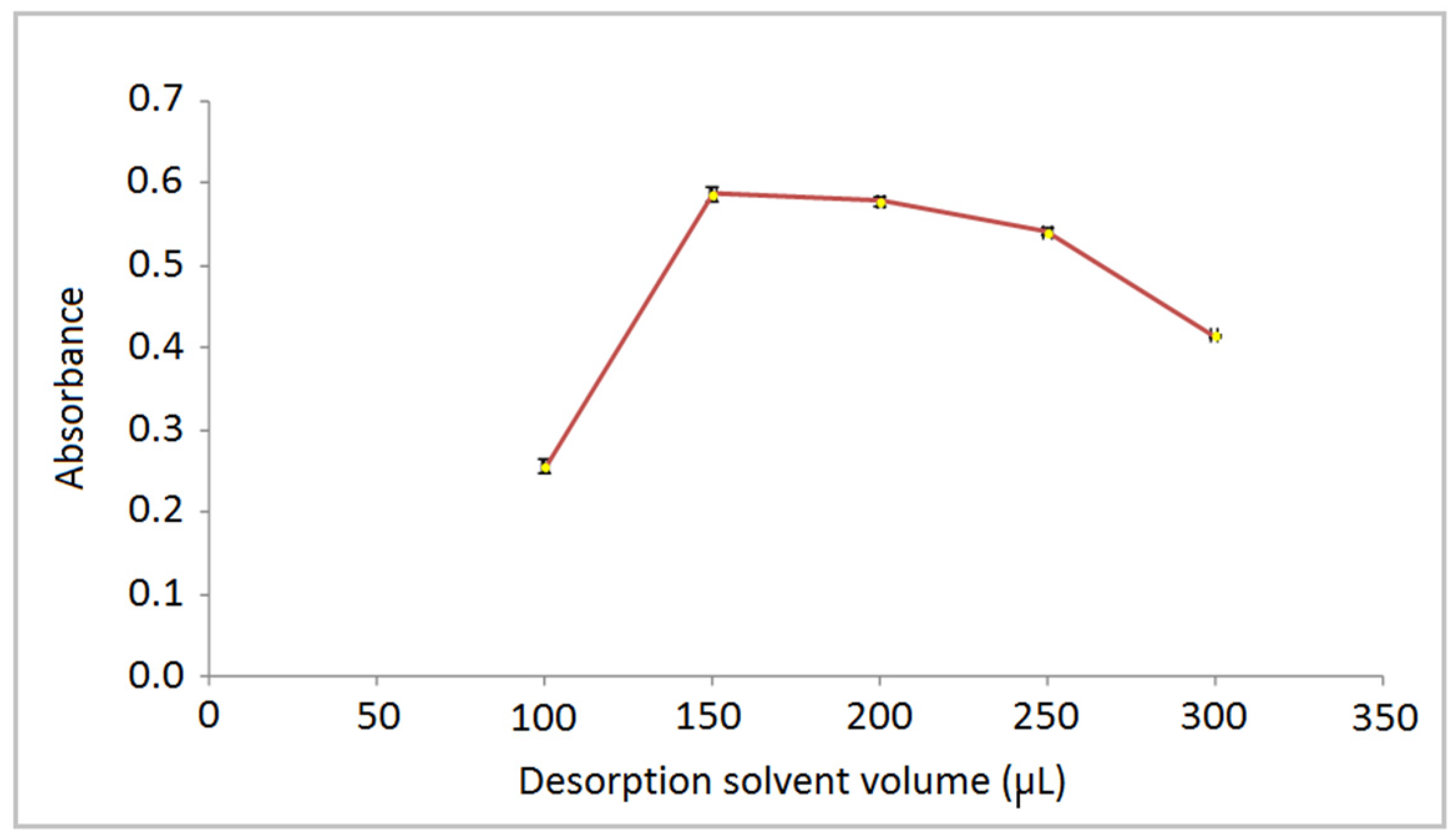
3.2. Effect of Desorption Solvents on Recovery
3.3. Optimization of the Procedure: A Central Composite Design
3.4. Salt Effect
3.5. Effect of Coexisting Ions
3.6. Adsorbent Reusability
3.7. Analytical Method Validation
3.8. Real Samples
3.9. Comparison with Similar Studies
3.10. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Weber, K.A. Natural Uranium Contamination in Major U.S. Aquifers Linked to Nitrate. Environ. Sci. Technol. Lett. 2015, 2, 215–220. [Google Scholar] [CrossRef]
- Della Betta, F.; Pereira, L.M.; Siqueira, M.A.; Valese, A.C.; Daguer, H.; Fett, R.; Vitali, L.; Costa, A.C.O. A sub-minute CZE method to determine nitrate and nitrite in meat products: An alternative for routine analysis. Meat Sci. 2016, 119, 62–68. [Google Scholar] [CrossRef]
- Alagha, I.; Doman, G.; Aouthmany, S. SIMULATION 1 Methemoglobinemia Empty Line Calibri Size 12 Empty Line Calibri Size 12. JETem 2022, 7, S1–S26. [Google Scholar] [CrossRef]
- Brender, J.D. Human Health Effects of Exposure to Nitrate, Nitrite, and Nitrogen Dioxide. In Just Enough Nitrogen; Springer International Publishing: Cham, Switzerland, 2020; pp. 283–294. [Google Scholar] [CrossRef]
- Bryan, N.S. Inorganic Nitrate and Nitrite: Dietary Nutrients or Poisons? In Nitrate Handbook; CRC Press: Boca Raton, FL, USA, 2022; pp. 357–373. [Google Scholar]
- Ackermann-Liebrich, U. Respiratory and Cardiovascular Effects of NO2 in Epidemiological Studies. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 840–844. [Google Scholar] [CrossRef]
- Tabrizi, A.B.; Sepehr, B. Extraction of ammonia and nitrite using modified magnetite iron oxide nanoparticles before spectrophotometric determination in different water samples. Int. J. Environ. Anal. Chem. 2015, 95, 833–846. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Analytical Chemistry of Nitric Oxide. Annu. Rev. Anal. Chem. 2009, 2, 409–433. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Simultaneous Determination of Nitrate and Nitrite in Fish Products with Improved Sensitivity by Sample Stacking-Capillary Electrophoresis. Food Anal. Methods 2015, 9, 706–711. [Google Scholar] [CrossRef]
- Lopez-Moreno, C.; Perez, I.V.; Urbano, A.M. Development and validation of an ionic chromatography method for the determination of nitrate, nitrite and chloride in meat. Food Chem. 2016, 194, 687–694. [Google Scholar] [CrossRef]
- He, L.; Zhang, K.; Wang, C.; Luo, X.; Zhang, S. Effective indirect enrichment and determination of nitrite ion in water and biological samples using ionic liquid-dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. J. Chromatogr. A 2011, 1218, 3595–3600. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, D.; Alvarez, M.; Robinson, K.; Ji, J.; Wang, Y.J.; Kao, Y.H.; Zhang, T. Mixed-mode and reversed-phase liquid chromatography–tandem mass spectrometry methodologies to study composition and base hydrolysis of polysorbate 20 and 80. J. Chromatogr. A 2011, 1218, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.H.I. Anodic Stripping Voltammetric Determination of Nitrite Using Carbon Paste Electrode Modified with Chitosan. Am. J. Analyt Chem. 2011, 2, 284–288. [Google Scholar] [CrossRef][Green Version]
- Alghamdi, A.H. Applications of stripping voltammetric techniques in food analysis. Arab. J. Chem. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Tarigh, G.D.; Shemirani, F. Development of a selective and pH-independent method for the analysis of ultra trace amounts of nitrite in environmental water samples after dispersive magnetic solid phase extraction by spectrofluorimetry. Talanta 2014, 128, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Turdean, G.L.; Szabo, G. Nitrite detection in meat products samples by square-wave voltammetry at a new single walled carbon naonotubes–myoglobin modified electrode. Food Chem. 2015, 179, 325–330. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Q.; Ma, W. Aqueous two-phase extraction and spectrophotometric determination of nitrite in food samples. J. Appl. Spectrosc. 2015, 82, 470–474. [Google Scholar] [CrossRef]
- Abdelshakour, M.A.; Attala, K.; Elsonbaty, A.; Salam, R.A.A.; Hadad, G.M.; Mostafa, A.E.; Eissa, M.S. Eco-Friendly UV-Spectrophotometric Methods Employing Magnetic Nano-Composite Polymer for the Extraction and Analysis of Sexual Boosters in Adulterated Food Products: Application of Computer-Aided Design. J. AOAC Int. 2023, 106, 1608–1619. [Google Scholar] [CrossRef]
- Markiewicz-Keszycka, M.; Cama-Moncunill, X.; Casado-Gavalda, M.P.; Dixit, Y.; Cama-Moncunill, R.; Cullen, P.J.; Sullivan, C. Laser-induced breakdown spectroscopy (LIBS) for food analysis: A review. Trends Food Sci. Technol. 2017, 65, 80–93. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, Q.; Chen, J.; Yang, S.; Zhu, Z.; Chen, D. Advancements in Sample Preparation Methods for the Chromatographic and Mass Spectrometric Determination of Zearalenone and Its Metabolites in Food: An Overview. Foods 2023, 12, 3558. [Google Scholar] [CrossRef]
- Bianchi, F.; Sereshti, H.; Karimi, M.; Karami, S.; Mahpishanian, S.; Bidhendi, M.E.; Rezania, S.; Mojiri, A.; Kamyab, H.; Nodeh, H.R. Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Analysis of Polycyclic Aromatic Hydrocarbons Using Magnetic Three-Dimensional Graphene Solid-Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry. Separations 2023, 10, 564. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, L.; Lv, R.; Wang, H.; Song, S.; Cao, M. Facile Cloud Point Extraction for the Separation and Determination of Phenolic Acids from Dandelion. ACS Omega 2021, 6, 13508–13515. [Google Scholar] [CrossRef]
- Pocurull, E.; Fontanals, N.; Calull, M.; Aguilar, C. Environmental Applications. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 591–641. [Google Scholar] [CrossRef]
- Hussain, C.M.; Keçili, R. Sampling and Sample preparation techniques for environmental analysis. In Modern Environmental Analysis Techniques for Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–119. [Google Scholar] [CrossRef]
- Sereshti, H.; Abdolhosseini, G.; Soltani, S.; Sadatfaraji, H.; Karami, S.; Nodeh, H.R. A green ternary polymeric deep eutectic solvent used in dispersive liquid-liquid microextraction technique for isolation of multiclass pesticides in fruit juice samples. J. Food Compos. Anal. 2023, 124, 105663. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lee; Lim, W.; Entsar; Rabea, I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Rosendo, L.M.; Brinca, A.T.; Pires, B.; Catarro, G.; Rosado, T.; Guiné, R.P.F.; Araújo, A.R.T.S.; Anjos, O.; Gallardo, E. Miniaturized Solid Phase Extraction Techniques Applied to Natural Products. Processes 2023, 11, 243. [Google Scholar] [CrossRef]
- Liu, W.-X.; Song, S.; Ye, M.-L.; Zhu, Y.; Zhao, Y.-G.; Lu, Y. Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials 2022, 12, 1845. [Google Scholar] [CrossRef]
- Zhang, B.T.; Zheng, X.; Li, H.F.; Lin, J.M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Central Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal−Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Ehrmann, C.; Li, N.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Materials Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Zeng, G.; Liu, S.; Hu, X.; Zhou, L.; Tan, X.; Liu, N.; Li, M.; Wen, J. Adsorption of estrogen contaminants (17β-estradiol and 17α-ethynylestradiol) by graphene nanosheets from water: Effects of graphene characteristics and solution chemistry. Chem. Eng. J. 2018, 339, 296–302. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, S.; Feng, X.; Wang, N.; Ola, O.; Zhu, Y. Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application. Nanomaterials 2023, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2015, 2, 40–53. [Google Scholar] [CrossRef]
- Asghari, Z.; Sereshti, H.; Soltani, S.; Taghizadeh, M.; Karami, S.; Bidhendi, M.E.; Rezania, S. An alginate-based eutectogel impregnated with polyvinylpyrrolidone/benzoic acid deep eutectic solvent and magnetic carboxylated multiwalled carbon nanotubes: Evaluated as sorbent in green microextraction of pesticides. J. Chromatogr. B 2023, 1229, 123865. [Google Scholar] [CrossRef] [PubMed]
- To, J.W.F.; Chen, Z.; Yao, H.; He, J.; Kim, K.; Chou, H.-H.; Pan, L.; Wilcox, J.; Cui, Y.; Bao, Z. Ultrahigh Surface Area Three-Dimensional Porous Graphitic Carbon from Conjugated Polymeric Molecular Framework. ACS Cent. Sci. 2015, 1, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water—A Review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef]
- Marlinda, A.R.; An’amt, M.N.; Yusoff, N.; Sagadevan, S.; Wahab, Y.A.; Johan, M.R. Recent progress in nitrates and nitrites sensor with graphene-based nanocomposites as electrocatalysts. Trends Environ. Anal. Chem. 2022, 34, e00162. [Google Scholar] [CrossRef]
- Ifa, L.; Syarif, T.; Sartia, S.; Juliani, J.; Nurdjannah, N.; Kusuma, H.S. Techno-economics of coconut coir bioadsorbent utilization on free fatty acid level reduction in crude palm oil. Heliyon 2022, 8, e09146. [Google Scholar] [CrossRef] [PubMed]
- Ifa, L.; Yani, S.; Nurjannah, N.; Darnengsih, D.; Rusnaenah, A.; Mel, M.; Mahfud, M.; Kusuma, H.S. Techno-economic analysis of bio-briquette from cashew nut shell waste. Heliyon 2020, 6, e05009. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Izzah, D.N.; Linggajati, I.W.L. Microwave-assisted drying of Ocimum sanctum leaves: Analysis of moisture content, drying kinetic model, and techno-economics. Appl. Food Res. 2023, 3, 100337. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Macroscopic Multifunctional Graphene-Based Hydrogels and Aerogels by a Metal Ion Induced Self-Assembly Process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Dziak, J.J.; Li, R. Design of Experiments with Multiple Independent Variables: A Resource Management Perspective on Complete and Reduced Factorial Designs. Psychol. Methods 2009, 14, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Karimi, M.; Samadi, S. Application of response surface method for optimization of dispersive liquid–liquid microextraction of water-soluble components of Rosa damascena Mill. essential oil. J. Chromatogr. A 2009, 1216, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Morales-Benítez, I.; Montoro-Leal, P.; García-Mesa, J.C.; Verdeja-Galán, J.; Alonso, E.I.V. Magnetic graphene oxide as a valuable material for the speciation of trace elements. TrAC Trends Anal. Chem. 2022, 157, 116777. [Google Scholar] [CrossRef]
- Hosseini, N.M.; Sheshmani, S.; Ashraf; Shahvelayati, S.; Ahmadi, R.; Adhami, F. Development and Characterization of Environmentally-Friendly Magnetically Graphene Oxide-Embedded Chitosan as a Recyclable Heterogeneous Photocatalyst. J. Polym. Environ. 2023, 32, 1952–1971. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Yang, M.-C.; Liao, H.-L.; Cheng, Y.-W.; Destyorini, F.; Irmawati, Y.; Liu, C.-M.; Yung, M.-C.; Hsu, C.-C.; Liu, T.-Y. Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field. Nanomaterials 2020, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Mccoy, T.M.; Brown, P.; Eastoe, J.; Tabor, R.F. Noncovalent Magnetic Control and Reversible Recovery of Graphene Oxide Using Iron Oxide and Magnetic Surfactants. ACS Appl. Mater. Interfaces 2015, 7, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, R.; Altunay, N.; Gürkan, N. Extraction, preconcentration and spectrophotometric determination of trace levels of thiosulfate in environmental waters. J. Iran. Chem. Soc. 2017, 14, 1033–1049. [Google Scholar] [CrossRef]
- Shariati-Rad, M.; Irandoust, M.; Mohammadi, S. Spectrophotometric determination of nitrite in soil and water using cefixime and central composite design. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 190–195. [Google Scholar] [CrossRef]



| Factor | Symbol | Levels | ||||
|---|---|---|---|---|---|---|
| −a a | −1 | 0 b | +1 | +a a | ||
| pH | A | 1.0 | 2.4 | 4.5 | 6.6 | 8.0 |
| Adsorbent (mg) | B | 5.0 | 10.0 | 17.5 | 24.9 | 30.0 |
| CTAB c (mg) | C | 1.0 | 2.8 | 5.5 | 8.2 | 10.0 |
| Source | Sum of Squares a | Df b | Mean Square c | F-Value d | p-Value e | Significance |
|---|---|---|---|---|---|---|
| Prob > F | ||||||
| Block | 1.2 × 10−3 | 1 | 1.2 × 10−3 | |||
| Model | 0.3 | 8 | 0.037 | 21.12 | <0.0001 | Significant |
| A f | 0.03 | 1 | 0.03 | 17.03 | 0.0012 | |
| B g | 0.051 | 1 | 0.051 | 28.67 | 0.0001 | |
| C h | 8.3 × 10−3 | 1 | 8.3 × 10−3 | 4.7 | 0.0493 | |
| AB | 8.6 × 10−3 | 1 | 8.6 × 10−3 | 4.87 | 0.0459 | |
| AC | 9.5 × 10−3 | 1 | 9.5 × 10−3 | 5.4 | 0.0369 | |
| A2 | 0.11 | 1 | 0.11 | 62.49 | <0.0001 | |
| B2 | 7.0 × 10−3 | 1 | 7.0 × 10−3 | 4 | 0.0670 | |
| C2 | 0.076 | 1 | 0.076 | 43.07 | <0.0001 | |
| Residual i | 0.023 | 13 | 1.8 × 10−3 | |||
| Lack of Fit j | 0.012 | 6 | 2.1 × 10−3 | 1.38 | 0.3388 | Not significant |
| Pure Error k | 0.01 | 7 | 1.5 × 10−3 | |||
| Cor total l | 0.32 | 22 |
| Coexisting Ions | Coexisting Ion/NO2− Ratio (w/w) | ER |
|---|---|---|
| NH4+ | 1000 | 101 |
| PO43− | 1000 | 98 |
| CO32− | 1000 | 102 |
| SO42− | 1000 | 97 |
| NO3− | 1000 | 99 |
| Cl− | 1000 | 103 |
| Fe3+ | 1000 | 98 |
| Hg2+ | 1000 | 91 |
| Cu2+ | 1000 | 96 |
| Na+ | 1000 | 102 |
| Ca2+ | 1000 | 96 |
| Sample | Added (ng mL−1) | Found (ng mL−1) | RSD (%) (n = 3) | RR a (%) |
|---|---|---|---|---|
| Tap water b | - | - | - | - |
| 20 | 20.02 | 1.04 | 100.1 | |
| 80 | 81.28 | 1.1 | 101.6 | |
| Rain water c | - | - | - | - |
| 20 | 19.29 | 0.99 | 96.45 | |
| 80 | 78.36 | 1.03 | 97.95 | |
| Mineral water d | - | - | - | - |
| 20 | 19.84 | 1.06 | 99.2 | |
| 80 | 80.4 | 1.25 | 100.5 | |
| Sewage e | - | 18.42 | 1 | - |
| 20 | 37.63 | 0.97 | 96.05 | |
| 80 | 98.85 | 1.09 | 100.54 | |
| Sea water f | - | - | - | - |
| 20 | 19.57 | 0.96 | 97.85 | |
| 80 | 79.04 | 1.34 | 98.8 |
| Analysis | Extraction | t (min) | V (mL) a | EF b | LOD c | LDR d | RSD (%) | R (%) e | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| UV-Vis | SPE f | >36 | 250 | 125 | 3.1 | 10–550 | <6.6 | 88–105 | [9] |
| UV-Vis | LPE g | >60 | - | - | 60 | 500–6000 | 2.2 | - | [20] |
| UV-Vis | - | >40 | - | - | 4.3 | 20–15000 | <10 | 94–108 | [54] |
| Fluorimetry | SPE h | 1.5 | 160 | 160 | 0.034 | 0.1–80 | 0.6 | 94–102 | [18] |
| Electrophoresis | - | 2.5 | - | - | 0.82 i | - | 0.99 | 89–104 | [12] |
| UV-Vis | SPE j | 10 | 25 | 167 | 5.12 | 20–100 | 1.01 | 93–110 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasheghani Farahani, M.; Karami, S.; Sereshti, H.; Mahpishanian, S.; Koupaei Malek, S.; Rezania, S. Magnetic Three-Dimensional Graphene: A Superior Adsorbent for Selective and Sensitive Determination of Nitrite in Water Samples by Ion-Pair Based-Surfactant-Assisted Solid-Phase Extraction Combined with Spectrophotometry. ChemEngineering 2024, 8, 47. https://doi.org/10.3390/chemengineering8030047
Vasheghani Farahani M, Karami S, Sereshti H, Mahpishanian S, Koupaei Malek S, Rezania S. Magnetic Three-Dimensional Graphene: A Superior Adsorbent for Selective and Sensitive Determination of Nitrite in Water Samples by Ion-Pair Based-Surfactant-Assisted Solid-Phase Extraction Combined with Spectrophotometry. ChemEngineering. 2024; 8(3):47. https://doi.org/10.3390/chemengineering8030047
Chicago/Turabian StyleVasheghani Farahani, Mina, Sajad Karami, Hassan Sereshti, Shokouh Mahpishanian, Somayeh Koupaei Malek, and Shahabaldin Rezania. 2024. "Magnetic Three-Dimensional Graphene: A Superior Adsorbent for Selective and Sensitive Determination of Nitrite in Water Samples by Ion-Pair Based-Surfactant-Assisted Solid-Phase Extraction Combined with Spectrophotometry" ChemEngineering 8, no. 3: 47. https://doi.org/10.3390/chemengineering8030047
APA StyleVasheghani Farahani, M., Karami, S., Sereshti, H., Mahpishanian, S., Koupaei Malek, S., & Rezania, S. (2024). Magnetic Three-Dimensional Graphene: A Superior Adsorbent for Selective and Sensitive Determination of Nitrite in Water Samples by Ion-Pair Based-Surfactant-Assisted Solid-Phase Extraction Combined with Spectrophotometry. ChemEngineering, 8(3), 47. https://doi.org/10.3390/chemengineering8030047







