Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
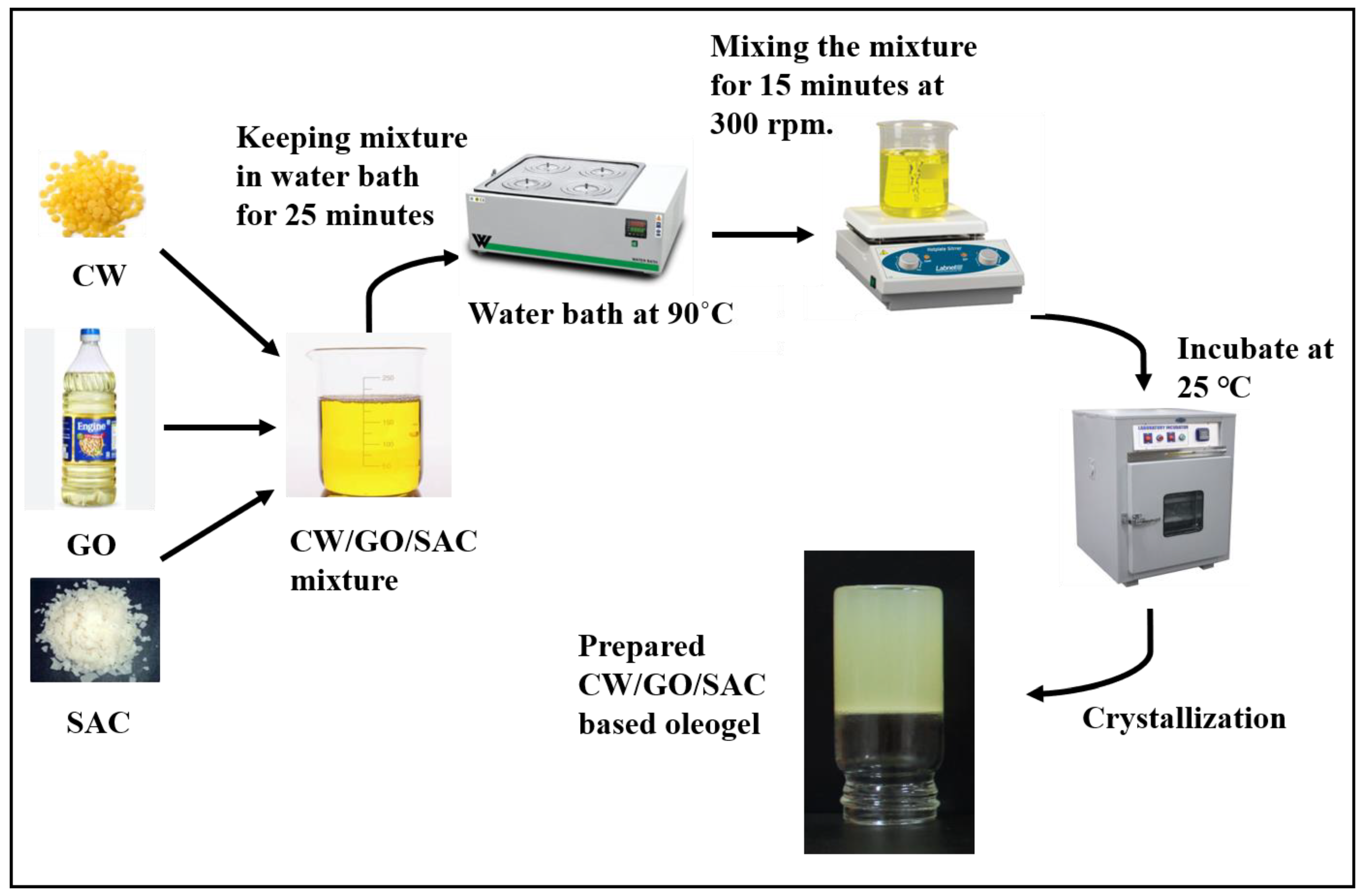
2.2. Oleogel Preparation
2.3. Oil Binding Capacity
2.4. Colorimetry
2.5. Polarized Light Microscopy (PLM)
2.6. FTIR Spectroscopy
2.7. Raman Spectroscopy
2.8. X-ray Diffraction (XRD)
2.9. Crystallization Kinetics
2.10. Texture Analysis
2.11. Curcumin Release Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance and OBC of the Prepared Oleogels
3.2. Colorimetry
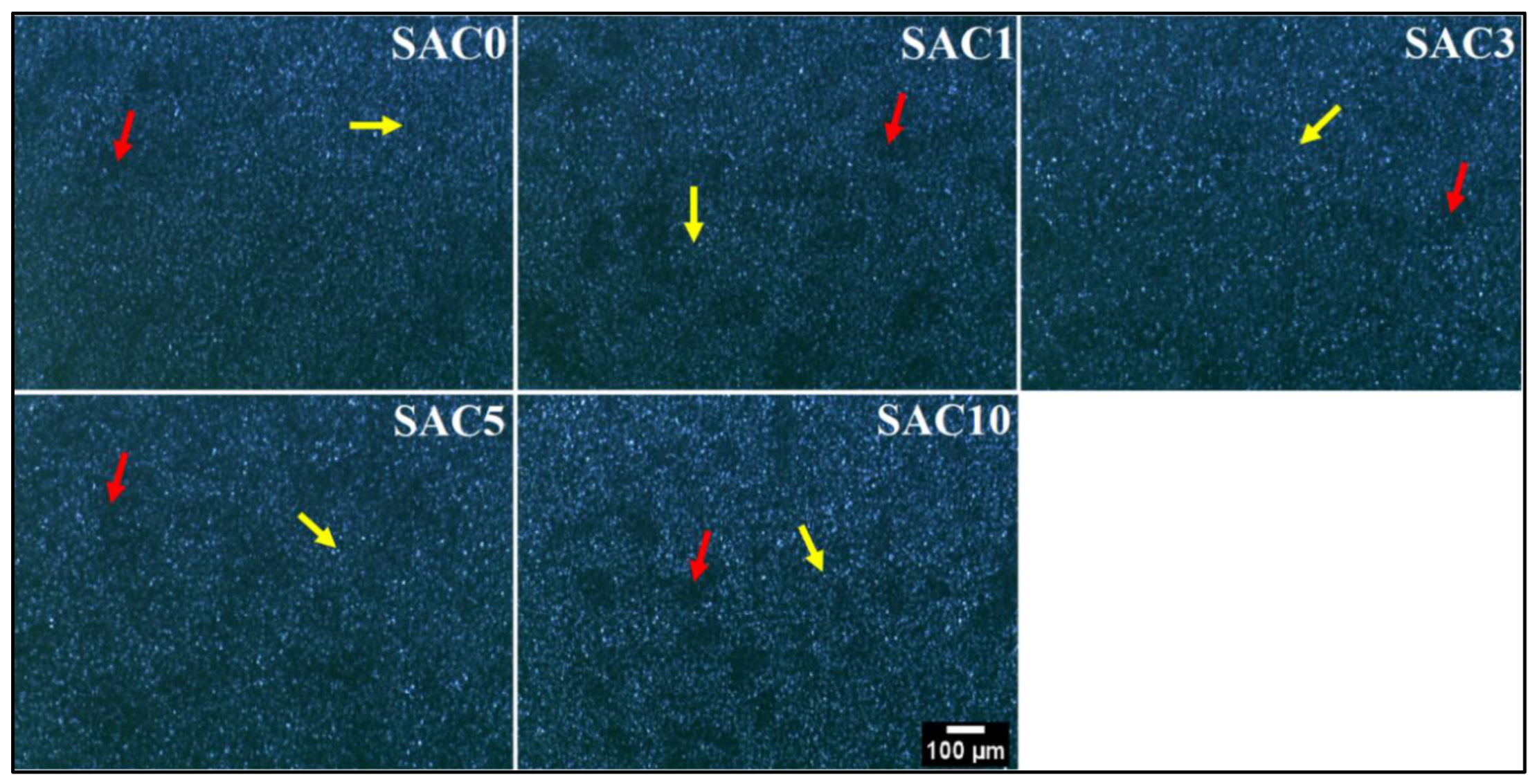
3.3. Polarized Light Microscopy
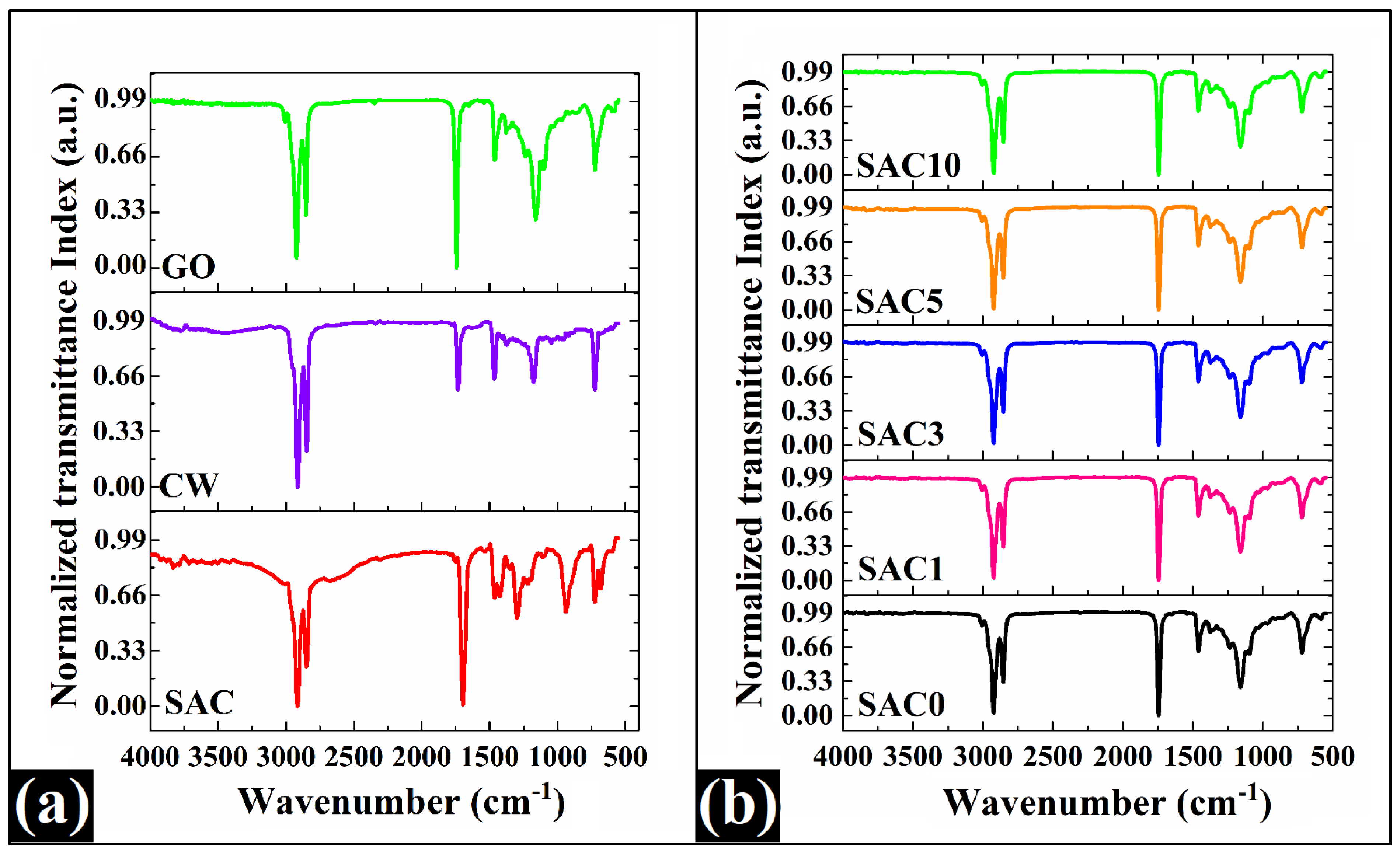
3.4. FTIR Analysis
3.5. Raman Spectroscopy
3.6. X-ray Diffraction
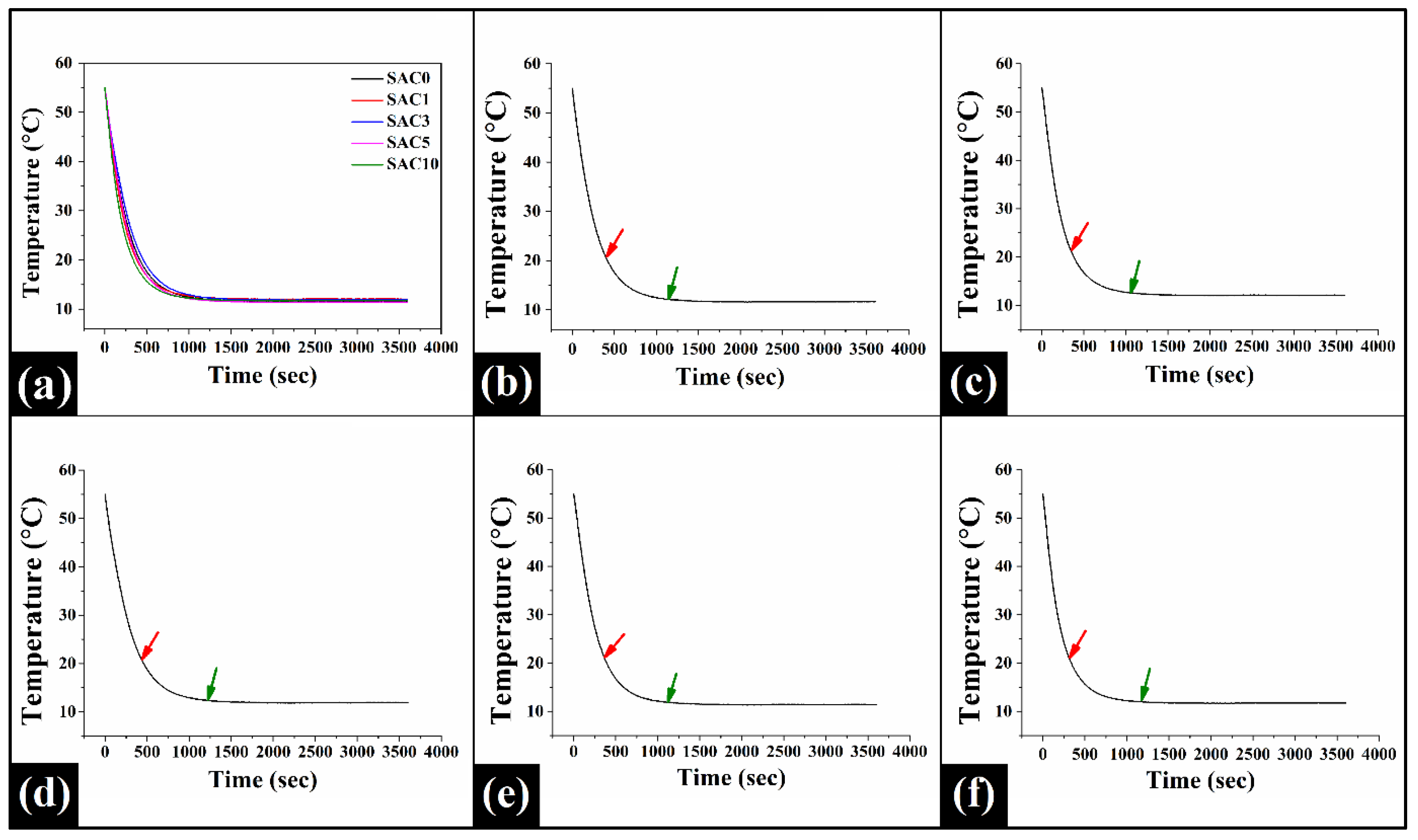
3.7. Crystallization Kinetics
3.8. Texture Analysis
3.9. Curcumin Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef]
- Yadav, E.; Sebastian, S.; Gupta, M.K. Aminopyridinyl Tricosanamide Based Pseudoplastic and Thermoreversible Oleogels for pH-Dependant in vitro Release of Metronidazole. ChemistrySelect 2022, 7, e202203014. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P.; Cunha, R.L. Tailoring properties of mixed-component oleogels: Wax and monoglyceride interactions towards flaxseed oil structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.; Laufer, S.; Rosen-Kligvasser, J.; Lesmes, U.; Davidovich-Pinhas, M. Impact of different oil gelators and oleogelation mechanisms on digestive lipolysis of canola oil oleogels. Food Hydrocoll. 2019, 97, 105218. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.-Q.; Guo, H.; Li, W.-H. Recent advances of organogels: From fabrications and functions to applications. Prog. Org. Coat. 2021, 159, 106417. [Google Scholar] [CrossRef]
- Banaś, K.; Harasym, J. Natural gums as oleogelators. Int. J. Mol. Sci. 2021, 22, 12977. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, G.; Bogojevic, O.; Pedersen, J.N.; Guo, Z.J.C.R.i.F.S.; Safety, F. Edible oleogels as solid fat alternatives: Composition and oleogelation mechanism implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2077–2104. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, J.; Zhou, Q.; Jiang, L.; Wang, L.; Dai, Y.; Yu, D.; Elfalleh, W. Crude wax extracted from rice bran oil improves oleogel properties and oxidative stability. Eur. J. Lipid Sci. Technol. 2021, 123, 2000091. [Google Scholar] [CrossRef]
- da Silva, T.L.; Arellano, D.B.; Martini, S. Physical properties of candelilla wax, monoacylglycerols, and fully hydrogenated oil oleogels. J. Am. Oil Chem. Soc. 2018, 95, 797–811. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; del Pilar Medina-Herrera, N.; Castro-López, C.; Martínez-Ávila, G.C.G. Candelilla wax: Prospective suitable applications within the food field. LWT 2022, 113170. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.; Sánchez-Becerril, M.; Marangoni, A.; Toro-Vazquez, J.; Ornelas-Paz, J.; Ibarra-Junquera, V. Structuration, elastic properties scaling, and mechanical reversibility of candelilla wax oleogels with and without emulsifiers. Food Res. Int. 2019, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K. Properties of margarines prepared from soybean oil oleogels with mixtures of candelilla wax and beeswax. J. Food Sci. 2020, 85, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ramirez, J.; Vernon-Carter, E.; Carrera-Tarela, Y.; Garcia, A.; Roldan-Cruz, C. Effects of candelilla wax/canola oil oleogel on the rheology, texture, thermal properties and in vitro starch digestibility of wheat sponge cake bread. LWT 2020, 130, 109701. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in microstructural and physicochemical properties of candelilla wax/rice bran oil–derived oleogels using sunflower lecithin and soya lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Li, L.; Taha, A.; Geng, M.; Zhang, Z.; Su, H.; Xu, X.; Pan, S.; Hu, H. Ultrasound-assisted gelation of β-carotene enriched oleogels based on candelilla wax-nut oils: Physical properties and in-vitro digestion analysis. Ultrason. Sonochemistry 2021, 79, 105762. [Google Scholar] [CrossRef]
- Tan, S.S.; Tan, C.X.; Tan, S.T. Bioactive Phytochemicals from Peanut Oil-Processing By-Products; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–11. [Google Scholar]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B.J.L. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT 2019, 112, 107648. [Google Scholar] [CrossRef]
- Mridula, D.; Saha, D.; Gupta, R.; Bhadwal, S.; Arora, S.; Kumar, S.R. Oil Expelling from Dehulled De-Skinned Groundnut Kernel using Screw Press: Optimization of Process Parameters and Physico-chemical Characteristics. Int. J. Curr. Microbiol. App. Sci 2020, 9, 1101–1115. [Google Scholar] [CrossRef]
- Pramanik, A.; Tiwari, S.; Tripathi, M.; Mandloi, S.; Tomar, R. Identification of groundnut germplasm lines for foliar disease resistance and high oleic traits using SNP and gene-based markers and their morphological characterization. Legume Res.-Int. J. 2022, 45, 305–310. [Google Scholar] [CrossRef]
- Saha, D.; Nanda, S.K.; Yadav, D.N. Optimization of spray drying process parameters for production of groundnut milk powder. Powder Technol. 2019, 355, 417–424. [Google Scholar] [CrossRef]
- Vishnu, V.S. Scope of Entrepreneurship Developments in Groundnut Processing. In Entrepreneurship Development in Food Processing; CRC Press: Boca Raton, FL, USA, 2021; pp. 187–215. [Google Scholar]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bajželj, B.; Laguzzi, F.; Röös, E. The role of fats in the transition to sustainable diets. Lancet Planet. Health 2021, 5, e644–e653. [Google Scholar] [CrossRef]
- Adlak, T.; Tiwari, S.; Gupta, N.; Tripathi, M.; Sikarwar, R.; Sastya, R.; Gupta, V. Assessment for yield and nutritional profiling of groundnut with the help of allele specific markers for desirable fatty acids. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1625–1637. [Google Scholar]
- Liu, C.; Zheng, Z.; Zaaboul, F.; Cao, C.; Huang, X.; Liu, Y. Effects of wax concentration and carbon chain length on the structural modification of fat crystals. Food Funct. 2019, 10, 5413–5425. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G. Vegetable Oil Oleogels Structured Using Mixtures of Stearyl Alcohol and Stearic Acid (SO: SA). In Edible Oleogels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 193–217. [Google Scholar]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Redondas, C.E.; Baümler, E.R.; Carelli, A.A. Sunflower wax recovered from oil tank settlings: Revaluation of a waste product from the oilseed industry. J. Sci. Food Agric. 2020, 100, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Flöter, E.; Wettlaufer, T.; Conty, V.; Scharfe, M. Oleogels—their applicability and methods of characterization. Molecules 2021, 26, 1673. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Muste, S. Application of analytical methods for the comprehensive analysis of oleogels—A review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef] [PubMed]
- Winkler-Moser, J.K.; Anderson, J.; Byars, J.A.; Singh, M.; Hwang, H.S. Evaluation of beeswax, candelilla wax, rice bran wax, and sunflower wax as alternative stabilizers for peanut butter. J. Am. Oil Chem. Soc. 2019, 96, 1235–1248. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. The effect of addition of high-melting monoacylglycerol and candelilla wax on pea and faba bean protein foam-templated oleogelation. J. Am. Oil Chem. Soc. 2020, 97, 1319–1333. [Google Scholar] [CrossRef]
- Monogarova, O.; Oskolok, K.; Apyari, V. Colorimetry in chemical analysis. J. Anal. Chem. 2018, 73, 1076–1084. [Google Scholar] [CrossRef]
- Lehnert, S.; Dubinina, A.; Deynichenko, H.; Khomenko, O.; Gapontseva, O.; Antoniuk, I.; Medvedeva, A.; Demichkovska, M.; Vasylieva, O. The study of influence of natural antioxidants on quality of peanut and linseed oil blends during their storage. East.-Eur. J. Enterp. Technol. 2018, 44–50. [Google Scholar] [CrossRef]
- Jedidi, B.; Mokbli, S.; Sbihi, H.M.; Nehdi, I.A.; Romdhani-Younes, M.; Al-Resayes, S.I. Effect of extraction solvents on fatty acid composition and physicochemical properties of Tecoma stans seed oils. J. King Saud Univ.-Sci. 2020, 32, 2468–2473. [Google Scholar] [CrossRef]
- Nurhasanah, S.; Setyadi, A.; Munarso, S.J.; Subroto, E.; Filianty, F. Shelf-life prediction of peanut oil (Arachis hypogaea L.) using an accelerated shelf-life testing (ASLT) method in the polypropylene packaging. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Shi, Z.; Xu, W.; Geng, M.; Chen, Z.; Meng, Z. Oil body-based one-step multiple phases and hybrid emulsion gels stabilized by sunflower wa and CMC: Application and optimization in 3D printing. Food Hydrocoll. 2023, 136, 108262. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Physical properties, microstructure, intermolecular forces, and oxidation stability of soybean oil oleogels structured by different cellulose ethers. Eur. J. Lipid Sci. Technol. 2018, 120, 1700287. [Google Scholar] [CrossRef]
- Sánchez-Becerril, M.; Marangoni, A.; Perea-Flores, M.; Cayetano-Castro, N.; Martínez-Gutiérrez, H.; Andraca-Adame, J.; Pérez-Martínez, J. Characterization of the micro and nanostructure of the candelilla wax organogels crystal networks. Food Struct. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed oil in new application: Assessment of structure of oleogels based on their physicochemical properties and microscopic observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Chen, Y.; Gavaliatsis, T.; Kuster, S.; Städeli, C.; Fischer, P.; Windhab, E.J. Crust treatments to reduce bread staling. Curr. Res. Food Sci. 2021, 4, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Pérez-Martínez, J.; Gallegos-Infante, J.; Toro-Vazquez, J.; Ornelas-Paz, J.J.C.P. Rheological properties of ethyl cellulose-monoglyceride-candelilla wax oleogel vis-a-vis edible shortenings. Carbohydr. Polym. 2021, 252, 117171. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Monou, P.-K.; Komis, G.; Bouropoulos, N.; Ritzoulis, C.; Delis, G.; Kiosis, E.; Arsenos, G.; Fatouros, D.G. Effect of Glyceryl Monoolein Addition on the Foaming Properties and Stability of Whipped Oleogels. Gels 2022, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.I.; Marangoni, A.G. The use of cooling rate to engineer the microstructure and oil binding capacity of wax crystal networks. Food Biophys. 2015, 10, 456–465. [Google Scholar] [CrossRef]
- Borriello, A.; Masi, P.; Cavella, S. Novel pumpkin seed oil-based oleogels: Development and physical characterization. LWT 2021, 152, 112165. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on thermal degradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Impact of roasting and extraction methods on chemical properties, oxidative stability and Maillard reaction products of peanut oils. J. Food Sci. Technol. 2019, 56, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Shasidhar, Y.; Vishwakarma, M.K.; Pandey, M.K.; Janila, P.; Variath, M.T.; Manohar, S.S.; Nigam, S.N.; Guo, B.; Varshney, R.K. Molecular mapping of oil content and fatty acids using dense genetic maps in groundnut (Arachis hypogaea L.). Front. Plant Sci. 2017, 8, 794. [Google Scholar] [CrossRef]
- Balamurugan, V.; Haritha, J.; ArunChendhuran, R.; Srivel, S.; AmalaJerrin, J.N.; Kishore, S.; Pachamuthu, M. Classification of Groundnut Oil Using Advanced ATR-MIR Spectroscopy and Chemometrics. Food Anal. Methods 2022, 15, 1778–1786. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B.; Tokatli, F. Use of FTIR and UV–visible spectroscopy in determination of chemical characteristics of olive oils. Talanta 2019, 201, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R.; Kumari, S.; Sharma, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib. Spectrosc. 2021, 113, 103222. [Google Scholar] [CrossRef]
- Lionetto, F.; López-Muñoz, R.; Espinoza-González, C.; Mis-Fernández, R.; Rodríguez-Fernández, O.; Maffezzoli, A. A study on exfoliation of expanded graphite stacks in candelilla wax. Materials 2019, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Núñez-García, I.C.; Rodríguez-Flores, L.G.; Guadiana-De-Dios, M.H.; González-Hernández, M.D.; Martínez-Ávila, G.C.; Gallegos-Infante, J.A.; González-Laredo, R.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Rojas, R. Candelilla Wax Extracted by Traditional Method and an Ecofriendly Process: Assessment of Its Chemical, Structural and Thermal Properties. Molecules 2022, 27, 3735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and green fabrication of a superhydrophobic surface by one-step immersion for continuous oil/water separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Hernández, Y.; Lozano, T.; Morales-Cepeda, A.B.; Navarro-Pardo, F.; Angeles, M.E.; Morales-Zamudio, L.; Melo-Banda, J.A.; Sánchez-Valdes, S.; Martínez-Colunga, G.; Rodríguez, F. Stearic acid as interface modifier and lubricant agent of the system: Polypropylene/calcium carbonate nanoparticles. Polym. Eng. Sci. 2019, 59, E279–E285. [Google Scholar] [CrossRef]
- Sagiri, S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic acid based oleogels: A study on the molecular, thermal and mechanical properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Pazinato, J.C.; Garcia, I.T. Sub-stoichiometric tungsten oxide by the stearic acid method. Ceram. Int. 2022, 48, 11971–11980. [Google Scholar] [CrossRef]
- Hasda, A.M.; Vuppaladadium, S.S.R.; Qureshi, D.; Prasad, G.; Mohanty, B.; Banerjee, I.; Shaikh, H.; Anis, A.; Sarkar, P.; Pal, K. Graphene oxide reinforced nanocomposite oleogels improves corneal permeation of drugs. J. Drug Deliv. Sci. Technol. 2020, 60, 102024. [Google Scholar] [CrossRef]
- Covaciu, F.-D.; Berghian-Grosan, C.; Feher, I.; Magdas, D.A. Edible oils differentiation based on the determination of fatty acids profile and Raman spectroscopy—A case study. Appl. Sci. 2020, 10, 8347. [Google Scholar] [CrossRef]
- Zhao, H.; Zhan, Y.; Xu, Z.; Nduwamungu, J.J.; Zhou, Y.; Powers, R.; Xu, C. The application of machine-learning and Raman spectroscopy for the rapid detection of edible oils type and adulteration. Food Chem. 2022, 373, 131471. [Google Scholar] [CrossRef]
- Esmonde-White, K.; Lewis, M.; Perilli, T.; Della Vedova, T.; Lewis, I. Raman Spectroscopy in Analyzing Fats and Oils in Foods. Raman Technol. Today’s Spectrosc. 2022, 37, 34–45. [Google Scholar] [CrossRef]
- Eshelman, E.; Daly, M.; Slater, G.; Dietrich, P.; Gravel, J.-F. An ultraviolet Raman wavelength for the in-situ analysis of organic compounds relevant to astrobiology. Planet. Space Sci. 2014, 93, 65–70. [Google Scholar] [CrossRef]
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.A.; Melo, M.J. Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J. Raman Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Tran, C.; O’Callaghan, T.; Hogan, S.A. Use of confocal Raman imaging to understand the microstructure of anhydrous milk fat-based oleogels. Food Struct. 2021, 30, 100228. [Google Scholar] [CrossRef]
- Okuro, P.K.; Tavernier, I.; Sintang, M.D.B.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic interactions between lecithin and fruit wax in oleogel formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Cerqueira, F.; Vicente, A.A.; Cunha, R.L.; Pastrana, L.M.; Cerqueira, M.A. Gelation Behavior and Stability of Multicomponent Sterol-Based Oleogels. Gels 2022, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, G. Corn oil-based oleogels with different gelation mechanisms as novel cocoa butter alternatives in dark chocolate. J. Food Eng. 2019, 263, 114–122. [Google Scholar] [CrossRef]
- Declerck, A.; Nelis, V.; Danthine, S.; Dewettinck, K.; Van der Meeren, P. Characterisation of fat crystal polymorphism in cocoa butter by time-domain NMR and DSC deconvolution. Foods 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of hydrogen bonds in TAG derivative-based oleogel structure and properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef]
- Chai, X.-h.; Meng, Z.; Cao, P.-r.; Liang, X.-y.; Piatko, M.; Campbell, S.; Lo, S.K.; Liu, Y.-f. Influence of indigenous minor components on fat crystal network of fully hydrogenated palm kernel oil and fully hydrogenated coconut oil. Food Chem. 2018, 255, 49–57. [Google Scholar] [CrossRef]
- Arita-Merino, N.; van Valenberg, H.; Gilbert, E.P.; Scholten, E. Quantitative Phase Analysis of Complex Fats during Crystallization. Cryst. Growth Des. 2020, 20, 5193–5202. [Google Scholar] [CrossRef]
- Kumar, M.; Velaga, S.; Singh, A. A study of thickness dependent microstructure of poly (3-hexylthiophene) thin films using grazing incidence x-ray diffraction. Soft Mater. 2022, 20, 24–34. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Determination of crystal structure of polyaniline and substituted polyanilines through powder X-ray diffraction analysis. Polym. Test. 2008, 27, 851–857. [Google Scholar] [CrossRef]
- Speakman, S.A. Estimating crystallite size using XRD. MIT Cent. Mater. Sci. Eng. 2014, 2, 14. [Google Scholar]
- Su, C.; Li, Y.; Zhu, J.; Gao, Y.; Li, Q.; Du, S.; Yu, X. Effect of flaxseed gum on the brittleness of oleogels based on candelilla wax. RSC Adv. 2022, 12, 30734–30741. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Dobson, S.; Marangoni, A.G. Hardness, plasticity, and oil binding capacity of binary mixtures of natural waxes in olive oil. Curr. Res. Food Sci. 2022, 5, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ramirez, I.S.A.; Yang, J.; Ciftci, O.N. Evaluation of oil-gelling properties and crystallization behavior of sorghum wax in fish oil. Food Chem. 2020, 309, 125567. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. The ternary solid state phase behavior of triclinic POP, POS, and SOS and its relationship to CB and CBE properties. Cryst. Growth Des. 2018, 19, 704–713. [Google Scholar] [CrossRef]
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Formation, characterization, and potential food application of rice bran wax oleogels: Expeller-pressed corn germ oil versus refined corn oil. Food Chem. 2020, 309, 125704. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Choudhary, B.; Mohanty, B.; Sarkar, P.; Anis, A.; Cerqueira, M.A.; Banerjee, I.; Maji, S.; Pal, K. Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels. Gels 2020, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Gillman, J.D.; Winkler-Moser, J.K.; Kim, S.; Singh, M.; Byars, J.A.; Evangelista, R.L. Properties of oleogels formed with high-stearic soybean oils and sunflower wax. J. Am. Oil Chem. Soc. 2018, 95, 557–569. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Gong, Y.; Wang, W.; Wang, X.; Yu, D.; Wu, F.; Wang, L. Effect of ultrasound on the structural characteristics and oxidative stability of walnut oil oleogel coated with soy protein isolate-phosphatidylserine. Ultrason. Sonochemistry 2022, 83, 105945. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Fawzia, S.; Madhujith, T.; Karim, A.J.C.R.i.F.S.; Safety, F. Synergistic effects of oleogelators in tailoring the properties of oleogels: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3507–3539. [Google Scholar] [CrossRef] [PubMed]
- Haj-Shafiei, S.; Workman, B.; Trifkovic, M.; Mehrotra, A.K. In-situ monitoring of paraffin wax crystal formation and growth. Cryst. Growth Des. 2019, 19, 2830–2837. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani, J.; Shuaib, D.; Mohammed, A.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, L. Nanoparticles fabricated from bulk solid lipids: Preparation, properties, and potential food applications. Adv. Colloid Interface Sci. 2019, 273, 102033. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, I.; Żbikowska, A.; Onacik-Gür, S. Candelilla wax-based oleogels versus palm oil: Evaluation of physical properties of innovative and conventional lipids using optical techniques. J. Sci. Food Agric. 2022, 102, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Sislioglu, K.; Gumus, C.E.; Koo, C.K.; Karabulut, I.; McClements, D.J. In vitro digestion of edible nanostructured lipid carriers: Impact of a Candelilla wax gelator on performance. Food Res. Int. 2021, 140, 110060. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Cerqueira, M.A.; Mohanty, B.; Habibullah, S.; Banerjee, I.; Pal, K. Effect of biodegradable hydrophilic and hydrophobic emulsifiers on the oleogels containing sunflower wax and sunflower oil. Gels 2021, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Sagiri, S.S.; Kasiviswanathan, U.; Shaw, G.S.; Singh, M.; Anis, A.; Pal, K. Effect of sorbitan monostearate concentration on the thermal, mechanical and drug release properties of oleogels. Korean J. Chem. Eng. 2016, 33, 1720–1727. [Google Scholar] [CrossRef]
- Sarkisyan, V.; Sobolev, R.; Frolova, Y.; Malinkin, A.; Makarenko, M.; Kochetkova, A. Beeswax fractions used as potential oil gelling agents. J. Am. Oil Chem. Soc. 2021, 98, 281–296. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Uvanesh, K.; Sagiri, S.; Senthilguru, K.; Pramanik, K.; Banerjee, I.; Anis, A.; Al-Zahrani, S.; Pal, K. Effect of span 60 on the microstructure, crystallization kinetics, and mechanical properties of stearic acid oleogels: An in-depth analysis. J. Food Sci. 2016, 81, E380–E387. [Google Scholar] [CrossRef]
- Wei, F.; Miao, J.; Tan, H.; Feng, R.; Zheng, Q.; Cao, Y.; Lan, Y. Oleogel-structured emulsion for enhanced oxidative stability of perilla oil: Influence of crystal morphology and cooling temperature. LWT 2021, 139, 110560. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study–delivery by design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of more efficacious curcumin delivery systems using colloid science: Enhanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.; Kasiviswanathan, U.; Soni, C.; Paul, S.R.; Nayak, S.K.; Sagiri, S.S.; Anis, A.; Pal, K. Stearic acid modified stearyl alcohol oleogel: Analysis of the thermal, mechanical and drug release properties. J. Surfactants Deterg. 2017, 20, 851–861. [Google Scholar] [CrossRef]
- Moydeen, A.M.; Padusha, M.; Thamer, B.M.; Ahamed, N.A.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M.H. Single-nozzle core-shell electrospun nanofibers of pvp/dextran as drug delivery system. Fibers Polym. 2019, 20, 2078–2089. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Kouidri, S.; Salahinejad, E.; Tcharkhtchi, A.; Bakir, F. Controlled release from polyurethane films: Drug release mechanisms. J. Appl. Polym. Sci. 2021, 138, 50083. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Madhar, N.A.; Al-Zahrani, S.M. Development of Bigels Based on Date Palm-Derived Cellulose Nanocrystal-Reinforced Guar Gum Hydrogel and Sesame Oil/Candelilla Wax Oleogel as Delivery Vehicles for Moxifloxacin. Gels 2022, 8, 330. [Google Scholar] [CrossRef] [PubMed]






| Samples | CW (g) | GO (g) | SAC Stock (g) | Final Weight (g) | SAC (mg) | Percentage of SAC (%) |
|---|---|---|---|---|---|---|
| SAC0 | 1 | 19 | 0 | 20 | 0 | 0 |
| SAC1 | 1 | 18 | 1 | 20 | 1 | 5 |
| SAC3 | 1 | 16 | 3 | 20 | 3 | 15 |
| SAC5 | 1 | 14 | 5 | 20 | 5 | 25 |
| SAC10 | 1 | 9 | 10 | 20 | 10 | 50 |
| Samples | Peaks | Peak Position (xc) | Peak Width (FWHM) | Height | d-Spacing (Å) | Crystallite Size (nm) | Lattice Strain | Dislocation Density (δ) × 1017 Lines/m2 |
|---|---|---|---|---|---|---|---|---|
| SAC0 | 1 | 22.18 | 4.74 | 603.44 | 4.65 | 2.07 | 0.11 | 0.23 |
| 2 | 24.15 | 3.43 | 297.96 | 4.28 | 2.88 | 0.07 | 0.12 | |
| 3 | 25.24 | 0.52 | 185.16 | 4.10 | 18.83 | 0.01 | 0.00 | |
| 4 | 27.29 | 3.55 | 193.85 | 3.79 | 2.80 | 0.06 | 0.13 | |
| 5 | 18.15 | 3.06 | 62.53 | 5.68 | 3.19 | 0.08 | 0.10 | |
| 6 | 26.12 | 0.94 | 21.52 | 3.96 | 10.49 | 0.02 | 0.01 | |
| Average | 2.71 | 4.41 | 6.71 | 0.06 | 0.10 | |||
| SAC1 | 1 | 23.13 | 4.30 | 670.36 | 4.46 | 2.29 | 0.09 | 0.19 |
| 2 | 24.39 | 0.58 | 22.62 | 4.24 | 16.87 | 0.01 | 0.00 | |
| 3 | 25.17 | 0.55 | 205.69 | 4.11 | 17.88 | 0.01 | 0.00 | |
| 4 | 26.04 | 1.20 | 45.06 | 3.97 | 8.25 | 0.02 | 0.01 | |
| 5 | 27.31 | 3.52 | 162.56 | 3.79 | 2.82 | 0.06 | 0.13 | |
| 6 | 19.95 | 4.43 | 190.38 | 5.17 | 2.21 | 0.11 | 0.20 | |
| Average | 2.43 | 4.29 | 8.39 | 0.05 | 0.09 | |||
| SAC3 | 1 | 22.96 | 3.26 | 211.03 | 4.50 | 3.02 | 0.07 | 0.11 |
| 2 | 25.23 | 3.43 | 108.26 | 4.10 | 2.89 | 0.07 | 0.12 | |
| 3 | 25.23 | 0.49 | 129.38 | 4.10 | 20.15 | 0.01 | 0.00 | |
| 4 | 27.81 | 5.19 | 115.45 | 3.72 | 1.92 | 0.09 | 0.27 | |
| 5 | 28.01 | 0.57 | 39.92 | 3.70 | 17.52 | 0.01 | 0.00 | |
| 6 | 22.43 | 6.38 | 398.81 | 4.60 | 1.54 | 0.14 | 0.42 | |
| Average | 3.22 | 4.12 | 7.84 | 0.06 | 0.15 | |||
| SAC5 | 1 | 23.14 | 3.71 | 306.60 | 4.46 | 2.65 | 0.08 | 0.14 |
| 2 | 24.21 | 0.84 | 23.05 | 4.27 | 11.74 | 0.02 | 0.01 | |
| 3 | 25.21 | 0.55 | 142.92 | 4.10 | 18.00 | 0.01 | 0.00 | |
| 4 | 25.99 | 0.71 | 18.23 | 3.98 | 13.88 | 0.01 | 0.01 | |
| 5 | 26.25 | 5.71 | 201.88 | 3.94 | 1.74 | 0.11 | 0.33 | |
| 6 | 21.07 | 4.83 | 190.37 | 4.89 | 2.03 | 0.11 | 0.24 | |
| Average | 2.73 | 4.27 | 8.34 | 0.06 | 0.12 | |||
| SAC10 | 1 | 22.90 | 3.54 | 359.25 | 4.51 | 2.78 | 0.08 | 0.13 |
| 2 | 24.42 | 2.28 | 75.77 | 4.23 | 4.33 | 0.05 | 0.05 | |
| 3 | 25.25 | 0.51 | 165.65 | 4.09 | 19.39 | 0.01 | 0.00 | |
| 4 | 26.04 | 0.52 | 24.00 | 3.97 | 19.14 | 0.01 | 0.00 | |
| 5 | 26.05 | 5.93 | 280.91 | 3.97 | 1.67 | 0.11 | 0.36 | |
| 6 | 21.04 | 5.57 | 279.80 | 4.90 | 1.76 | 0.13 | 0.32 | |
| Average | 3.06 | 4.28 | 8.18 | 0.06 | 0.14 |
| Temp vs. Time | Exponential Decay Model | ||
|---|---|---|---|
| Samples | Onset of Secondary Crystallization (s) | Time to Reach Thermal Equilibrium (s) | Initial Rate of Crystallization (k) (°C/ms) |
| SAC0 | 393.33 ± 41.63 ab | 1143.33 ± 136.50 ab | 2.62 ± 0.42 a |
| SAC1 | 343.33 ± 30.55 a | 1053.33 ± 37.86 a | 2.93 ± 0.30 a |
| SAC3 | 430 ± 20 b | 1220 ± 79.37 b | 2.41 ± 0.19 a |
| SAC5 | 370 ± 45.83 ab | 1106.67 ± 87.37 ab | 2.75 ± 0.38 a |
| SAC10 | 310 ± 34.64 a | 1013.33 ± 37.86 a | 3.31 ± 0.65 a |
| Sample | Kd | Kr | Kd/Kr | m | R2 |
|---|---|---|---|---|---|
| SAC0 | 1.23 a | 0.25 b | 5.02 a | 0.34 a | 0.99 |
| SAC1 | 4.74 d | 0.79 c | 6.17 a | 0.30 a | 0.99 |
| SAC3 | 2.64 c | 0.26 b | 10.18 b | 0.42 b | 0.99 |
| SAC5 | 1.89 bc | 0.09 a | 22.41 c | 0.48 c | 0.99 |
| SAC10 | 1.66 b | 0.30 b | 5.52 a | 0.36 ab | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaturvedi, D.; Bharti, D.; Dhal, S.; Sahu, D.; Behera, H.; Sahoo, M.; Kim, D.; Jarzębski, M.; Anis, A.; Mohanty, B.; et al. Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels. ChemEngineering 2023, 7, 96. https://doi.org/10.3390/chemengineering7050096
Chaturvedi D, Bharti D, Dhal S, Sahu D, Behera H, Sahoo M, Kim D, Jarzębski M, Anis A, Mohanty B, et al. Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels. ChemEngineering. 2023; 7(5):96. https://doi.org/10.3390/chemengineering7050096
Chicago/Turabian StyleChaturvedi, Diksha, Deepti Bharti, Somali Dhal, Deblu Sahu, Haladhar Behera, Minaketan Sahoo, Doman Kim, Maciej Jarzębski, Arfat Anis, Biswaranjan Mohanty, and et al. 2023. "Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels" ChemEngineering 7, no. 5: 96. https://doi.org/10.3390/chemengineering7050096
APA StyleChaturvedi, D., Bharti, D., Dhal, S., Sahu, D., Behera, H., Sahoo, M., Kim, D., Jarzębski, M., Anis, A., Mohanty, B., Sagiri, S. S., & Pal, K. (2023). Role of Stearic Acid as the Crystal Habit Modifier in Candelilla Wax-Groundnut Oil Oleogels. ChemEngineering, 7(5), 96. https://doi.org/10.3390/chemengineering7050096










