Abstract
Natural gas liquids are a by-product of natural gas preparation, one of the most common and environmentally friendly energy sources. In natural gas fields located in remote areas, there is no resource-efficient way to use natural gas liquids. However, natural gas liquids are a valuable hydrocarbon feedstock for the production of motor fuels, in particular motor gasolines. The aim of this work is to develop a method for obtaining motor gasolines by processing natural gas liquids on a zeolite catalyst, taking into account the influence of the particle size of the zeolite catalyst, the technological parameters of the process, and the composition of the feedstock. As part of the work, for the first time, regularities of the influence of zeolite catalyst particle size, technological parameters of the process and the composition of feedstock on the composition and characteristics of the resulting processed products were revealed. A database about the composition and characteristics of natural gas liquids, obtained from various gas fields in Western Siberia of the Russian Federation, has been accumulated. During the study, it was found that the optimal particle size of the zeolite catalyst is 0.50–1.00 mm; optimal technological parameters are a temperature of 375 °C, pressure 2.5 atm. and the feedstock space velocity 2 h−1. It is shown that the processing of natural gas liquids of various compositions on a zeolite catalyst, on average, makes it possible to increase their detonation resistance by more than 16 points. The results obtained indicate the prospects of using the process for the production of motor gasoline. The paper presents a number of blending recipes for obtaining fuels, both within the framework of production at the fields and at processing plants.
1. Introduction
One of the most environmentally friendly and applicable sources of energy in the world now is natural gas. Gas is used to generate heat and electricity, and also as a fuel for transport [1]. The popularity and environmental friendliness of natural gas are the reasons for the steadily growing demand for this type of fuel [2,3]. That is why the search for and development of new natural gas fields are being actively conducted. Basically, large gas fields are located in areas with harsh climatic conditions, in particular in the Arctic regions [4,5]. The Russian Federation is one of the leaders in terms of explored reserves of natural gas, its production and supply to world markets [6,7].
At the same time, an increase in gas production leads to an increase in the volume of products obtained in the process of its preparation and bringing it to commercial quality. One such product is natural gas liquids (NGLs). NGLs in the process of preparation of commercial natural gas are obtained in the course of low-temperature separation, low-temperature condensation, rectification, or by combining these processes [8,9,10]. NGLs consist of hydrocarbons C5-C8 with more than 50% vol. The composition of NGLs includes paraffinic hydrocarbons of normal and iso-structure; the content of naphthenes usually varies from 5% to 30% vol., the content of aromatic hydrocarbons usually does not exceed 10% vol., and the content of olefins usually does not exceed 5% vol. The presence of olefinic hydrocarbons in the composition of NGLs is explained by the peculiarity of the preparation of natural gas in the process of adsorption drying [11,12]. The described composition of NGLs makes them a promising feedstock for the production of motor gasoline components. This direction, in contrast to the existing methods of using NLGs, will allow using them most rationally. Now, in oil and gas fields, NLGs are usually added to oil to increase the yield of light oil fractions, while in the case of gas fields, they are simply burned or pumped back into the reservoir to maintain pressure, since it is unprofitable to store and export the produced volumes in batches, and there are no pipelines for transportation. At the same time, as mentioned earlier, NLGs are obtained in areas with a harsh climate, which also need to be provided with motor fuels, in particular motor gasolines. Now, such territories are provided with gasoline from the so-called “big land” which multiplies the final cost of fuel for consumers.
In addition, there is the problem of fuel delivery. Sometimes fuel delivery is simply impossible due to the lack of infrastructure, or is available, for example, only in the winter months, when it is possible to drive along the winter roads [13,14,15]. In this case, fuel must be stored, for which it is necessary to build and maintain fuel storage facilities, which also does not reduce the cost of fuel. The solution to this problem can be the production of fuel, in particular gasoline from NGLs using small-tonnage autonomous installations [16]. Such processes for light hydrocarbon feedstock can be implemented on zeolite catalysts, the use of which, due to their resistance to catalytic poisons, does not require additional preparation of feedstock, as well as the use of hydrogen [17]. Chemical reactions that allow increasing the octane number of products occur on zeolites [18,19,20]. There are examples of work on the production of motor gasoline from feedstock similar in composition (straight-run gasolines) [21]. In addition, there are works on obtaining gasoline from gas condensates; however, in these, the authors managed to obtain only low-octane gasolines [22]. In addition, NLGs can be used as feedstock for the steam cracking process, which is an energy-intensive process used to convert NLGs into ethylene and propylene, as well as other chemicals. It is the primary source of ethylene, one of the most important building blocks for the chemical and plastics industry. Steam cracking also co-produces hydrogen which is typically combusted with the tail gas on site for process heat, but alternatively could be separated and sold as a by-product [23].
Speaking about NGLs as a feedstock, it is not possible to operate with statistical data on their quantities; however, on average, in the process of preparing 1 million m3 of natural gas, about 50–55 tons of NGLs are obtained. In 2019, the production of natural and associated petroleum gas in the Russian Federation amounted to 738.4 billion m3, which indicates significant volumes of NLGs produced and allows us to consider them as a feedstock for the production of motor gasoline [24].
The aim of this work is to develop a method for obtaining motor gasolines by processing natural gas liquids on a zeolite catalyst, taking into account the influence of the particle size of the zeolite catalyst, the technological parameters of the process, and the composition of the feedstock.
2. Materials and Methods
The objects of study in the work are 7 samples of NGLs and products of their processing on a zeolite catalyst. NGL samples were obtained from various gas fields in Western Siberia of the Russian Federation (in accordance with the oil and gas geological zoning, the fields are located within the Pudinsky oil and gas region, which is part of the Vasyugan oil and gas region). Table 1 shows labeling of NGL samples and products of their processing on a zeolite catalyst (ZP).

Table 1.
Labeling of NGL samples and products of their processing on a zeolite catalyst.
For processing, a zeolite catalyst of the KN-30 brand, structural type ZSM-5, produced by PJSC “Novosibirsk Chemical Concentrates Plant”, was used [25]. Table 2 presents the main characteristics of the catalyst.

Table 2.
Main characteristics of the zeolite catalyst 1.
Table 3 shows the equipment and methods used for determination of the composition and characteristics of NGL samples and products of their processing on a zeolite catalyst.

Table 3.
Research methods.
3. Experimental Section
Processing of NGL samples on a zeolite catalyst was carried out on a laboratory flow-type catalytic unit. Figure 1 shows the basic technological scheme of the laboratory catalytic unit.
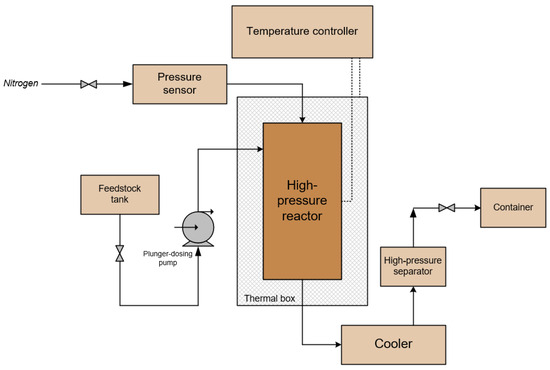
Figure 1.
Basic technological scheme of the laboratory catalytic unit.
The laboratory catalytic unit consists of a plunger-dosing pump, a vertical flow-type reactor, a heat exchanger, a high-pressure separator, and control instrumentation (pressure sensor, elastic element pressure gauge, and thermocouple). The reactor is located in a thermal box for minimizing temperature losses to the atmosphere and to eliminate the risk of contact with the hot parts of the reactor. A thermocouple is installed in the bottom flange of the reactor through a thermowell to control the temperature inside the reactor.
The feedstock is poured into the feedstock tank, from where it is fed to the suction line of the plunger-dosing pump. Feedstock, getting into the upper zone of the reactor, passes through a layer of catalyst. At the exit from the reactor, the product heated to a certain temperature is cooled, and the vapors formed are partially condensed in the condenser at 20 °C. The condenser is a shell-and-tube heat exchanger, in which cooling occurs due to the supply of distilled water at a given temperature into the shell side. The product, after passing through the heat exchanger, enters the high-pressure separator, where partial cooling also occurs due to the supply of water to the separator jacket. The gas phase from the separator is discharged from the system to the atmosphere through a pressure control valve. The liquid product is taken from the high-pressure separator into a specially prepared container for further analysis.
The flow-type reactor is a vertical cylinder surrounded by a heating element, which is enclosed in a copper band, over which thermal insulation is applied. The reactor is equipped with top and bottom flanges with thermowells for thermocouples. The feedstock is fed through the top flange and discharged through the bottom one.
In this work, before loading into a laboratory reactor, an industrial zeolite catalyst was crushed mechanically. The crushed zeolite catalyst was sieved in laboratory sieves of a certain size to obtain the desired fraction of the catalyst. Three fractions of the catalyst were used in the work. Table 4 shows detailed characteristics of the catalyst fractions.

Table 4.
Characteristics of the used catalyst fractions.
The prepared fraction of the zeolite catalyst was loaded into the reactor. The loaded catalyst was calcined in a reactor for 6 h at a temperature of 500 °C in a nitrogen atmosphere. In the process of calcination, all moisture is removed from the pores of the catalyst, which it could have absorbed from the environment during its crushing. Having passed all the above stages, the catalyst is considered ready for use in the processing of feedstock in a catalytic unit.
The experiment was carried out under the conditions of varying the following technological parameters of the process:
- Temperature (in the range from 350 to 425 °C by 25 °C steps);
- Pressure (in the range from 2.5 to 4.5 atm. by 1 atm. steps);
- Feedstock space velocity to the reactor (in the range from 2 to 4 h−1 by 1 h−1 steps).
Table 5 shows sets of technological parameters at which the processing of NGLs on a zeolite catalyst was carried out (with the example of ZP 1).

Table 5.
Sets of technological parameters.
In the product labeling, the first digit indicates the NGL from which the product was obtained, and the second is the technological parameters under which the product was obtained. For example: ZP 1-5 means that the product was obtained from NGL No. 1 under process conditions No. 5; ZP 4-2 means that the product was obtained from NGL No. 4 under process conditions No. 2.
4. Results and Discussion
4.1. Composition and Characteristics of NGL Samples
The results of determining the composition and characteristics of NGL samples are presented in Table A1, Table A2, Table A3 and Table A4 of Appendix A. The average values of the characteristics and composition of the NGL samples are presented in Table 6. The characteristics and composition were determined according to modern standards [26,27,28,29,30,31].

Table 6.
Average values of the characteristics and composition of the NGL samples.
Analysis of the characteristics and composition of NGL samples was carried out in comparison with traditional straight-run blending components of motor gasoline—straight-run gasoline fractions, as well as feedstock of catalytic processes, aimed at obtaining blending components of motor gasolines.
It can be concluded that the NGL samples are characterized by relatively low sulfur content. The processing of NGLs on a zeolite catalyst will make it possible to obtain blending components of motor gasolines that meet the requirements for motor gasolines of the highest environmental classes [32]. The resulting products will not require additional hydrotreatment; the minimum sulfur content will save the resource of catalysts at all stages of processing. The obtained density values of the NGL samples are close to the density values of such common blending components of gasoline as gas gasoline (650–660 kg/m3), alkylates (680–700 kg/m3) and isomerate (640–680 kg/m3). It should be noted that the NGL samples are characterized by a relatively high evaporation temperature, which makes it possible to use the products of their processing on a zeolite catalyst to increase the SVP of motor gasolines in winter periods. The fractional composition of the NGL samples is extremely close to the fractional composition of straight-run gasoline fractions. As can be seen, the NGL samples do not have high RON values. However, the knock resistance of this hydrocarbon feedstock is on average 10 points higher than the knock resistance of straight-run gasoline fractions.
From the results of determining the hydrocarbon composition, it can be concluded that, by analogy with the total sulfur content, the NGL samples are characterized by a relatively low content of benzene and aromatic and olefinic hydrocarbons. The processing of NGLs on a zeolite catalyst will make it possible to obtain blending components of motor gasolines that meet the requirements for motor gasolines of the highest environmental classes [32]. The low content of hydrocarbons, the content of which is strictly limited, will make it possible to involve NGL processing products on a zeolite catalyst in the blending of motor gasolines in a significant amount.
Thus, the results obtained in this section indicate that the use of NGLs as blending components without processing is inappropriate (comparatively low detonation resistance, high evaporation, low density). However, the characteristics and composition of NGLs and their outstanding environmental properties make them a promising feedstock for processing on a zeolite catalyst in order to obtain blending components of motor gasoline.
4.2. NGL Processing on Zeolite under Conditions of Variable Catalyst Particle Size
The task of the first study stage was to reveal the regularities of the influence of catalyst particle size on the composition and characteristics of the products of NGL processing on zeolite, as well as to determine the most preferable catalyst particle size from the viewpoint of involving the resulting products in the blending of motor gasoline.
NGL 4 was used for this stage of study. The variation in the zeolite catalyst particle size was carried out according to Table 4, with a set of technological parameters of the process 2 (Table 5).
Table 7, Figure 2 and Table A4 of the Appendix A show the results of determining the composition and characteristics of NGL processing products on zeolite under conditions of varying catalyst particle size [26,27,28,29,30].

Table 7.
Characteristics of NGL processing products on zeolite under conditions of varying catalyst particle size.
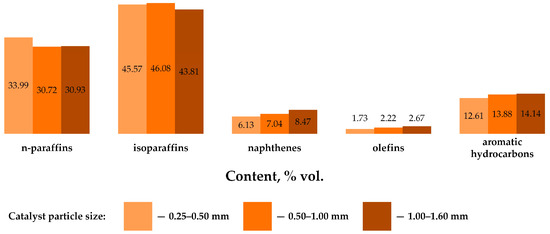
Figure 2.
Group hydrocarbon composition of NGL processing products on zeolite under conditions of varying catalyst particle size.
From the results presented in Table 7, it follows that the use of any considered fractions of the zeolite catalyst allows an increase in the octane number of the obtained products relative to the feedstock by more than 15 points. It can be seen from the data that an extremum (maximum) of RON (86.1 points) is observed at a zeolite catalyst particle size of 0.50–1.00 mm. It is also worth noting that with an increase in the particle size of the zeolite catalyst, the SVP value decreases (by 37.2 kPa) with a simultaneous increase in density (by 19.6 kg/m3).
From the data presented in Figure 2, it can be seen that the extremum (maximum) content of paraffins of normal and iso-structure is observed when the zeolite catalyst particle size is 0.50–1.00 mm. Also, with an increase in the zeolite catalyst particle size, an increase in the content of naphthenic (by 2.34% vol.), olefinic (by 0.94% vol.) and aromatic hydrocarbons (by 1.53% vol.) is observed.
Analyzing the data presented in Table A4 of the Appendix A, it can be seen that the predominant hydrocarbon in the composition of the obtained products is isopentane. The content of propane and butanes of normal and iso-structure decreases with increasing zeolite catalyst particle size, which explains the decrease in SVP value. The content of benzene varies in the range of 0.92–1.05% vol., and practically does not depend on the use of catalyst particle size.
Thus, analyzing the obtained data about the composition and characteristics of NGL processing products on zeolite under conditions of varying the size of the catalyst particles, it can be concluded that the most preferred size of the catalyst particles is 0.50–1.00 mm. The resulting product is characterized by the highest octane numbers and has a composition optimal for involvement in the blending of motor gasolines.
Products obtained using a smaller and larger particle size of the catalyst are inferior in their characteristics to the obtained product using a zeolite catalyst particle size of 0.50–1.00 mm, due to a decrease in the active surface area. In the case of a particle size of 1.00–1.60 mm, this is due to insufficient grinding; in the case of a particle size of 0.25–0.50 mm it is due to caking of too-crushed particles of the zeolite catalyst.
Further studies in the work were carried out using the zeolite catalyst particle size, determined as the most preferred (0.50–1.00 mm).
4.3. NGL Processing on a Zeolite Catalyst under Conditions of Varying Process Parameters
The task of the next study stage was to identify regularities of the influence of the technological parameters of processing on the composition and characteristics of NGL processing products on a zeolite catalyst, as well as to determine the most preferable process parameters from the viewpoint of involving the resulting products in the blending of motor gasoline.
NGL 4 was used for the study. The variation in the technological parameters of the process was carried out according to Table 5. The variation in the technological parameters was carried out using sample NGL No. 4 as a feedstock, since the product of processing this sample NGL (ZP 4-2) of all the obtained products is characterized by an average content of aromatic hydrocarbons and the highest value of SVP.
The results of determining the composition and characteristics of NGL processing products on a zeolite catalyst under conditions of varying the technological parameters of the process are presented in Table 8, Figure 3 and Table A5 of the Appendix A [26,27,28,29,30].

Table 8.
Characteristics of NGL processing products on a zeolite catalyst under conditions of varying the technological parameters of the process.
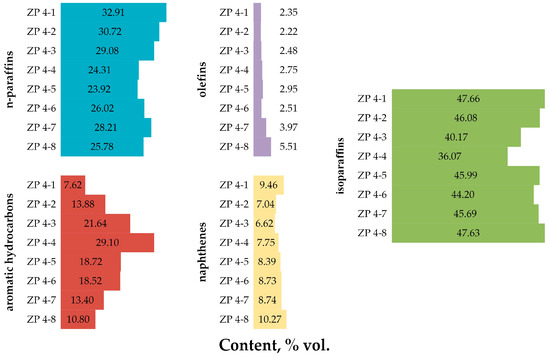
Figure 3.
Group hydrocarbon composition of NGL-processing products on a zeolite catalyst under conditions of varying the technological parameters of the process.
From the data presented in Figure 2, it can be seen that an increase in the temperature of NGL processing on a zeolite catalyst from 350 to 425 °C leads to an increase in the RON of the obtained products by 6.7 points, MON by 5.0 points and density by 60.7 kg/m3. The SVP of the obtained products has an extremum (maximum) at a process temperature of 375 °C.
An increase in the density of products with a simultaneous increase in SVP at a temperature of 375 °C indicates the occurrence of cracking reactions and condensation of aromatic hydrocarbons, which simultaneously give compounds with a higher molecular weight and light hydrocarbon gases.
It can also be seen that an increase in the pressure of NGL processing on a zeolite catalyst from 2.5 atm. to 4.5 atm. leads to a decrease in the RON of the obtained products by 2.5 points, MON by 2.7 points, a decrease in SVP by 67.0 kPa and an increase in density by 23.0 kg/m3. When the processing pressure is varied, an extremum (maximum) of RON is observed at a pressure of 3.5 atm., which is associated with a simultaneously relatively high content of isoparaffins and aromatic hydrocarbons in the product (Figure 3). However, it is worth noting the low SVP value of the processing product on a zeolite catalyst, obtained at a processing pressure of 3.5 atm., which makes it a promising component for involvement in the blending of motor gasolines.
In addition, analyzing the data presented in Table 8, with an increase in the feedstock space velocity from 2 h−1 to 4 h−1 SVP by 70.5 kPa, a density increase of 9.9 kg/m3 can be seen. There is a downward trend in RON as the feedstock space velocity increases by an average of 1.5 points every 1 h−1, which is explained by a decrease in the content of aromatic hydrocarbons in the composition of the obtained products (Figure 3).
As can be seen from the results presented in Figure 3, with an increase in the processing temperature on a zeolite catalyst from 350 to 425 °C, a decrease in the content of n-paraffins (by 8.40% vol.) and isoparaffins (by 11.59% vol.) in the obtained products is observed, in contrast to aromatic hydrocarbons, the content of which in the resulting products increases with increasing the processing temperature (by 21.48% vol.). The content of olefins increases with the increasing temperature, but has an extremum (minimum) at a processing temperature of 375 °C. The content of naphthenes decreases with the increasing temperature and has an extremum (minimum) at a processing temperature of 400 °C. The predominant group of hydrocarbons in all obtained products of NGL 4 processing on a zeolite catalyst is isoparaffins.
The regularity of the decrease in the content of n-paraffins and naphthenes in the obtained products, with a simultaneous increase in the content of aromatic and olefinic hydrocarbons with an increase in the processing temperature, finds the following explanation: an increase in the processing temperature leads to an intensification of cracking reactions with the formation of olefins, the hydrogen transfer in which then leads to more intensive formation of aromatic hydrocarbons. An increase in the role of cracking reactions with increasing temperature leads to a decrease in the role of isomerization reactions, which leads to a decrease in the content of isoparaffins in the obtained products. The increase in the octane number of obtained products with an increase in the processing temperature is explained by the increase in the content of high-octane aromatic hydrocarbons in them.
It can also be seen that an increase in pressure of NGL processing on a zeolite catalyst from 2.5 atm. to 4.5 atm. leads to a decrease in the content of n-paraffins (by 4.70% vol.) and isoparaffins (by 1.88% vol.) in the obtained products, as well as an increase in the content of naphthenes (by 1.69% vol.), olefins (by 0.29% vol.) and aromatic hydrocarbons (by 4.64% vol.). The content of normal structure paraffins has an extremum (minimum) at a processing pressure of 3.5 atm. The predominant group of hydrocarbons is isoparaffins; the group of hydrocarbons with the lowest content is olefins.
The identified regularities of the influence of the pressure increase on the composition of the obtained products from processing on a zeolite catalyst can be explained as follows. In conformity with the Le Chatelier principle, a change in the processing pressure in a big way leads to a change in the equilibrium of chemical reactions towards a smaller volume (number of moles). The cracking reactions proceed with a raise in volume and pressure (increased number of moles) which is why a raise in processing pressure inhibits the course of these reactions. On the inhibition of cracking reactions with a raise in the processing pressure from 2.5 atm. up to 4.5 atm. also indicates an increase in the content of naphthenes in the obtained products. Meanwhile, some reactions of the formation of aromatic hydrocarbons by hydrogen transfer in olefins proceed with a reduction in volume and pressure (reduction in number of moles). So, for the reactions of aromatic hydrocarbon formation by the hydrogen transfer in olefins, proceeding with a reduction in volume and pressure (a reduction in the number of moles), raising the pressure according to the Le Chatelier principle will shift the equilibrium towards the formation of reaction products and, accordingly, an increase in the content of aromatic hydrocarbons in the products.
A decrease in the products’ SVP with an increase in the processing pressure on a zeolite catalyst indicates the suppression of cracking reactions, which proceed with the formation of light hydrocarbons. The increase in product density is explained by the formation of heavier aromatic hydrocarbons.
Also, from the data presented in Figure 3, it can be concluded that an increase in the feedstock space velocity from 2 h−1 to 4 h−1 leads to a decrease in the content of n-paraffins in the resulting products (by 4.94% vol.) and aromatic hydrocarbons (by 3.08% vol.), as well as an increase in the content of isoparaffins (by 1.55% vol.), naphthenes (by 3.23% vol.) and olefins (by 3.29% vol.). The predominant group in the composition of the obtained products is isoparaffins; the group of hydrocarbons with the lowest content is olefins.
The identified regularities of the effect of an increase in the feedstock space velocity on the composition of the products obtained by processing on a zeolite catalyst can be explained as follows. An increase in the feedstock space velocity leads to a decrease in the time of contact of the feedstock with the zeolite catalyst; the contact time is sufficient only for the reactions of n-paraffins cracking (the observed decrease in content of n-paraffins) and the reactions of n-paraffins’ isomerization (an observed increase in the content of isoparaffins), as well as reactions of hydrogen transfer in olefins with the formation of naphthenes from long-chain olefins which do not have time to crack again (an observed increase in the content of naphthenes). In addition, it can be seen that the reduced feedstock–catalyst contact time is not enough for the reactions of hydrogen transfer in olefins with the formation of aromatic hydrocarbons, in particular benzene (Table 5 of the Appendix A) (observed decrease in the content of aromatic hydrocarbons and benzene, as well as an increase in the content of olefins).
Thus, it can be concluded that the most preferred technological parameters for processing NGLs on a zeolite catalyst are the following: temperature 375 °C, pressure 2.5 atm. and feedstock space velocity 2 h−1. These technological parameters are optimal, because they make it possible to obtain a product most suitable for use as a blending component of motor gasolines.
The following changes in processing parameters lead to products that are less suitable for use in blending motor gasolines:
- Increasing the process temperature to more than 375 °C will lead to an increase in the content of aromatic hydrocarbons in the obtained products, the content of which is strictly regulated. However, when the temperature drops below 375 °C, the RON of the product decreases significantly (to 81.3 points).
- Increasing the pressure of the process leads to an increase in the content of benzene in the obtained products. At a pressure of 2.5 atm. the content of benzene is minimal (1.05% vol.).
- An increase in the feedstock space velocity leads to a significant decrease in the RON of the product (by 3.1 points). RON has a maximum value at a feedstock space velocity of 2 h−1.
Further studies in the work were carried out using the technological parameters of the process determined as the most preferable (temperature 375 °C; pressure 2.5 atm. and feedstock space velocity 2 h−1).
4.4. Processing of NGLs with Various Composition on a Zeolite Catalyst
The task of the next stage of the study was to evaluate the possibility of obtaining blending components of motor gasoline by processing NGLs of various compositions, as well as to identify regularities of the influence of NGL composition on the composition and characteristics of the products of processing on a zeolite catalyst.
The results of determining the composition and characteristics of products obtained by processing various NGL compositions on a zeolite catalyst are presented in Table 9, Figure 4 and Table A6 of the Appendix A [26,27,28,29,30].

Table 9.
Characteristics of products obtained by processing various NGL compositions on a zeolite catalyst.
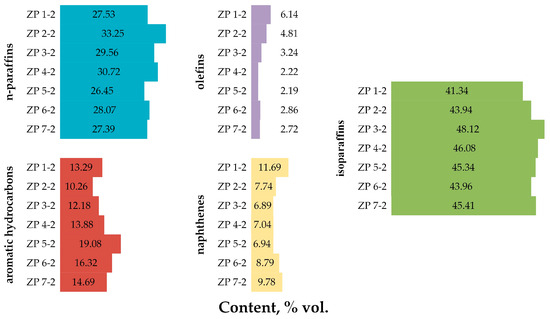
Figure 4.
Group hydrocarbon composition of products obtained by processing various NGL compositions on a zeolite catalyst.
Analyzing the data presented in Table 9, it can be noted that the RON of the NGL-processing product on a zeolite catalyst is on average 84.8 points, which meets the required RON values of RON-80 gasoline. The value of the density and SVP of the obtained products do not meet the requirements of standard [32], which indicates that it is not possible to use the products of NGL processing on a zeolite catalyst as a finished motor fuel; however, the obtained products can be used as the main blended component of motor gasoline.
Comparing the characteristics of products obtained from processing NGLs of various compositions on a zeolite catalyst (Table 9) with the characteristics of feed NGL samples (Table A1 of the Appendix A), the following can be noted:
- Increase in the RON of products relative to feedstock by an average of 16.6 points;
- Growth of SVP of products by an average of 56.7 kPa;
- A slight increase in the density of products by an average of 15.8 kg/m3.
From the data presented in Figure 4, it can be seen that the predominant group of hydrocarbons in the composition of products obtained from processing NGLs of various compositions on a zeolite catalyst are paraffins (normal and iso-structure), but it should be noted that there are many more isoparaffins in the composition products than normal paraffins. There is a group of hydrocarbons whose volume content in the products of NGL processing on a zeolite catalyst is minimal—olefins.
Analyzing the group composition of the feedstock and obtained products, it is worth noting the decrease in the volume share of n-paraffins in the products of NGL processing on a zeolite catalyst (on average by 10.81% vol.) and a slight increase in the share of isoparaffins (on average by 4.40% vol.).
Compared with the feedstock, in the corresponding products of NGL processing on a zeolite catalyst, the content of naphthenes on average decreased by more than 2 times, and the content of olefins increased on average by more than 3 times.
The largest increase in the volume share in the products of NGL processing on a zeolite catalyst compared to the feedstock is observed in aromatic hydrocarbons—the content increased by more than 13 times.
At the same time, it can be seen that all the obtained products of NGL processing on a zeolite catalyst in terms of the content of olefinic and aromatic hydrocarbons meet the requirements of standard [32].
Analyzing the data presented in Table A6 of the Appendix A, it should be noted that isopentane is the predominant compound in all products of NGL processing on a zeolite catalyst. Also, there is a significant component content in the NGL-processing products of butanes of normal and iso-structure, n-pentane, toluene, dimethylbenzene, 2-methylpentane and 3-methylpentane.
The volume fraction of benzene in the obtained products increased by a factor of 10 on average compared to its content in the feed NGLs. The content of benzene in the obtained products only slightly exceeds the requirements of standard [32], which again allows us to conclude that the obtained products are promising for use as the main blended component of motor gasoline.
The change in the composition of NGL samples during processing on a zeolite catalyst can be explained as follows. The decrease in the content of n-paraffins and naphthenes in the products is due to the occurrence of cracking reactions; the occurrence of the same reactions explains the increase in the content of olefins in the obtained products. The increase in the content of isoparaffins finds an explanation in the proceeds of n-paraffin isomerization reactions. The increase in the content of aromatic hydrocarbons and benzene in the obtained products is explained by the reactions of hydrogen transfer in olefins, which also lead to the formation of paraffins. The occurrence of hydrogen transfer reactions in olefins with the formation of aromatic hydrocarbons and paraffins also explains the fact that, despite the occurrence of cracking reactions, the content of n-paraffins did not decrease to a minimum, and the content of olefins in the products did not increase so significantly.
The increase in the octane number of NGL processing products on a zeolite catalyst compared to the feedstock is due to a significant increase in the content of high-octane aromatic hydrocarbons and isoparaffins. The increase in SVP with a simultaneous increase in the density of the obtained products is explained by the occurrence of cracking reactions, which simultaneously lead to the formation of the lightest and heaviest products.
Analyzing the influence of the composition of the feed NGLs on the composition and characteristics of products obtained on a zeolite catalyst, we can conclude the following:
- The more aromatic hydrocarbons are present in the feedstock, the more aromatic hydrocarbons will be present in the products of processing on the zeolite catalyst;
- An increased content of normal paraffins in the feedstock will give an increased content of olefinic and naphthenic hydrocarbons in the obtained products on a zeolite catalyst.
4.5. Development of Blending Recipes for Motor Gasoline Production
To demonstrate the possibility of using the products obtained by processing NGLs of various compositions on a zeolite catalyst as blending components of motor gasoline, blending recipes were developed. Recipes were developed by using the “Compounding” software [31].
In the developed recipes, the products of NGL processing of various compositions on a zeolite catalyst, obtained at optimal technological parameters of the process, are used as the main blending components.
As additional blending components, products of downstream processing, available only at refineries, include: catalytic cracking gasoline (FCC gasoline), reformate, isomerate, alkylate, and components that can be available at the fields; feed NGL, straight-run gasoline fraction (SRGF) and also oil products available on the market; toluene, methyl tertiary butyl ether (MTBE). Characteristics of additional blending components, calculated using “Compounding” software, are presented in Table A7 of the Appendix A.
Figure 5 and Figure 6 shows the blending recipes of RON92 motor gasolines, the most common brand for use worldwide.
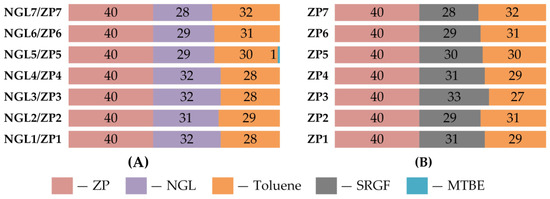
Figure 5.
Blending recipes for motor gasoline production, % wt. (for fields). (A) Recipes with NGL, (B) Recipes with SRGF.
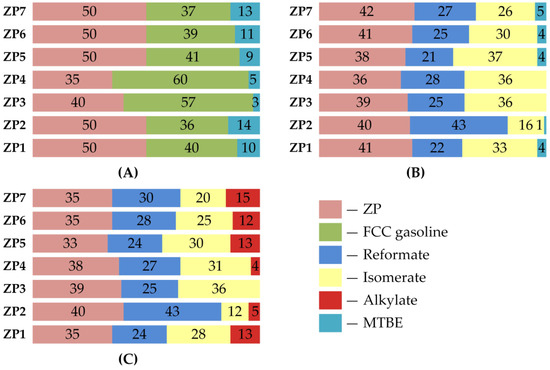
Figure 6.
Blending recipes for motor gasoline production, % wt. (for refinery). (A) Recipes with FCC gasoline, (B) Recipes with reformate, isomerate and MTBE, (C) Recipes with reformate, isomerate and alkylate.
The recipes presented in Figure 5 demonstrate the use of NGL processing products on a zeolite catalyst to produce motor gasolines in the field.
As can be seen, the production of motor gasoline is possible on the basis of all the obtained processed products, regardless of the composition of the feed NGL, while in all blending recipes, the involvement of the obtained processed products is 40% wt. Feed NGL and SRGF were used as a low-octane component and toluene was used to increase the octane number. In the case of a recipe based on NGL 5/ZP 5, there was an additional 1% vol. MTBE, due to the high content of aromatic hydrocarbons in ZP 5 and the impossibility of further increasing the octane number of the blend by the involvement of toluene.
Table A8 of the Appendix A shows the characteristics of the obtained gasolines. By all characteristics, the obtained gasolines meet the requirements of standard [32]. It can be seen that motor gasolines blending with the feed NGLs compared to gasolines blending with SRGF are characterized by higher evaporation and lower density, aromatics and benzene content. That makes these recipes the most preferable for the production of motor gasoline operating in the winter. In addition, these motor gasolines are more environmentally friendly.
The recipes presented in Figure 6 demonstrate the use of NGL products processed on a zeolite catalyst to produce motor gasolines at refineries.
As you can see, it is possible to obtain motor gasoline on the basis of all obtained products, regardless of the composition of the feed NGL. In the blending recipes, the involvement of the obtained products is: for recipes involving FCC gasoline—35–50% wt.; for recipes involving reformate, isomerate and MTBE—36–42% wt.; for recipes involving reformate, isomerate and alkylate—33–40% wt. Alkylate and MTBE were used as octane boosters. The use of blending recipes involving FCC gasoline allows the fullest use of NGL processing products on a zeolite catalyst, but at the same time requires the involvement of high-octane and expensive MTBE.
Table A9 of the Appendix A shows the characteristics of the obtained gasolines. By all characteristics, the obtained gasolines meet the requirements of standard [32]. It can be seen that motor gasolines obtained by blending recipes with the involvement of reformate are characterized by practically the maximum allowable benzene content. In addition, it can be noted that the involvement of products of NGL 3 and NGL 4 processing on the zeolite, which are characterized by the highest octane numbers, in blending allows for minimizing the involvement of high-octane and expensive components—alkylate and MTBE.
5. Conclusions
- The composition (sulfur content, fractional, group and component hydrocarbon compositions) and properties (RON, MON, SVP, and density) of NGL samples obtained from various gas fields in Western Siberia were determined and analyzed. It is shown that the use of NGLs as blending components without processing is inappropriate (comparatively low detonation resistance, high evaporation, low density). However, the characteristics and composition of NGLs and their outstanding environmental properties make them a promising feedstock for processing on a zeolite catalyst in order to obtain blending components of motor gasoline.
- Processing of NGLs on zeolite, under conditions of varying the particle size of the catalyst, has been implemented. Regularities of the influence of the particle size of the zeolite catalyst on the composition and characteristics of the obtained products are revealed. It has been established that the use of any of the considered fractions of the zeolite catalyst makes it possible to increase the RON of the obtained products relative to the feedstock by more than 15 points. It has been established that the most preferred fraction of the catalyst is 0.50–1.00 mm, since the resulting product is characterized by the highest octane numbers and has a composition that is optimal for involving motor gasoline in blending.
- Processing of NGLs on a zeolite catalyst under conditions of varying technological parameters of the process (temperature, pressure and feedstock space velocity) has been implemented. Regularities of the influence of the technological parameters of the process on the composition and characteristics of the obtained products are identified. It has been shown that the most preferred technological parameters of NGL processing on a zeolite catalyst are the following: temperature 375 °C; pressure 2.5 atm. and feedstock space velocity 2 h−1. These technological parameters are optimal, because they make it possible to obtain a product most suitable for use as a blending component of motor gasolines.
- The processing of NGL samples of various compositions on zeolite was implemented with technological parameters and using the particle size of the catalyst determined as the most preferable. It is shown that the processing of NGLs of various compositions on a zeolite catalyst made it possible to increase the RON of the product by an average of 16.6 points. Regularities of the influence of NGL composition on the composition and characteristics of the obtained products are identified. It has been established that with an increase in the content of aromatic hydrocarbons in the composition of feed NGL, their content in the products of processing increases many times over, and an increase in the content of n-paraffins in the feedstock will give an increased content of olefinic and naphthenic hydrocarbons in the obtained products.
- The possibility is shown of using the products obtained from processing NGLs of various compositions on a zeolite catalyst as blending components of motor gasolines. RON 92 gasoline blending recipes have been developed for fields (two options) and refineries (three options). In the developed recipes, products obtained from processing NGLs with various compositions on a zeolite catalyst are used as the main blended components: the share of involvement in the recipes for fields is 40% wt., and for refineries 33–50% wt. It has been shown that motor gasolines obtained according to the developed blending recipes fully meet the requirements of standard [32].
In future works it is planned to conduct deactivation tests to identify regularities of the influence of the processed feedstock composition and technological parameters of the process on the mechanism and degree of zeolite catalyst deactivation. This will make it possible to choose the technology of catalyst regeneration. The results obtained in the work will allow for moving on to the development of a mathematical model for the process, for the selection and calculation of the necessary technological equipment. In the future, the proposed process will find applications in fields and processing enterprises, and will be especially relevant for enterprises located in remote areas for the autonomous provision of motor gasoline for their own needs.
Author Contributions
Investigation, A.A., I.B. and D.L.; formal analysis, A.A.; Writing—review and editing, A.A. and M.K.; methodology, I.B.; Writing—original draft, I.B.; conceptualization, M.K.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by Russian Science Foundation grant No. 21-73-00095, https://rscf.ru/en/project/21-73-00095/ and the TPU development program.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank PJSC “Novosibirsk Chemical Concentrates Plant” for providing samples of catalysts.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Composition and Characteristics of NGL Samples

Table A1.
NGL sample characteristics.
Table A1.
NGL sample characteristics.
| Sample | Total Sulfur Content, mg/kg | Density at 15 °C, kg/m3 | SVP, kPa | RON, Point | MON, Point |
|---|---|---|---|---|---|
| NGL 1 | 28.0 | 719.1 | 104.3 | 69.7 | 66.9 |
| NGL 2 | 30.0 | 651.6 | 97.4 | 70.6 | 67.8 |
| NGL 3 | 17.0 | 674.0 | 71.4 | 66.4 | 63.2 |
| NGL 4 | 20.0 | 685.4 | 67.2 | 69.0 | 65.7 |
| NGL 5 | 7.0 | 692.5 | 65.5 | 67.2 | 64.0 |
| NGL 6 | 0.0 | 667.0 | 86.0 | 67.8 | 65.1 |
| NGL 7 | 0.0 | 682.4 | 58.7 | 66.5 | 63.4 |

Table A2.
NGL sample fractional composition.
Table A2.
NGL sample fractional composition.
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| Volume,% | NGL 1 | NGL 2 | NGL 3 | NGL 4 | NGL 5 | NGL 6 | NGL 7 |
| Temperature, °C | |||||||
| IBP | 28 | 7 | 29 | 31 | 37 | 28 | 29 |
| 50 | 57 | 49 | 61 | 69 | 72 | 55 | 61 |
| 90 | 103 | 98 | 95 | 168 | 144 | 100 | 112 |
| EBP | 140 | 145 | 117 | 168 | 185 | 114 | 123 |
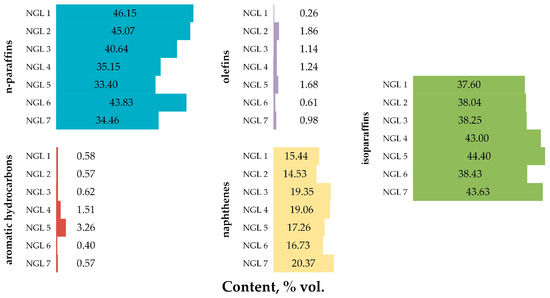
Figure A1.
NGL sample group hydrocarbon composition.

Table A3.
Components whose content in NGL samples is significant.
Table A3.
Components whose content in NGL samples is significant.
| Component | Content, % vol. | ||||||
|---|---|---|---|---|---|---|---|
| NGL 1 | NGL 2 | NGL 3 | NGL 4 | NGL 5 | NGL 6 | NGL 7 | |
| n-pentane | 19.38 | 21.01 | 18.21 | 18.75 | 18.37 | 19.92 | 18.04 |
| isopentane | 15.53 | 17.14 | 13.33 | 18.94 | 24.93 | 15.61 | 17.06 |
| n-bytane | 12.57 | 10.27 | 5.71 | 2.84 | 3.11 | 7.86 | 0.66 |
| 2-methylpentane | 7.56 | 7.76 | 8.53 | 7.10 | 4.43 | 8.22 | 8.08 |
| n-heptane | 3.38 | 3.41 | 4.94 | 4.38 | 7.26 | 4.07 | 5.28 |
| benzene | 0.11 | 0.11 | 0.14 | 0.10 | 0.00 | 0.13 | 0.14 |
From the results presented in Table A1, it can be seen that the total sulfur content in NGL samples averages 14.6 mg/kg, and in some cases (NGL 6, 7) it is 0 mg/kg (trace amount, less than the device sensitivity limits). NGL 2 is characterized by the highest total sulfur content. The density of NGL samples averages 681.7 kg/m3. NGL 1 is characterized as having the highest density, NGL 2 is characterized by the lowest. The SVP of the NGL samples averages 78.6 kPa. NGL 1 is characterized by the highest SVP, and NGL 7 has the lowest. The RON of the NGL samples averages 68.2 points, and the MON is 65.2 points. NGL 2 is characterized by the highest RON and MON, NGL 3 is characterized by the lowest ones.
According to Table A2, NGL 5 is characterized by the heaviest and widest fractional composition, and NGL 6 has the narrowest.
According to the results of chromatographic analysis of the NGL samples, shown in Table A3, it can be noted that the predominant group of hydrocarbons in all samples is paraffinic hydrocarbons (the average content of n-paraffins is 39.81% vol., isoparaffins—40.48% vol.). Considering paraffinic hydrocarbons, it is worth noting that in the predominant number of samples, the content of normal paraffins prevails over isostructural paraffins (NGL 1-3 and NGL 6). In NGL 4, 5, 7, an inversion is observed. NGL 1 is characterized by the highest content of n-paraffins, NGL 5 has the lowest. NGL 5 is characterized by the highest content of isoparaffins, NGL 1 has the lowest.
The hydrocarbon groups characterized by the lowest content in the NGL samples are olefinic (average content 1.11% vol.) and aromatic (average content 1.07% vol.) hydrocarbons. An intermediate position is occupied by naphthenic hydrocarbons (average content 17.53% vol.). NGL 7 is characterized by the highest content of naphthenes, NGL 2 is characterized by the lowest content. NGL 2 is characterized by the highest content of olefins, NGL 1 is the lowest. NGL 5 is characterized by the highest content of aromatic hydrocarbons, NGL 6 is the lowest.
Based on the data presented in Figure A1, it can be said that the predominant components in all NGL samples are normal and isopentanes. The content of benzene in NGL samples averages 0.10% vol. NGLs 3 and 7 are characterized by the highest content of benzene, NGL 5 (absence of benzene) is characterized by the lowest content.
Appendix A.2. NGL Processing on Zeolite under Conditions of Variable Catalyst Particle Size

Table A4.
Components whose content in NGL processing products on zeolite under conditions of varying catalyst particle size is significant.
Table A4.
Components whose content in NGL processing products on zeolite under conditions of varying catalyst particle size is significant.
| Component | Content, % vol. | ||
|---|---|---|---|
| Catalyst Particle Size, mm | |||
| 0.25–0.50 | 0.50–1.00 | 1.00–1.60 | |
| isopentane | 18.56 | 19.60 | 16.09 |
| n-butane | 15.16 | 12.09 | 11.55 |
| n-pentane | 8.82 | 8.58 | 9.26 |
| isobutane | 8.84 | 7.97 | 6.55 |
| 2-methylpentane | 5.27 | 4.62 | 5.82 |
| 3-methylpentane | 4.60 | 3.75 | 4.76 |
| toluene | 4.66 | 1.67 | 4.84 |
| dimethylbenzene | 5.31 | 5.34 | 5.86 |
| propane | 5.32 | 5.80 | 4.13 |
| benzene | 0.97 | 1.05 | 0.92 |
Appendix A.3. NGL Processing on a Zeolite Catalyst under Conditions of Varying Process Parameters

Table A5.
Components whose content in NGL processing products on zeolite under conditions of varying the technological parameters of the process is significant.
Table A5.
Components whose content in NGL processing products on zeolite under conditions of varying the technological parameters of the process is significant.
| Component | Content, % vol. | |||||||
|---|---|---|---|---|---|---|---|---|
| ZP 4–1 | ZP 4-2 | ZP 4-3 | ZP 4-4 | ZP 4-5 | ZP 4-6 | ZP 4-7 | ZP 4-8 | |
| isopentane | 19.79 | 19.60 | 16.19 | 13.85 | 19.00 | 18.63 | 19.10 | 18.12 |
| n-pentane | 13.58 | 8.58 | 5.58 | 4.69 | 8.21 | 7.74 | 10.50 | 10.63 |
| n-butane | 9.73 | 12.09 | 11.27 | 8.39 | 10.48 | 10.04 | 10.09 | 9.55 |
| 2-methylpentane | 6.38 | 4.62 | 3.37 | 3.25 | 4.72 | 4.47 | 5.43 | 5.30 |
| isobutane | 5.72 | 7.97 | 7.80 | 5.58 | 5.86 | 5.19 | 5.88 | 5.70 |
| 3-methylpentane | 4.15 | 3.75 | 2.98 | 2.71 | 3.94 | 3.85 | 3.94 | 3.58 |
| propane | 3.28 | 5.80 | 8.88 | 11.81 | 7.77 | 7.98 | 5.75 | 3.92 |
| dimethylbenzene | 2.51 | 5.34 | 8.20 | 11.37 | 6.64 | 7.00 | 4.62 | 3.61 |
| 2-methylhexane | 2.39 | 1.61 | 1.23 | 1.41 | 1.83 | 1.69 | 1.98 | 1.93 |
| dimethylcyclopentane | 2.38 | 1.12 | 0.78 | 0.96 | 1.24 | 1.15 | 1.51 | 1.60 |
| toluene | 2.27 | 1.67 | 1.37 | 1.40 | 1.94 | 1.83 | 2.05 | 1.95 |
| benzene | 0.38 | 1.05 | 1.82 | 2.63 | 1.30 | 1.31 | 0.87 | 0.65 |
From Table A5 it can be seen that the predominant compound in the composition of the NGL 4 processing products on a zeolite catalyst at various temperatures is isopentane. Its content, as well as the total proportion of isoparaffins in the products of processing on a zeolite catalyst, decreases with increasing process temperatures. It is also worth noting that, compared with the feedstock, the proportions of normal and isopentane and 2-methylpentane decreased in the obtained products, while the content of normal and isobutane, 3-methylpentane, propane, dimethylbenzene, 2-methylhexane, dimethylcyclopentane, toluene and benzene increased. The content of benzene increased with an increase in the process temperature, similarly to an increase in the total content of aromatic hydrocarbons, which is undesirable from the viewpoint of the involvement of the obtained products in the blending of motor gasoline.
It can also be seen that the predominant compound in the composition of NGL 4 processing products obtained at various process pressures is also isopentane, the share of which, similarly to the total content of isoparaffins, decreases with increasing pressure. The reverse trend is observed for the content of benzene, the proportion of which increases with increasing the processing pressure. The content of normal and isobutane increased on average by five and six times relative to feedstock, respectively; the share of propane increased by six times, which causes an increased value of SVP for NGL 4 processing products on a zeolite catalyst.
In addition, analyzing the data presented in Table A5, it can be noted that isopentane is also the predominant compound in the composition of NGL 4 processing products on a zeolite catalyst obtained at various feedstock space velocity. Despite the fact that the content of n-butane, isobutane, and propane increases compared to the feed NGL 4, with an increase in the feedstock space velocity, the share of these components decreases, which leads to a decrease in the SVP of NGL 4 processing products on a zeolite catalyst.
Appendix A.4. Processing of NGLs with Various Composition on a Zeolite Catalyst

Table A6.
Components whose content in products obtained by processing of various compositions of NGL on a zeolite catalyst is significant.
Table A6.
Components whose content in products obtained by processing of various compositions of NGL on a zeolite catalyst is significant.
| Component | Content, % vol. | ||||||
|---|---|---|---|---|---|---|---|
| ZP 1-2 | ZP 2-2 | ZP 3-2 | ZP 4-2 | ZP 5-2 | ZP 6-2 | ZP 7-2 | |
| isopentane | 12.81 | 19.12 | 19.70 | 19.60 | 18.26 | 17.16 | 16.43 |
| n-butane | 9.12 | 15.95 | 14.65 | 12.09 | 9.79 | 11.31 | 7.88 |
| n-pentane | 7.46 | 10.19 | 9.88 | 8.58 | 6.98 | 8.50 | 9.42 |
| toluene | 5.04 | 7.44 | 5.10 | 5.34 | 7.53 | 5.78 | 5.27 |
| isobutane | 4.98 | 0.08 | 7.90 | 7.97 | 5.81 | 5.82 | 5.51 |
| dimethylbenzene | 4.50 | 1.88 | 4.63 | 5.80 | 6.58 | 7.23 | 6.63 |
| 2-methylpentane | 3.95 | 6.40 | 6.12 | 4.62 | 4.62 | 5.56 | 6.14 |
| 3-methylpentane | 3.25 | 5.60 | 5.47 | 3.75 | 3.72 | 4.95 | 4.64 |
| benzene | 1.25 | 0.07 | 1.14 | 1.05 | 1.42 | 1.06 | 0.93 |
Appendix A.5. Development of Blending Recipes for Motor Gasoline Production

Table A7.
Characteristics of additional blending components.
Table A7.
Characteristics of additional blending components.
| Component | Density at 15 °C, kg/m3 | SVP, kPa | RON | MON | Content, % vol. | ||
|---|---|---|---|---|---|---|---|
| Point | Olefins | Aromatic Hydrocarbons | Benzene | ||||
| FCC gasoline | 760.5 | 53.6 | 93.0 | 85.9 | 22.04 | 28.81 | 0.62 |
| Reformate | 829.1 | 24.3 | 104.7 | 93.7 | 0.00 | 72.24 | 2.48 |
| Isomerate | 661.2 | 62.8 | 89.8 | 87.9 | 0.00 | 0.04 | 0.02 |
| Alkylate | 701.6 | 27.8 | 97.9 | 95.5 | 0.00 | 0.68 | 0.00 |
| SRGF | 733.7 | 24.3 | 69.3 | 63.7 | 0.00 | 1.24 | 1.11 |
| Toluene | 870.4 | 7.2 | 121.0 | 104.0 | 0.00 | 100.00 | 0.00 |
| MTBE | 737.3 | 40.3 | 125.0 | 110.0 | 0.00 | 0.00 | 0.00 |

Table A8.
Characteristics of gasoline (blending recipes for fields).
Table A8.
Characteristics of gasoline (blending recipes for fields).
| Component | Density at 15 °C, kg/m3 | SVP, kPa | RON | MON | Content, % vol. | ||
|---|---|---|---|---|---|---|---|
| Point | Olefins | Aromatic Hydrocarbons | Benzene | ||||
| (A) Recipes with NGL | |||||||
| ZP1 | 740.0 | 87.4 | 92.4 | 84.3 | 2.64 | 29.01 | 0.56 |
| ZP2 | 735.1 | 81.7 | 92.3 | 84.4 | 2.63 | 28.43 | 0.06 |
| ZP3 | 733.3 | 86.3 | 92.4 | 84.5 | 1.82 | 28.53 | 0.55 |
| ZP4 | 739.3 | 90.9 | 92.0 | 83.8 | 1.39 | 29.64 | 0.48 |
| ZP5 | 754.5 | 68.4 | 92.3 | 83.4 | 1.50 | 34.41 | 0.65 |
| ZP6 | 742.5 | 77.8 | 92.2 | 83.7 | 1.42 | 33.08 | 0.49 |
| ZP7 | 751.7 | 69.6 | 92.2 | 83.4 | 1.45 | 33.47 | 0.44 |
| (B) Recipes with SRGF | |||||||
| ZP1 | 763.1 | 61.6 | 92.5 | 83.5 | 2.65 | 31.14 | 0.89 |
| ZP2 | 758.8 | 58.7 | 92.5 | 83.6 | 2.11 | 31.37 | 0.36 |
| ZP3 | 749.9 | 71.4 | 92.4 | 83.8 | 1.44 | 28.54 | 0.87 |
| ZP4 | 756.1 | 77.0 | 92.3 | 83.3 | 0.99 | 31.12 | 0.81 |
| ZP5 | 766.6 | 56.3 | 92.2 | 82.7 | 0.94 | 34.51 | 0.96 |
| ZP6 | 760.7 | 60.0 | 92.3 | 83.0 | 1.27 | 34.27 | 0.80 |
| ZP7 | 765.3 | 60.0 | 92.5 | 83.1 | 1.16 | 34.33 | 0.72 |

Table A9.
Characteristics of gasoline (blending recipes for refinery).
Table A9.
Characteristics of gasoline (blending recipes for refinery).
| Component | Density at 15 °C, kg/m3 | SVP, kPa | RON | MON | Content, % vol. | ||
|---|---|---|---|---|---|---|---|
| Point | Olefins | Aromatic Hydrocarbons | Benzene | ||||
| (A) Recipes with FCC gasoline | |||||||
| ZP1 | 731.9 | 90.5 | 92.3 | 84.9 | 11.71 | 17.99 | 0.89 |
| ZP2 | 722.2 | 86.7 | 92.0 | 84.8 | 10.07 | 15.21 | 0.25 |
| ZP3 | 728.3 | 93.2 | 92.2 | 85.5 | 13.41 | 20.91 | 0.83 |
| ZP4 | 734.9 | 93.1 | 92.3 | 85.2 | 13.57 | 21.83 | 0.75 |
| ZP5 | 734.9 | 84.2 | 92.0 | 84.4 | 9.85 | 21.26 | 0.98 |
| ZP6 | 725.2 | 88.7 | 92.1 | 84.6 | 9.76 | 19.29 | 0.79 |
| ZP7 | 728.8 | 88.7 | 92.1 | 84.4 | 9.24 | 17.83 | 0.70 |
| (B) Recipes with reformate, isomerate and MTBE | |||||||
| ZP1 | 720.3 | 81.0 | 92.0 | 86.0 | 2.57 | 19.35 | 1.00 |
| ZP2 | 745.8 | 70.3 | 92.1 | 84.8 | 2.06 | 32.14 | 0.99 |
| ZP3 | 720.2 | 88.6 | 92.5 | 86.9 | 1.34 | 20.39 | 1.00 |
| ZP4 | 721.8 | 90.1 | 92.3 | 86.3 | 0.84 | 22.65 | 1.00 |
| ZP5 | 722.4 | 74.5 | 92.3 | 86.1 | 0.84 | 20.47 | 1.00 |
| ZP6 | 720.1 | 78.5 | 92.2 | 85.8 | 1.22 | 22.63 | 0.99 |
| ZP7 | 728.0 | 78.4 | 92.1 | 85.3 | 1.18 | 23.45 | 0.99 |
| (C) Recipes with reformate, isomerate and alkylate | |||||||
| ZP1 | 723.1 | 72.6 | 92.1 | 86.6 | 2.20 | 19.98 | 0.97 |
| ZP2 | 747.1 | 68.8 | 92.0 | 84.9 | 2.06 | 32.27 | 0.99 |
| ZP3 | 720.2 | 88.6 | 92.5 | 86.9 | 1.34 | 20.39 | 1.00 |
| ZP4 | 721.4 | 91.2 | 92.3 | 86.5 | 0.86 | 22.25 | 0.99 |
| ZP5 | 724.0 | 67.0 | 92.4 | 86.7 | 0.74 | 21.62 | 1.00 |
| ZP6 | 724.9 | 70.2 | 92.4 | 86.4 | 1.03 | 23.74 | 1.00 |
| ZP7 | 732.3 | 68.6 | 92.3 | 86.1 | 0.99 | 24.57 | 1.00 |
For all characteristics, the obtained gasolines meet the requirements of standard [32]: RON not less than 92.0 points, MON not less than 83.0 points, SVP in the range of 45.0–100.0 kPa, density at 15 °C within 720.0–780.0 kg/m3; the content of olefins is not more than 18.00% vol., aromatic hydrocarbons is not more than 35.00% vol., benzene is not more than 1.00% vol.
References
- Elyakov, A.L. A Comprehensive Evaluation Method the Effectiveness of the Prospect of Inter-Fuel Competition for the Production of Electric and Thermal Energy in the Arctic Regions of the Republic of Sakha (Yakutia). IOP Conf. Ser. Earth Environ. Sci. 2021, 720, 012126. [Google Scholar] [CrossRef]
- Najibullah Khan, N.B.; Barifcani, A.; Tade, M.; Pareek, V. A case study: Application of energy and exergy analysis for enhancing the process efficiency of a three stage propane pre-cooling cycle of the cascade LNG process. J. Nat. Gas Sci. Eng. 2016, 29, 125–133. [Google Scholar] [CrossRef]
- Xu, J.; Lin, W.; Chen, X.; Zhang, H. Review of Unconventional Natural Gas Liquefaction Processes. Front. Energy Res. 2022, 10, 915893. [Google Scholar] [CrossRef]
- Cherepovitsyn, A.; Evseeva, O. Parameters of sustainable development: Case of arctic liquefied natural gas projects. Resources 2021, 10, 1–27. [Google Scholar] [CrossRef]
- Zhukov, O.V.; Cherepovitsyn, A.E. Project Implementation Efficiency: Developing Natural Gas Resources of the Western Arctic Shelf. IOP Conf. Ser. Earth Environ. Sci. 2021, 808, 012032. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Bogoviz, A.V.; Goncharenko, L.P.; Sybachin, S.A. A critical review of Russia’s energy strategy in the period until 2035. Int. J. Energy Econ. Policy 2019, 9, 95–102. [Google Scholar] [CrossRef]
- Geng, Z. Russian energy strategies in the natural gas market for energy security. Int. J. Energy Econ. Policy 2021, 11, 62–66. [Google Scholar] [CrossRef]
- Mandis, M.; Baratti, R.; Chebeir, J.; Tronci, S.; Romagnoli, J.A. Performance assessment of control strategies with application to NGL separation units. J. Nat. Gas Sci. Eng. 2022, 106, 104763. [Google Scholar] [CrossRef]
- Tronci, S.; Chebeir, J.A.; Mandis, M.; Baratti, R.; Romagnoli, J.A. Control Strategies for Natural Gas Liquids Recovery Plants. Comput. Aided Chem. Eng. 2020, 48, 1291–1296. [Google Scholar] [CrossRef]
- Devold, H. Oil and Gas Production Handbook, An Introduction to Oil and Gas Production, Transport, Refining and Petrochemical Industry, 3rd ed.; ABB Oil and Gas: Zürich, Switzerland, 2013; ISBN 978-82-997886-3-2. [Google Scholar]
- Shafiei, M.; Fatemi, S. C4+ liquid recovery from natural gas by temperature swing adsorption followed by liquefaction of heavy extracted product. Sep. Purif. Technol. 2022, 302, 121976. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, W.; Zhang, D.; Na, P.; Tang, Z. Recovery of light hydrocarbons from natural gas by vacuum pressure swing adsorption process. J. Nat. Gas Sci. Eng. 2019, 68, 102895. [Google Scholar] [CrossRef]
- Abildgaard, M.S.; Ren, C.; Leyva-Mayorga, I.; Stefanovic, C.; Soret, B.; Popovski, P. Arctic Connectivity: A Frugal Approach to Infrastructural Development. Arctic 2022, 75, 72–85. [Google Scholar] [CrossRef]
- Kvitko, A.V.; Shendrik, V.A.; Simonova, A.S. Ice Crossings as the Basis for Transport Development in the Arctic. In Proceedings of ARCTD 2021: Arctic Territorial Development; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Nalivaychenko, E.; Volkov, A.; Tishkov, S. Fuel and Energy Complex of the Arctic Zone of Russia and Its Transport Infrastructure. IOP Conf. Ser. Earth Environ. Sci. 2020, 918, 012238. [Google Scholar] [CrossRef]
- Belinskaya, N.; Altynov, A.; Bogdanov, I.; Popok, E.; Kirgina, M.; Simakov, D.S.A. Production of Gasoline Using Stable Gas Condensate and Zeoforming Process Products as Blending Components. Energy Fuels 2019, 33, 4202–4210. [Google Scholar] [CrossRef]
- Soltanali, S.; Mohaddecy, S.R.S.; Mashayekhi, M.; Rashidzadeh, M. Catalytic upgrading of heavy naphtha to gasoline: Simultaneous operation of reforming and desulfurization in the absence of hydrogen. J. Environ. Chem. Eng. 2020, 8, 104548. [Google Scholar] [CrossRef]
- Kirgina, M.; Belinskaya, N.; Altynov, A.; Bogdanov, I.; Temirbolat, A. Transformations of stable gas condensate hydrocarbons into high-octane gasoline components over ZSM-5 zeolite catalyst. J. Nat. Gas Sci. Eng. 2020, 84, 103605. [Google Scholar] [CrossRef]
- Korobitsyna, L.L.; Travkina, O.S.; Velichkina, L.M.; Vosmerikov, A.V.; Kutepov, B.I. Catalytic Conversion of Methanol and Straight-Run Gasoline over Granulated Catalysts with Different Concentrations of H-Form ZSM-5 Zeolite. Pet. Chem. 2022, 62, 544–551. [Google Scholar] [CrossRef]
- Velázquez, H.D.; Cerón-Camacho, R.; Mosqueira-Mondragón, M.L.; Hernández-Cortez, J.G.; Montoya de la Fuente, J.A.; Hernández-Pichardo, M.L.; Beltrán-Oviedo, T.A.; Martínez-Palou, R. Recent progress on catalyst technologies for high quality gasoline production. Catal. Rev. Sci. Eng. 2022, 65, 4. [Google Scholar] [CrossRef]
- Stepanov, V.G. Low-tonnage production of motor fuels at remote fields. Chem. Technol. Fuels Oils 2005, 41, 1–15. [Google Scholar] [CrossRef]
- Stepanov, V.G.; Ione, K.G.; Snytnikova, G.P. Zeolite Catalysts in the Upgrading of Low-Octane Hydrocarbon Feedstocks to Unleaded Gasolines. Stud. Surf. Sci. Catal. 1996, 100, 477–482. [Google Scholar] [CrossRef]
- Statistical Compendium. Fuel and Energy Complex of Russia—2019. Analytical Center under the Government of the Russian Federation. Available online: https://www.ac.gov.ru/uploads/2-Publications/TEK_annual/TEK.2019.pdf (accessed on 1 April 2023).
- Young, B.; Hawkins, T.R.; Chiquelin, C.; Sun, P.; Gracida-Alvarez, U.R.; Elgowainy, A. Environmental Life Cycle Assessment of Olefins and By-Product Hydrogen from Steam Cracking of Natural Gas Liquids, Naphtha, and Gas Oil. J. Clean. Prod. 2022, 359, 13184. [Google Scholar] [CrossRef]
- PJSC. Novosibirsk Chemical Concentrates Plant. Available online: http://www.nccp.ru/products/zeolite_catalyst (accessed on 1 April 2023).
- ASTM D4294-16; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2010.
- EN 14517:2004; Liquid Petroleum Products—Determination of Hydrocarbon Types and Oxygenates in Petrol—Multidimensional Gas Chromatography Method. ISO: Geneva, Switzerland, 2004.
- ISO 12185:1996; Crude Petroleum and Petroleum Products—Determination of Density—Oscillating U-tube Method. ISO: Geneva, Switzerland, 1996.
- ISO 3405:2011; Petroleum Products—Determination of Distillation Characteristics at Atmospheric Pressure. ISO: Geneva, Switzerland, 2011.
- ISO 3007:1999; Petroleum Products and Crude Petroleum—Determination of Vapor Pressure—Reid Method. ISO: Geneva, Switzerland, 2012.
- Ivanchina, E.D.; Kirgina, M.V.; Chekantsev, N.V.; Sakhnevich, B.V.; Sviridova, E.V.; Romanovskiy, R.V. Complex modeling system for optimization of compounding process in gasoline pool to produce high-octane finished gasoline fuel. Chem. Eng. J. 2015, 282, 194–205. [Google Scholar] [CrossRef]
- EN 228-2017; Automotive Fuels. Unleaded Petrol. Requirements and Test Methods. iTeh Standards: Toronto, ON, Canada, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).