Time Crystal Synthon: The Way to Integrate Cascade Reactions for Advancing Multistep Flow Synthesis
Abstract
:1. Introduction
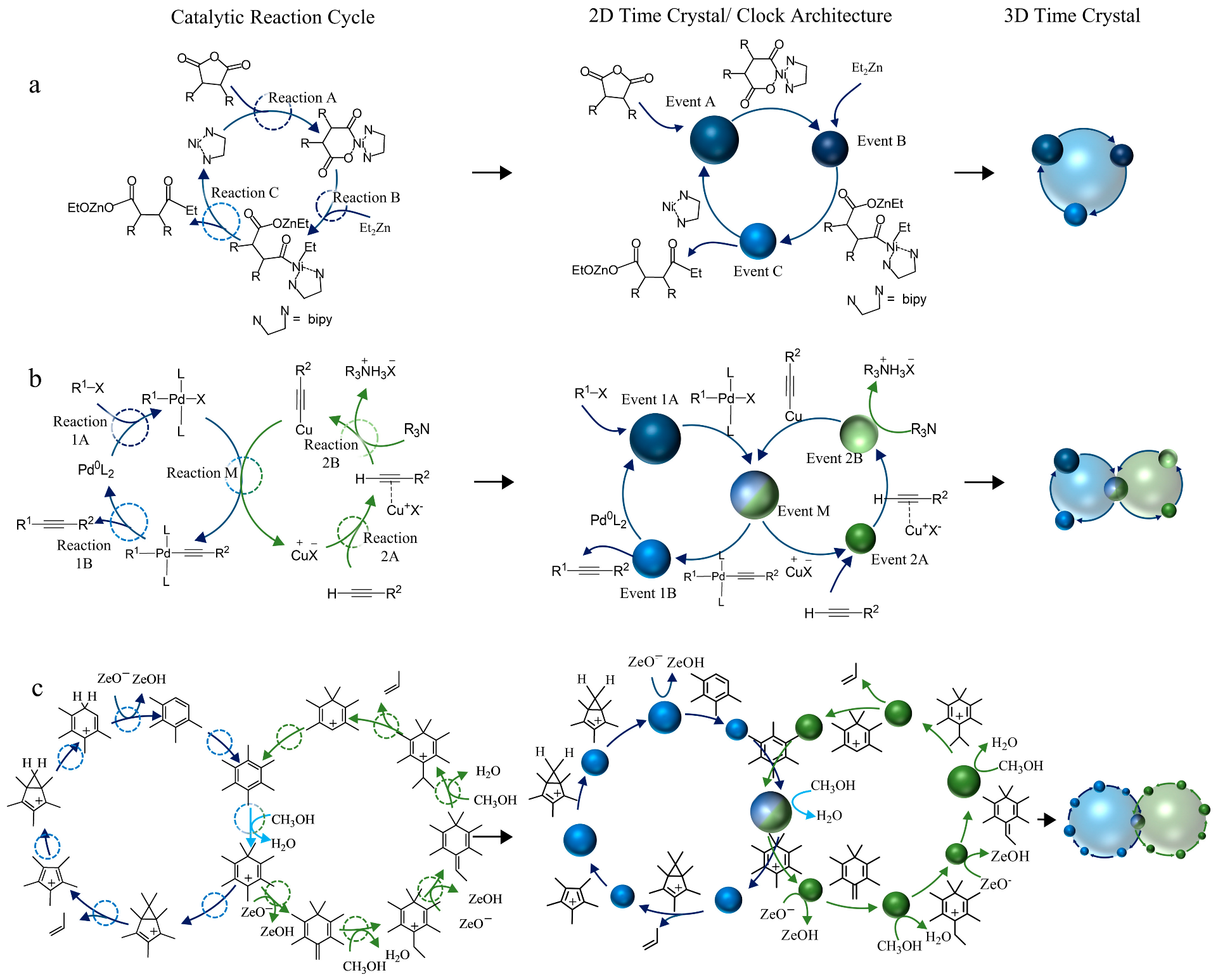
2. Time-Crystal Synthon (TCS)
2.1. Types of Time-Crystal Synthons
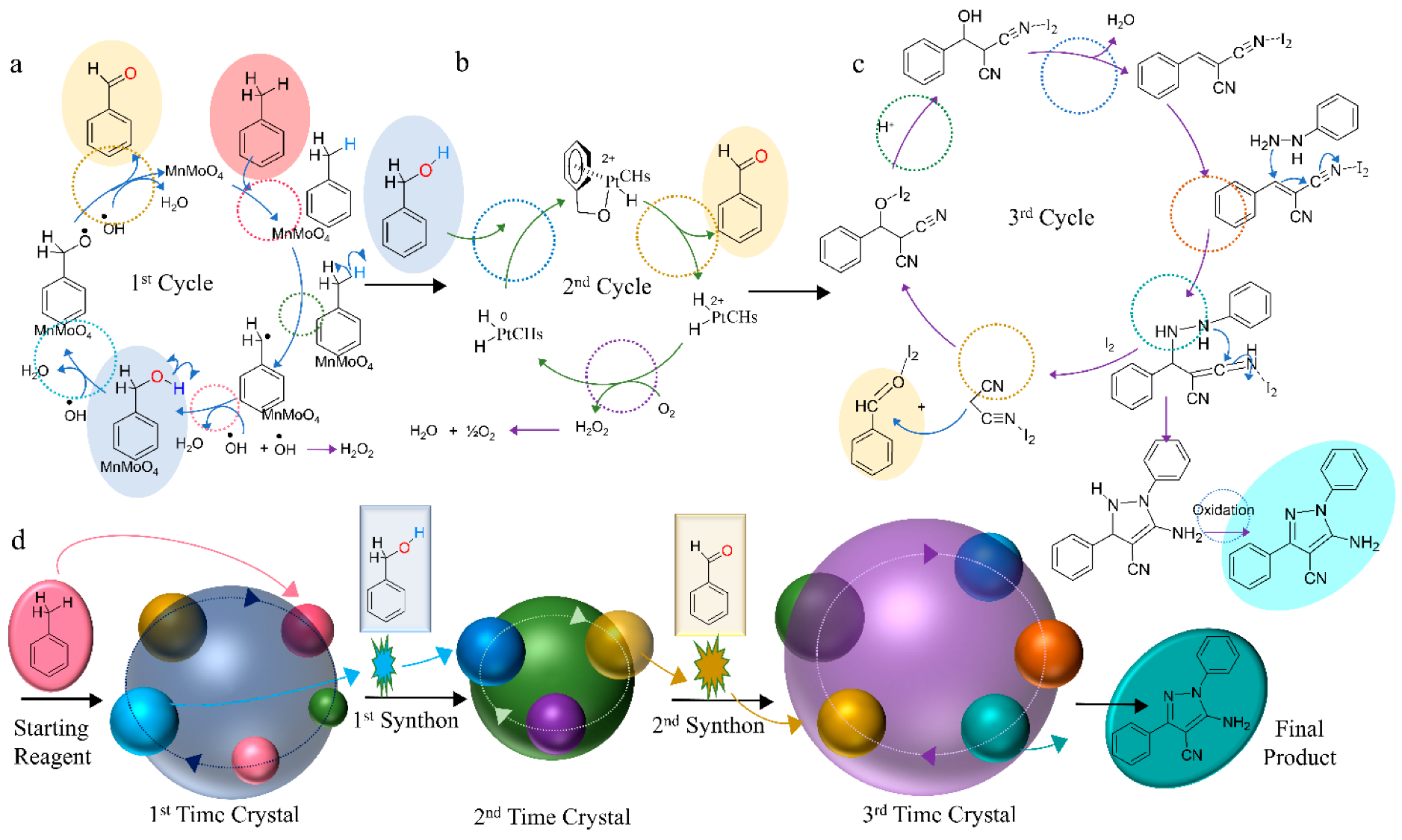
2.2. Formation of a Cascade of the Reaction Cycle
3. Time-Crystal Synthons in Developing Model Cascade Reaction Cycles
4. Advantages of Time-Crystal Synthon Concept
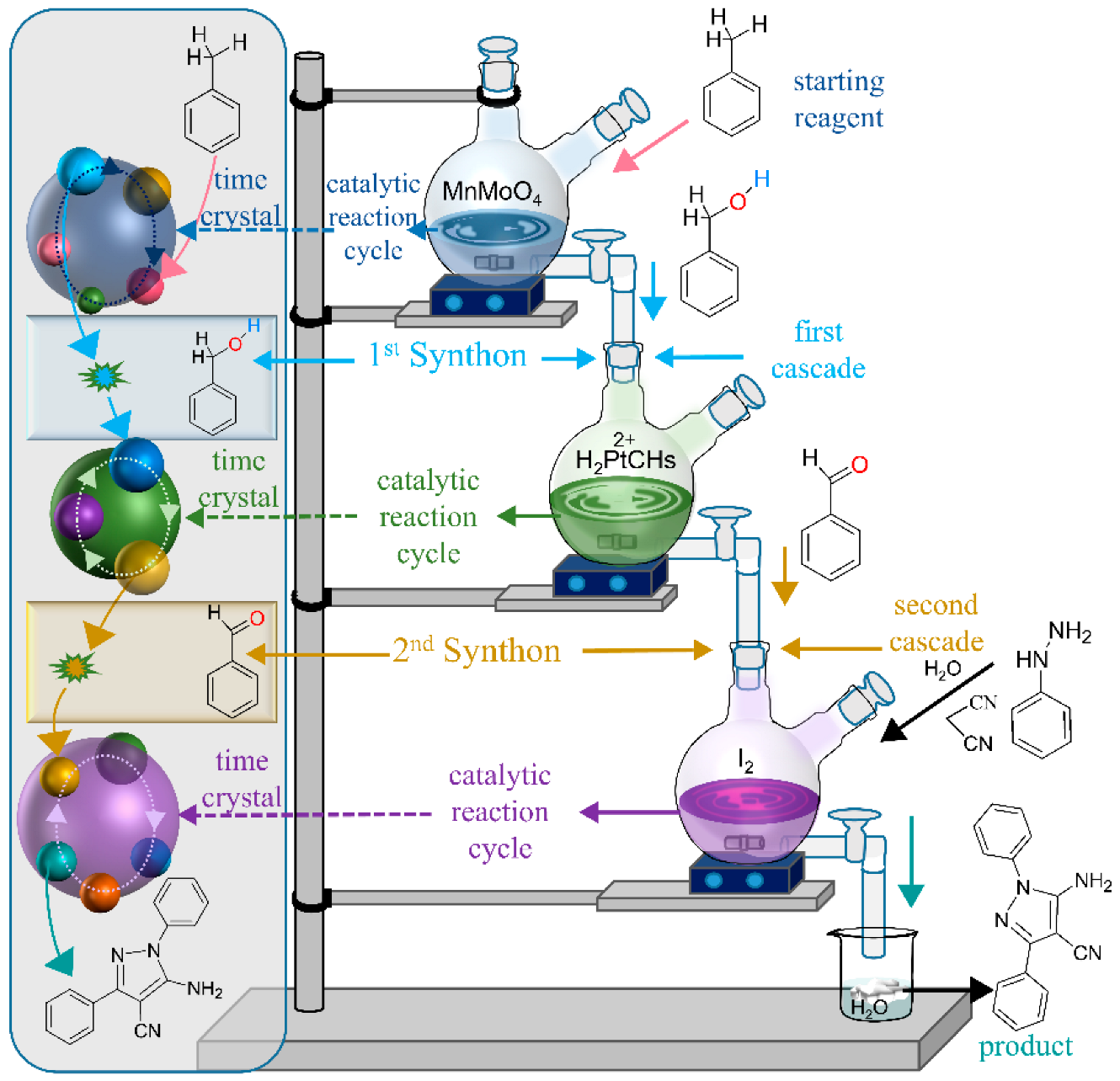
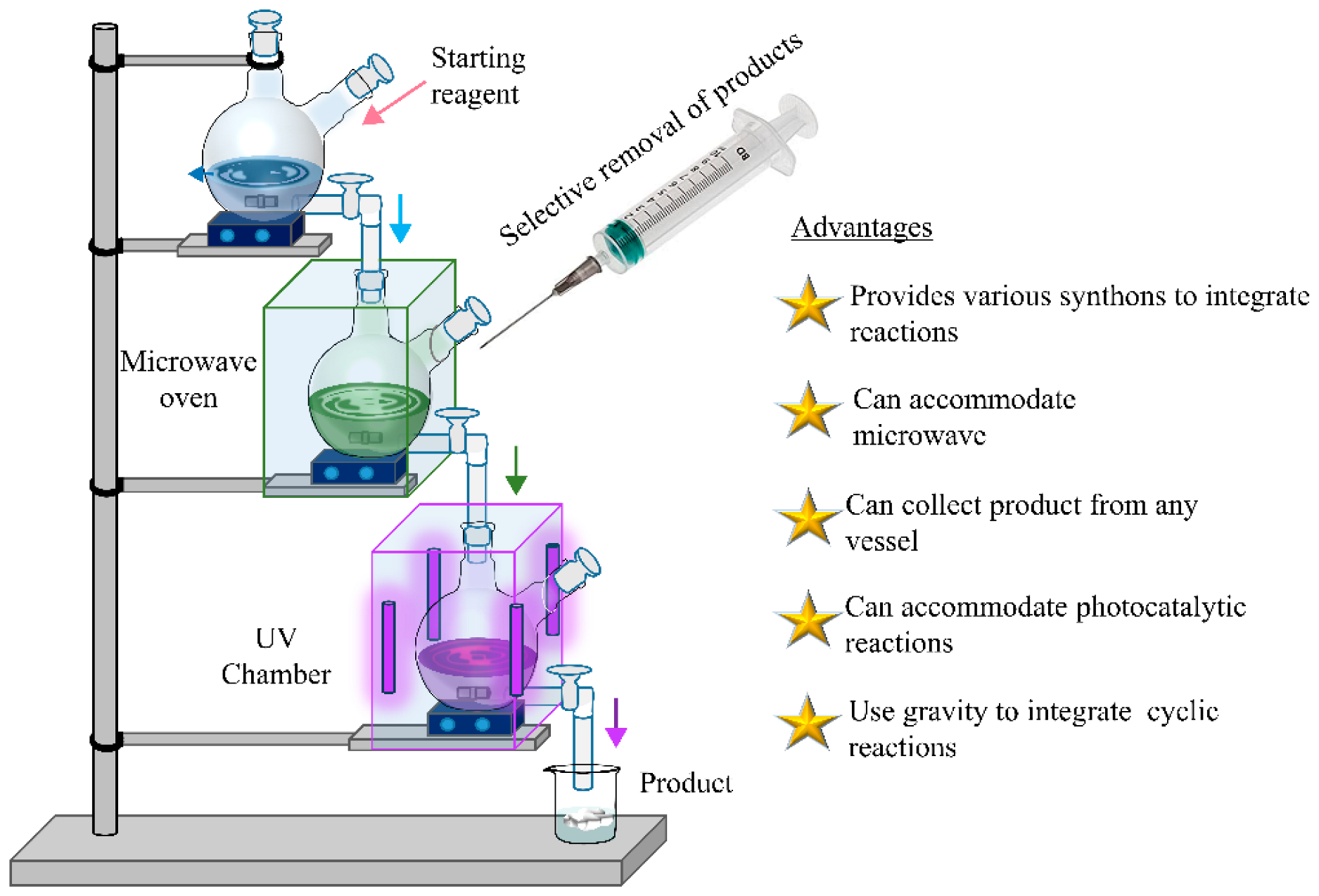
4.1. Hybridized Facilities of Multistep Flow Synthesis and Step-by-Step Synthesis
4.2. Extracting Specific Products as per Requirements
4.3. Rhythm That Sustains the Cascade of Cyclic Reaction
4.4. Application of TCS in Other Fields
5. Challenges
5.1. Heterogeneous Catalysis
5.2. Complete Conversion
5.3. Solvent Similarity
5.4. Specific Product Carrier Channel in the Reaction Chamber Joining Tubes
6. Conclusions
Funding
Conflicts of Interest
References
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Ley, S.V.; Baxendale, I.R. New tools and concepts for modern organic synthesis. Nat. Rev. Drug Discov. 2002, 1, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Geyer, K.; Codée, J.D.C.; Seeberger, P.H. Microreactors as tools for synthetic chemists—The chemists’ round-bottomed flask of the 21st century? Chemistry 2006, 12, 8434–8442. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding whether to go with the flow: Evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef]
- Sagmeister, P.; Lebl, R.; Castillo, I.; Rehrl, J.; Kruisz, J.; Sipek, M.; Horn, M.; Sacher, S.; Cantillo, D.; Williams, J.D.; et al. Advanced Real-Time Process Analytics for Multistep Synthesis in Continuous Flow. Angew. Chem. Int. Ed. 2021, 60, 8139–8148. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Tsubogo, T.; Oyamada, H.; Kobayashi, S. Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 2015, 520, 329. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.O.M.A.; Miranda, L.S.M.; Bornscheuer, U.T. A Retrosynthesis Approach for Biocatalysis in Organic Synthesis. Chem. Eur. J. 2017, 23, 12040–12063. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S. Flow fine synthesis with heterogeneous catalysts. Tetrahedron 2018, 74, 1705–1730. [Google Scholar] [CrossRef]
- Sahoo, P.; Ghosh, S. Time Crystal Engineering in Catalytic Reaction cycles. In Rhythmic Oscillations in Proteins to Human Cognition; Bandyopadhyay, A., Ray, K., Eds.; Springer Book Series ‘Studies in Rhythm Engineering’; Springer: New York, NY, USA, 2021; Chapter 4; pp. 103–134. [Google Scholar]
- Sahoo, P.; Ghosh, S. Space and Time Crystal Engineering in Developing Futuristic Chemical Technology. ChemEngineering 2021, 5, 67. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Zimmermann, S.; Kumar, K. Cascade reaction based synthetic strategies targeting biologically intriguing indole polycycles. Org. Biomol. Chem. 2019, 17, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Shlesinger, M.F. Fractal time in condensed matter. Ann. Rev. Phys. Chem. 1988, 39, 269–290. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, K.; Singhania, A.; Sahoo, P.; Ghosh, S.; Chhajed, R.; Ray, K.; Fujita, D.; Bandyopadhyay, A. A Self-Operating Time Crystal Model of the Human Brain: Can We Replace Entire Brain Hardware with a 3D Fractal Architecture of Clocks Alone? Information 2020, 11, 238. [Google Scholar] [CrossRef]
- Corey, E.J. General methods for the construction of complex molecules. Pure Appl. Chem. 1967, 14, 19. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: A Holistic View. Angew. Chem. Int. Ed. 2007, 46, 8342. [Google Scholar] [CrossRef]
- Lloyd, G.O.; Steed, J.W. Anion-Tuning of Supramolecular Gel Properties. Nat. Chem. 2009, 1, 437. [Google Scholar] [CrossRef]
- Sahoo, P. Unveiling Discotic Liquid Crystalline Phase Changes through Supramolecular Disc Polymorphism at Their Disc-driven Gel State. Cryst. Growth Des. 2023, 23, 6238–6243. [Google Scholar] [CrossRef]
- Nachon, F.; Brazzolotto, X.; Dias, J.; Courageux, C.; Drożdż, W.; Cao, X.-Y.; Stefankiewicz, A.R.; Lehn, J.-M. Grid-Type Quaternary Metallosupramolecular Compounds Inhibit Human Cholinesterases through Dynamic Multivalent Interactions. ChemBioChem 2022, 23, e202200456. [Google Scholar] [CrossRef]
- Oda, R.; Artzner, F.; Laguerre, M.; Huc, I. Molecular Structure of Self-Assembled Chiral Nanoribbons and Nanotubules Revealed in the Hydrated State. J. Am. Chem. Soc. 2008, 130, 14705. [Google Scholar] [CrossRef]
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-Bonding-Triggered Supramolecular Gel Formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef]
- Dash, R.K.; Li, Y.; Kim, J.; Beard, D.A.; Saidel, G.M.; Cabrera, M.E. Metabolic Dynamics in Skeletal Muscle during Acute Reduction in Blood Flow and Oxygen Supply to Mitochondria: In-Silico Studies Using a Multi-Scale, Top-Down Integrated Model. PLoS ONE 2008, 3, e3168. [Google Scholar] [CrossRef]
- Sahoo, P. Cocrystallizing and Codelivering Complementary Drugs to Multidrug-Resistant Tuberculosis Bacteria in Perfecting Multidrug Therapy. Curr. Top. Med. Chem. 2023, 23, 1850–1858. [Google Scholar] [CrossRef]
- Hanson, A.D.; McCarty, D.R.; Henry, C.S.; Xian, X.; Joshi, J.; Patterson, J.A.; García-García, J.D.; Fleischmann, S.D.; Tivendale, N.D.; Millar, A.H. The number of catalytic cycles in an enzyme’s lifetime and why it matters to metabolic engineering. Proc. Natl. Acad. Sci. USA 2021, 118, e2023348118. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Hamid, N.A.A.; Rahiman, W.; Mohamad, A.A. Electrode polymer binders for supercapacitor applications: A review. J. Mater. Res. Technol. 2023, 23, 3470–3491. [Google Scholar] [CrossRef]
- Shoukat, H.; Altaf, A.A.; Hamayun, M.; Ullah, S.; Kausar, S.; Hamza, M.; Muhammad, S.; Badshah, A.; Rasool, N.; Imran, M. Catalytic Oxidation of Toluene into Benzaldehyde and Benzyl Alcohol Using Molybdenum-Incorporated Manganese Oxide Nanomaterials. ACS Omega 2021, 6, 19606–19615. [Google Scholar] [CrossRef]
- Göksu, H.; Burhan, H.; Mustafov, S.D.; Şen, F. Oxidation of Benzyl Alcohol Compounds in the Presence of Carbon Hybrid Supported Platinum Nanoparticles (Pt@CHs) in Oxygen Atmosphere. Sci. Rep. 2020, 10, 5439. [Google Scholar] [CrossRef]
- Srivastava, M.; Rai, P.; Singh, J.; Singh, J. Efficient iodine-catalyzed one-pot synthesis of highly functionalised pyrazoles in water. New J. Chem. 2014, 38, 302–307. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Annunziata, R.; Puglisi, A.; Celentano, G. Solid supported chiral N-picolylimidazolidinones: Recyclable catalysts for the enantioselective, metal-and hydrogen-free reduction of imines in batch and in flow mode. Adv. Synth. Catal. 2017, 359, 2375–2382. [Google Scholar] [CrossRef]
- Barnard, T.M.; Vanier, G.S.; Collins, M.J., Jr. Scale-Up of the Green Synthesis of Azacycloalkanes and Isoindolines under Microwave Irradiation. Org. Process Res. Dev. 2006, 10, 1233–1237. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225. [Google Scholar] [CrossRef]
- Loones, K.T.J.; Maes, B.U.W.; Rombouts, G.; Hostyn, S.; Diels, G. Microwave-assisted organic synthesis: Scale-up of palladium-catalyzed aminations using single-mode and multi-mode microwave equipment. Tetrahedron 2005, 61, 10338. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P. Hydrogen-Producing Photocatalyst at Sunscreen for Athletes in Preventing and Healing Muscle-Nerve-Skin Injuries. Curr. Top. Med. Chem. 2023, 23, 249–256. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a novel therapeutic molecule, regulate oxidative stress, inflammation, and apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, M.; Song, J.; Chen, X.; Yang, H. Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano 2019, 13, 8505–8511. [Google Scholar] [CrossRef]
- Li, S.; Liao, R.; Sheng, X.; Luo, X.; Zhang, X.; Wen, X.; Zhou, J.; Peng, K. Hydrogen gas in cancer treatment. Front. Oncol. 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Xie, F.; Li, J.; Zhang, Y.; Liu, M.; Zhao, P.; Ma, X.; Lebaron, T.W. Therapeutic potential of molecular hydrogen in ovarian cancer. Transl. Cancer Res. 2018, 7, 988–995. [Google Scholar] [CrossRef]
- Pizzi, A.; Knolle, J.; Nunnenkamp, A. Higher-order and fractional discrete time crystals in clean long-range interacting systems. Nat. Commun. 2021, 12, 2341. [Google Scholar] [CrossRef]
- Solfanelli, A.; Ruffo, S.; Succic, S.; Defenu, N. Logarithmic, fractal and volume-law entanglement in a Kitaev chain with long-range hopping and pairing. J. High Energy Phys. 2023, 2023, 66. [Google Scholar] [CrossRef]
- Saxena, K.; Singh, P.; Sahoo, P.; Ghosh, S.; Krishnanda, D.; Ray, K.; Fujita, D.; Bandyopadhyay, A. Proceedings of Trends in Electronics and Health Informatics; Lecture Notes in Networks and Systems; Springer: Singapore, 2022; Volume 376, p. 243. [Google Scholar]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O. Separation techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [PubMed]
- Arnold, T.; Linke, D. Phase separation in the isolation and purification of membrane proteins. BioTechniques 2007, 43, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Burke, J.M.; Ivory, C.F. Influence of the semi-permeable membrane on the performance of dynamic field gradient focusing. Electrophoresis 2010, 31, 893–901. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, P. Time Crystal Synthon: The Way to Integrate Cascade Reactions for Advancing Multistep Flow Synthesis. ChemEngineering 2023, 7, 88. https://doi.org/10.3390/chemengineering7050088
Sahoo P. Time Crystal Synthon: The Way to Integrate Cascade Reactions for Advancing Multistep Flow Synthesis. ChemEngineering. 2023; 7(5):88. https://doi.org/10.3390/chemengineering7050088
Chicago/Turabian StyleSahoo, Pathik. 2023. "Time Crystal Synthon: The Way to Integrate Cascade Reactions for Advancing Multistep Flow Synthesis" ChemEngineering 7, no. 5: 88. https://doi.org/10.3390/chemengineering7050088
APA StyleSahoo, P. (2023). Time Crystal Synthon: The Way to Integrate Cascade Reactions for Advancing Multistep Flow Synthesis. ChemEngineering, 7(5), 88. https://doi.org/10.3390/chemengineering7050088





