Abstract
Red pepper powder is used as a spice added to various types of foods to improve the spiciness and aroma of foods. The unique aroma and spiciness of red pepper are related to the contents of bioactive compounds, including alkaloids, phenolic compounds, terpenes, and flavonoids. These phytochemical compounds have extensively provided many biological activities, such as antioxidant, anti-inflammatory, and antimicrobial. The assessment of bioactive compounds in red pepper is crucial to evaluate the quality of red pepper powder. Therefore, the objective of this study aimed to analyze total phenolic and total flavonoid compounds for further red peppercorn powder application. To assess the contents of bioactive compounds, Response Surface Methodology (RSM) with Box–Behnken Design (BBD) was applied to design the experiment and analyze the data. Furthermore, extraction conditions such as extraction time (30 to 150 min), temperature (35 to 65 °C), and solid-to-solvent ratio (0.5:10 to 0.5:20 g/mL) were investigated for their effects on the yield of total phenolic and total flavonoid contents. The result of this study found that all extraction parameters significantly affected the extraction yields of phenolic and flavonoid compounds. The aroma and taste of red pepper powder can be adjusted by changing extraction conditions such as temperature, time, and solid-to-solvent ratio because changing these conditions allowed the bioactive compounds to be extracted from red pepper at different concentrations. Overall, the assessment of bioactive compounds in red peppercorns holds significant importance for their application as red peppercorn powder.
1. Introduction
Piper nigrum, a member of the Piperaceae family, is a highly esteemed medicinal plant. It is widely utilized as a spice and is recognized as one of the most prominent spices [1,2]. Moreover, pepper has been utilized not only just to improve the taste of food, but also for preserving food and for medicinal reasons because of its diverse range of beneficial and nutritional components. There are four main types of pepper available in the market—green, black, red, and white peppers. Green pepper is obtained from unripe peppercorns, harvested while still young, and has a fresh citrus taste with less spiciness compared to dried varieties. Black pepper is harvested when the berries transition from green to yellow, and then dried to remove moisture. Red pepper is made from fully ripe berries and can be presented as whole berries. It has a sweeter and less spicy taste than black pepper but offers a more rounded flavor. White pepper is derived from mature red berries, and its flavor profile is distinct due to the removal of the fruit’s outer skin after soaking, resulting in a taste reminiscent of fresh grass and lime [3,4]. In Cambodia, Kampot pepper is highly praised globally for its exceptional quality, characterized by a gentle spice, delightful aromas with rich layers of freshness and complexity, and a long-lasting, remarkable flavor. It has gained international recognition as one of the finest pepper varieties worldwide. Kampot pepper has been accredited by Geographical Indication (GI) due to its quality, and it stands out due to its connection with the agro-environment, which includes the impact of weather patterns, coastal climate, and distinctive soil conditions [3].
The development of red peppercorn powder provides a convenient means of incorporating bioactive components into various food formulations. Most spices produced in large quantities through industrial processes are typically in powder form. In the spice sector, powdered black pepper obtained from grinding is widely utilized as a valuable ingredient in various food applications [5]. It is utilized as a flavor enhancer to be added to dry spice blends (such as curry powder), drink mixes, and prepared or raw meals. However, the three varieties of peppers (green, white, and red peppercorns) could be the best value-added product for powder industrial processing, especially red peppercorns. Likewise, establishing this role would require the identification of distinct bioactive compounds to aid in pinpointing specific advantages of red peppercorns [6] before concluding that red peppercorns would be a new alternative source for pepper powder products. Therefore, a comprehensive analysis of the bioactive compounds in red peppercorns is crucial for optimizing the application of red peppercorn powder in different food products.
The selection of an appropriate extraction method is crucial for conducting qualitative and quantitative studies on bioactive compounds derived from plant materials [7,8]. Extraction serves as the initial and pivotal step in any medicinal plant study, significantly influencing the final results and outcomes. The most common factors affecting extraction processes are matrix properties of the plant part, solvent, temperature, pressure, and time [9]. Therefore, the objective of this study aimed to determine the bioactive compounds of Kampot red peppercorns at various extraction conditions and then consider the possibility to develop red peppercorns powder by adjusting its bioactive contents.
2. Materials and Methods
2.1. Sample Preparation
In this study, dried red peppercorns (see Figure 1) were used as samples obtained from a farm in Kampot province, Cambodia (10°36′50.69″ N 104°18′52.29″ E). The red peppercorns were ground as a sample to extract the bioactive compounds.
Figure 1.
Kampot red peppercorns: (a) dried red peppercorns; (b) red pepper powder.
2.2. Design of Experiment and Extraction of Bioactive Compounds
Extraction conditions (three parameters and three levels) for extracting bioactive compounds from the red peppercorns were designed using the Box–Behnken Design (BBD) approach (see Table 1). Through this applied approach, 15 experimental runs with different conditions of extraction time, extraction temperature, and solid-to-solvent ratio were randomly generated by the statistical software.

Table 1.
Experimental condition designed by RSM with BBD approach.
In this work, solvent extraction using absolute ethanol as the extractant was applied to extract a bioactive compound from red peppercorns powder. Figure 2 illustrates the summary of the extraction procedure for bioactive compounds from the red peppercorns. Firstly, red peppercorn powder was mixed with ethanol at the designed solid-to-solvent ratio as seen in Table 1 and placed in a water bath (SSB-18, SciLab, Seoul, Republic of Korea) at various temperatures (35 to 65 °C), and times (30 to 150 min) and at 250 rpm of shaking speed. After completion of extraction, the solid–liquid mixture was centrifuged at 2000 rpm for 15 min to separate the extract from the solid residue, then subjected to filtration through a Nylon filter (D = 0.45 μm). The extract was obtained and subjected to the analysis of total phenolic and flavonoid contents.
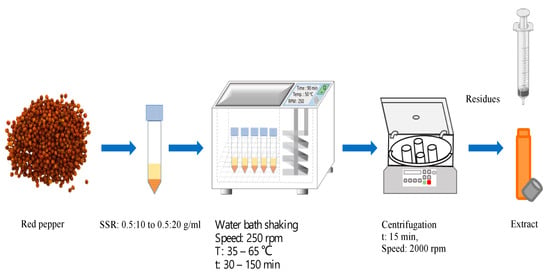
Figure 2.
Basic process for the extraction of bioactive compounds from red pepper used in this work.
2.3. Bioactive Compounds Analysis
The red peppercorn extracts were used to analyze bioactive compounds such as total phenolic content (TPC) and total flavonoid content (TFC), with some modification from the study of [10,11], respectively. Gallic acid and quercetin were utilized as the standard equivalent concentration to the determination of TPC and TFC, respectively. The prepared samples and series of standard solutions were measured using UV spectrophotometer (UV-1280, Shimadzu, Kyoto, Japan) at 765 nm and 415 nm for TPC and TFC, respectively. Following that, the yield of TPC and TFC was computed using Equation (1). TPC and TFC were expressed as mg gallic acid equivalent per gram dry weight (mg GAE/g DW) and mg quercetin equivalent per gram dry weight (mg QE/g DW), respectively.
where Yi: Yield of TPC and TFC (mg GAE/g DW; mg QE/g DW); Mext: Mass extract (g); Ci: Concentration of TPC and TFC (mg/g); DF: Dilution factor; Ms: Mass of the sample (g)
Yi = (Mext × Ci × DF)/Ms
2.4. Optimization of Extraction Process
The design of the experiment (DOE) was performed to observe the effect of three independent variables, including extraction time (30, 90, and 150 min), extraction temperature (35, 50, and 65 °C), and solid-to-solvent ratio (0.5:10, 0.5:15, and 0.5:20 g/mL) on the yield of TPC and TFC. The optimization of the yield of TPC and TFC was analyzed through the quadratic polynomial model, as seen in the following equation:
where Yi is the response; β0, βi, βii, and βij are the regression coefficient of the intercepts, linear, quadratic, and interaction terms, respectively; Xi and Xj are the independent variables.
2.5. Statistical Analysis
Total phenolic and total flavonoid contents (TPC and TFC) were statistically computed as Analysis of Variance (ANOVA). The ANOVA was utilized to assess the quality of a fit model of the experimental data, the significance of the model, and the effect of extraction parameters on total phenolic and total flavonoid contents in the extraction process. It is also noted that the significance of the model and the independent variables were determined by a p-value of less than 0.05.
3. Results
3.1. Extraction Yield of Total Phenolic and Flavonoid Contents
In this study, total phenolic contents (TPC) and total flavonoid contents (TFC) extracted from red peppercorns (Piper nigrum L.) were identified. There were three parameters such as extraction temperature (35, 50, and 65 °C), extraction time (30, 90, and 150 min), and solid-to-solvent ratio (0.5:10, 0.5:15, and 0.5:20 g/mL). Within the levels of each extraction parameter, yields of TPC and TFC ranged from 3.26 to 8.38 mg GAE/g DW and 8.03 to 15.51 mg QE/g DW, respectively (see Table 2).

Table 2.
Extraction yields of TPC and TFC obtained from red peppercorns (Piper nigrum L.) at different conditions designed by RSM with BBD.
Moreover, the data in the table show that the highest yield of TPC (8.38 mg GAE/g DW) was at the highest level of extraction temperature (65 °C), and highest extraction time (150 min), however, it was at the center level of solid-to-solvent ratio (0.5:15 mL g/mL). It is remarkable that the highest level of solid-to-solvent ratio (0.5:20 g/mL) decreased the yield of TFC and TPC. In addition, this result indicated the effect of extraction time, extraction temperature, and solid-to-solvent ratio on the yields of both TPC and TFC. This means that optimization of the extraction process is necessary for obtaining the best yields of either TPC or TFC extracted from biomass materials. Furthermore, when using ethanol for extraction, its concentration, temperature, and extraction time are important factors to consider. Higher ethanol concentrations generally result in increased extraction efficiency, but there is a point beyond which further concentration may lead to diminishing returns or loss of selectivity. The extraction temperature and time also play significant roles in determining the yield and composition of extracted compounds.
3.2. Linear Effect of Extraction Parameters on Total Phenolic and Total Flavonoid Contents
Extraction parameters have the potential to influence the extraction yields of total phenolic compounds (TPC) and total flavonoid compounds (TFC) at various degrees, ranging from minor to substantial effects. Additionally, it is important to consider that during the extraction process, the yield can be influenced by individual parameters as well as interactions among multiple parameters. In this study, the individual impact of extraction temperature, extraction time, and solid-to-solvent ratio was investigated systematically to establish a linear relationship between these three parameters and extraction efficiency. Figure 3 and Figure 4 present the multiple linear regression of extraction temperature (35, 50, and 65 °C), extraction time (30, 90, and 150 min), and solid-to-solvent ratio (0.5:10, 0.5:15, and 0.5:20 g/mL) on the yields of TPC and TFC.
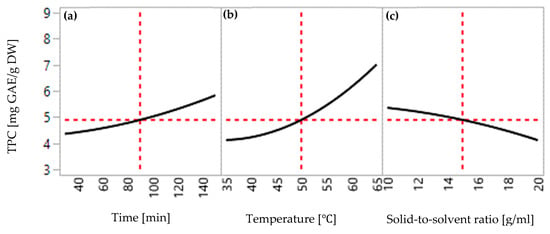
Figure 3.
Effect of extraction conditions on the yield of TPC extracted from red peppercorns: (a) extraction time (30 to 150 min); (b) temperature (36 to 65 °C); (c) solid-to-solvent ratio (0.5:10 to 0.5:20 g/mL).
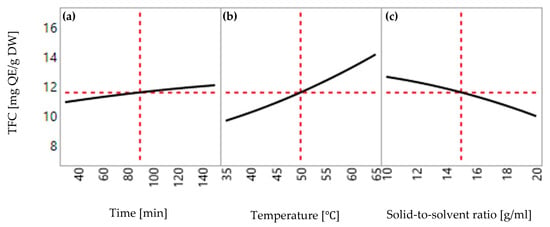
Figure 4.
Effect of extraction conditions on the yield of TFC extracted from red peppercorns: (a) extraction time (30 to 150 min); (b) temperature (36 to 65°C); (c) solid-to-solvent ratio (0.5:10 to 0.5:20 g/mL).
According to Table 3 and Table 4, it was seen that independent variables were statistically significant in the response. Furthermore, it is remarkable that extraction time was highly significant on the yield of TPC (p-value = 0.0004), while it had a less significant effect on the yield of TFC (p-value = 0.038). Extraction temperature showed a strong effect on the yield of TPC (p-value < 0.0001) and TFC (p-value < 0.0001). Meanwhile, the solid-to-solvent ratio was dominantly significant for both yields of TPC (p-value = 0.003) and TFC (p-value < 0.0001). More importantly, coefficient estimates generated from the regression analysis provided information about the magnitude and direction of the linear effect. Based on the data from Analysis of Variance (ANOVA), positive coefficients of extraction time (0.6 for TPC and 0.57 for TFC) and extraction temperature have positive coefficients (1.59 for TPC and 2.24 for TFC) which indicated a positive linear relationship, resulting in an increase in extraction efficiency with increasing levels of extraction parameters. On the other hand, the solid-to-solvent ratio provided a negative linear relationship due to the negative coefficient (−0.47 for TPC and −1.33 for TFC), thereby resulting in decreasing the extraction yield with rising levels of the solid-to-solvent ratio.

Table 3.
Analysis of variance (ANOVA) for response surface model for TPC.

Table 4.
Analysis of variance (ANOVA) for response surface model for TFC.
3.3. Interaction Effects of Extraction Parameters on Total Phenolic and Total Flavonoid Contents
The impact of the combination of parameters (interaction effect) on the yields of TPC and TFC extracted from red peppercorns was determined in this study. Figure 5a,b provide information about the interaction effect between extraction time and extraction temperature (X1X2), extraction time and solid-to-solvent ratio (X1X3), and extraction temperature and solid-to-solvent ratio (X2X3) on the yields of TPC and TFC. The result found that there was a correlation between X1X2 because the p-value is smaller than 0.05 (p-value = 0.0088 for TPC and p-value = 0.016) (See Table 3 and Table 4). Moreover, there was no interaction effect between X1X3 and X2X3 due to p-values higher than 0.05. It means that these two parameters do not have any interactions during the extraction process of TPC and TFC from red peppercorns using the solvent extraction in the setting value.
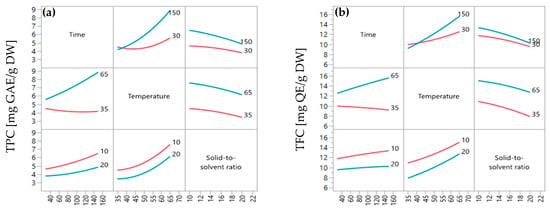
Figure 5.
Interaction effect of extraction conditions on extraction yield: (a) total phenolic contents (TPC) and (b) total flavonoid contents (TFC).
3.4. Optimization of Extraction of Total Phenolic and Total Flavonoid Contents
The summarized data for the analysis of variance (ANOVA) of the model, lack of fit, independent variables, and interaction factor for each parameter of the extraction yields of TPC and TFC are presented in Table 3 and Table 4, respectively. Evaluating the response surface method and predicting ideal variances, as well as determining the correlations between selected parameters and their corresponding response, are important aspects of model fitting from a statistical perspective. A quadratic polynomial model was employed to fit the experimental values of TPC and TFC yields. This model facilitated the investigation of correlations between independent variables (extraction time, temperature, and solid-to-solvent ratio) and their corresponding responses (yields of TPC and TFC). Additionally, it helped identify the optimal conditions for maximizing the extraction of bioactive compounds from red peppercorn.
Figure 6 present the comparison between the predicted and actual values of TPC and TFC. The analysis showed a high correlation between the experimental data and the model’s predicted values, as indicated by the coefficient of multiple determination (R2) of 0.89 for TPC yield and 0.85 for TFC yield. Furthermore, the model was found to be statistically significant with a p-value smaller than 0.0001.
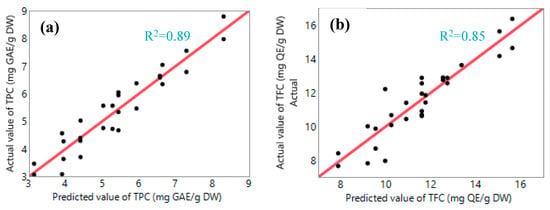
Figure 6.
Comparison of experimental and predicted values of (a) TPC and (b) TFC.
In addition, the lack-of-fit test revealed that the p-value is greater than 0.05, indicating that the models adequately fit the experimental data for both TPC and TFC yields. These results collectively demonstrate that the fitted model effectively explains the relationship between the response and input variables, making it suitable for predicting the yields of TPC and TFC obtained from red peppercorns. The equation models for TPC and TFC represent the second-order polynomial equations derived for the predicted model of TPC and TFC yields, respectively. These equations depict the empirical relationship between the yield of TPC and TFC and the parameters under investigation. However, the mathematical model was altered by excluding the interaction terms (X1X3) and (X2X3) and the quadratic terms of X12, X22, and X32 from the model. The decision to remove these factors was based on their lack of significant influence on the yield of TPC and TFC, as indicated by their p-values exceeding 0.05. Therefore, the final equations (Equations (3) and (4)) for predicting the yields of TPC and TFC in relation to extraction time, extraction temperature, and solid-to-solvent ratio can be formulated as follows:
where X1 is the extraction time, X2 is the extraction temperature, and X3 is the solid-to-solvent ratio
TPC (mg GAE/g DW) = 5.34 + 0.60X1 + 1.59X2 − 0.47X3 + 0.58X1X2
TFC (mg QE/g DW) = 11.59 + 0.57X1 + 2.24X2 − 1.33X3 + 0.96X1X2
Figure 7 and Figure 8 depict the surface plots illustrating the extraction yields of TPC and TFC concerning extraction time, extraction temperature, and solid-to-solvent ratio. By examining these plots, the optimal conditions for extracting bioactive compounds from red peppercorns using solvent extraction were determined as follows: an extraction time of 150 min, extraction temperature of 65 °C, and a solid-to-solvent ratio of 0.5:10 g/mL. Employing these optimum conditions resulted in TFC and TPC yields of 18.87 ± 1.28 mg QE/g DW and 9.85 ± 0.07 mg GAE/g DW, respectively.
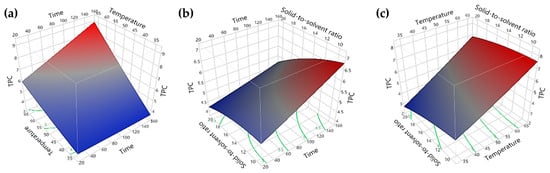
Figure 7.
Surface plot for the yield of TPC (mg GAE/g DW): (a) time (min) and temperature (°C); (b) time (min) and solid-to-solvent ratio (g/mL); (c) temperature (°C) and solid-to-solvent ratio (g/mL).
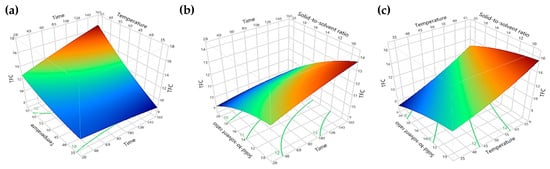
Figure 8.
Surface plot for the yield of TFC: (a) time (min) with temperature (°C); (b) time (min) with solid-to-solvent ratio (g/mL); (c) temperature (°C) with solid-to-solvent ratio (g/mL).
4. Discussion
Phenolic compounds are natural substances that are not directly involved in the growth and development of organisms. They consist of a phenol group in their structure and can be categorized into two types: extractable, which can dissolve in solvents, and non-extractable, which cannot dissolve. Extractable polyphenols can be dissolved in water or organic solvents, while non-extractable polyphenols remain in the residue after extracting EP from the food [12,13]. The primary active components found in peppercorns include phenolics, different types of lignan derivatives, terpenes, chalcones, flavonoids, alkaloids, and steroids [14,15]. Moreover, the solubility of phenolic compounds in the extraction solvent affects the retrieval of polyphenols from plant materials [16]. The polarity of the solvent will be essential in enhancing the solubility of phenolic compounds [17]. In this study, absolute ethanol was used as an extraction solvent and its volume varied from 10 mL to 20 mL. Ethanol was chosen as the extraction solvent due to its numerous advantages compared to other organic solvents. It is relatively safer and less toxic. Ethanol is an organic solvent with a polarity index value of 5.2 and a dielectric constant of 24.55. In terms of extraction yield, other inorganic solvents such as methanol are better due to high polarity compared to ethanol, however, the extraction yield is comparable. Therefore, ethanol was highly recommended by previous studies for the extraction of bioactive compounds from plant materials due to the current trends of using bioactive compounds for further applications, such as the pharmaceutical industries and food industries. Furthermore, it also can reduce the operation cost due to removing ethanol not being needed after the extraction process. Ethanol is a GRAS solvent, while methanol is toxic [18,19].
The result found that the extraction of phenolic compounds benefits from a higher proportion of solids to solvent. This finding aligns with the mass transfer principle, which states that the concentration gradient between the solid and solvent drives mass transfer. A higher solid-to-solvent ratio enhances the concentration gradient, leading to faster diffusion and increased extraction of solids by the solvent [20]. This statement agrees with [21], who claimed that as the amount of extracting solvent increases, there is a higher chance of bioactive components coming into contact with it. However, once equilibrium is reached, this chance does not continue to increase further. Overall, the primary impact of the solid-to-solvent ratio was to alter the solubility and equilibrium constant, leading to an increase in the overall yields of phenolic and flavonoid compounds. These yields reached their highest point at the optimal solid-to-solvent ratio [22].
Together with the solid-to-solvent ratio, extraction time and temperature play an important role in the recovery of bioactive components from red peppercorns, which refers to the opposing processes of solubilization and analyte degradation through oxidation that is apparent. Raising the extraction temperature enhances analyte solubility and mass transfer rate and lowers solvent viscosity and surface tension, facilitating better penetration into sample matrices for improved extraction speed. Nevertheless, phenolic compounds are easy to be hydrolyzed and oxidized. Prolonged extraction durations and higher temperatures raise the risk of phenolic oxidation, leading to reduced phenolic yield in the extracts [19]. Furthermore, the higher temperature does not only affect the solvent properties, resulting in increasing TPC and TFC yields, but it could also weaken the phenolic–protein and phenolic–polysaccharide linkage, thereby increasing the migration of TPC and TFC into the extraction solvent [23,24].
Results in this study indicated that extraction conditions (time, temperature, solid-to-solvent ratio) affect the extraction yields of phenolic and flavonoid compounds. The leaching amount and behavior of this bioactive compound from red peppercorn to solvent are possibly adjusted by changing the extraction condition. Since bioactive compounds play important roles in the taste and aroma of peppercorns, this means that adjusting the aroma and taste of peppercorn powder can be conducted by applying extraction as one of the processing steps.
5. Conclusions
This study assessed the contents of total phenolic and flavonoid compounds of red peppercorn by applying various extraction conditions. Response Surface Methodology (RSM) with Box–Behnken Design was applied to understand the relationship of extraction conditions and the leaching amount of the phenolic and flavonoid compounds from red peppercorn to solvent. It was found that all independent variables significantly influenced the leaching amount of the bioactive compounds, highlighting the importance of the extraction parameters studied in the optimization process. The optimized conditions were derived from RSM (extraction temperature of 65 °C, extraction time of 150 min, and solid-to-solvent ratio 0.5:10 g:mL), and employing these optimum conditions resulted in TFC and TPC yields of 18.87 ± 1.28 mg QE/g DW and 9.85 ± 0.07 mg GAE/g DW, respectively, which can be applied for future large-scale extractions of red peppercorns considering temperature, extraction time, and solvent ratio for cost-effectiveness evaluation. At this condition, the aroma of red peppercorn powder was greatly changed because a significant number of bioactive compounds have been leached into the solvent. This study holds potential value in the development of red peppercorn powder applications by considering extraction as one of the processing steps to adjust the taste and aroma of red peppercorn powder. After identification of the presence of bioactive compounds in red peppercorns, it could provide the information a practical application of red peppercorns powder through its use in food industries because it may enhance the food flavor due to the distinct sweet and spicy flavor of red peppercorns, making them a popular ingredient in culinary preparations. Extracted bioactive compounds can be used to create natural flavorings, spice blends, and seasoning mixes for enhancing the taste of various food products.
Further research should take into account other factors (type of extraction solvents) that may affect extraction efficiency and ensure a more comprehensive understanding of the extraction process. Moreover, Liquid Chromatography-Mass Spectrophotometry (LC-MS) should be performed in order to identify the major bioactive compounds present in the red peppercorns.
Author Contributions
S.L.: designed the experiment, collected and analyzed the data, performed statistical analysis, interpreted the result, and wrote the manuscript. S.S.: collected and analyzed the data, and performed statistical analysis. P.H.: conceptualized the study, reviewed and edited, provided critical feedback, and supervised the research process. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cambodia Higher Education Improvement Project (HIEP Credit No: 6221-KH).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Damanhouri, Z.A. A Review on Therapeutic Potential of Piper nigrum L. (Black Pepper): The King of Spices. Med. Aromat. Plants 2014, 3, 3. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A Systematic Review on Black Pepper (Piper nigrum L.): From Folk Uses to Pharmacological Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Pisith, S.O.K. An Analysis of Pepper (Piper nigrum) Value Chains in Cambodia. N. Am. Acad. Res. NAAR J. 2021, 4, 107–127. [Google Scholar] [CrossRef]
- Morm, E.; Ma, K.; Horn, S.; Debaste, F.; Haut, B.; In, S. Experimental Characterization of the Drying of Kampot Red Pepper (Piper nigrum L.). Foods 2020, 9, 1532. [Google Scholar] [CrossRef]
- Ghodki, B.M.; Goswami, T.K. Effect of Grinding Temperatures on Particle and Physicochemical Characteristics of Black Pepper Powder. Powder Technol. 2016, 299, 168–177. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health Benefits of Herbs and Spices: The Past, the Present, the Future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Smith, R.M. Before the Injection—Modern Methods of Sample Preparation for Separation Techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, Isolation And Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lobo, M.; Gonzalez, M. Factors Affecting Sample Extraction in the Liquid Chromatographic Determination of Organic Acids in Papaya and Pineapple. Food Chem. 2009, 114, 734–741. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.; Khatib, A.; Abdull Razis, A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Sulaiman, C.; Balachandran, I. Total Phenolics and Total Flavonoids in Selected Indian Medicinal Plants. Indian J. Pharm. Sci. 2012, 74, 258. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Silván, J.M.; Saura-Calixto, F. Nonextractable Polyphenols, Usually Ignored, Are the Major Part of Dietary Polyphenols: A Study on the Spanish Diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Hosseininejad, S.; González, C.M.; Hernando, I.; Moraga, G. Valorization of Persimmon Fruit Through the Development of New Food Products. Front. Food Sci. Technol. 2022, 2, 914952. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the Genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Halligudi, N.; Bhupathyraaj, M.; Hakak, M.H.S. Therapeutic Potential of Bioactive Compounds of Piper nigrum L. (Black Pepper): A Review. Asian J. Appl. Chem. Res. 2022, 3, 17–23. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.-G.; Morcuende, D.; Andrade, M.-J.; Kylli, P.; Estévez, M. Avocado (Persea americana Mill.) Phenolics, In Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Ermi Hikmawanti, N.P.; Fatmawati, S.; Asri, A.W. The Effect of Ethanol Concentrations as The Extraction Solvent on Antioxidant Activity of Katuk (Sauropus androgynus (L.) Merr.) Leaves Extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of Phenolics and Dietary Fibre Extraction from Date Seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, H.; Liu, C. Extraction of Bio-Active Components from Rhodiola Sachalinensis under Ultrahigh Hydrostatic Pressure. Sep. Purif. Technol. 2007, 57, 277–282. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass Transfer Process during Extraction of Phenolic Compounds from Milled Berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus Nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).