Solid-Oxide Amperometric Sensor for Hydrogen Detection in Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Characterization of CZS Ceramics
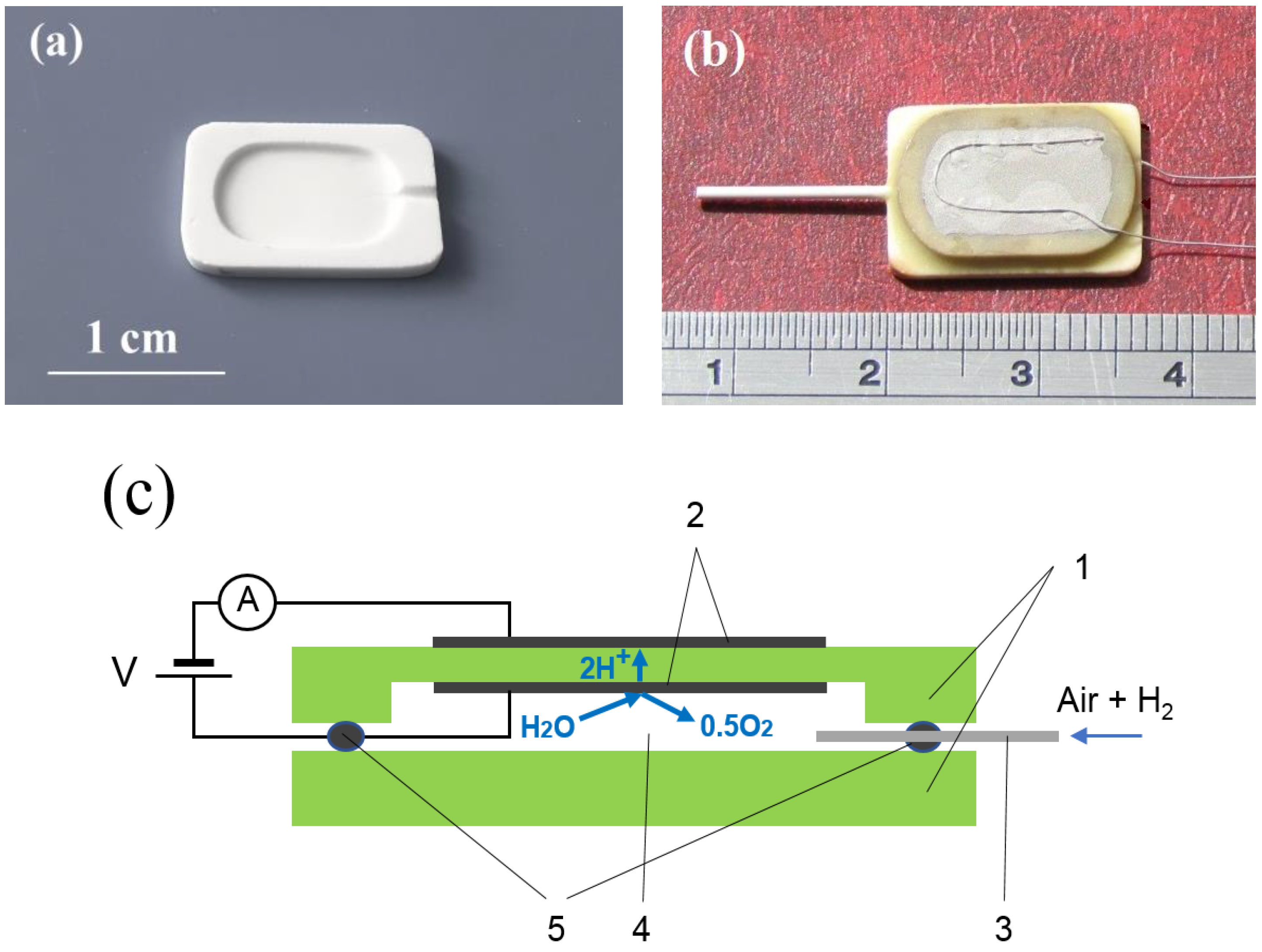
2.2. Fabrication of Sensor
2.3. Sensor Operation
3. Results and Discussion
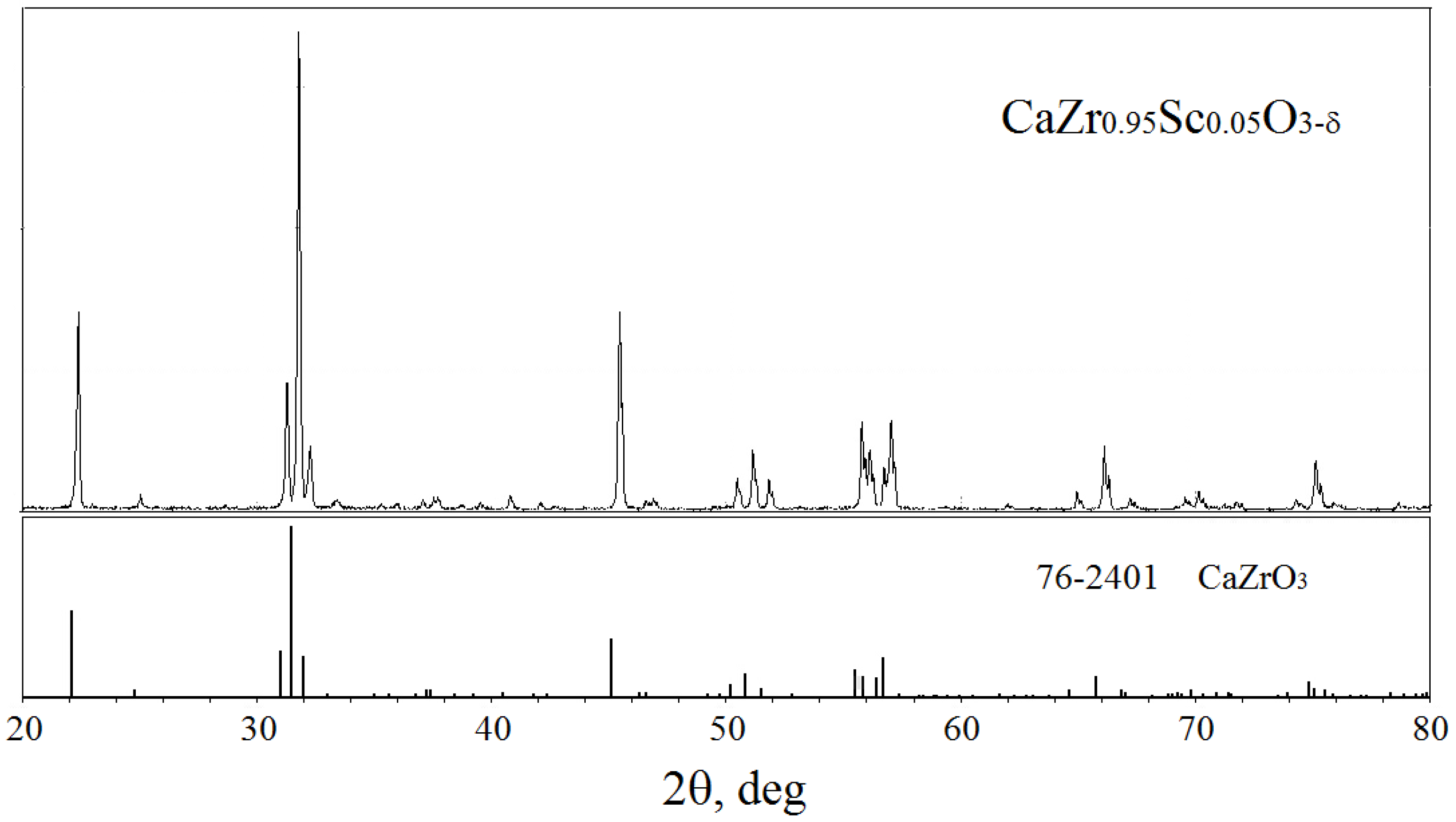
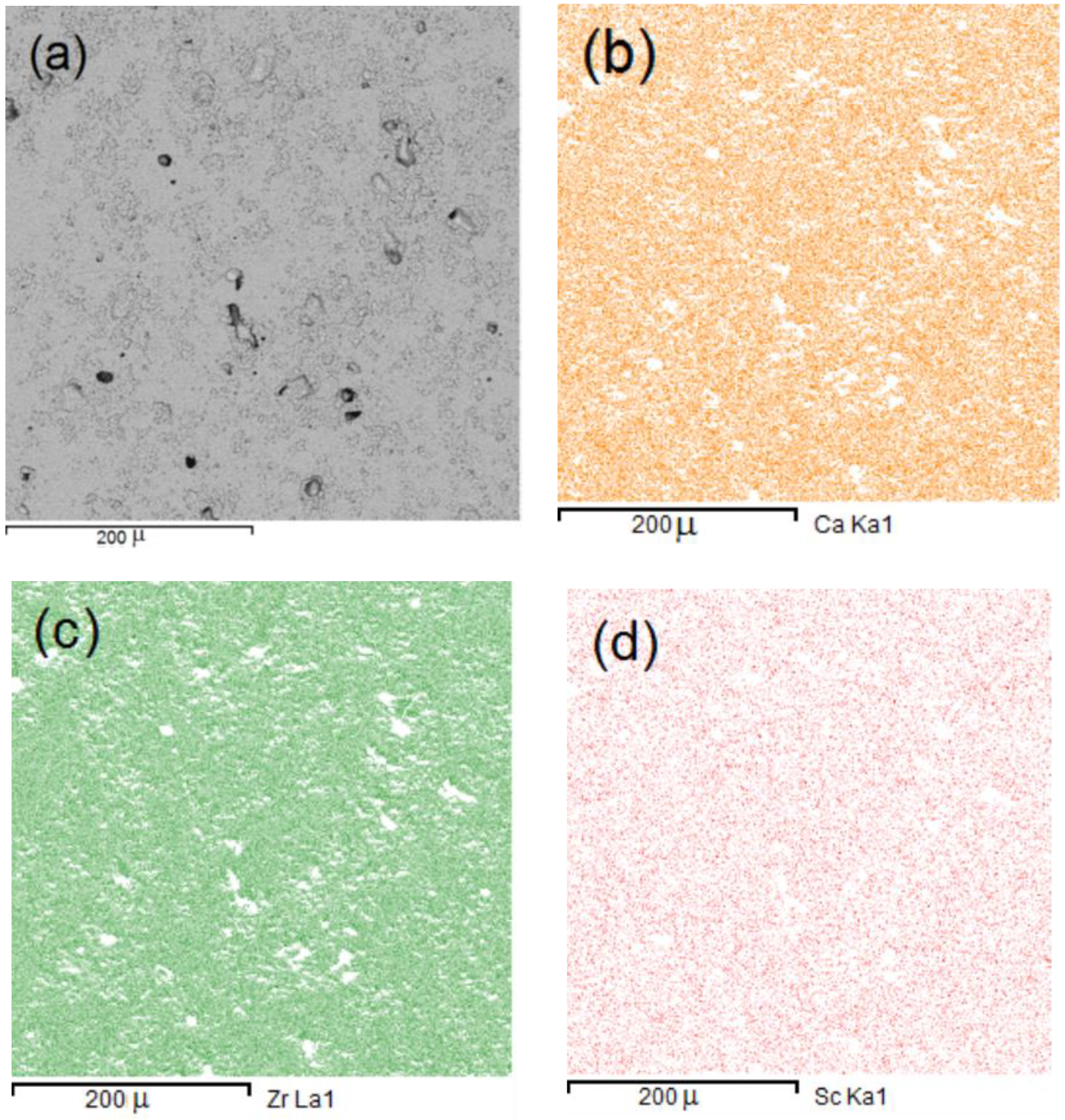
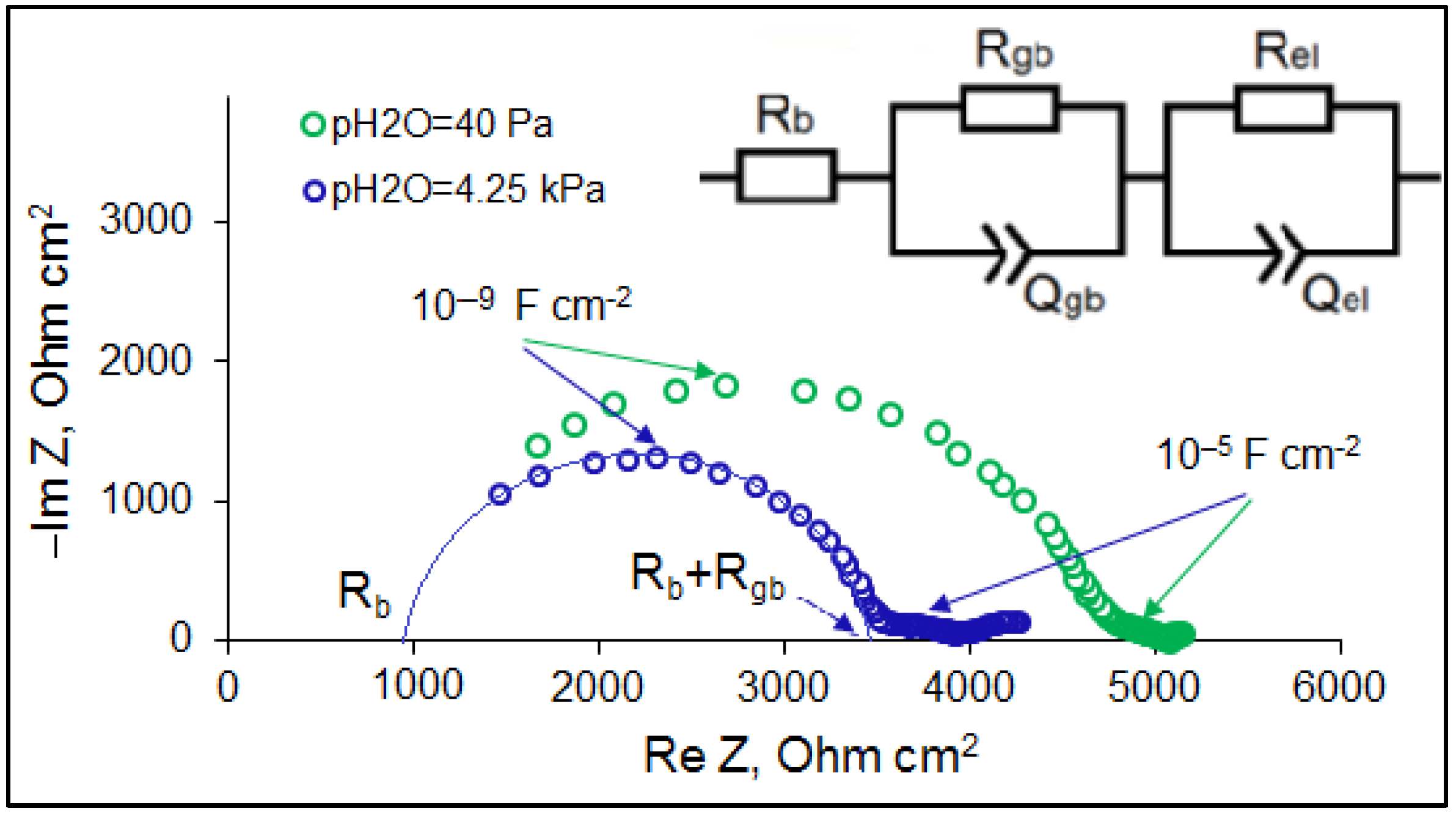
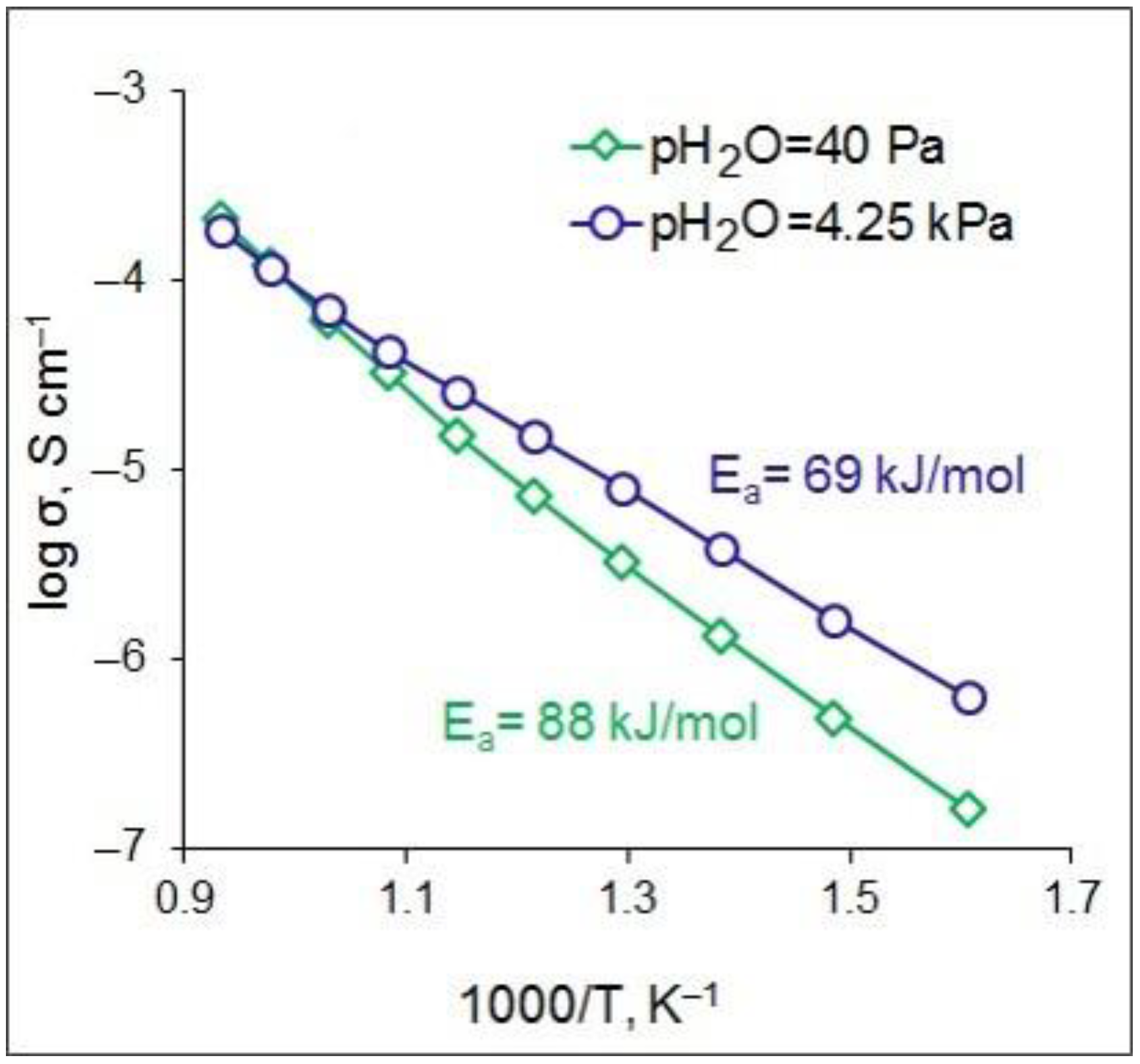
3.1. Fabrication and Characterization of CZS Ceramics
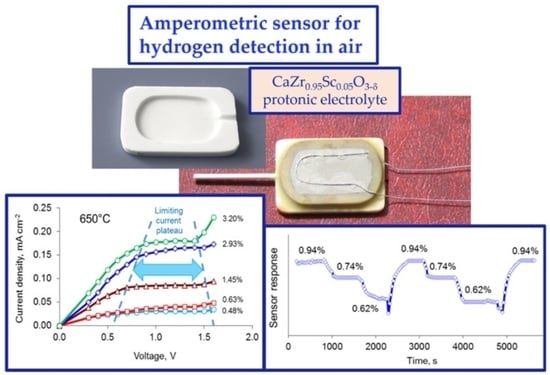
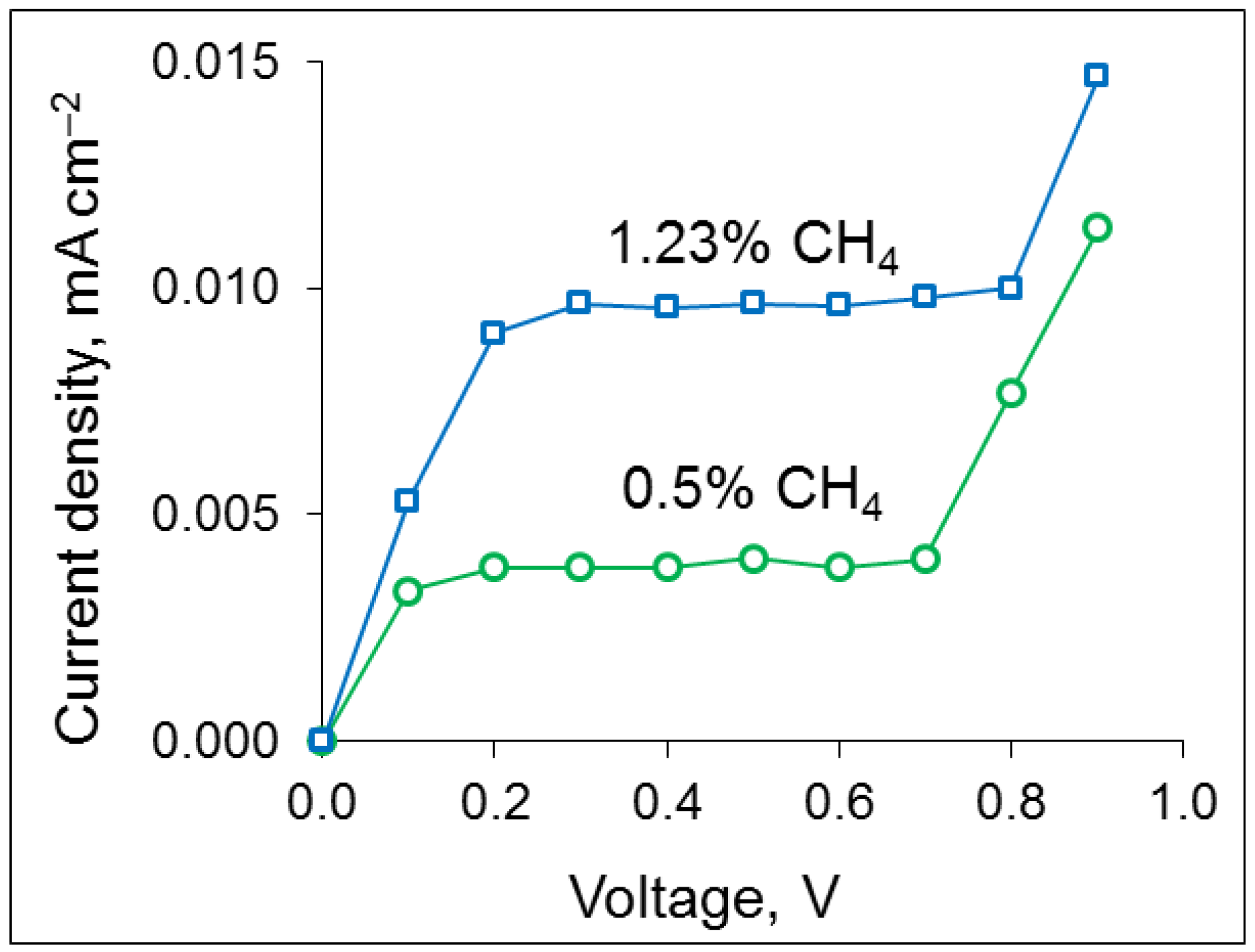
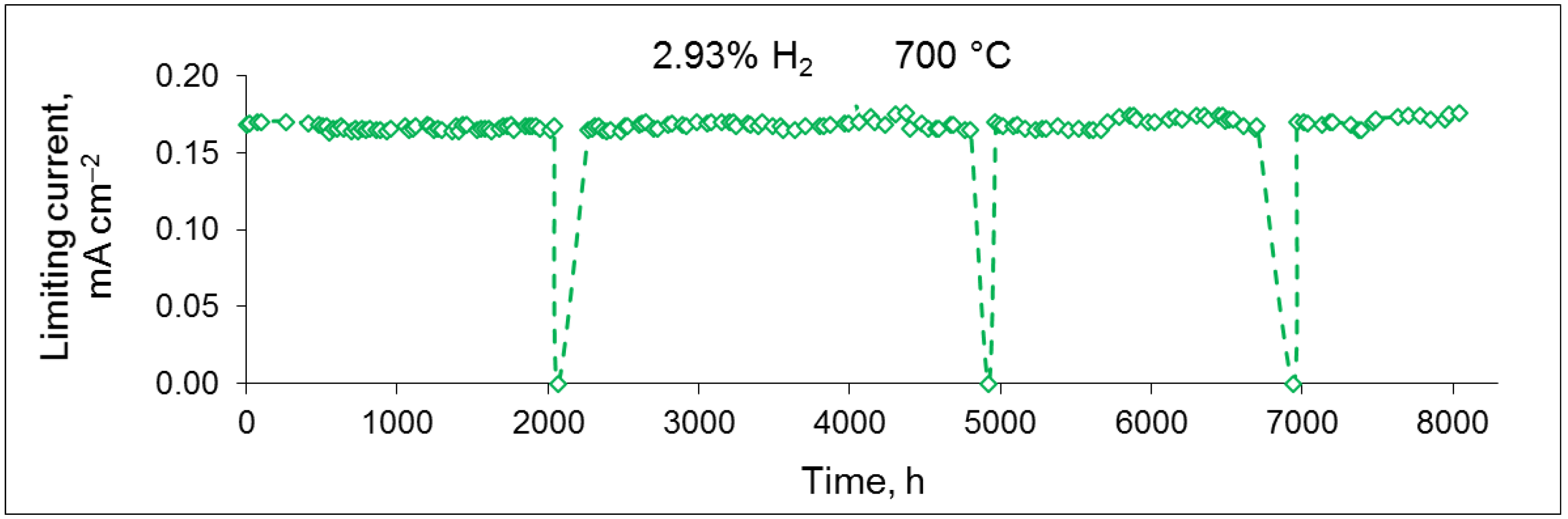
3.2. Sensor Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Ndaya, C.C.; Javahiraly, N.; Brioude, A. Recent advances in palladium nanoparticles-based hydrogen sensors for leak detection. Sensors 2019, 19, 4478. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Carriere, N.; Bahrami, F.; Mojahedi, M.; Aitchison, J.S. Pd-based integrated optical hydrogen sensor on a silicon-on-insulator platform. Opt. Lett. 2013, 38, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-performance nanostructured palladium-based hydrogen sensors—Current limitations and strategies for their mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Lim, W.; Norton, D.P.; Pearton, S.J.; Ren, F.; Johnson, J.L.; Ural, A. Nitride and oxide semiconductor nanostructured hydrogen gas sensors. Semicond. Sci. Technol. 2010, 25, 024002. [Google Scholar] [CrossRef]
- Engel, M.; Baumbach, M.; Kammerer, T.; Schütze, A. Preparation of microstructured pellistors and their application for fast fuel vapor discrimination. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems. Maastricht MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; pp. 268–271. [Google Scholar]
- Gorbova, E.; Tzorbatzoglou, F.; Molochas, C.; Chloros, D.; Demin, A.; Tsiakaras, P. Fundamentals and principles of solid-state electrochemical sensors for high temperature gas detection. Catalysts 2021, 12, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919. [Google Scholar] [CrossRef]
- Akbar, S.; Dutta, P.; Lee, C. High-temperature ceramic gas sensors: A review. Int. J. Appl. Ceram. Technol. 2006, 3, 302–311. [Google Scholar] [CrossRef]
- Ivers-Tiffee, E.; Hardtl, K.; Menesklou, W.; Riegel, J. Principles of solid state oxygen sensors for lean combustion gas control. Electrochim. Acta 2001, 47, 807–814. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakano, H.; Takase, S.; Song, J.-H. Solid electrolyte impedancemetric NOx sensor attached with zeolite receptor. Sens. Actuators B 2018, 264, 177–183. [Google Scholar] [CrossRef]
- Kondo, M.; Muroga, T.; Katahira, K.; Oshima, T. Sc-doped CaZrO3 hydrogen sensor for liquid blanket system. Fusion Eng. Des. 2008, 83, 1277–1281. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. Solid-state potentiometric gas sensors—Current status and future trends. J. Solid State Electrochem. 2009, 13, 3–25. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Park, J.; Kim, J.; Choi, A.; Park, C.O. The study of the voltage drift in high-temperature proton conductor-based hydrogen sensors adopting the solid reference electrode. Sens. Actuators B 2009, 140, 273–277. [Google Scholar] [CrossRef]
- Kalyakin, A.; Demin, A.K.; Gorbova, E.; Volkov, A.; Tsiakaras, P.E. Sensor based on a solid oxide electrolyte for measuring the water-vapor and hydrogen content in air. Catalysts 2022, 12, 1558. [Google Scholar] [CrossRef]
- Fadeyev, G.; Kalyakin, A.; Gorbova, E.; Brouzgou, A.; Demin, A.; Volkov, A.; Tsiakaras, P. A simple and low-cost amperometric sensor for measuring H2, CO and CH4. Sens. Actuators B 2015, 221, 879–883. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Li, Y.; Zhou, H.; He, Z.; Liu, H.; Dai, L. A limiting current hydrogen sensor based on BaHf0.8Fe0.2O3−δ dense diffusion barrier and BaHf0.7Sn0.1In0.2O3−δ protonic conductor. Ceram. Int. 2022, 48, 22072–22082. [Google Scholar] [CrossRef]
- Kalyakin, A.; Fadeyev, G.; Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Application of solid oxide proton-conducting electrolytes for amperometric analysis of hydrogen in H2+N2+H2O gas mixtures. Electrochim. Acta 2014, 141, 120–125. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B.; Kuzmin, A.V.; Plaksin, S.V. Electrical conductivity of CaZr1−xScxO3−d (x = 0.01 − 0.20) in dry and humid air. Inorg. Mater. 2014, 50, 495–502. [Google Scholar] [CrossRef]
- Løken, A.; Kjølseth, C.; Haugsrud, R. Electrical conductivity and TG-DSC study of hydration of Sc-doped CaSnO3 and CaZrO3. Solid State Ion. 2014, 267, 61–67. [Google Scholar] [CrossRef]
- Tian, Z.; Ruan, F.; Bao, J.; Song, X.; An, S.; Wu, R.; Jing, Q.; Lv, H.D.; Zhou, F.; Xie, M. Preparation and electrochemical properties of CaZr1-xScxO3-α. J. Electrochem. Soc. 2019, 166, B441–B448. [Google Scholar] [CrossRef]
- Gopel, W.; Reinhardt, G.; Rosch, M. Trends in the development of solid state amperometric and potentiometric high temperature sensors. Solid State Ion. 2000, 136, 519–531. [Google Scholar] [CrossRef]
- Kalyakin, A.S.; Danilov, N.A.; Volkov, A.N. Determining humidity of nitrogen and air atmospheres by means of a protonic ceramic sensor. J. Electroanal. Chem. 2021, 895, 115523. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyakin, A.; Volkov, A.; Dunyushkina, L. Solid-Oxide Amperometric Sensor for Hydrogen Detection in Air. ChemEngineering 2023, 7, 45. https://doi.org/10.3390/chemengineering7030045
Kalyakin A, Volkov A, Dunyushkina L. Solid-Oxide Amperometric Sensor for Hydrogen Detection in Air. ChemEngineering. 2023; 7(3):45. https://doi.org/10.3390/chemengineering7030045
Chicago/Turabian StyleKalyakin, Anatoly, Alexander Volkov, and Liliya Dunyushkina. 2023. "Solid-Oxide Amperometric Sensor for Hydrogen Detection in Air" ChemEngineering 7, no. 3: 45. https://doi.org/10.3390/chemengineering7030045
APA StyleKalyakin, A., Volkov, A., & Dunyushkina, L. (2023). Solid-Oxide Amperometric Sensor for Hydrogen Detection in Air. ChemEngineering, 7(3), 45. https://doi.org/10.3390/chemengineering7030045