Ti/Zr/O Mixed Oxides for the Catalytic Transfer Hydrogenation of Furfural to GVL in a Liquid-Phase Continuous-Flow Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Subsection
2.2. Synthesis of Catalysts
2.3. Characterization of Catalysts
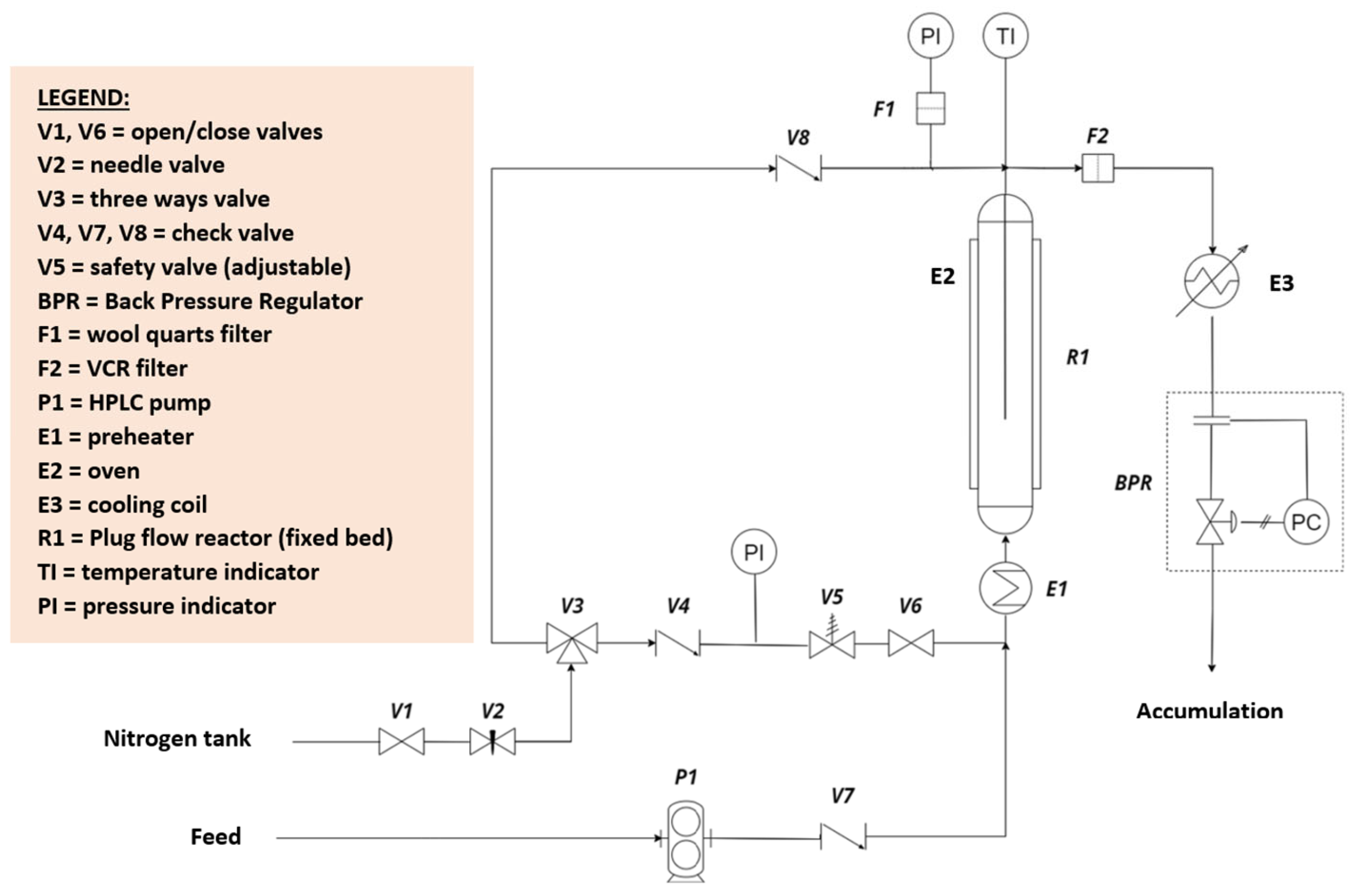
2.4. Catalytic Tests
3. Results and Discussion
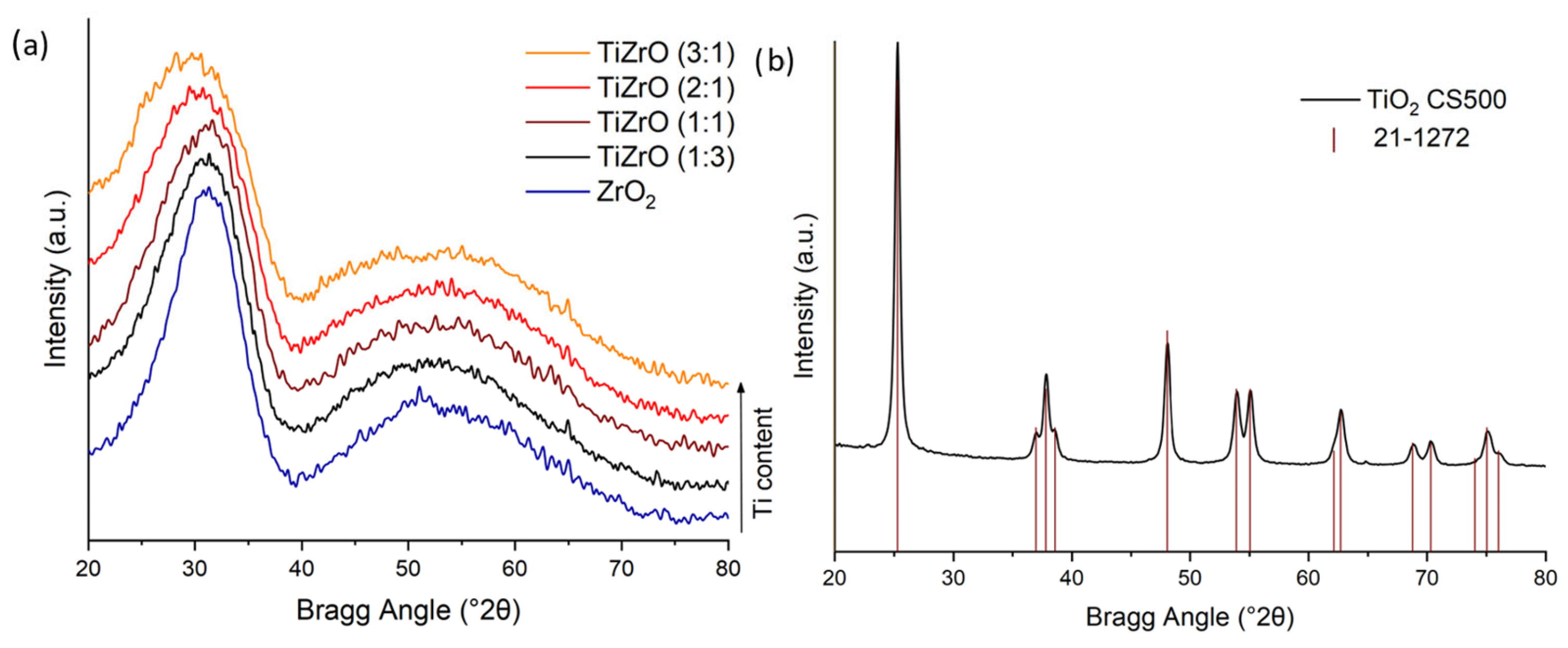
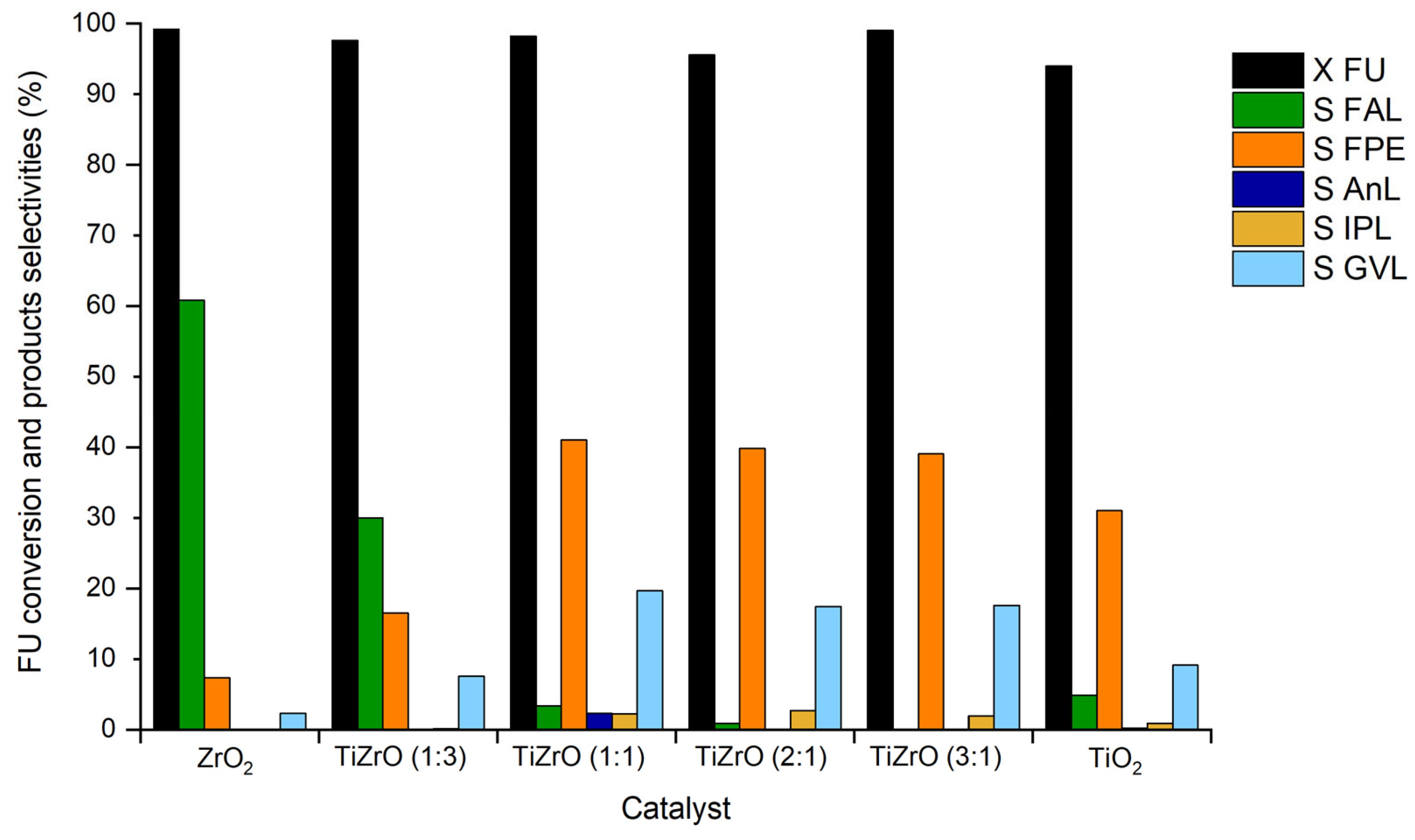
3.1. Physicochemical Properties of the Catalysts
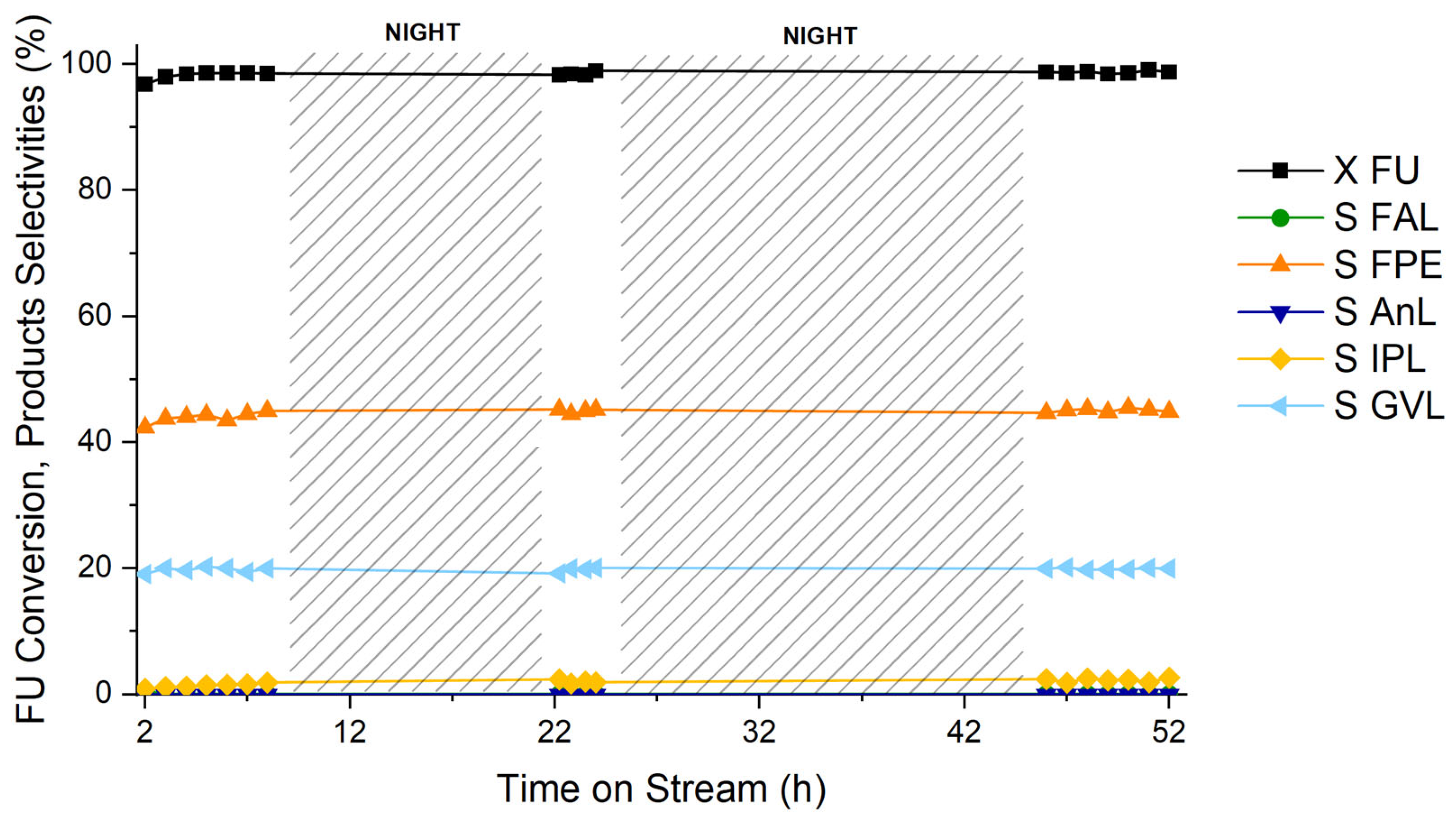
3.2. Catalytic Results
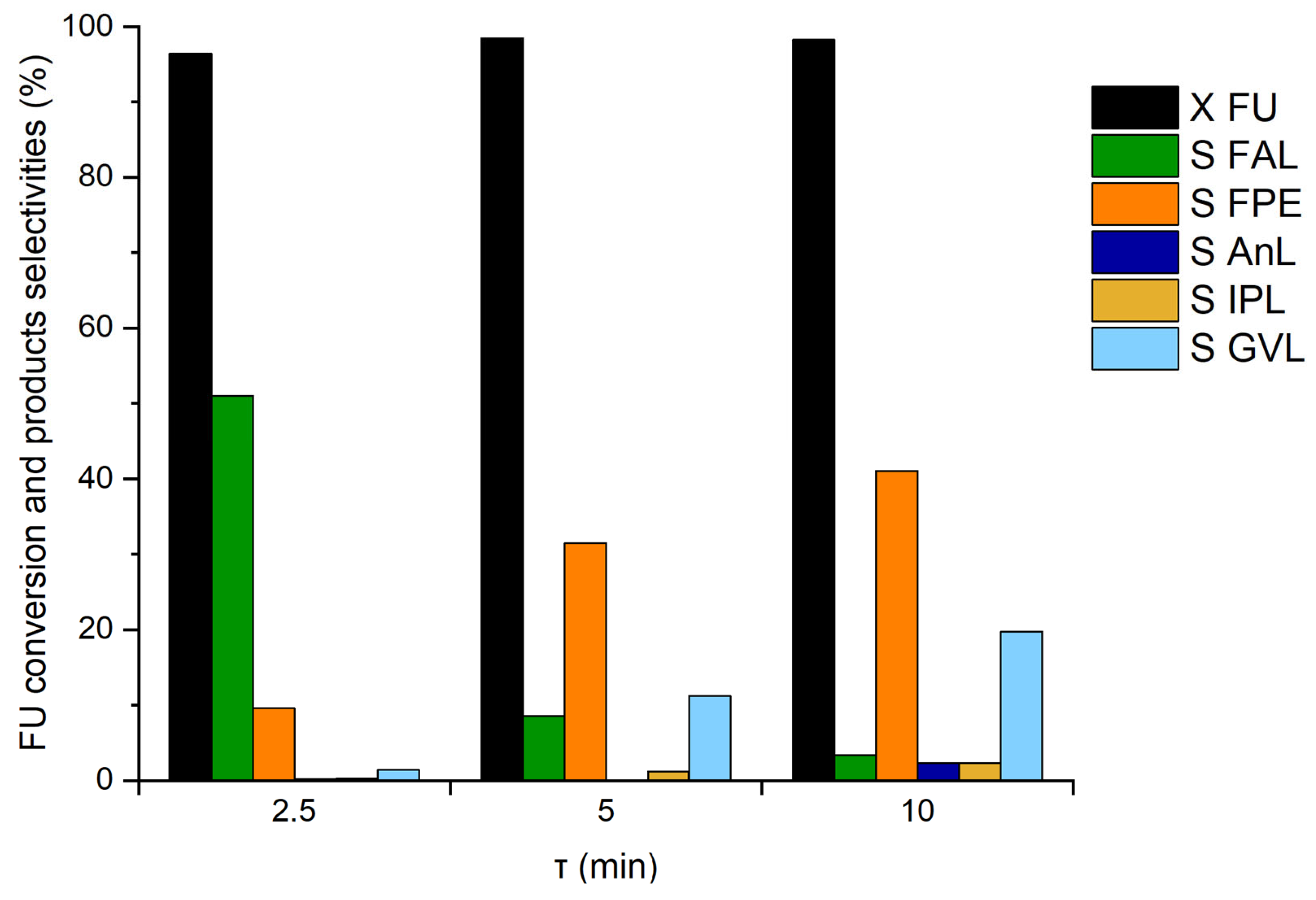
3.3. Influence of the Reaction Conditions
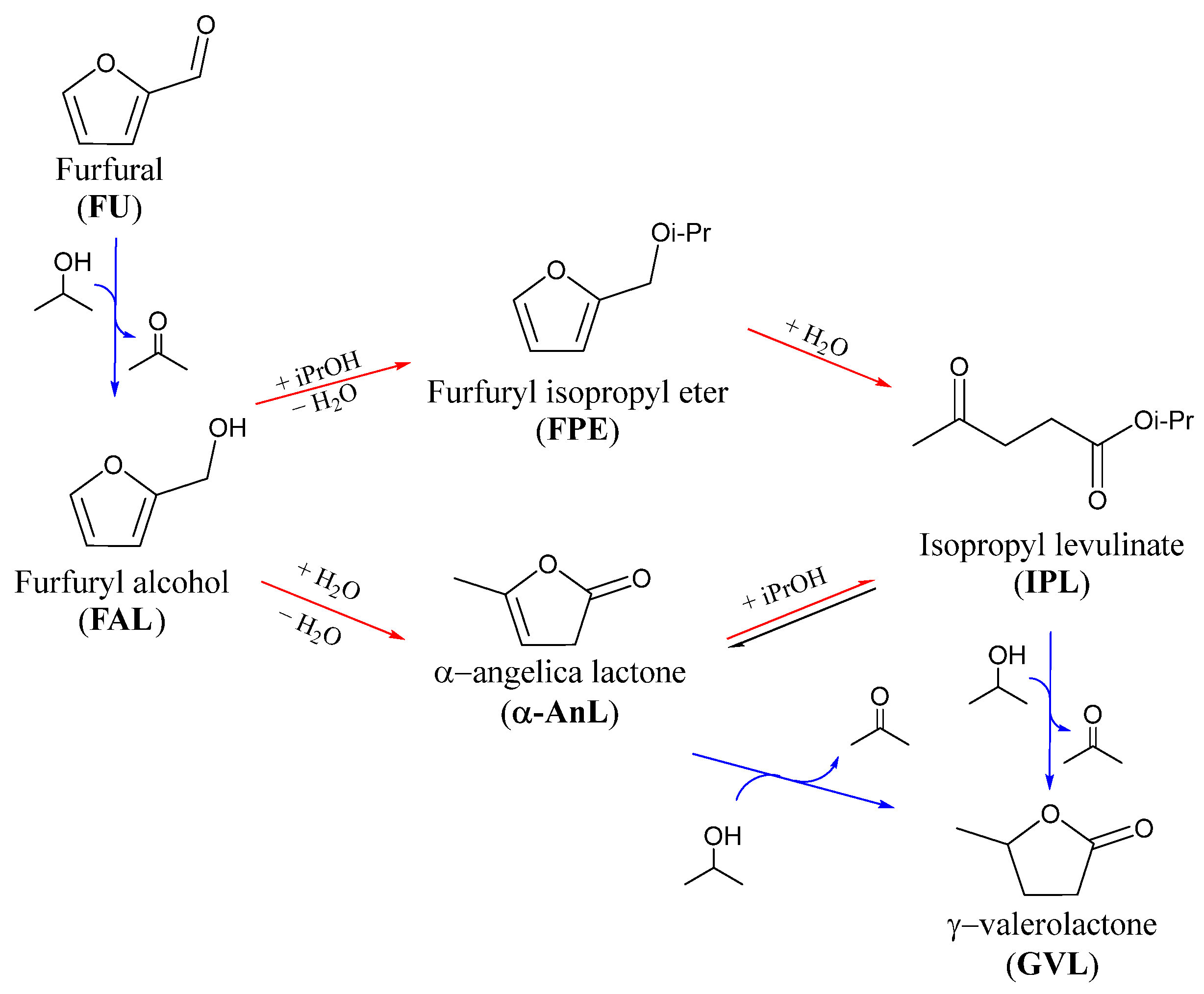
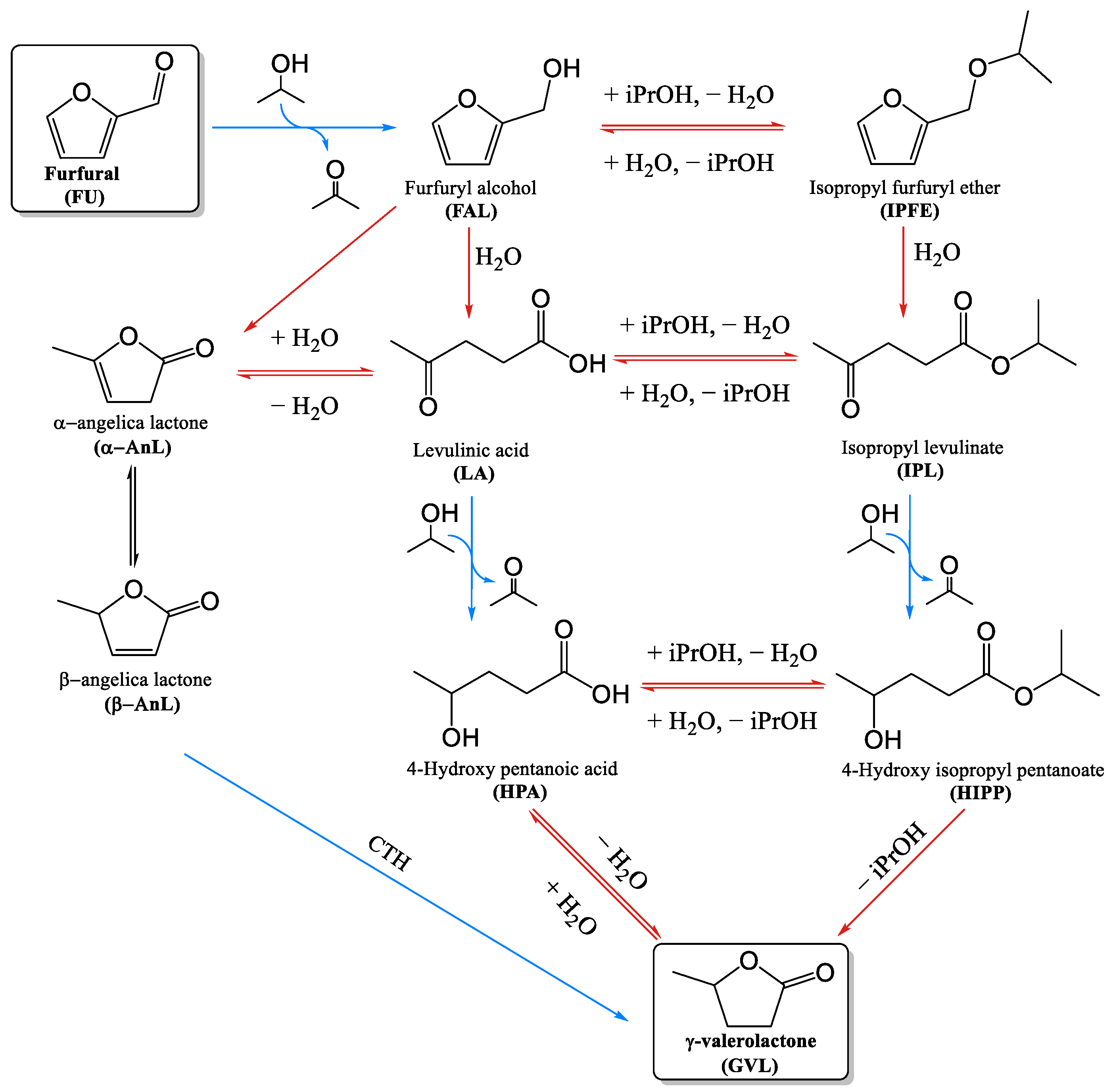
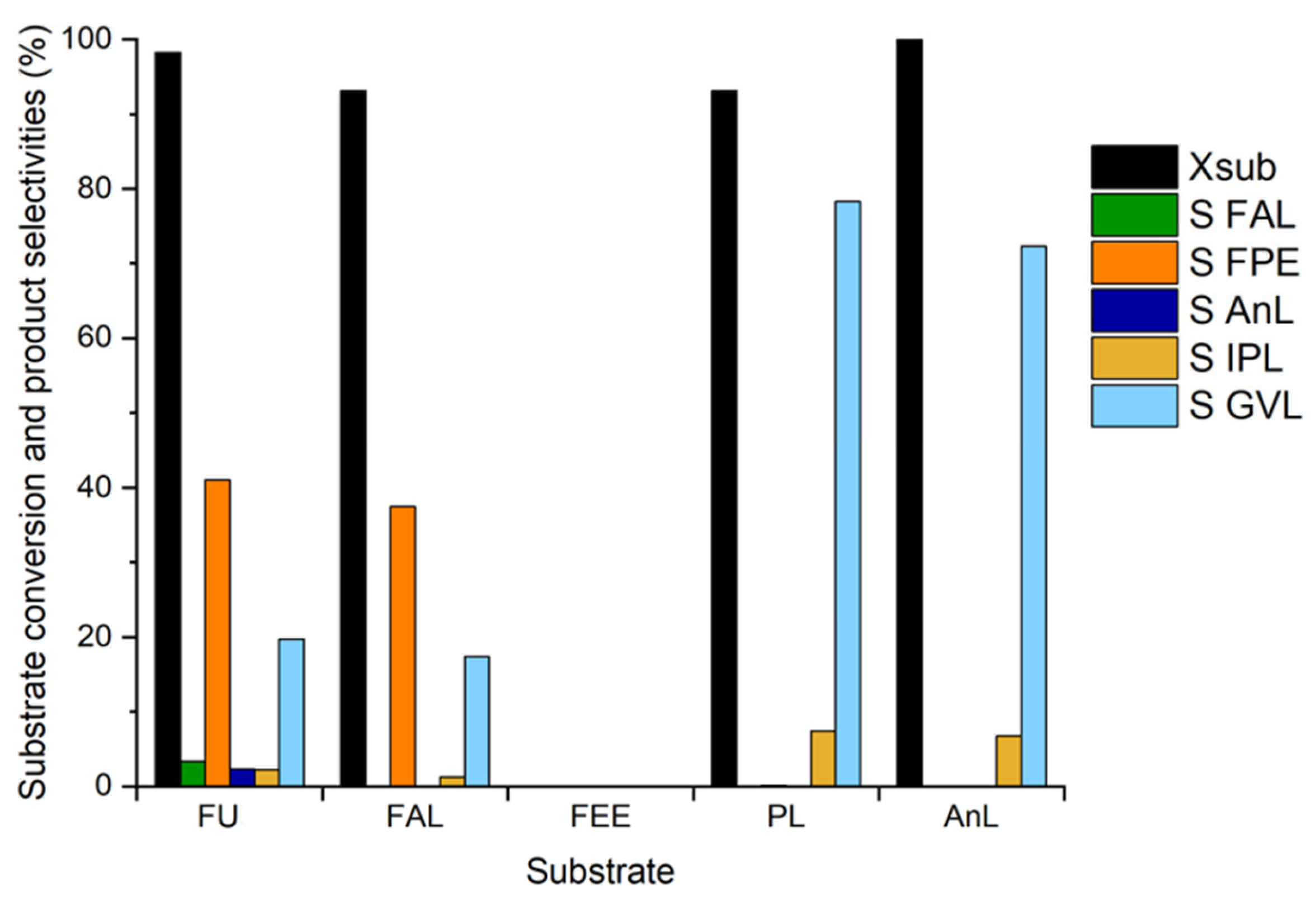
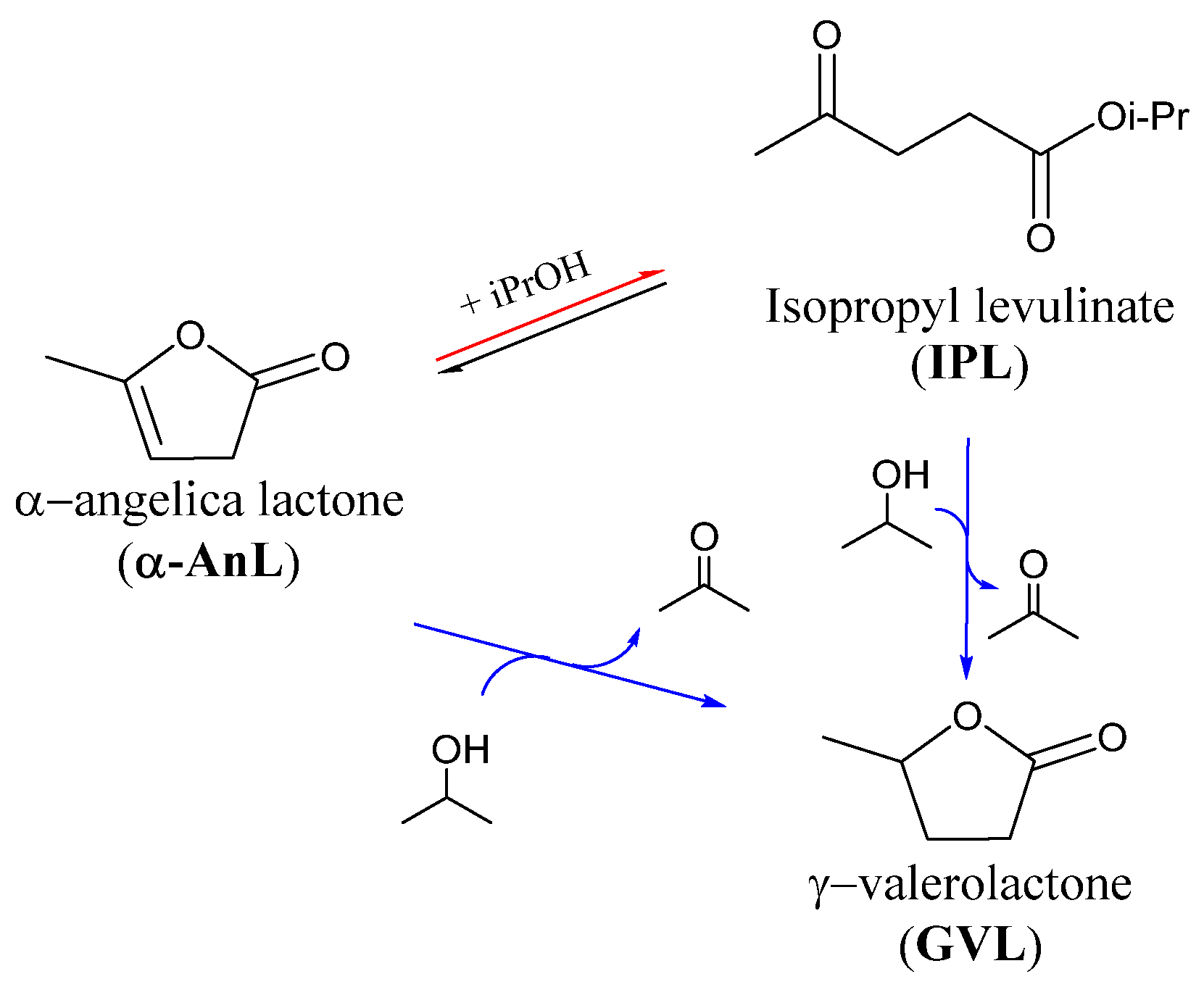
3.4. Study of the Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Asensio, R.; Cecilia, R.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Moreno-Tost, R.; Mairelles-Torres, P. Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl. Catal. A Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A Versatile, Biomass-Derived Platform Chemical for the Production of Renewable Chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Marçon, H.M.; Pastre, J.C. Continuous Flow Meerwein–Ponndorf–Verley Reduction of HMF and Furfural Using Basic Zirconium Carbonate. RSC Adv. 2022, 12, 7980–7989. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xiao, H.; Jin, Y.; Wu, S. Recent Progress in Direct Production of Furfural from Lignocellulosic Residues and Hemicellulose. Bioresour. Technol. 2022, 354, 127126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, Z.; Wu, T.Y.; Zhang, L. Furfural from Pyrolysis of Agroforestry Waste: Critical Factors for Utilisation of C5 and C6 Sugars. Renew. Sustain. Energy Rev. 2023, 176, 113194. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Liang, R.; Triantafyllidis, K.S.; Yang, W.; Len, C. Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Modified Zr-Based Catalysts Using Primary Alcohols as H-Donors. Mol. Catal. 2021, 499, 111199. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Martin Alonso, D.; Wettstein, S.G.; Dumesic, J.A. Gamma-Valerolacton, A Sustainable Platform Molecule Derived from Lignocellulosic Biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; López-Aguado, C.; Wilson, K.; Osatiashtiani, A. Efficient One-Pot Production of γ-Valerolactone from Xylose over Zr-Al-Beta Zeolite: Rational Optimization of Catalyst Synthesis and Reaction Conditions. Green Chem. 2017, 19, 5114–5121. [Google Scholar] [CrossRef]
- García, A.; Miguel, P.J.; Ventimiglia, A.; Dimitratos, N.; Solsona, B. Optimization of the Zr-loading on siliceous support catalysts leads to a suitable Lewis/Brønsted acid sites ratio to produce high yields to γ-valerolactone from furfural in one-pot. Fuel 2022, 324, 124549. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, F.; Wang, J.; Cao, L.; Han, Q. A Review on Solid Acid Catalysis for Sustainable Production of Levulinic Acid and Levulinate Esters from Biomass Derivatives. Bioresour. Technol. 2021, 342, 125977. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, H.; Lu, Y.-M.; Liu, Y.-X.; Wu, Z.-B.; Hu, D.-Y.; Yang, S. Cascade Catalytic Transfer Hydrogenation–Cyclization of Ethyl Levulinate to γ-Valerolactone with Al–Zr Mixed Oxides. Appl. Catal. Gen. 2016, 510, 11–19. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an Effective Tool for the Reductive Upgrading of Cellulose, Hemicellulose, Lignin, and Their Derived Molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- Zhang, Y.; Gyngazova, M.S.; Lolli, A.; Grazia, L.; Tabanelli, T.; Cavani, F.; Albonetti, S. Chapter 10—Hydrogen Transfer Reaction as an Alternative Reductive Process for the Valorization of Biomass-Derived Building Blocks. In Studies in Surface Science and Catalysis; Albonetti, S., Perathoner, S., Quadrelli, E.A., Eds.; Horizons in Sustainable Industrial Chemistry and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; Volume 178, pp. 195–214. [Google Scholar] [CrossRef]
- Islam, M.J.; Granollers Mesa, M.; Osatiashtiani, A.; Taylor, M.J.; Manayil, J.C.; Parlett, C.M.A.; Isaacs, M.A.; Kyriakou, G. The Effect of Metal Precursor on Copper Phase Dispersion and Nanoparticle Formation for the Catalytic Transformations of Furfural. Appl. Catal. B Environ. 2020, 273, 119062. [Google Scholar] [CrossRef]
- Assary, R.S.; Curtiss, L.A.; Dumesic, J.A. Exploring Meerwein–Ponndorf–Verley Reduction Chemistry for Biomass Catalysis Using a First-Principles Approach. ACS Catal. 2013, 3, 2694–2704. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A. Recent Advances on TiO2-ZrO2 Mixed Oxides as Catalysts and Catalyst Supports. Catal. Rev. 2005, 47, 257–296. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Yu, X.; Li, H.; Zhang, J.; He, L. Facile and High-Yield Synthesis of Alkyl Levulinate Directly from Furfural by Combining Zr-MCM-41 and Amberlyst-15 without External H2. Energy Fuels 2018, 33, 330–339. Available online: https://pubs.acs.org/doi/10.1021/acs.energyfuels.8b03422 (accessed on 22 January 2023). [CrossRef]
- Zhang, J.; Liu, Y.; Yang, S.; Wei, J.; He, L.; Peng, L.; Tang, X.; Ni, Y. Highly Selective Conversion of Furfural to Furfural Alcohol or Levulinate Ester in One Pot over ZrO2@SBA-15 and Its Kinetic Behavior. ACS Sustain. Chem. Eng. 2020, 8, 5584–5594. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Zhang, J.; Huang, R.; Wang, Y.; Cao, S.; He, L.; Peng, L. Acetic Acid-Regulated Mesoporous Zirconium-Furandicarboxylate Hybrid with High Lewis Acidity and Lewis Basicity for Efficient Conversion of Furfural to Furfuryl Alcohol. Renew. Energy 2022, 184, 115–123. [Google Scholar] [CrossRef]
- Xie, W.; Gao, C.; Li, J. Sustainable Biodiesel Production from Low-Quantity Oils Utilizing H6PV3MoW8O40 Supported on Magnetic Fe3O4/ZIF-8 Composites. Renew. Energy 2021, 168, 927–937. [Google Scholar] [CrossRef]
- Ronda-Leal, M.; Osman, S.M.; Won Jang, H.; Shokouhimehr, M.; Romero, A.A.; Luque, R. Selective Hydrogenation of Furfural Using TiO2-Fe2O3/C from Ti-Fe-MOFs as Sacrificial Template: Microwave vs. Continuous Flow Experiments. Fuel 2023, 333, 126221. [Google Scholar] [CrossRef]
- Lolli, A.; Zhang, Y.; Basile, F.; Cavani, F.; Albonetti, S. Beyond H2: Exploiting H-Transfer Reaction as a Tool for the Catalytic Reduction of Biomass. In Chemicals and Fuels from Bio-Based Building Blocks; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 349–378. [Google Scholar] [CrossRef]
- Vásquez, P.B.; Tabanelli, T.; Monti, E.; Albonetti, S.; Bonincontro, D.; Dimitratos, N.; Cavani, F. Gas-Phase Catalytic Transfer Hydrogenation of Methyl Levulinate with Ethanol over ZrO2. ACS Sustain. Chem. Eng. 2019, 7, 8317–8330. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of γ-Valerolactone from Furfural by a Single-Step Process Using Sn-Al-Beta Zeolites: Optimizing the Catalyst Acid Properties and Process Conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Hernández, B.; Iglesias, J.; Morales, G.; Paniagua, M.; López-Aguado, C.; García Fierro, J.L.; Wolf, P.; Hermans, I.; Melero, J.A. One-Pot Cascade Transformation of Xylose into γ-Valerolactone (GVL) over Bifunctional Brønsted–Lewis Zr–Al-Beta Zeolite. Green Chem. 2016, 18, 5777–5781. [Google Scholar] [CrossRef]
- López-Aguado, C.; Paniagua, M.; Melero, J.A.; Iglesias, J.; Juárez, P.; López Granados, M.; Morales, G. Stable Continuous Production of γ-Valerolactone from Biomass-Derived Levulinic Acid over Zr–Al-Beta Zeolite Catalyst. Catalysts 2020, 10, 678. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; López-Aguado, C. Rational Optimization of Reaction Conditions for the One-Pot Transformation of Furfural to γ-Valerolactone over Zr–Al-Beta Zeolite: Toward the Efficient Utilization of Biomass. Ind. Eng. Chem. Res. 2018, 57, 11592–11599. [Google Scholar] [CrossRef]
- Al Azri, N.; Patel, R.; Ozbuyukkaya, G.; Kowall, C.; Cormack, G.; Proust, N.; Enick, R.; Veser, G. Batch-to-Continuous Transition in the Specialty Chemicals Industry: Impact of Operational Differences on the Production of Dispersants. Chem. Eng. J. 2022, 445, 136775. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Reductive Amination of Levulinic Acid or Its Derivatives to Pyrrolidones over Heterogeneous Catalysts in the Batch and Continuous Flow Reactors: A Review. Renew. Sustain. Energy Rev. 2021, 143, 110876. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Komanoya, T.; Nakajima, K.; Kitano, M.; Hara, M. Synergistic Catalysis by Lewis Acid and Base Sites on ZrO2 for Meerwein–Ponndorf–Verley Reduction. J. Phys. Chem. C 2015, 119, 26540–26546. [Google Scholar] [CrossRef]
- Schiller, R.; Weiss, C.K.; Landfester, K. Phase Stability and Photocatalytic Activity of Zr-Doped Anatase Synthesized in Miniemulsion. Nanotechnology 2010, 21, 405603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Su, T.; Rodríguez-Padrón, D.; Lü, H.; Len, C.; Luque, R.; Yang, Z. Efficient Transfer Hydrogenation of Alkyl Levulinates to γ-Valerolactone Catalyzed by Simple Zr–TiO2 Metal Oxide Systems. Mater. Today Chem. 2022, 24, 100745. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Critical Design of Heterogeneous Catalysts for Biomass Valorization: Current Thrust and Emerging Prospects. Catal. Sci. Technol. 2016, 6, 7364–7385. [Google Scholar] [CrossRef]
- Wan, J.; Fu, L.; Yang, H.; Wang, K.; Xi, F.; Pan, L.; Li, Y.; Liu, Y. TiO2–ZrO2 Composite Oxide as an Acid–Base Bifunctional Catalyst for Self-Condensation of Cyclopentanone. Ind. Eng. Chem. Res. 2020, 59, 19918–19928. [Google Scholar] [CrossRef]
- Rao, K.N.; Reddy, B.M.; Park, S.-E. Novel CeO2 Promoted TiO2–ZrO2 Nano-Oxide Catalysts for Oxidative Dehydrogenation of p-Diethylbenzene Utilizing CO2 as Soft Oxidant. Appl. Catal. B Environ. 2010, 100, 472–480. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, X.; Boreriboon, N.; Song, C.; Wang, Z.; Cen, K. CO2 Hydrogenation to Methanol over Bimetallic Pd-Cu Catalysts Supported on TiO2-CeO2 and TiO2-ZrO2. Catal. Today 2021, 371, 150–161. [Google Scholar] [CrossRef]
- Reddy, B.M.; Lee, S.-C.; Han, D.-S.; Park, S.-E. Utilization of Carbon Dioxide as Soft Oxidant for Oxydehydrogenation of Ethylbenzene to Styrene over V2O5–CeO2/TiO2–ZrO2 Catalyst. Appl. Catal. B Environ. 2009, 87, 230–238. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Viswanathan, B. Effect of Surface Area, Pore Volume and Particle Size of P25 Titania on the Phase Transformation of Anatase to Rutile. Indian J. Chem. 2009, 48, 1378–1382. [Google Scholar]
- Watanabe, S.; Ma, X.; Song, C. Characterization of Structural and Surface Properties of Nanocrystalline TiO2−CeO2 Mixed Oxides by XRD, XPS, TPR, and TPD. J. Phys. Chem. C 2009, 113, 14249–14257. Available online: https://pubs.acs.org/doi/10.1021/jp8110309# (accessed on 24 January 2023). [CrossRef]
- Lu, M.; Du, H.; Wei, B.; Zhu, J.; Li, M.; Shan, Y.; Shen, J.; Song, C. Hydrodeoxygenation of Guaiacol on Ru Catalysts: Influence of TiO2–ZrO2 Composite Oxide Supports. Ind. Eng. Chem. Res. 2017, 56, 12070–12079. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S. Synthesis of Nano-Particles of Anatase-TiO2 and Preparation of Its Optically Transparent Film in PVA. Mater. Lett. 2007, 61, 4725–4730. [Google Scholar] [CrossRef]
- Tajima, M.; Niwa, M.; Fujii, Y.; Koinuma, Y.; Aizawa, R.; Kushiyama, S.; Kobayashi, S.; Mizuno, K.; Ohuchi, H. Decomposition of Chlorofluorocarbons on TiO2ZrO2. Appl. Catal. B Environ. 1997, 12, 263–276. [Google Scholar] [CrossRef]
- Manríquez, M.E.; López, T.; Gómez, R.; Navarrete, J. Preparation of TiO2–ZrO2 Mixed Oxides with Controlled Acid–Basic Properties. J. Mol. Catal. Chem. 2004, 220, 229–237. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Roslan, I.I.; Jaenicke, S.; Chuah, G.-K. A Combo Zr-HY and Al-HY Zeolite Catalysts for the One-Pot Cascade Transformation of Biomass-Derived Furfural to γ-Valerolactone. J. Catal. 2019, 375, 56–67. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.F.; Wilson, K. ZrO2-SBA-15 Catalysts for the One-Pot Cascade Synthesis of GVL from Furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef]
- García, A.; Miguel, P.J.; Pico, M.P.; Álvarez-Serrano, I.; López, M.L.; García, T.; Solsona, B. γ-Valerolactone from Levulinic Acid and Its Esters: Substrate and Reaction Media Determine the Optimal Catalyst. Appl. Catal. Gen. 2021, 623, 118276. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kelbichová, V.; Vitvarová, D.; Kubů, M.; Kubička, D. Aldol Condensation of Furfural and Acetone on Zeolites. Catal. Today 2014, 227, 154–162. [Google Scholar] [CrossRef]
- García-Olmo, A.; Yepez, A.; Balu, A.; Romero, A.; Luque, R. Insights into the Activity, Selectivity and Stability of Heterogeneous Catalysts in the Continuous Flow Hydroconversion of Furfural to Valuable Products. Catal. Sci. Technol. 2016, 6, 4705–4711. [Google Scholar] [CrossRef]



| Sample | Ti Atomic Content (%) | Zr Atomic Content (%) | BET SSA (m2/g) | Acid Site Density (mmol/g) | Tmax NH3 Desorption (°C) |
|---|---|---|---|---|---|
| ZrO2 | 0 | 100 | 250 | 0.49 | 291 |
| Ti/Zr/O (1:3) | 27.8 | 72.2 | 268 | 0.51 | 278 |
| Ti/Zr/O (1:1) | 46.5 | 53.5 | 279 | 0.67 | 237 |
| Ti/Zr/O (2:1) | 67 | 33 | 287 | 0.54 | 267 |
| Ti/Zr/O (3:1) | 74.5 | 25.5 | 330 | 0.62 | 263 |
| TiO2 | 100 | 0 | 60 | 0.25 | 367 |
| Sample | BET SSA (m2/g) | Vpores (m3/g) | Dpores (nm) | |||
|---|---|---|---|---|---|---|
| Fresh | Spent | Fresh | Spent | Fresh | Spent | |
| ZrO2 | 279 | 248 | 0.37 | 0.31 | 4.6 | 4.3 |
| Ti/Zr/O (1:3) | 268 | 184 | 0.62 | 0.31 | 8.5 | 6.1 |
| Ti/Zr/O (1:1) | 279 | 220 | 0.66 | 0.34 | 8.4 | 5.6 |
| Ti/Zr/O (2:1) | 287 | 213 | 0.81 | 0.39 | 9.9 | 6.4 |
| Ti/Zr/O (3:1) | 330 | 190 | 0.56 | 0.28 | 5.8 | 5.0 |
| TiO2 | 78 | 61 | 0.16 | 0.11 | 7.0 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saotta, A.; Allegri, A.; Liuzzi, F.; Fornasari, G.; Dimitratos, N.; Albonetti, S. Ti/Zr/O Mixed Oxides for the Catalytic Transfer Hydrogenation of Furfural to GVL in a Liquid-Phase Continuous-Flow Reactor. ChemEngineering 2023, 7, 23. https://doi.org/10.3390/chemengineering7020023
Saotta A, Allegri A, Liuzzi F, Fornasari G, Dimitratos N, Albonetti S. Ti/Zr/O Mixed Oxides for the Catalytic Transfer Hydrogenation of Furfural to GVL in a Liquid-Phase Continuous-Flow Reactor. ChemEngineering. 2023; 7(2):23. https://doi.org/10.3390/chemengineering7020023
Chicago/Turabian StyleSaotta, Anna, Alessandro Allegri, Francesca Liuzzi, Giuseppe Fornasari, Nikolaos Dimitratos, and Stefania Albonetti. 2023. "Ti/Zr/O Mixed Oxides for the Catalytic Transfer Hydrogenation of Furfural to GVL in a Liquid-Phase Continuous-Flow Reactor" ChemEngineering 7, no. 2: 23. https://doi.org/10.3390/chemengineering7020023
APA StyleSaotta, A., Allegri, A., Liuzzi, F., Fornasari, G., Dimitratos, N., & Albonetti, S. (2023). Ti/Zr/O Mixed Oxides for the Catalytic Transfer Hydrogenation of Furfural to GVL in a Liquid-Phase Continuous-Flow Reactor. ChemEngineering, 7(2), 23. https://doi.org/10.3390/chemengineering7020023










