Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating
Abstract
1. Introduction
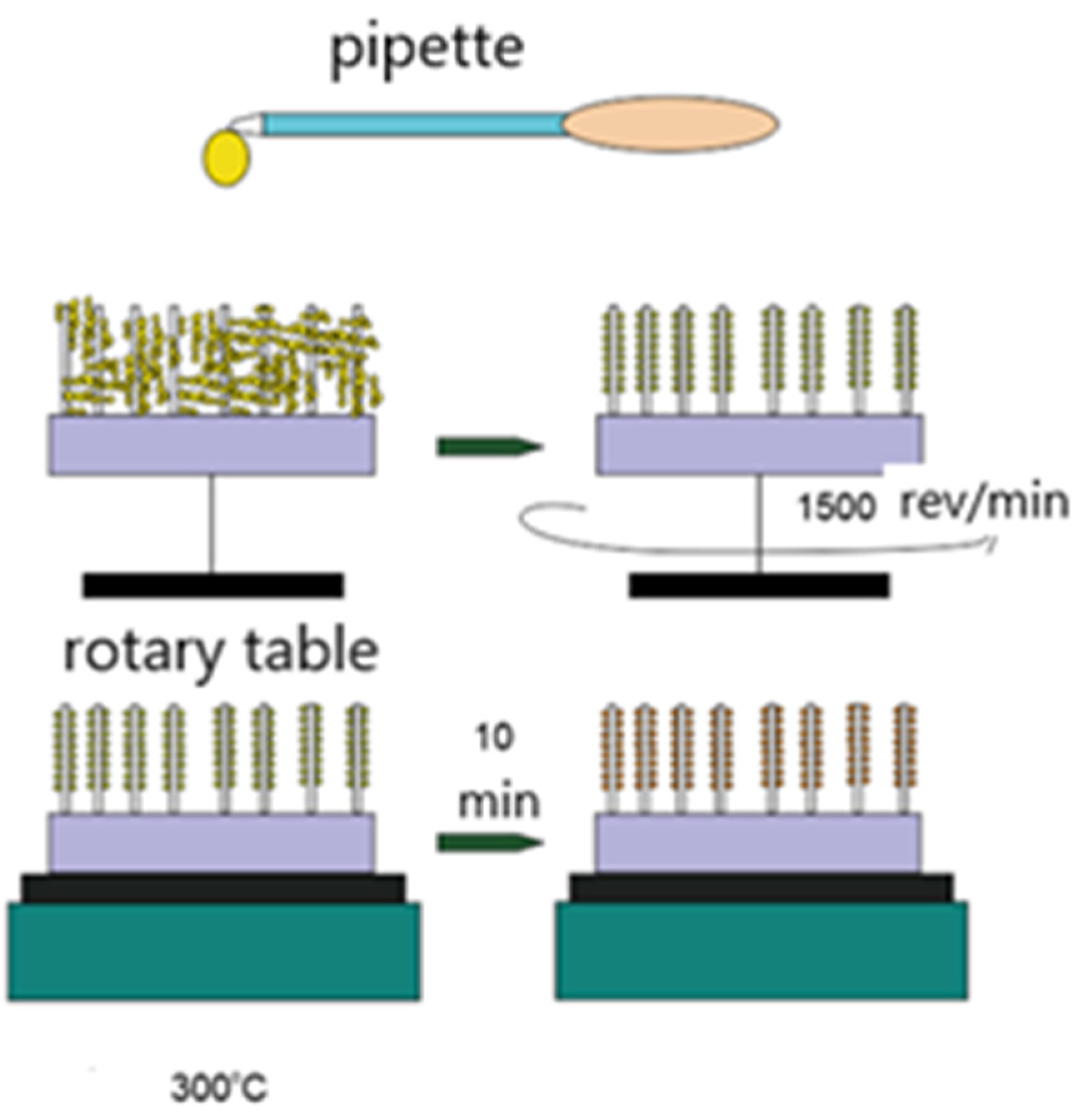
2. Materials and Methods
3. Results and Discussion
3.1. Sample Characterization
3.2. Photocatalytic Activity
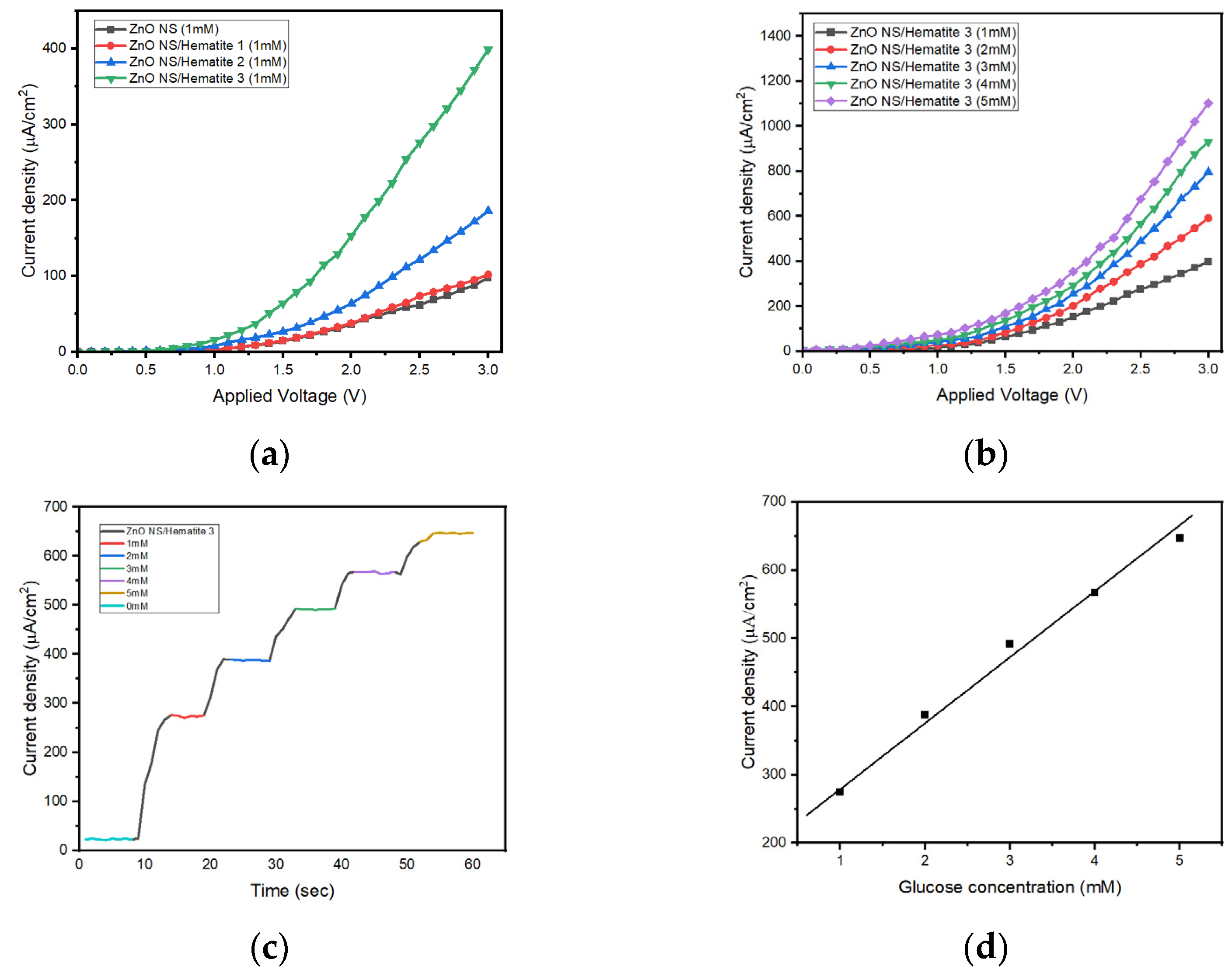
3.3. Bio-Sensing of Glucose Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanez-Sedeno, P.; Gonzalez-Cortes, A.; Campuzano, S.; Pingarron, J.M. Multimodal/Multifunctional Nanomaterials in (Bio)electrochemistry: Now and in the Coming Decade. Nanomaterials 2020, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Huang, D.H.; Rigby, K.; Chu, C.H.; Wu, X.H.; Kim, J.H. Environmental Materials beyond and below the Nanoscale: Single-Atom Catalysts. ACS EsT Eng. 2021, 1, 157–172. [Google Scholar] [CrossRef]
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W.S. Treatment of wastewater from petroleum industry: Current practices and perspectives. Environ. Sci. Pollut. Res. 2020, 27, 27172–27180. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.S. Effect of humidity on the photocatalytic degradation of gaseous hydrocarbons mixture. Mater. Chem. Phys. 2017, 197, 1–9. [Google Scholar] [CrossRef]
- Tian, L.; Yang, X.F.; Cui, X.K.; Liu, Q.Q.; Tang, H. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance. Appl. Surf. Sci. 2019, 463, 9–17. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Pan, F.; Hao, W.; Ge, Q.; Wang, T.M. Light-storing photocatalyst. Appl. Phys. Lett. 2004, 85, 5778–5780. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Sun, S.D.; Yu, X.J.; Yang, Q.; Yang, Z.M.; Liang, S.H. Mesocrystals for photocatalysis: A comprehensive review on synthesis engineering and functional modifications. Nanoscale Adv. 2019, 1, 34–63. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Vattikuti, S.V.P.; Devarayapalli, K.C.; Yoo, K.; Shim, J.; Sreekanth, T.V.M. Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Han, Z.Z.; Ren, L.L.; Cui, Z.H.; Chen, C.Q.; Pan, H.B.; Chen, J.Z. Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl. Catal. B Environ. 2012, 126, 298–305. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. a-Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Chen, R.; Zhou, D.; Xiang, L. A Review on the Fabrication of Hierarchical ZnO Nanostructures for Photocatalysis Application. Crystals 2016, 6, 148. [Google Scholar] [CrossRef]
- Becheri, A.; Durr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc-Oxide varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Nanto, H.; Minami, T.; Takata, S. Zinc-Oxide thin-film ammonia gas sensors with high-sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, Z.; Yan, X.Q.; Liao, Q.L. ZnO nanostructures in enzyme biosensors. Sci. China Mater. 2015, 58, 60–76. [Google Scholar] [CrossRef]
- Yuan, M.; Li, J.; Yu, Y.; Fu, Y.; Fong, A.; Hu, J. Fabrication of a Fe2O3 Nanoparticles Implantation-modified Electrode and its Applications in Electrochemical Sensing. Electroanalysis 2016, 28, 954–961. [Google Scholar] [CrossRef]
- Riha, S.C.; Vermeer, M.J.D.; Pellin, M.J.; Hupp, J.T.; Martinson, A.B.F. Hematite-based Photo-oxidation of Water Using Transparent Distributed Current Collectors. Acs Appl. Mater. Interfaces 2013, 5, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Yin, P.; Zhu, X.; OuYang, C.Z.; Xie, Y. Synthesis of hematite (alpha-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Glasscock, J.A.; Barnes, P.R.F.; Plumb, I.C.; Bendavid, A.; Martin, P.J. Structural, optical and electrical properties of undoped polycrystalline hematite thin films produced using filtered arc deposition. Thin Solid Films 2008, 516, 1716–1724. [Google Scholar] [CrossRef]
- Hu, Y.S.; Kleiman-Shwarsctein, A.; Forman, A.J.; Hazen, D.; Park, J.N.; McFarland, E.W. Pt-doped alpha-Fe2O3 thin films active for photoelectrochemical water splitting. Chem. Mater. 2008, 20, 3803–3805. [Google Scholar] [CrossRef]
- Bjorksten, U.; Moser, J.; Gratzel, M. Photoelectrochemical Studies on Nanocrystalline Hematite Films. Chem. Mater. 1994, 6, 858–863. [Google Scholar] [CrossRef]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M.; Merkle, M.G.; Dahmen, U.; Wang, L.W.; Alivisatos, A.P. Selective Facet Reactivity during Cation Exchange in Cadmium Sulfide Nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef]
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Graetzel, M. Influence of Feature Size, Film Thickness, and Silicon Doping on the Performance of Nanostructured Hematite Photoanodes for Solar Water Splitting. J. Phys. Chem. C 2009, 113, 772–782. [Google Scholar] [CrossRef]
- Zhang, F.J.; Song, N.N.; Zhang, S.Y.; Zou, S.; Zhong, S. Synthesis of sponge-loaded Bi2WO6/ZnFe2O4 magnetic photocatalyst and application in continuous flow photocatalytic reactor. J. Mater. Sci. Mater. Electron. 2017, 28, 8197–8205. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chang, J.S.; Lee, D.J. Synthesis of a novel solid mediator Z-scheme heterojunction photocatalysis CuFe2O4/Cu/UiO-66-NH2 for oxidation of dye in water. Chemosphere 2022, 296, 134080. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Narayanan, N.; Harish, S.K.; Hayakawa, Y.; Lee, B.K.; Neppolian, B. Environmentally Sustainable Synthesis of CoFe2O4-TiO2/rGO Ternary Photocatalyst: A Highly Efficient and Stable Photocatalyst for High Production of Hydrogen (Solar Fuel) (vol 4, pg 880, 2019). Acs Omega 2019, 4, 2980. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Chen, Y.C.; Lin, Y.G. Novel ZnO/Fe2O3 Core-Shell Nanowires for Photoelectrochemical Water Splitting. Acs Appl. Mater. Interfaces 2015, 7, 14157–14162. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.S.; Zhang, L.; Zhang, J.J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, Y.-S.; Ho, S.-C.; Lee, Y.-J. Investigation of a Photoelectrochemical Passivated ZnO-Based Glucose Biosensor. Sensors 2011, 11, 4648–4655. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakranova, D.; Seitov, B.; Bakranov, N. Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating. ChemEngineering 2023, 7, 22. https://doi.org/10.3390/chemengineering7020022
Bakranova D, Seitov B, Bakranov N. Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating. ChemEngineering. 2023; 7(2):22. https://doi.org/10.3390/chemengineering7020022
Chicago/Turabian StyleBakranova, Dina, Bekbolat Seitov, and Nurlan Bakranov. 2023. "Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating" ChemEngineering 7, no. 2: 22. https://doi.org/10.3390/chemengineering7020022
APA StyleBakranova, D., Seitov, B., & Bakranov, N. (2023). Photocatalytic and Glucose Sensing Properties of ZnO-Based Nanocoating. ChemEngineering, 7(2), 22. https://doi.org/10.3390/chemengineering7020022






