Graphite/β-PbO2 Composite Inert Anode Synthesis Using Electrochemical Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Material Synthesis
2.3. Material Characteristics
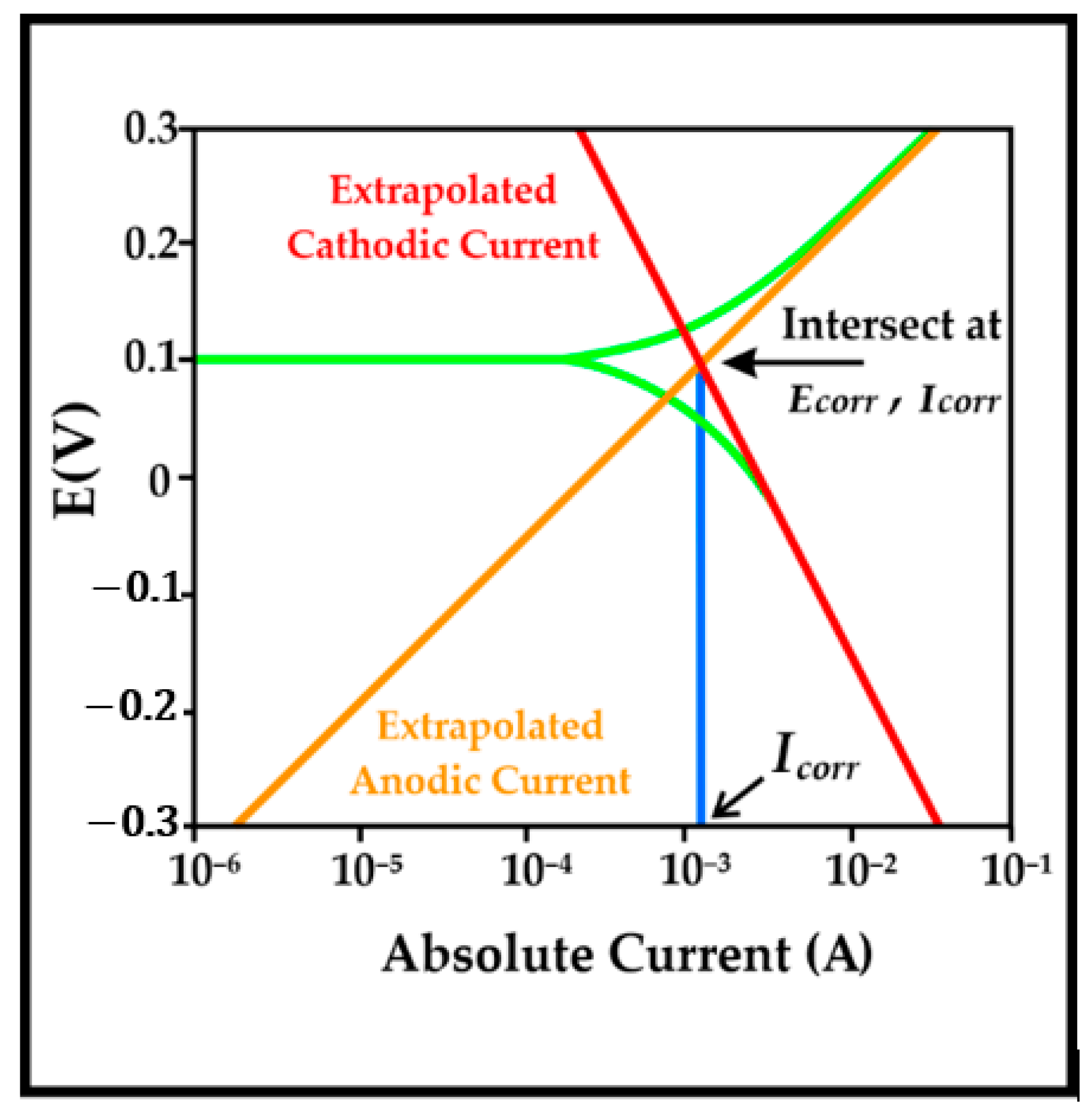
2.3.1. Potentiodynamic Polarization
2.3.2. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy
3. Results
3.1. Potentiodynamic Polarization Test
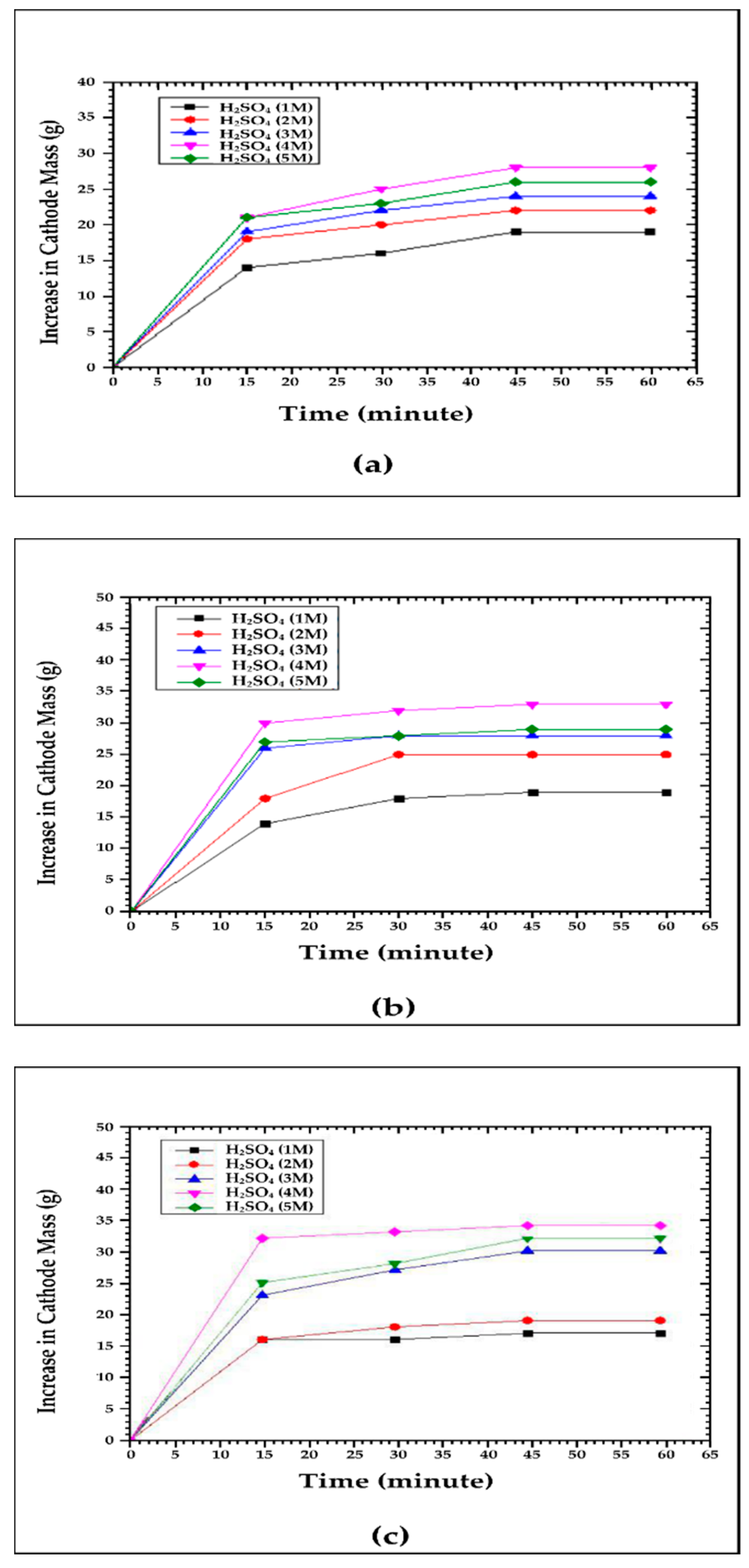
3.2. Effect of Voltage and Sulfuric Acid Concentration on Cathode Mass Increase
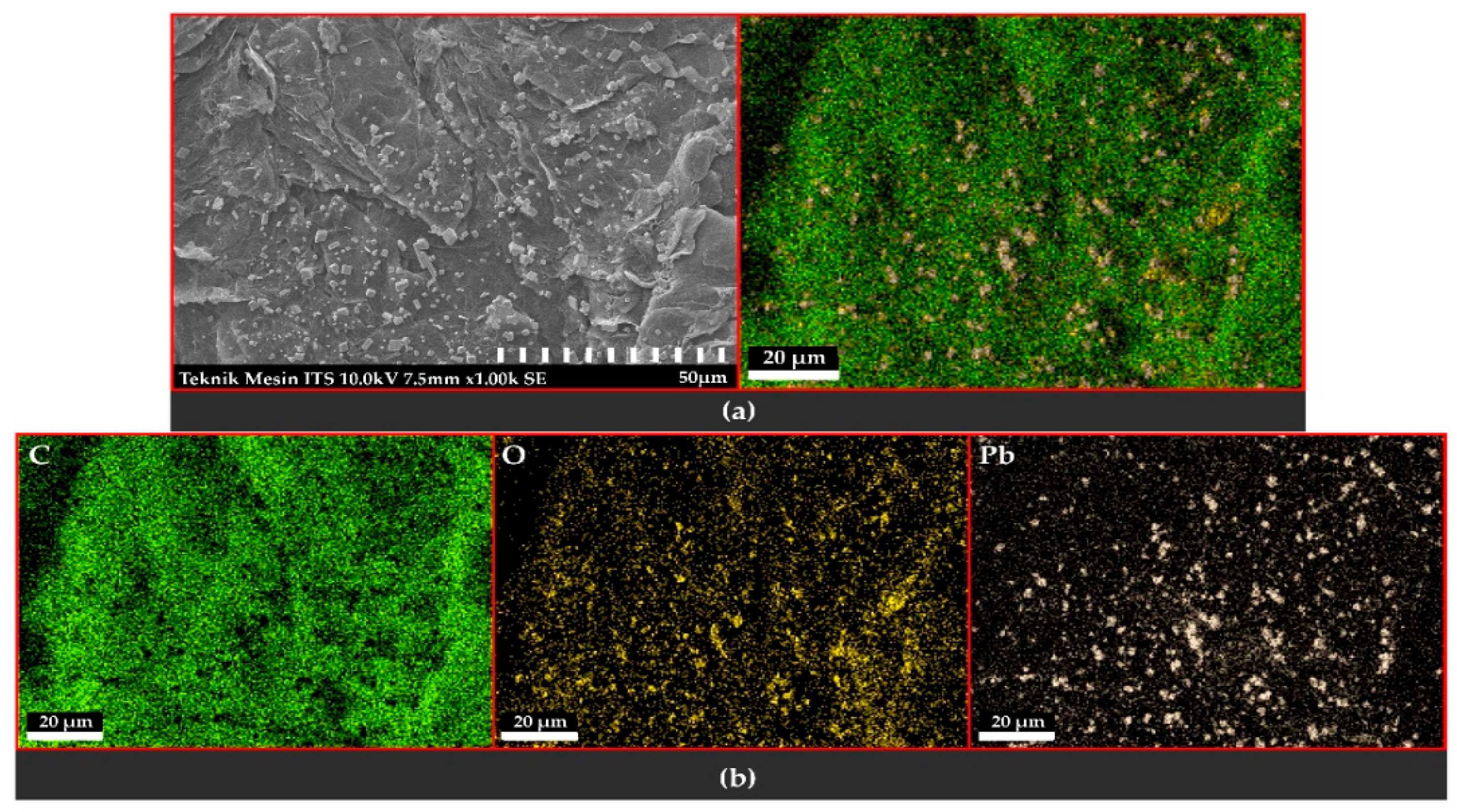
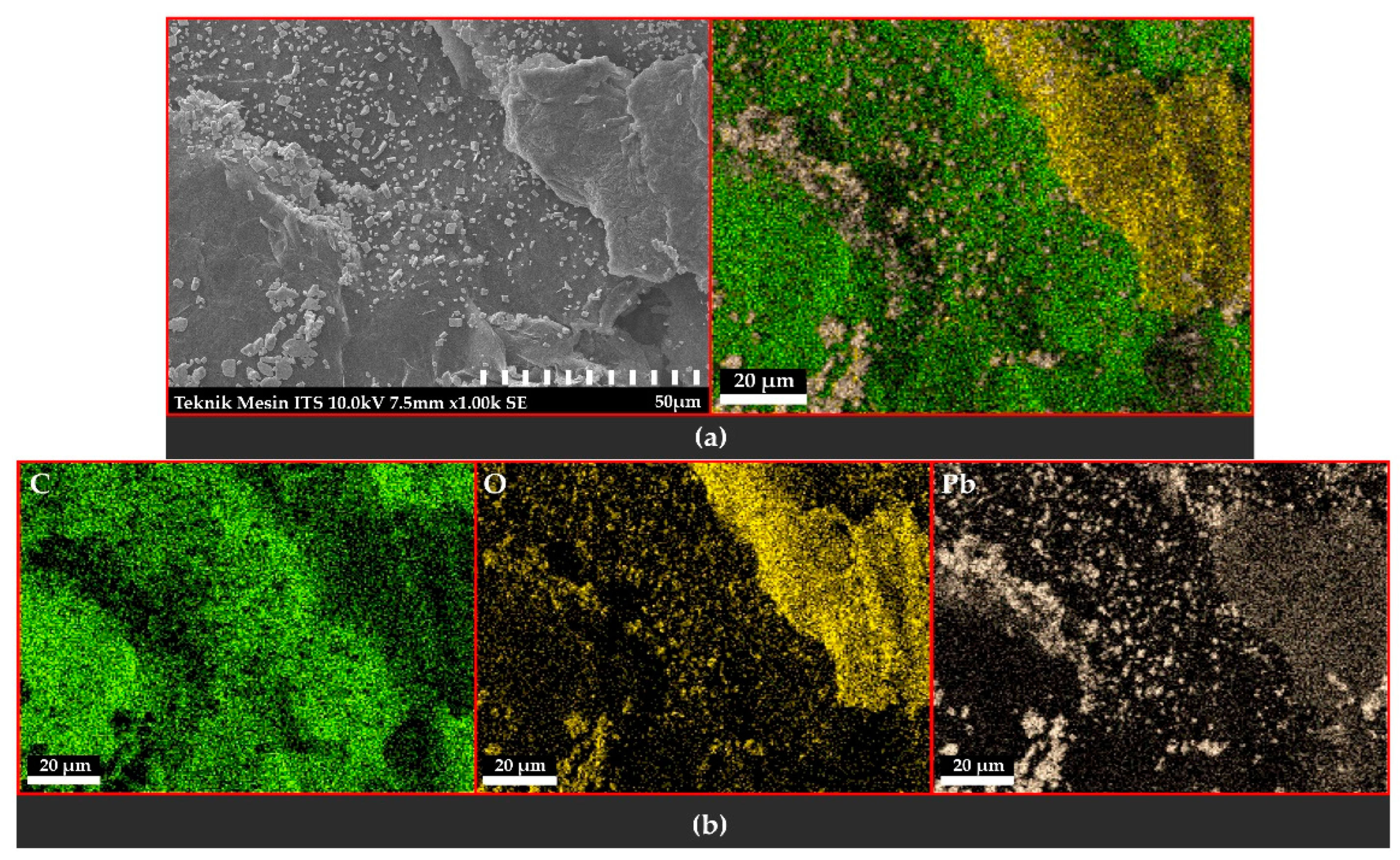
3.3. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy
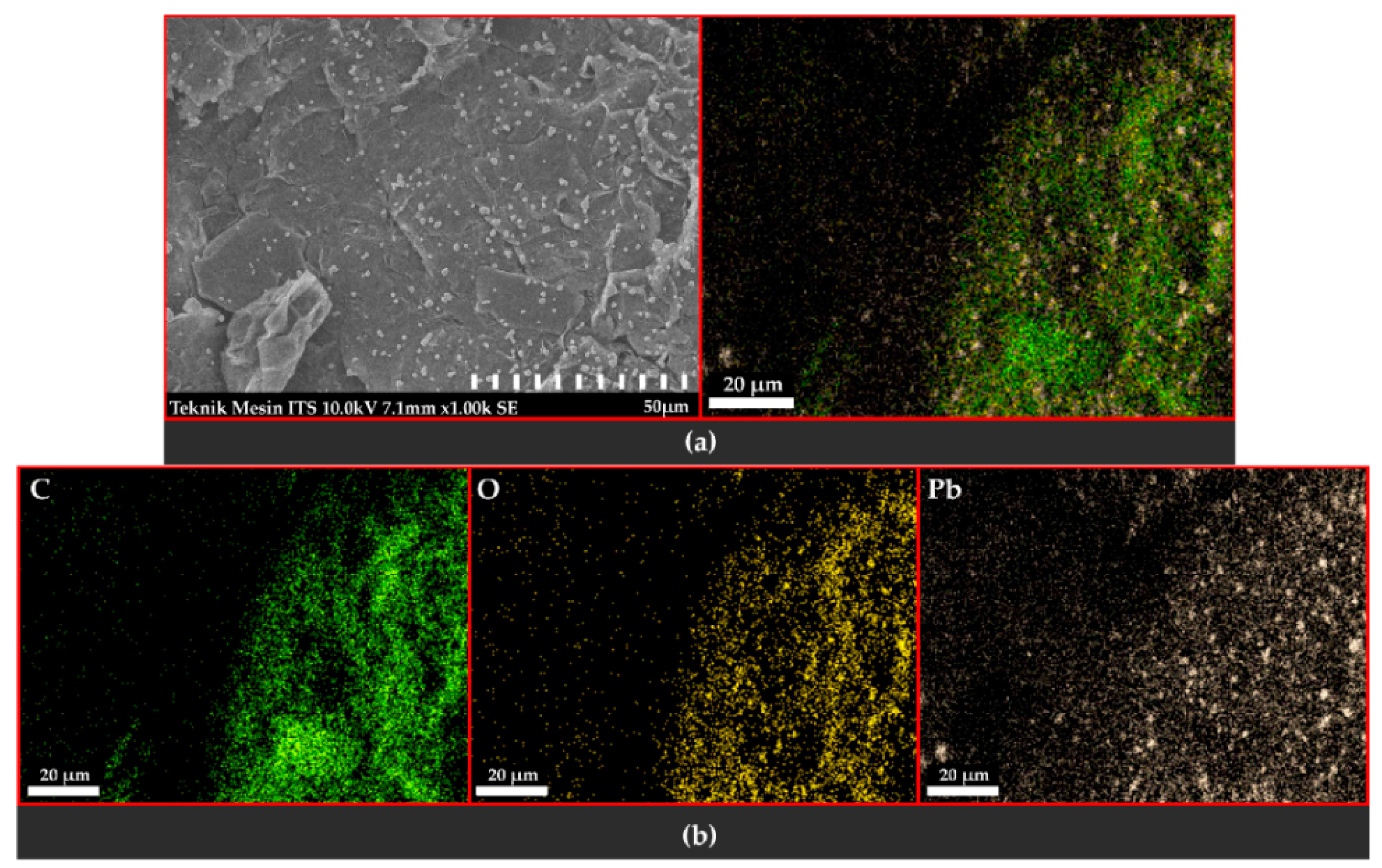
3.3.1. Observation of the Topography and Morphology of the Sample
3.3.2. Distribution and Elemental Composition
Voltage of 6 V
Voltage of 9 V
Voltage of 12 V
4. Discussion
4.1. Potentiodynamic Polarization
4.2. Effect of Voltage and Sulfuric Acid Concentration on Cathode Mass Increase
4.3. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moskalyk, R.; Alfantazi, A.; Tombalakian, A.; Valic, D. Anode effects in electrowinning. Miner. Eng. 1999, 12, 65–73. [Google Scholar] [CrossRef]
- Dobrev, T.; Valchanova, I.; Stefanov, Y.; Magaeva, S. Investigations of new anodic materials for zinc electrowinning. Trans. IMF 2009, 87, 136–140. [Google Scholar] [CrossRef]
- Zhan, P.; Xu, R.-D.; Huang, L.-P.; Chen, B.-M.; Zhou, J.-F. Effects of polyaniline on electrochemical properties of composite inert anodes used in zinc electrowinning. Trans. Nonferrous Met. Soc. China 2012, 22, 1693–1700. [Google Scholar] [CrossRef]
- Kumar, R.S.; Karthikeyan, S.; Ramakrishnan, S.; Vijayapradeep, S.; Kim, A.R.; Kim, J.-S.; Yoo, D.J. Anion dependency of spinel type cobalt catalysts for efficient overall water splitting in an acid medium. Chem. Eng. J. 2023, 451, 138471. [Google Scholar] [CrossRef]
- Hu, G.; Xu, R.-D.; He, S.-W.; Chen, B.-M.; Yang, H.-T.; Yu, B.-H.; Liu, Q. Electrosynthesis of Al/Pb/α-PbO2 composite inert anode materials. Trans. Nonferrous Met. Soc. China 2015, 25, 2095–2102. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, R.; Yu, B.; He, Y.; Li, Y.; Qin, Z. Study on the properties of Pb–Co3O4–PbO2 composite inert anodes prepared by vacuum hot pressing technique. RSC Adv. 2017, 7, 49166–49176. [Google Scholar] [CrossRef]
- He, S.; Xu, R.; Hu, G.; Chen, B. Study on the Electrosynthesis of Pb-0.3%Ag/α-PbO2 Composite Inert Anode Materials. Electrochemistry 2015, 83, 974–978. [Google Scholar] [CrossRef]
- Gedam, N.; Neti, N.R.; Kormunda, M.; Subrt, J.; Bakardjieva, S. Novel Lead dioxide-Graphite-Polymer composite anode for electrochemical chlorine generation. Electrochimica Acta 2015, 169, 109–116. [Google Scholar] [CrossRef]
- Hakimi, F.; Rashchi, F.; Dolati, A.; Astaraei, F.R. Anodizing Pb Electrode for Synthesis of β-PbO2 Nanoparticles: Optimization of Electrochemical Parameters. J. Electrochem. Soc. 2019, 166, D617–D625. [Google Scholar] [CrossRef]
- Mandal, P.; Gupta, A.K.; Dubey, B.K. A novel approach towards multivariate optimization of graphite/PbO2 anode synthesis conditions: Insight into its enhanced oxidation ability and physicochemical characteristics. J. Environ. Chem. Eng. 2018, 6, 4438–4451. [Google Scholar] [CrossRef]
- Poll, C.G.; Payne, D.J. Electrochemical Synthesis of PbO2, Pb3O4 and PbO Films on a Transparent Conducting Substrate. Electrochimica Acta 2015, 156, 283–288. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Chen, B. Effect of Ce(NO3)4 on the electrochemical properties of Ti/PbO2–TiO2–Ce(NO3)4 electrode for zinc electrowinning. Appl. Phys. A 2019, 125, 150. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Feng, S.; Chen, C.; Chen, B. Facile one-step synthesis of a Co3O4- and CNT-doped 3D-Ti/PbO2 electrode with a high surface for zinc electrowinning. Hydrometallurgy 2020, 199, 105529. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Chen, B. Effect of CeO2 and graphite powder on the electrochemical performance of Ti/PbO2 anode for zinc electrowinning. Ceram. Int. 2018, 44, 19735–19742. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Zhang, K.; Rossi, C.; Rodriguez, G.A.A.; Tenailleau, C.; Alphonse, P. Development of a nano-Al∕CuO based energetic material on silicon substrate. Appl. Phys. Lett. 2007, 91, 113117. [Google Scholar] [CrossRef]
- Khachatryan, H.; Lee, S.-N.; Kim, K.-B.; Kim, H.-K.; Kim, M. Al thin film: The effect of substrate type on Al film formation and morphology. J. Phys. Chem. Solids 2018, 122, 109–117. [Google Scholar] [CrossRef]
- He, G.; Lu, S.X.; Xu, W.; Ye, P.; Liu, G.; Wang, H.; Dai, T. Stable superhydrophobic Zn/ZnO surfaces fabricated via electrodeposition on tin substrate for self-cleaning behavior and switchable wettability. J. Alloys Compd. 2018, 747, 772–782. [Google Scholar] [CrossRef]
- Yue, W.; Ding, C.; Qin, H.; Gong, C.; Zhang, J. Crystallographic Characteristic Effect of Cu Substrate on Serrated Cathode Dissolution in Cu/Sn–3.0Ag–0.5Cu/Cu Solder Joints during Electromigration. Materials 2021, 14, 2486. [Google Scholar] [CrossRef]
- Broda, B.; Inzelt, G. Microgravimetric study of electrodeposition and dissolution of lead dioxide on gold and platinum substrates. J. Solid State Electrochem. 2018, 22, 3921–3931. [Google Scholar] [CrossRef]
- Omar, N.I.; Selvami, S.; Kaisho, M.; Yamada, M.; Yasui, T.; Fukumoto, M. Deposition of Titanium Dioxide Coating by the Cold-Spray Process on Annealed Stainless Steel Substrate. Coatings 2020, 10, 991. [Google Scholar] [CrossRef]
- Abedini, A.; Valmoozi, A.A.E.; Afghahi, S.S.S. Anodized graphite as an advanced substrate for electrodeposition of PbO2. Mater. Today Commun. 2022, 31, 103464. [Google Scholar] [CrossRef]
- Li, H. Electrocatalytic Activity of Ti/Al/Ti/PbO2-WC Rod Composite Electrodes During Zinc Electrowinning. Int. J. Electrochem. Sci. 2018, 13, 4367–4378. [Google Scholar] [CrossRef]
- Yu, B.; Xu, R.; He, S.; Qin, Z.; Wang, W. Preparations and Performances Testing of α/β-PbO2 Phase Compositions Prepared in Methanesulfonic Acid in Order to Provide More Appropriate Environmentally Sustainable Electrodes. Electrochemistry 2019, 87, 197–203. [Google Scholar] [CrossRef]
- Zheng, S.; Jia, G.; Xue, W.; Huang, B.; Chen, J.; Wang, J. Performance of a lead–carbon negative electrode based on a Pb–C electrodeposited porous graphite/Pb conductive net skeleton. Micro Nano Lett. 2016, 11, 264–268. [Google Scholar] [CrossRef]
- Perez, N. Electrochemistry and Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pavlov, D.; Kirchev, A.; Stoycheva, M.; Monahov, B. Influence of H2SO4 concentration on the mechanism of the processes and on the electrochemical activity of the Pb/PbO2/PbSO4 electrode. J. Power Sources 2004, 137, 288–308. [Google Scholar] [CrossRef]
- Selpiana, S.; Haryati, S.; Bustan, M.D. Cathode current efficiency of lead electrowinning in sulphate electrolyte. AIP Conf. Proc. 2022, 2391, 040007. [Google Scholar] [CrossRef]
- Clancy, M.; Bettles, C.; Stuart, A.; Birbilis, N. The influence of alloying elements on the electrochemistry of lead anodes for electrowinning of metals: A review. Hydrometallurgy 2013, 131–132, 144–157. [Google Scholar] [CrossRef]
- Ivanov, I.; Stefanov, Y.; Noncheva, Z.; Petrova, M.; Dobrev, T.; Mirkova, L.; Vermeersch, R.; Demaerel, J.-P. Insoluble anodes used in hydrometallurgy Part II. Anodic behaviour of lead and lead-alloy anodes. Hydrometallurgy 2000, 57, 125–139. [Google Scholar] [CrossRef]
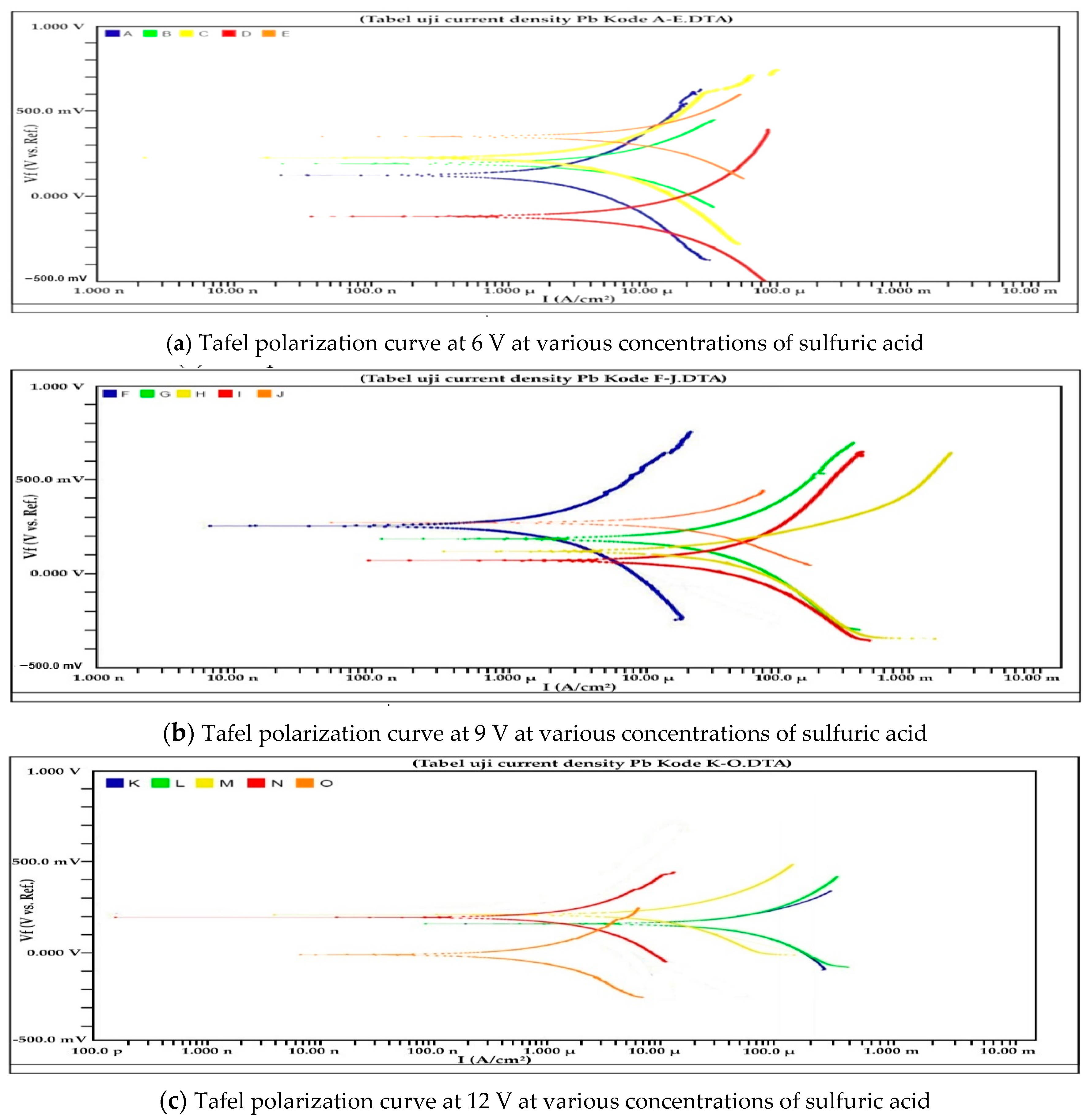

| Sample | Ecorr (mV) | Current Density (μA/cm2) | Corrosion Rate (mpy) |
|---|---|---|---|
| A (1 M) | 129.1 | 1.85 | 2.17 |
| B (2 M) | 185.6 | 2.81 | 3.30 |
| C (3 M) | 230.9 | 2.83 | 3.33 |
| D (4 M) | 143.5 | 6.03 | 7.10 |
| E (5 M) | 350.2 | 3.92 | 4.61 |
| Sample | Ecorr (mV) | Current Density (μA/cm2) | Corrosion Rate (mpy) |
|---|---|---|---|
| F (1 M) | 252.2 | 1.29 | 1.52 |
| G (2 M) | 66.93 | 23.44 | 27.55 |
| H (3 M) | 187.4 | 26.67 | 31.35 |
| I (4 M) | 122.2 | 28.14 | 33.08 |
| J (5 M) | 271.3 | 11.91 | 14.00 |
| Sample | Ecorr (mV) | Current Density (μA/cm2) | Corrosion Rate (mpy) |
|---|---|---|---|
| K (1 M) | 203.0 | 8.461 | 9.95 |
| L (2 M) | 132.1 | 24.08 | 28.31 |
| M (3 M) | 196.2 | 25.89 | 30.44 |
| N (4 M) | 181.2 | 0.863 | 1.015 |
| O (5 M) | 24.22 | 0.538 | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selpiana, S.; Haryati, S.; Bustan, M.D. Graphite/β-PbO2 Composite Inert Anode Synthesis Using Electrochemical Methods. ChemEngineering 2023, 7, 20. https://doi.org/10.3390/chemengineering7020020
Selpiana S, Haryati S, Bustan MD. Graphite/β-PbO2 Composite Inert Anode Synthesis Using Electrochemical Methods. ChemEngineering. 2023; 7(2):20. https://doi.org/10.3390/chemengineering7020020
Chicago/Turabian StyleSelpiana, Selpiana, Sri Haryati, and Muhammad Djoni Bustan. 2023. "Graphite/β-PbO2 Composite Inert Anode Synthesis Using Electrochemical Methods" ChemEngineering 7, no. 2: 20. https://doi.org/10.3390/chemengineering7020020
APA StyleSelpiana, S., Haryati, S., & Bustan, M. D. (2023). Graphite/β-PbO2 Composite Inert Anode Synthesis Using Electrochemical Methods. ChemEngineering, 7(2), 20. https://doi.org/10.3390/chemengineering7020020






