Effects of Lithium Source and Content on the Properties of Li-Rich Layered Oxide Cathode Materials
Abstract
:1. Introduction
2. Materials and Methods
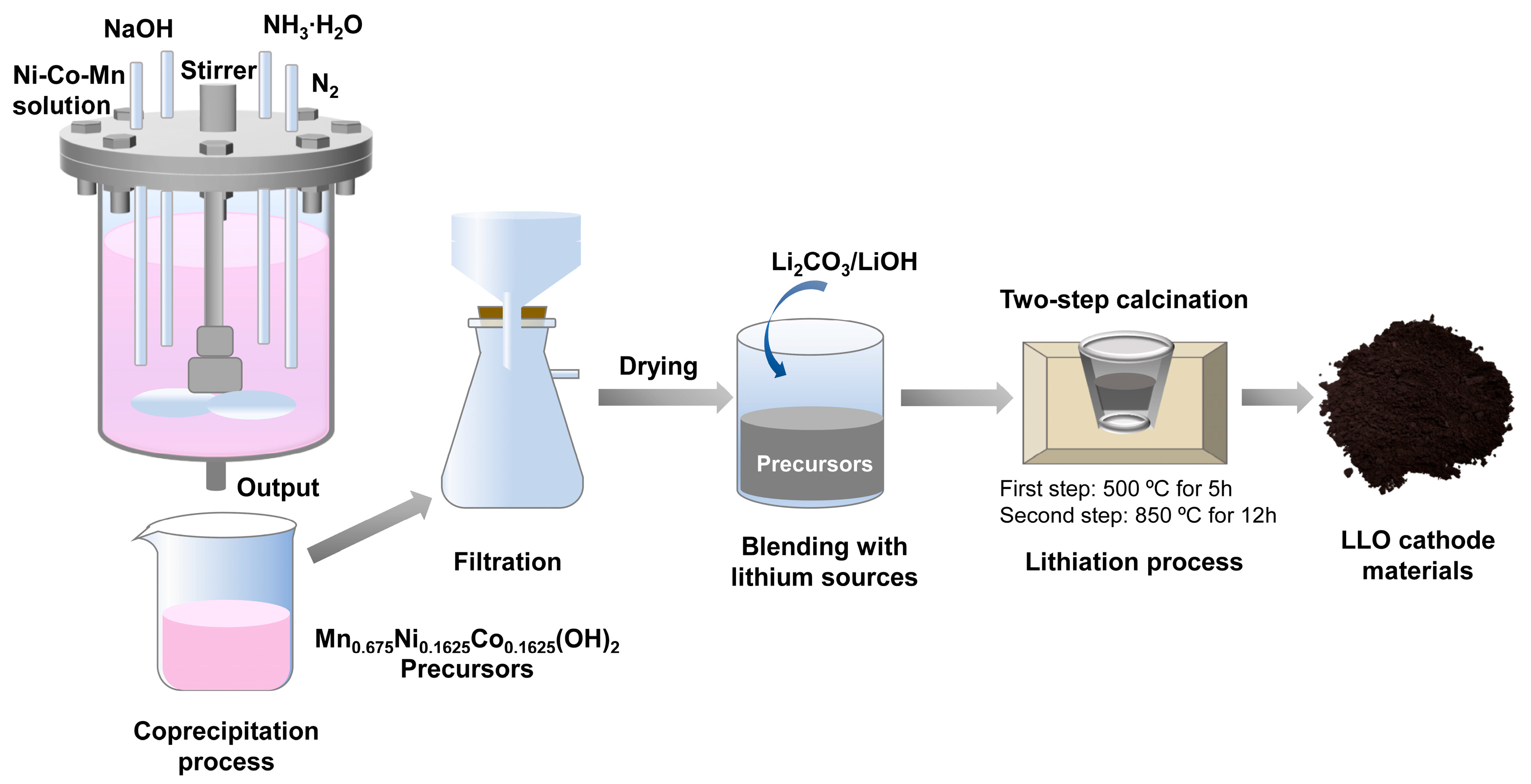
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
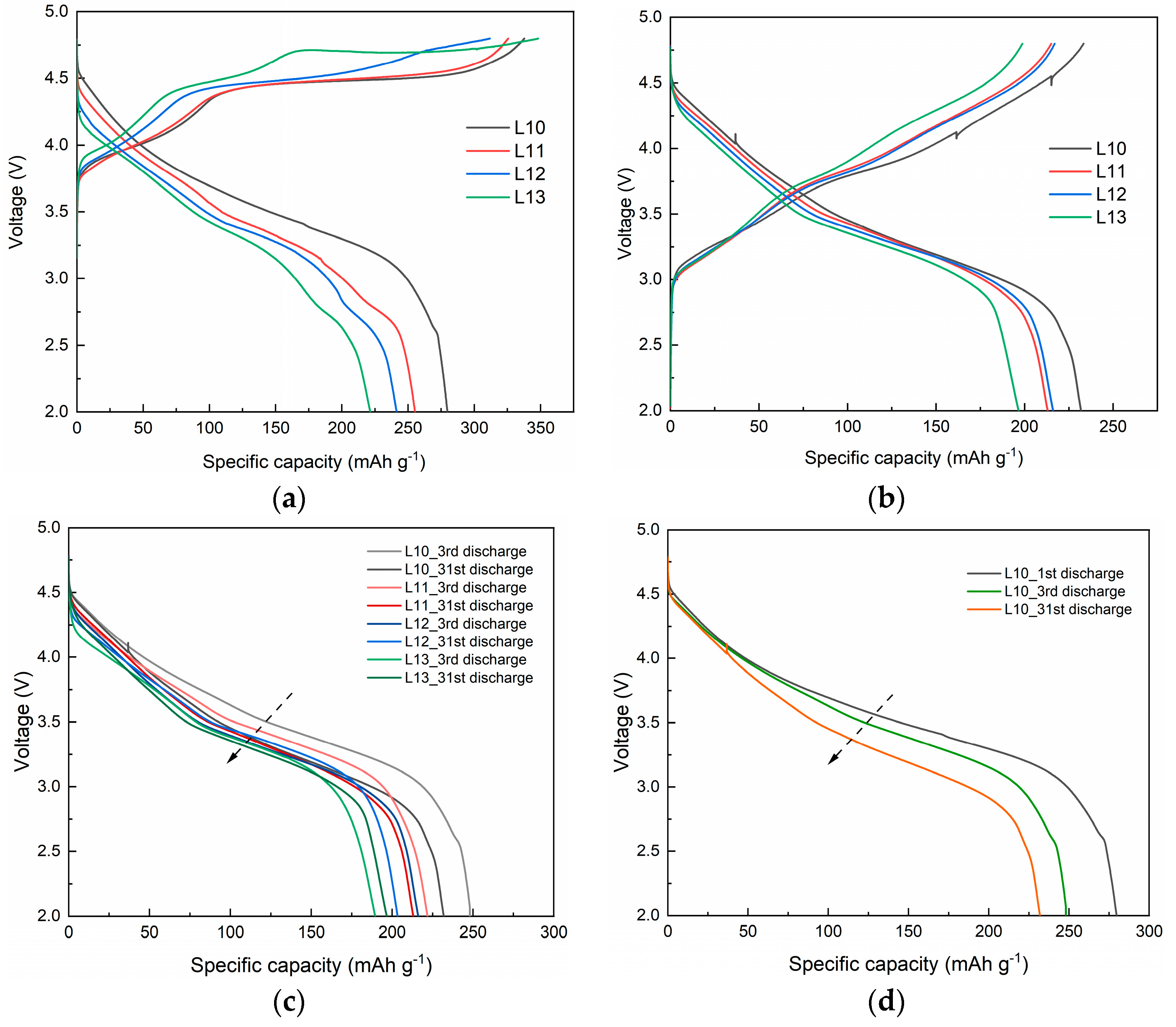
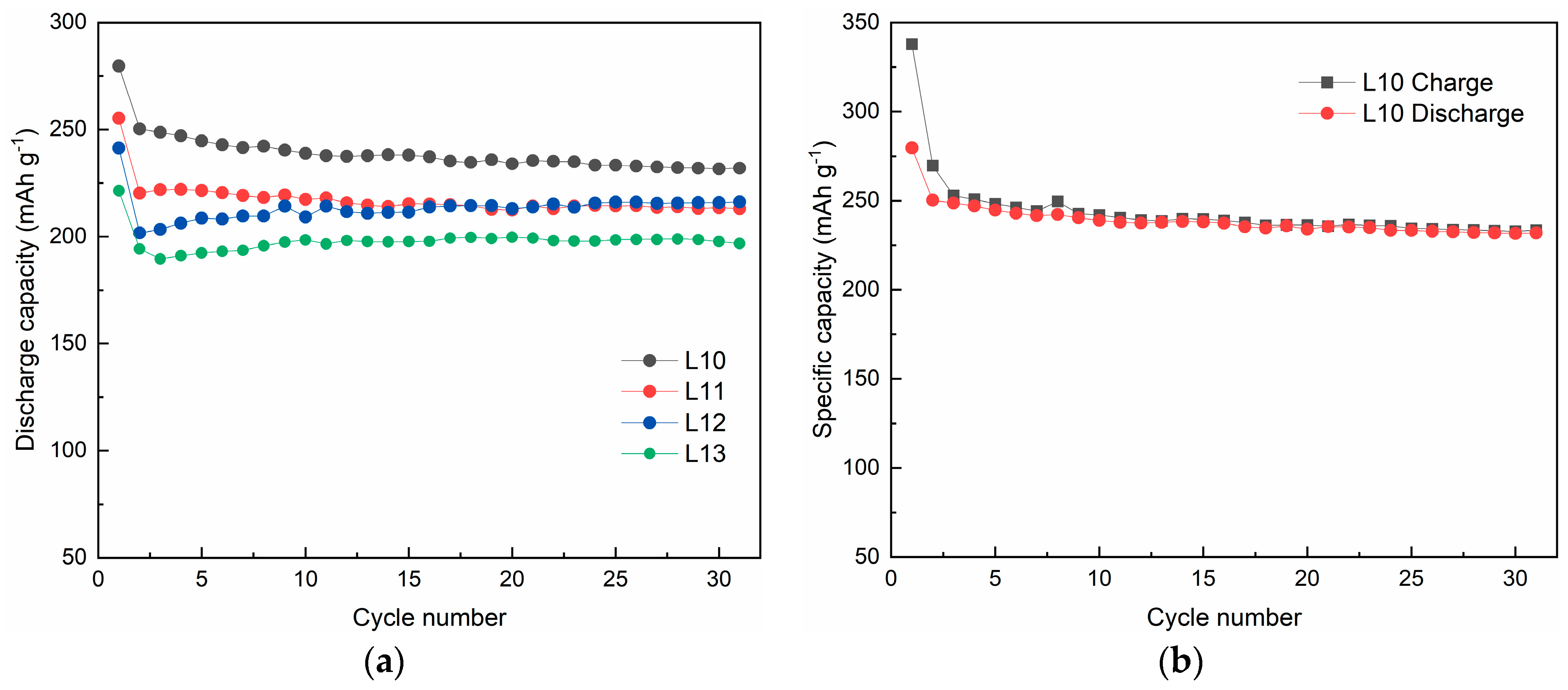
3.1. Effect of Lithium Content on Structure and Electrochemical Performance
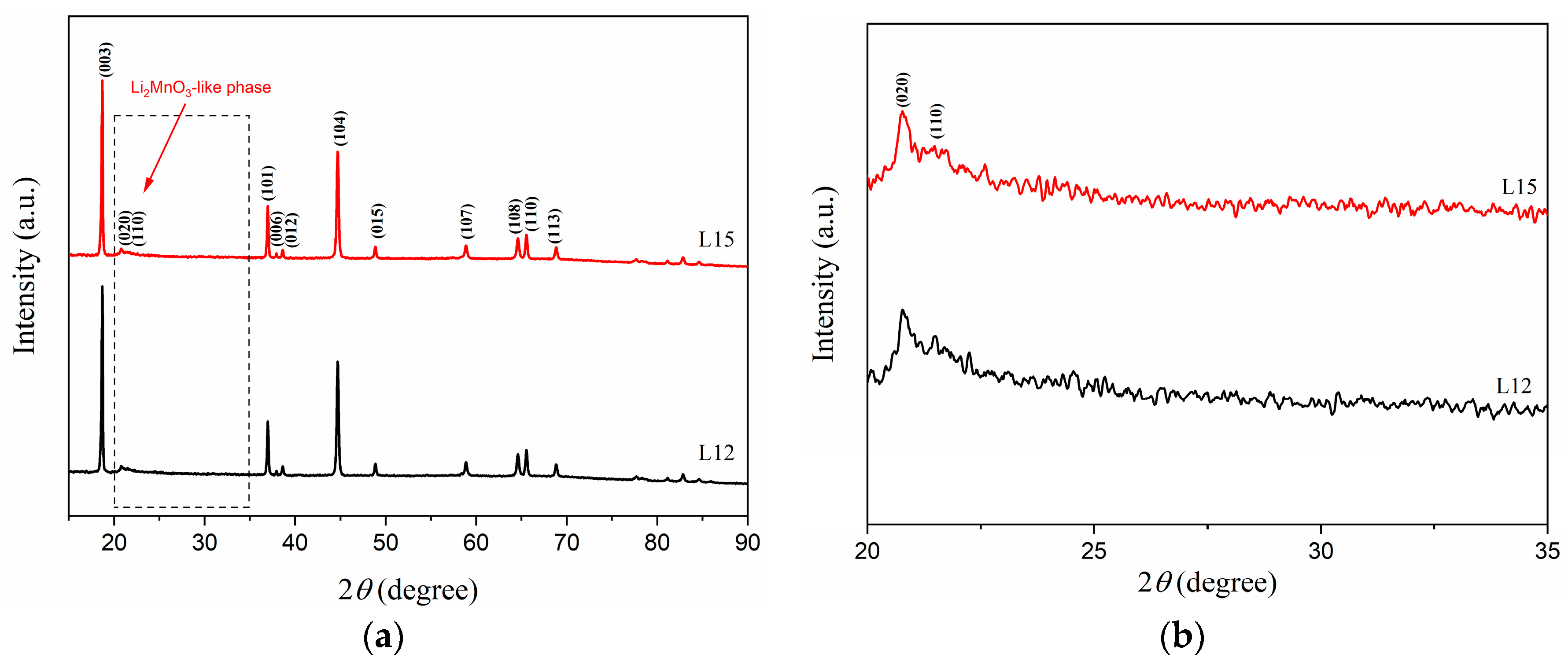
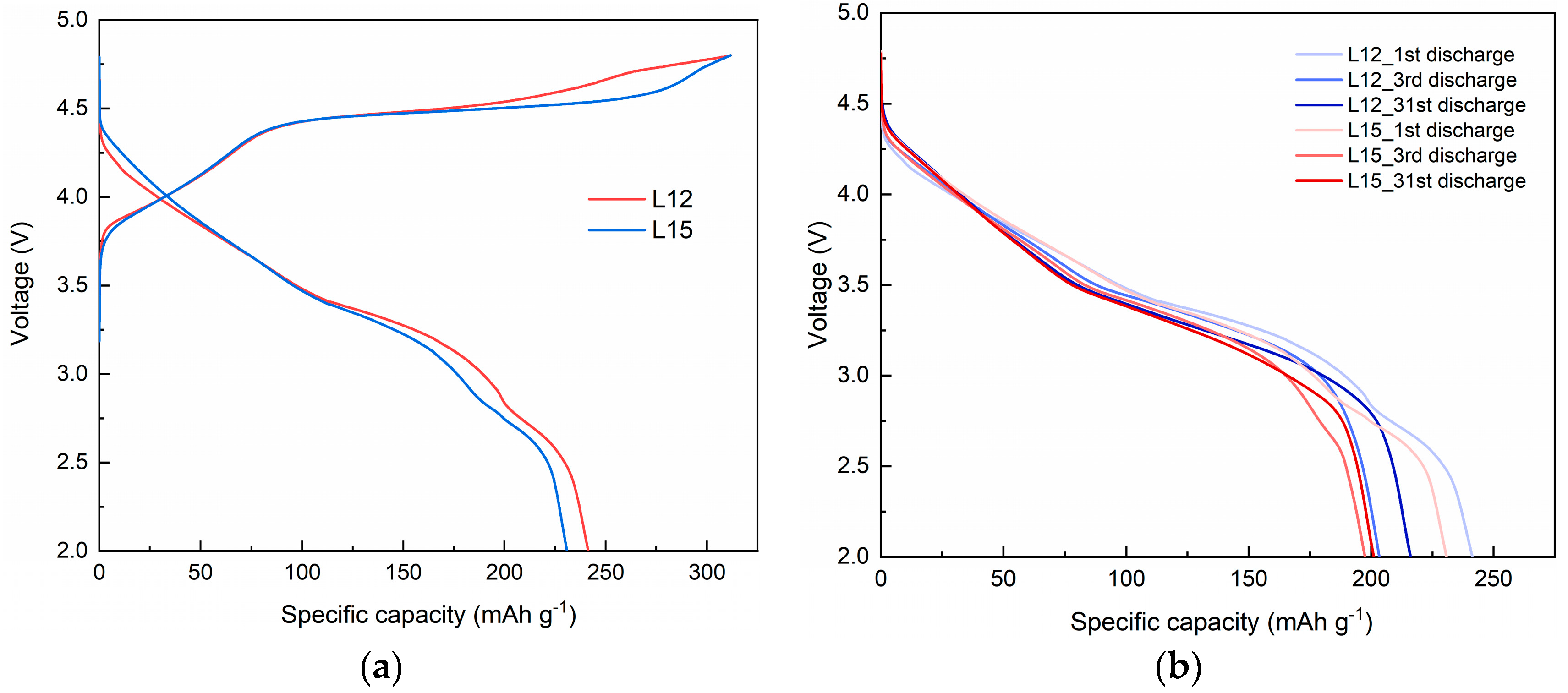
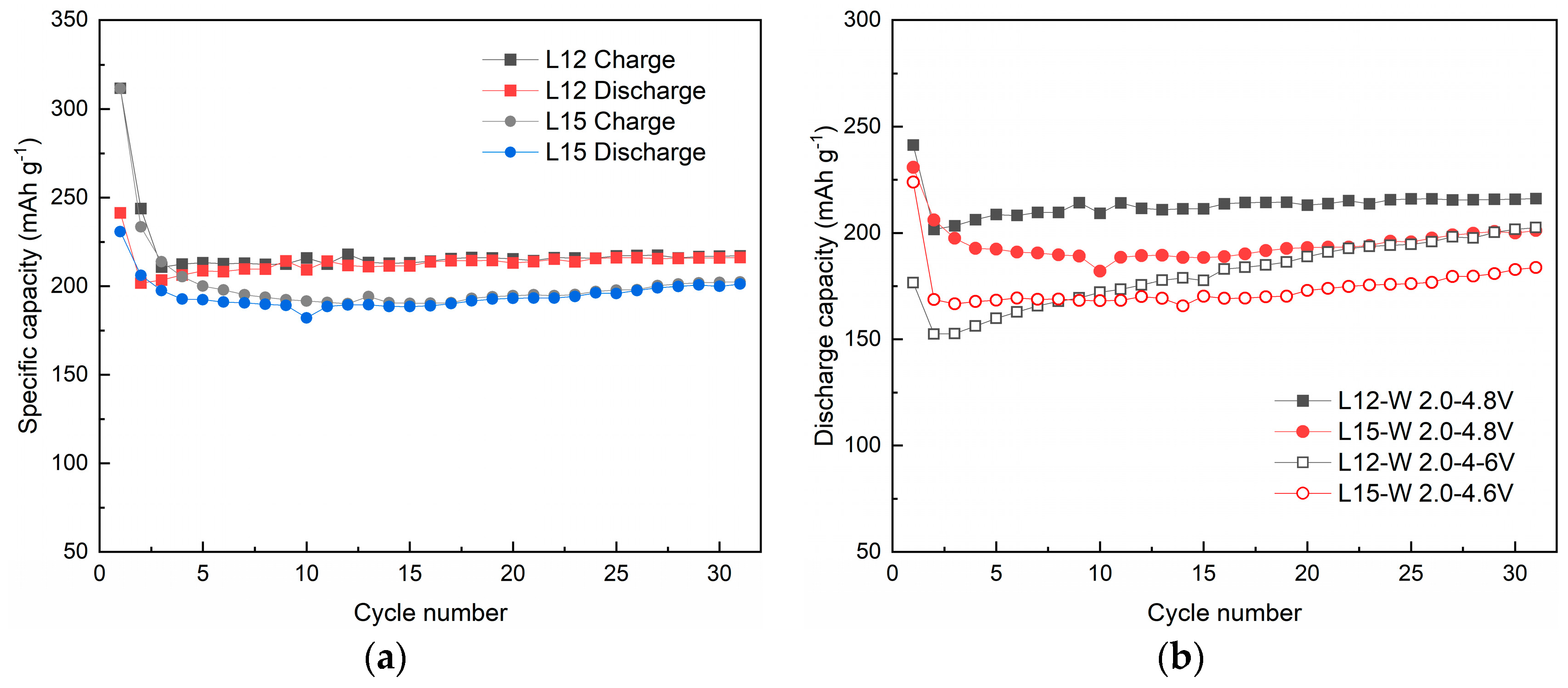
3.2. Effect of Lithium Sources on Structure and Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuo, W.; Luo, M.; Liu, X.; Wu, J.; Liu, H.; Li, J.; Winter, M.; Fu, R.; Yang, W.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv. 2014, 4, 63268–63284. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H. High-Energy Cathode Materials (Li2MnO3-LiMO2) for Lithium-Ion Batteries. J. Phys. Chem. Lett. 2013, 4, 1268–1280. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2016, 7, 1601284. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface Modification of Li-Rich Mn-Based Layered Oxide Cathodes: Challenges, Materials, Methods, and Characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Hy, S.; Liu, H.; Zhang, M.; Qian, D.; Hwang, B.-J.; Meng, Y.S. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 2016, 9, 1931–1954. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, S.; Chen, J.; Gao, M.; Liu, Y.; Zhu, T.; Jiang, Y. Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: A review from fundamentals to research progress and applications. Mol. Syst. Des. Eng. 2018, 3, 748–803. [Google Scholar] [CrossRef]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, e2005937. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Thackeray, M.M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–473. [Google Scholar] [CrossRef]
- Numata, K.; Sakaki, C.; Yamanaka, S. Synthesis of Solid Solutions in a System of LiCoO2-Li2MnO3 for Cathode Materials of Secondary Lithium Batteries. Chem. Lett. 1997, 26, 725–726. [Google Scholar] [CrossRef]
- Numata, K.; Sakaki, C.; Yamanaka, S. Synthesis and characterization of layer structured solid solutions in the system of LiCoO2–Li2MnO3. Solid State Ion. 1999, 117, 257–263. [Google Scholar] [CrossRef]
- Kalyani, P.; Chitra, S.; Mohan, T.; Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 1999, 80, 103–106. [Google Scholar] [CrossRef]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Cathode Materials Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A191. [Google Scholar] [CrossRef]
- Lu, Z.; Dahn, J.R. Structure and Electrochemistry of Layered Li[CrxLi(1/3−x/3)Mn(2/3−2x/3)]O2. J. Electrochem. Soc. 2002, 149, A1454. [Google Scholar] [CrossRef]
- Kim, J.-S.; Johnson, C.S.; Vaughey, J.T.; Thackeray, M.M.; Hackney, S.A.; Yoon, W.; Grey, C.P. Electrochemical and Structural Properties of xLi2M‘O3·(1−x)LiMn0.5Ni0.5O2 Electrodes for Lithium Batteries (M‘ = Ti, Mn, Zr; 0 ≤ x ⩽ 0.3). Chem. Mater. 2004, 16, 1996–2006. [Google Scholar] [CrossRef]
- Jarvis, K.A.; Deng, Z.; Allard, L.F.; Manthiram, A.; Ferreira, P.J. Atomic Structure of a Lithium-Rich Layered Oxide Material for Lithium-Ion Batteries: Evidence of a Solid Solution. Chem. Mater. 2011, 23, 3614–3621. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Mannessiez, P.; Ménétrier, M.; Weill, F.; Bourgeois, L.; Duttine, M.; Suard, E.; Delmas, C. Li1.20Mn0.54Co0.13Ni0.13O2 with Different Particle Sizes as Attractive Positive Electrode Materials for Lithium-Ion Batteries: Insights into Their Structure. J. Phys. Chem. C 2012, 116, 13497–13506. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Duncan, H.; Hage, F.; Chen, G. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat. Commun. 2015, 6, 8711. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, X.; Wang, C.; Bi, H.; Zhang, J.; Li, S.; Wang, M.; Che, R. Insight into the atomic structure of Li2MnO3 in Li-rich Mn-based cathode materials and the impact of its atomic arrangement on electrochemical performance. J. Mater. Chem. A 2017, 5, 11214–11223. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshii, K.; Myung, S.T.; Nakai, I.; Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo(1/3)Ni(1/3)Mn(1/3)O2. J. Am. Chem. Soc. 2011, 133, 4404–4419. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kim, J.S.; Lefief, C.; Li, N.; Vaughey, J.T.; Thackeray, M.M. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 2004, 6, 1085–1091. [Google Scholar] [CrossRef]
- Johnson, C.S.; Li, N.; Lefief, C.; Thackeray, M.M. Anomalous capacity and cycling stability of xLi2MnO3·(1−x)LiMO2 electrodes (M=Mn, Ni, Co) in lithium batteries at 50 °C. Electrochem. Commun. 2007, 9, 787–795. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Genc, A.; Xiao, J.; Xu, P.; Chen, X.; Zhu, Z.; Zhao, W.; Pullan, L.; Wang, C.; et al. Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution. Nano Lett. 2014, 14, 2628–2635. [Google Scholar] [CrossRef]
- Fu, F.; Yao, Y.; Wang, H.; Xu, G.L.; Amine, K.; Sun, S.; Shao, M. Structure dependent electrochemical performance of Li-rich layered oxides in lithium-ion batteries. Nano Energy 2017, 35, 370–378. [Google Scholar] [CrossRef]
- Pimenta, V.; Sathiya, M.; Batuk, D.; Abakumov, A.M.; Giaume, D.; Cassaignon, S.; Larcher, D.; Tarascon, J.-M. Synthesis of Li-Rich NMC: A Comprehensive Study. Chem. Mater. 2017, 29, 9923–9936. [Google Scholar] [CrossRef]
- Cao, K.; Shen, T.; Wang, K.; Chen, D.; Wang, W. Influence of different lithium sources on the morphology, structure and electrochemical performances of lithium-rich layered oxides. Ceram. Int. 2017, 43, 8694–8702. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Zhang, H.; Song, D.; Shi, X.; Zhang, L. The effect of lithium content on the structure, morphology and electrochemical performance of Li-rich cathode materials Li1+x(Ni1/6Co1/6Mn4/6)1−xO2. New J. Chem. 2017, 41, 10048–10053. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Wei, Q.; Shu, H.; Liu, L.; Yang, S.; Hu, B.; Song, Y.; Zou, G.; Hu, L.; et al. Synthesis and characterization of a Li-rich layered cathode material Li1.15[(Mn1/3Ni1/3Co1/3)0.5(Ni1/4Mn3/4)0.5]0.85O2 with spherical core–shell structure. J. Mater. Chem. 2012, 22, 19666–19672. [Google Scholar] [CrossRef]
- Meng, Y.S.; Ceder, G.; Grey, C.P.; Yoon, W.S.; Jiang, M.; Bréger, J.; Shao-Horn, Y. Cation Ordering in Layered O3 Li[NixLi1/3-2x/3Mn2/3-x/3]O2 (0 ≤ x ≤ 1/2) Compounds. Chem. Mater. 2005, 17, 2386–2394. [Google Scholar] [CrossRef]
- Boulineau, A.; Croguennec, L.; Delmas, C.; Weill, F. Structure of Li2MnO3 with different degrees of defects. Solid State Ion. 2010, 180, 1652–1659. [Google Scholar] [CrossRef]
- Shunmugasundaram, R.; Arumugam, R.S.; Dahn, J.R. A Study of Stacking Faults and Superlattice Ordering in Some Li-Rich Layered Transition Metal Oxide Positive Electrode Materials. J. Electrochem. Soc. 2016, 163, A1394–A1400. [Google Scholar] [CrossRef]
- Wang, M.-J.; Shao, A.-F.; Yu, F.-D.; Sun, G.; Gu, D.-M.; Wang, Z.-B. Simple Water Treatment Strategy To Optimize the Li2MnO3 Activation of Lithium-Rich Cathode Materials. ACS Sustain. Chem. Eng. 2019, 7, 12825–12837. [Google Scholar] [CrossRef]
- Xiang, Y.; Yin, Z.; Zhang, Y.; Li, X. Effects of synthesis conditions on the structural and electrochemical properties of the Li-rich material Li[Li0.2Ni0.17Co0.16Mn0.47]O2 via the solid-state method. Electrochim. Acta 2013, 91, 214–218. [Google Scholar] [CrossRef]
- Shunmugasundaram, R.; Senthil Arumugam, R.; Dahn, J.R. High Capacity Li-Rich Positive Electrode Materials with Reduced First-Cycle Irreversible Capacity Loss. Chem. Mater. 2015, 27, 757–767. [Google Scholar] [CrossRef]
- Wenfeng, R.; Zhao, Y.; Hu, X.; Xia, M. Preparation-microstructure-performance relationship of Li-rich transition metal oxides microspheres as cathode materials for lithium ion batteries. Electrochim. Acta 2016, 191, 491–499. [Google Scholar] [CrossRef]

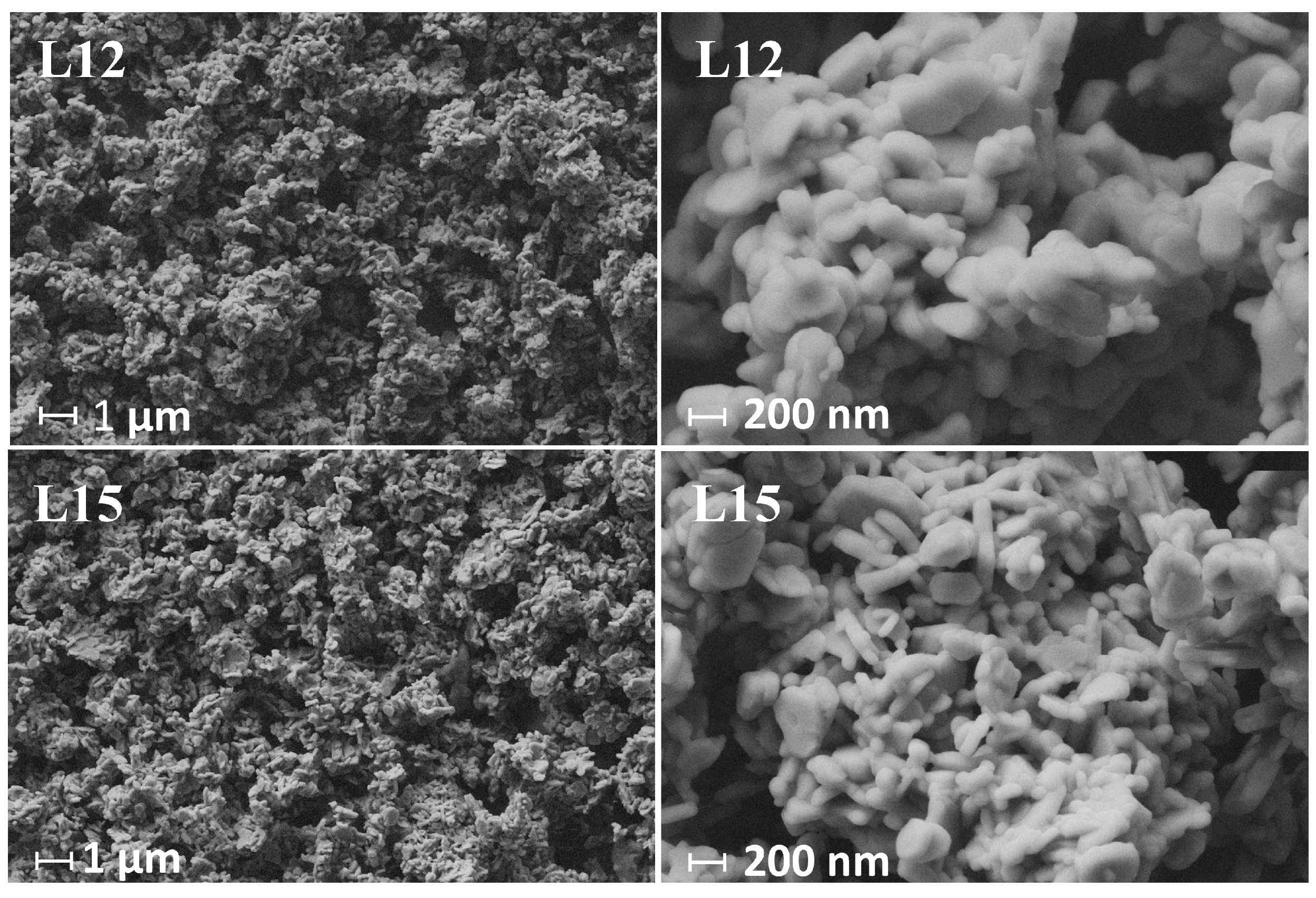
| Sample | Experimental Li/Mn/Ni/Co Molar Ratio | Li/TM Ratio | Chemical Formula | |||
|---|---|---|---|---|---|---|
| Li | Mn | Ni | Co | |||
| L10 | 1.31 | 0.57 | 0.15 | 0.15 | 1.52 | Li1.31Mn0.57Ni0.15Co0.15O2 |
| L11 | 1.31 | 0.53 | 0.14 | 0.14 | 1.64 | Li1.31Mn0.53Ni0.14Co0.14O2 |
| L12 | 1.23 | 0.47 | 0.12 | 0.12 | 1.73 | Li1.23Mn0.47Ni0.12Co0.12O2 |
| L13 | 0.89 | 0.32 | 0.08 | 0.08 | 1.86 | Li0.89Mn0.32Ni0.08Co0.08O2 |
| Sample | a/Å | b/Å | c/Å | Alpha/° | Gamma/° | Beta/° |
|---|---|---|---|---|---|---|
| L10 | 4.937 | 8.534 | 5.028 | 90 | 90 | 109.64 |
| L11 | 4.928 | 8.527 | 5.017 | 90 | 90 | 109.31 |
| L12 | 4.916 | 8.518 | 5.033 | 90 | 90 | 109.46 |
| L13 | 4.916 | 8.511 | 5.027 | 90 | 90 | 109.41 |
| Sample | Initial Charge Capacity/mAh g−1 | Initial Discharge Capacity/mAh g−1 | Irreversible Capacity/mAh g−1 | Initial Coulumbic Efficiency/% |
|---|---|---|---|---|
| L10 | 337.77 | 279.65 | 58.12 | 82.8 |
| L11 | 325.66 | 255.23 | 70.43 | 78.4 |
| L12 | 311.75 | 241.34 | 70.41 | 77.4 |
| L13 | 348.28 | 221.44 | 126.84 | 63.6 |
| Sample | Lithium Source | Experimental Li/Mn/Ni/Co Molar Ratio | Chemical Formula | |||
|---|---|---|---|---|---|---|
| Li | Mn | Ni | Co | |||
| L12 | Li2CO3 | 1.23 | 0.47 | 0.12 | 0.12 | Li1.23Mn0.47Ni0.12Co0.12O2 |
| L15 | LiOH | 1.24 | 0.49 | 0.12 | 0.12 | Li1.24Mn0.49Ni0.12Co0.12O2 |
| Sample | Lithium Source | a/Å | b/Å | c/Å | Alpha/° | Gamma/° | Beta/° |
|---|---|---|---|---|---|---|---|
| L12 | Li2CO3 | 4.916 | 8.518 | 5.033 | 90 | 90 | 109.46 |
| L15 | LiOH | 4.920 | 8.516 | 5.035 | 90 | 90 | 109.41 |
| Sample | Lithium Source | Initial Charge Capacity/mAh g−1 | Initial Discharge Capacity/mAh g−1 | Irreversible Capacity/mAh g−1 | Initial Coulumbic Efficiency/% |
|---|---|---|---|---|---|
| L12 | Li2CO3 | 311.75 | 241.34 | 70.41 | 77.4 |
| L15 | LiOH | 311.66 | 230.86 | 80.80 | 74.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hietaniemi, M.; Välikangas, J.; Hu, T.; Tynjälä, P.; Lassi, U. Effects of Lithium Source and Content on the Properties of Li-Rich Layered Oxide Cathode Materials. ChemEngineering 2023, 7, 15. https://doi.org/10.3390/chemengineering7010015
Wang Y, Hietaniemi M, Välikangas J, Hu T, Tynjälä P, Lassi U. Effects of Lithium Source and Content on the Properties of Li-Rich Layered Oxide Cathode Materials. ChemEngineering. 2023; 7(1):15. https://doi.org/10.3390/chemengineering7010015
Chicago/Turabian StyleWang, Yufan, Marianna Hietaniemi, Juho Välikangas, Tao Hu, Pekka Tynjälä, and Ulla Lassi. 2023. "Effects of Lithium Source and Content on the Properties of Li-Rich Layered Oxide Cathode Materials" ChemEngineering 7, no. 1: 15. https://doi.org/10.3390/chemengineering7010015
APA StyleWang, Y., Hietaniemi, M., Välikangas, J., Hu, T., Tynjälä, P., & Lassi, U. (2023). Effects of Lithium Source and Content on the Properties of Li-Rich Layered Oxide Cathode Materials. ChemEngineering, 7(1), 15. https://doi.org/10.3390/chemengineering7010015







