Extraction of Noble Metals from Pyrite Cinders
Abstract
:1. Introduction
- (a)
- A higher rate of gold leaching by chloride solutions due to the use of high concentrations of an oxidizing agent (molecular chlorine);
- (b)
- The possibility of obtaining hydrochloric acid solutions rich in Au content, from which it is convenient to subsequently extract gold by direct electrolysis;
- (c)
- The effectiveness of the application of the chlorinated leaching of gold to various ore materials that are difficult to cyanidate, for example, antimony, arsenic, copper and telluride concentrates;
- (d)
- A fairly simple and effective method for the subsequent adsorption extraction of gold on activated carbon. Unlike cyanidation, gold from chloride solutions is deposited on coal granules in the form of metal:
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beregovsky, V.I.; Bergman, R.V.; Danilova, L.A.; Kozyrev, B.C.; Tarasov, B.E.; Teper, B.C.; Fominykh, E.G. Complex Use of Pyrite cinders; Metallurgizdat: Moscow, Russia, 1963; 70p. [Google Scholar]
- Abikak, Y.; Kenzhaliev, B.; Gladyshev, S.; Abdulvaliev, R. Development of Technology for Processing Pyrite Cinder to Produce Non-Ferrous Metal Concentrate. Teknomekanik 2022, 5, 35–41. [Google Scholar]
- Kosmukhambetov, A.R. Scientific Foundations of Oxyhydrochlorination, Electromembrane Processes and Their Application in Hydrometallurgy of Non-Ferrous, Rare and Noble Metals. Ph.D. Thesis, Satbayev University, Almaty, Kazakhtan, 2005; 352p. [Google Scholar]
- Litvinenko, L.G.; Litvinenko, V.G.; Shchelkonogov, M.A.; Morozov, A.A. The Method of Complex Processing of Pyrite Cinders. Patent 2623948 RU, 6 December 2017. [Google Scholar]
- Mukhamedshin, I.K.; Bashlykova, T.V.; Fadina, I.B.; Zhivaeva, A.B. Method for Deep Processing of Pyrite Cinders. Patent 2397260 RU, 13 March 2020. [Google Scholar]
- Abikak, E.B.; Kenzhaliev, B.K. Development of an Integrated Technology Intended to Process Pyrite Slag Using Chemical Pre-activation. NEWS of the National Academy of Sciences of the Republic of Kazakhstan Series of Geology and Technical Sciences. 2022. Available online: http://geolog-technical.kz/assets/20223/3.%2032-51.pdf (accessed on 1 December 2022).
- Abdulvaliev, R.A.; Gladyshev, S.V.; Pozmogov, V.A.; Kasymzhanova, A.K. Hydrochemical technology for processing the ferruginous fraction of bauxites. Enrich. Ores 2019, 310, 44–49. [Google Scholar] [CrossRef]
- Ivanik, S.A.; Ilyukhin, D.A. Hydrometallurgical technology for gold recovery from refractory gold-bearing raw materials and the solution to problems of subsequent dehydration processes. J. Ind. Pollut. Control 2017, 33, 891–897. [Google Scholar]
- Vazhnikova, E.A.; Tarchigina, N.F. Ecological and economic analysis of chemical technologies for processing solid wastes of sulfuric acid production. Int. J. Humanit. Nat. Sci. 2019, 12, 78. [Google Scholar] [CrossRef]
- Shin, S.N.; Gulyaeva, R.I. Method for Processing Pyrite Cinders. Patent 2172788 EN, 27 August 2001. [Google Scholar]
- Vlasov, O.A.; Mechev, V.V. Method of Processing Pyrite Stubs. Patent 2394924 RU, 10 July 2020. [Google Scholar]
- Bakov, A.A.; Arzhannikov, G.I. A method for Processing Pyrite Stubs. Patent 2149706 RU, 19 March 2013. [Google Scholar]
- Kurdyumov, G.E.; Galeru, K.E.; Parshin. Method of Processing Pyrite Stubs. Patent 2716440 RU, 20 March 2020. [Google Scholar]
- Evdokimov, A.V.; Voychinikov, G.I.; Khmelnitskaya, O.D.; Mullov, V.M. Kinetics of dissolution of gold in the area of high concentration of sodium cyanide. (JSC “Irgiridmet”). Modern methods of technological mineralogy in the processes of complex and deep processing of mineral raw materials. In Proceedings of the International Meeting “Plaksinsky Readings-2012”, Petrozavodsk, Russia, 10–14 September 2012; p. 253. [Google Scholar]
- Elshin, V.V.; Kolodin, A.A.; Ovsyukov, A.E.; Malchikhin, A.S. Peculiarities of cyanide leaching of gold in the grinding cycle. Metallurgist 2013, 7, 86–90. [Google Scholar]
- Bogorodsky, A.V.; Balikov, S.V.; Emelyanov, Y.E.; Kopylova, N.V. Autoclave oxidation of sulfide gold-bearing concentrates. Non-Ferr. Metall. 2011, 4, 68–72. [Google Scholar]
- Sinev, L.A.; Sazhin, Y.G. Heat treatment of sulfide gold-arsenic concentrates in an oxygen-free atmosphere. In Proceedings of the 16th International Scientific and Technical Conference “Scientific Basis and Practice for Processing Ores from Technogenic Raw Materials”, Ekaterinburg, Russia, 7–8 April 2011; pp. 240–244. [Google Scholar]
- Lodeyshchikov, V.V. Technology for extracting gold and silver from refractory ores. Irkutsk 1999, 2, 786. [Google Scholar]
- Kotlyar, Y.A.; Meretukov, M.A.; Strizhko, L.S. Metallurgy of Noble Metals: Textbook in 2 Books; MISIS: Moscow, Russia, 2005; 392p. [Google Scholar]
- Ghobeiti, H.M.; Shahram, R.; Fereshten, R. Chloride-hypochlorite leaching of gold from a mechanically activated refractory sulfide concentrate. Hydrometallurgy 2013, 138, 59–64. [Google Scholar]
- Gudkov, A.S.; Zhuchkov, I.A.; Mineev, G.G. Mechanism and kinetics of sulfite-thiosulfate dissolution of gold. Izvestiya vuzov. Russ. J. Non-Ferr. Met. 2010, 51, 393–397. [Google Scholar] [CrossRef]
- Senanayake, G.; Zhang, X.M. Gold leaching by copper (II) in ammoniacalthiosulphate solutions in the presence of additives. Part II. Effect of residual Cu (II), pH and redox potentials on reactivity of colloidal gold. Hydrometallurgy 2012, 115–116, 21–29. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper (II) in ammoniacalthiosulphate solutions in the presence of additives. Partt I. A review of hard-soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Koizhanova, A.; Osipovskaya, L.; Erdenova, M. Study of precious metals extraction recovery from technogenic wastes. In Proceedings of the 12th International Multidisciplinary Scientific GeoConference and EXPO—Modern Management of Mine Producing, Geology and Environmental Protection, SGEM, Albena, Bulgaria, 17–23 June 2012; Volume 1, pp. 843–846. [Google Scholar]
- Koizhanova, A.; Erdenova, M.; Sedelnikova, G.; Kamalov, E.; Zhanabai, Z. Comparative Study of the Effectiveness of Methods for Extracting Gold from Stale Tailings of Sorption. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, 2018; Volume 18, p. 43. Available online: https://www.proquest.com/openview/82dd569f2440d213ef876b92f525b2b4/1?pq-origsite=gscholar&cbl=1536338 (accessed on 1 December 2022).
- Seitkan, A.; Redfren, S. Arsenic in refractory gold ore processing. Kompleks. Ispolz. Miner. Syra 2021, 317, 5–13. [Google Scholar] [CrossRef]
- Yessengarayev, Y.K.; Surimbayev, B.N.; Baimbetov, B.S.; Mamyachenkov, S.V.; Kanaly, T.S. Ore treatment hydrogen peroxide during heap leaching of gold. Kompleks. Ispolz. Miner. Syra 2021, 316, 5–14. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 2009; 412p. [Google Scholar]
- Yurchenko, E.N.; Kustova, G.N.; Batsanov, S.S. Vibrational Spectra of Inorganic Compounds; Nauka: Novosibirsk, Russia, 1981; 145p. [Google Scholar]
- Thermo Fisher Scientific’s Nicolet FT-IR spectrometer simplifies infrared spectroscopy for QA/QC and investigative analytical laboratories. Microelectron. Int. 2008, 25. [CrossRef]
- Moenke, H. Mineralspektren; Akademie Verlag: Berlin, Germany, 1962; 394p. [Google Scholar]
- Vlasov, A.; Florinskaya, V.; Venediktov, A.A.; Dutova, K.; Morozov, V.; Smirnova, E. Infrared Spectra of Inorganic Glasses and Crystals; Chemistry: Leningrad, Russia, 1972; 304p. [Google Scholar]
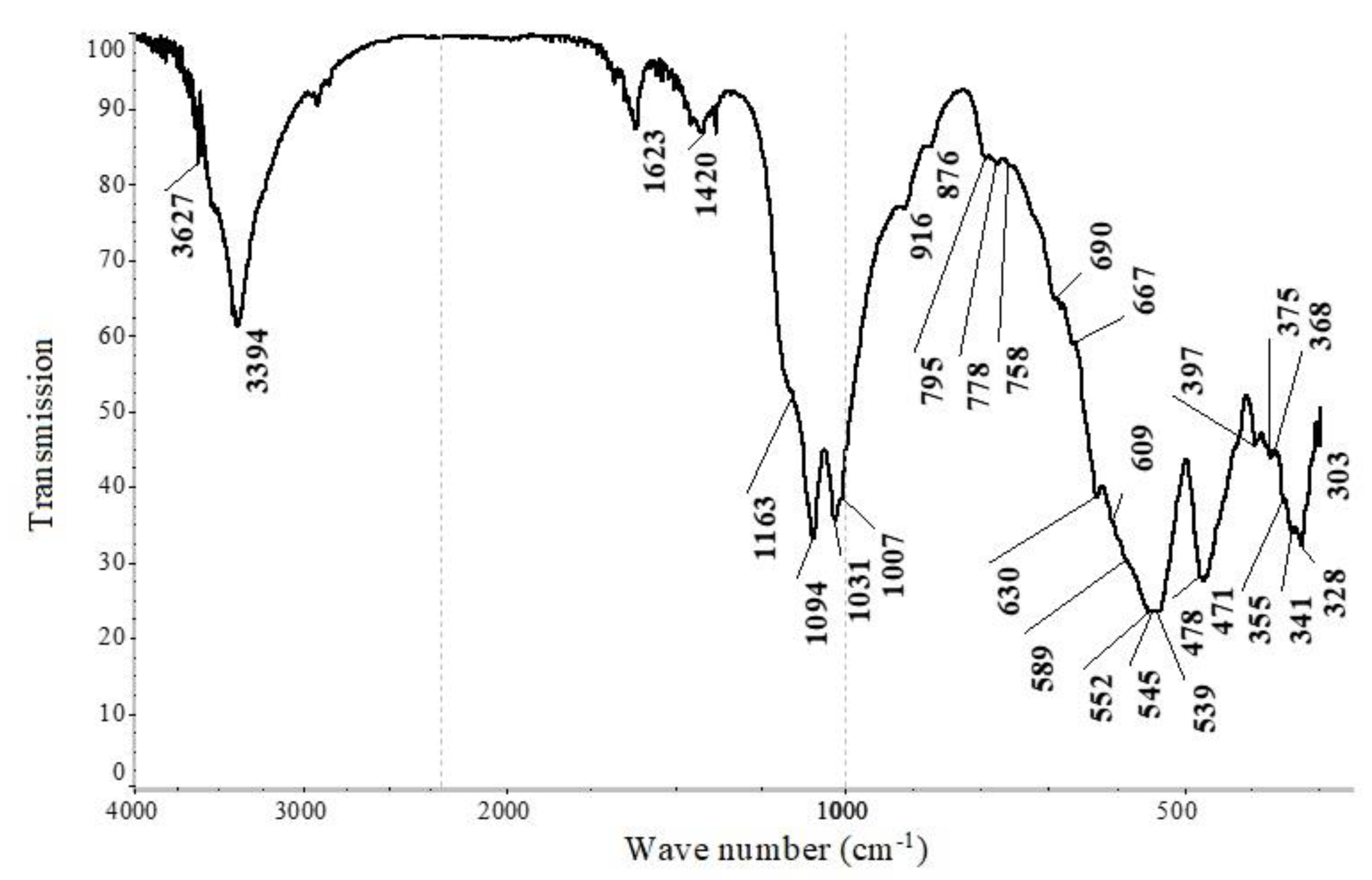
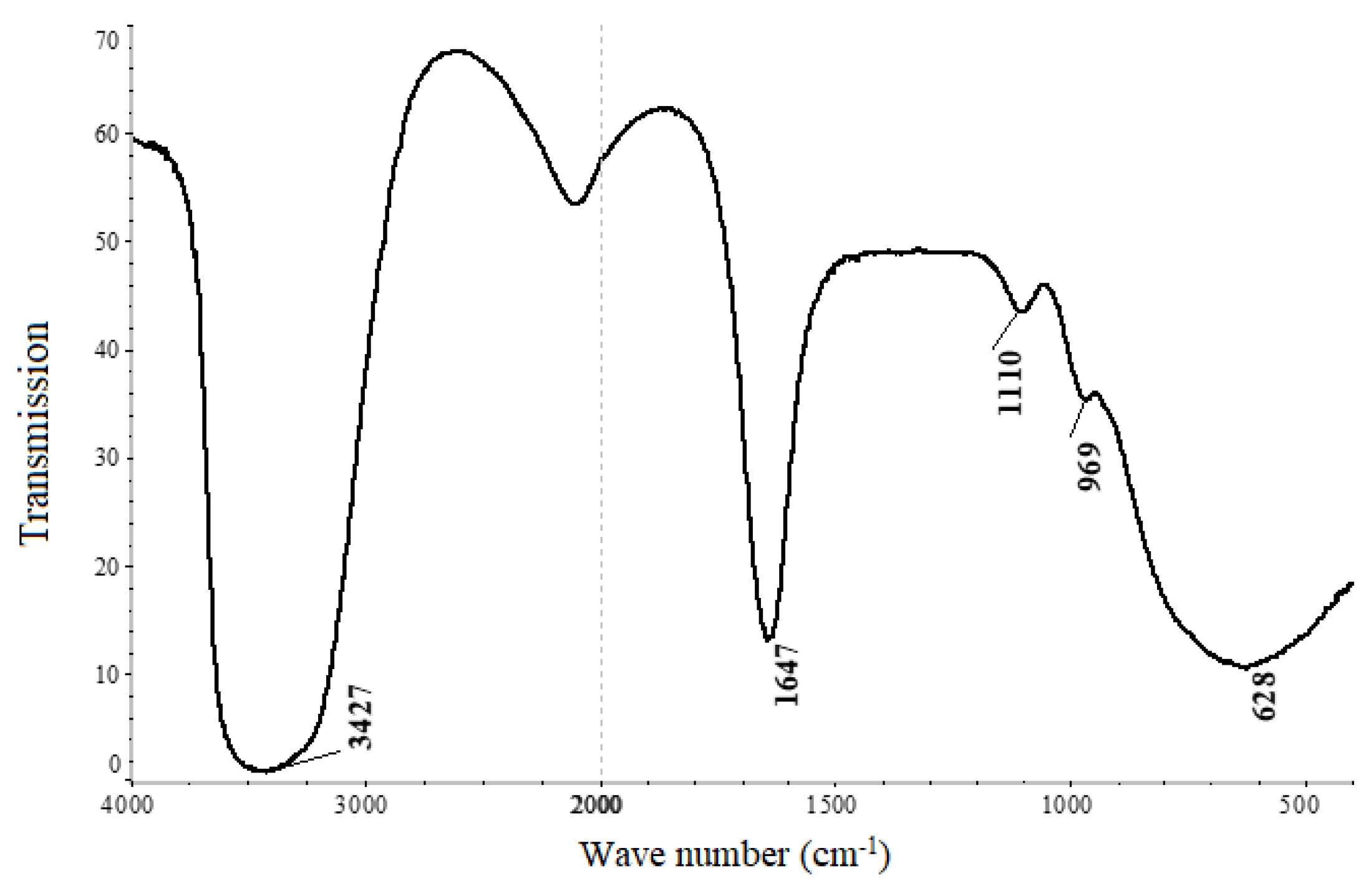
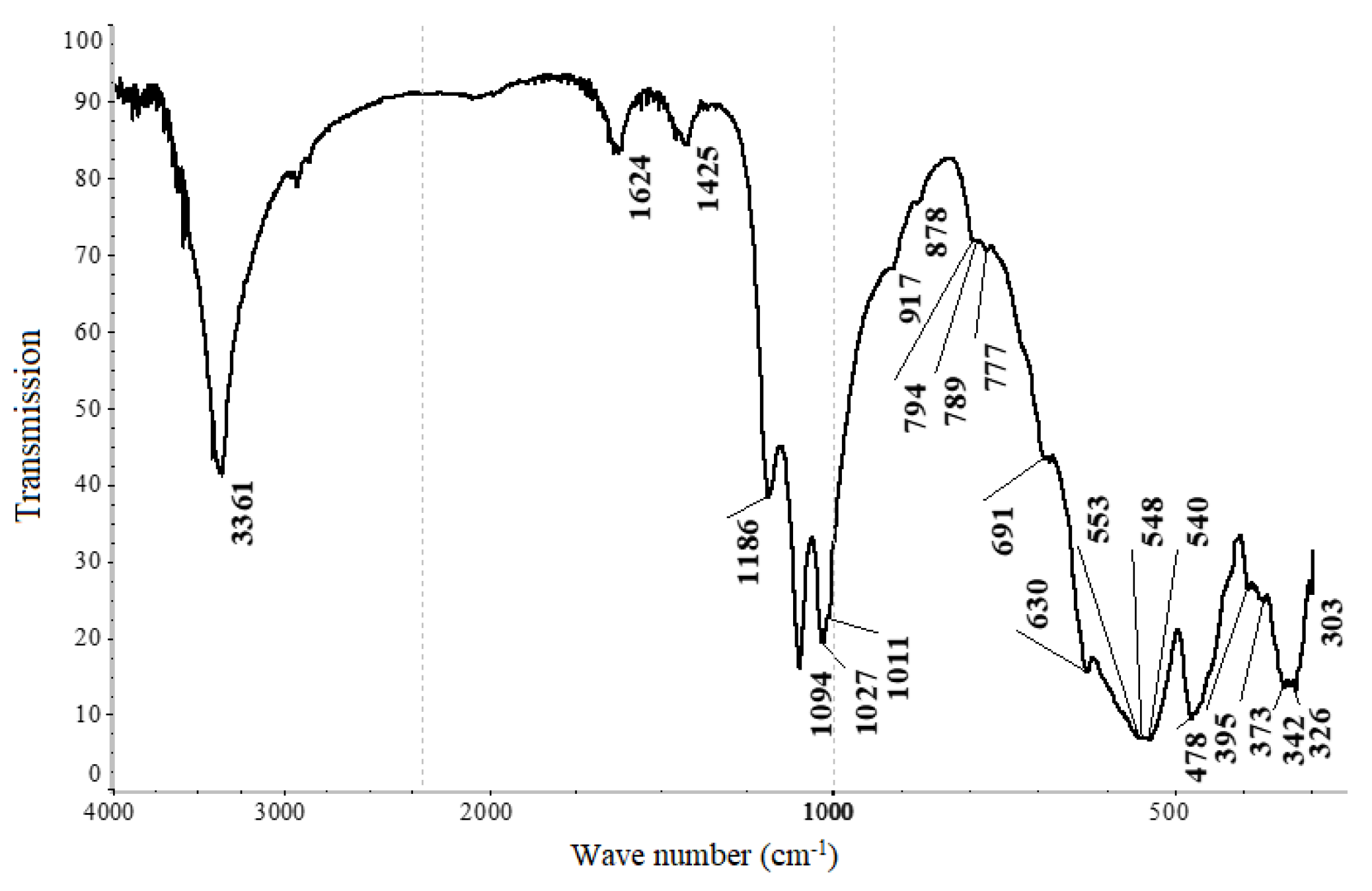
| Composition of Noble Metal Concentrate, % | |||||
|---|---|---|---|---|---|
| Na | Mg | Al | Si | P | Ti |
| 0.613 | 0.137 | 1.691 | 6.364 | 0.070 | 0.097 |
| Ca | K | Cl | S | Mn | Fe |
| 0.904 | 0.207 | 0.020 | 3.105 | 0.015 | 42.647 |
| Co | Cu | Zn | As | Se | Sr |
| 0.066 | 0.152 | 0.376 | 0.099 | 0.223 | 0.027 |
| Zr | Ba | Hg | Pb | ||
| 0.008 | 2.586 | 0.062 | 0.188 | ||
| Compound Name | Formula | S-Q |
|---|---|---|
| Maghemite, syn | Fe2O3 | 62.5% |
| Hematite, syn | Fe2O3 | 20.9% |
| Quartz, syn | SiO2 | 7.5% |
| Szomolnokite, syn | FeSO4·H2O | 5.8% |
| Barium tetraaluminate|Barium Aluminum Oxide | BaAl4O7 | 3.3% |
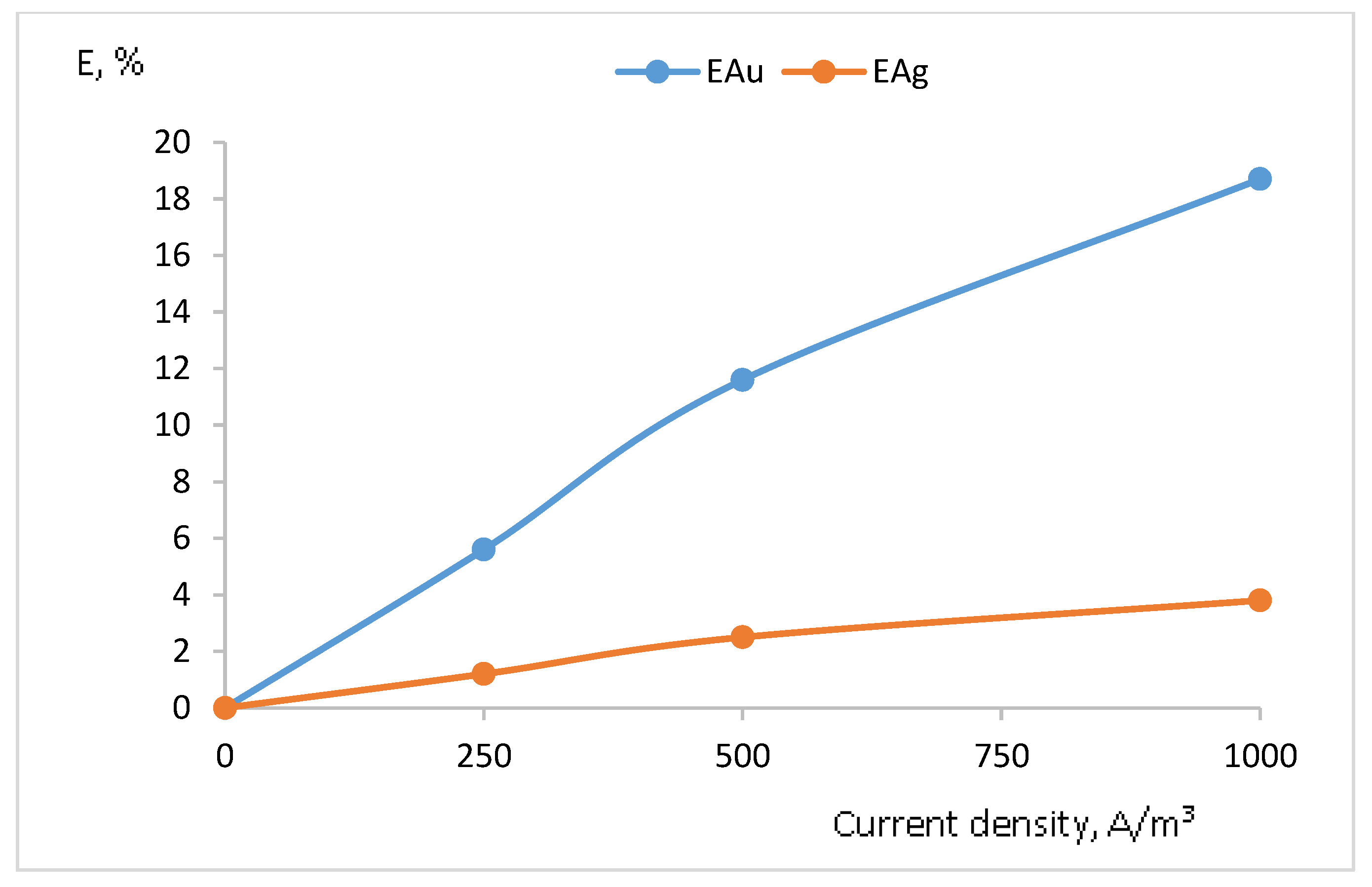
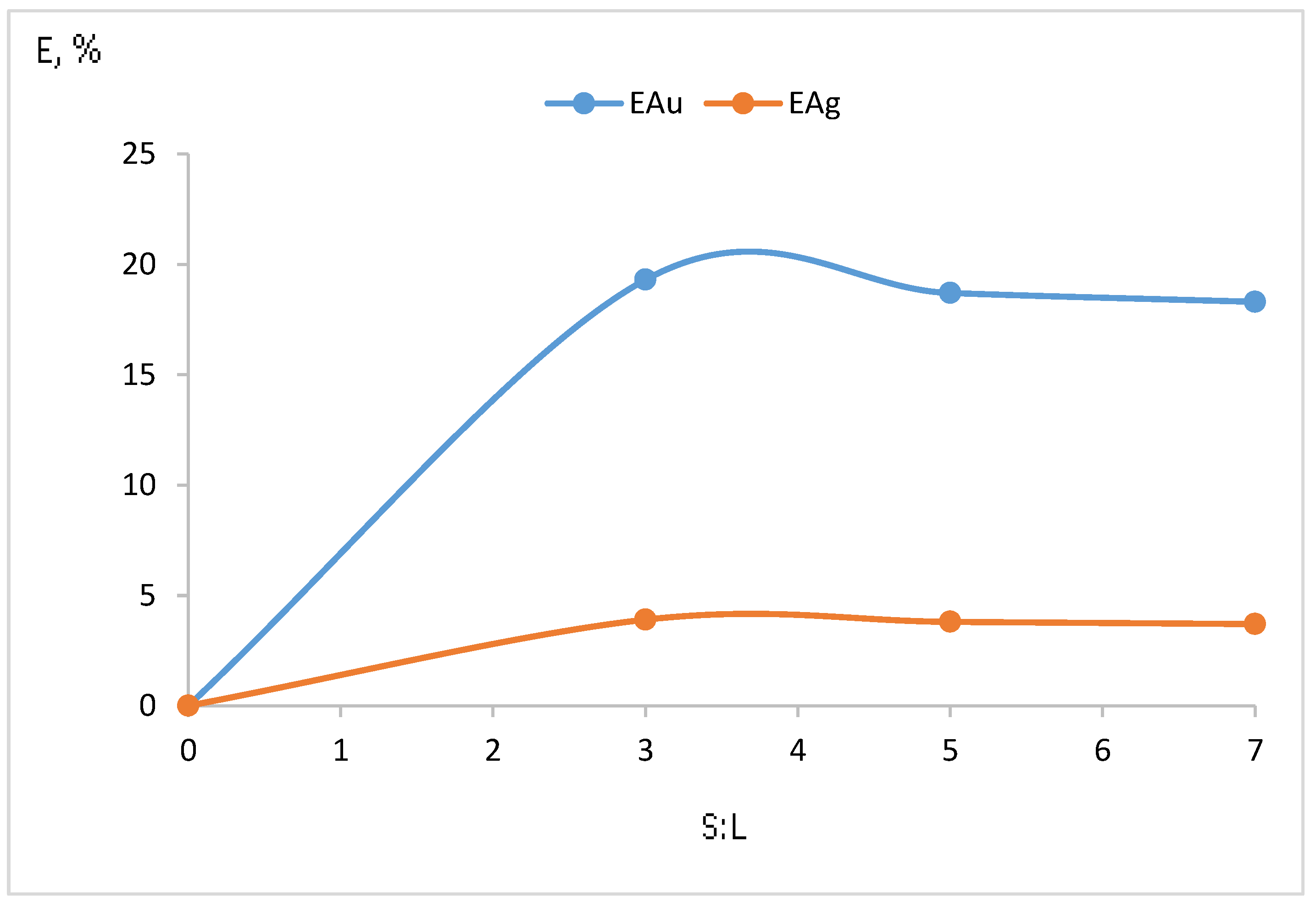
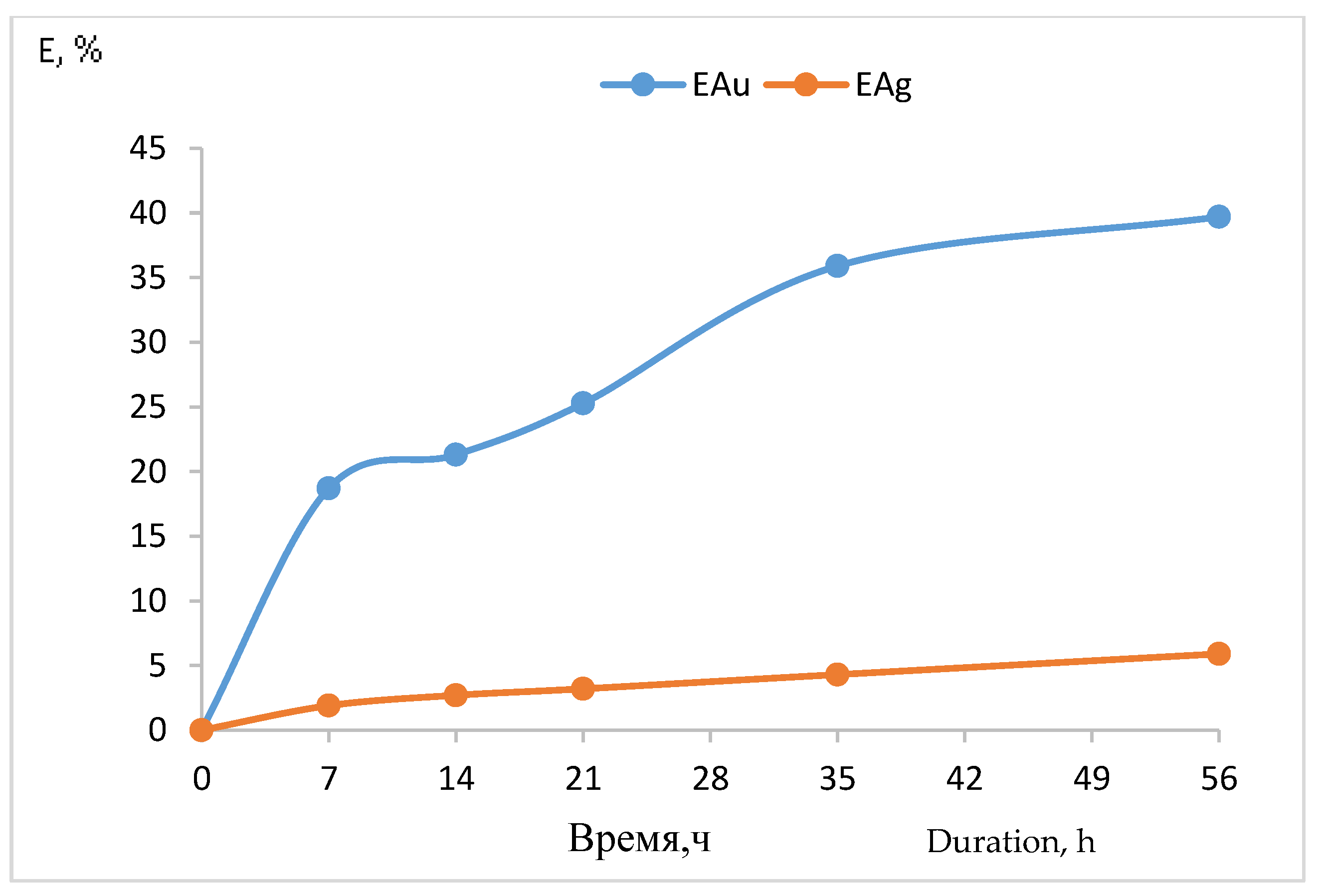
| Experimental Conditions | Time, h | S:L. | Current Density, A/m3 | Au Content, mg/m3 | Extraction Au,% | Ag Content, mg/m3 | Extraction Ag,% |
|---|---|---|---|---|---|---|---|
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 7 | 1:5 | 250 | 0.084 | 5.6 | 0.212 | 1.2 |
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 7 | 1:5 | 500 | 0.174 | 11.6 | 0.443 | 2.5 |
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 7 | 1:5 | 1000 | 0.280 | 18.7 | 0.673 | 3.8 |
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 7 | 1:3 | 1000 | 0.289 | 19.3 | 0.690 | 3.9 |
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 7 | 1:7 | 1000 | 0.275 | 18.3 | 0.655 | 3.7 |
| Pulp: concentrate and sodium chloride 5 g/dm3 + chlorine gas | 56 | 1:5 | 1000 | 0.596 | 39.7 | 1.044 | 5.9 |
| Pulp: concentrate and anolyte from the previous experiment + chlorine gas | 7 | 1:5 | 1000 | 0.465 | 31.0 | 2.389 | 13.5 |
| Electrochlorination | 7 | - | 1000 | 1.360 | 89.7 | 7.274 | 41.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhaliyev, B.; Surkova, T.; Yessimova, D.; Baltabekova, Z.; Abikak, Y.; Abdikerim, B.; Dosymbayeva, Z. Extraction of Noble Metals from Pyrite Cinders. ChemEngineering 2023, 7, 14. https://doi.org/10.3390/chemengineering7010014
Kenzhaliyev B, Surkova T, Yessimova D, Baltabekova Z, Abikak Y, Abdikerim B, Dosymbayeva Z. Extraction of Noble Metals from Pyrite Cinders. ChemEngineering. 2023; 7(1):14. https://doi.org/10.3390/chemengineering7010014
Chicago/Turabian StyleKenzhaliyev, Bagdaulet, Tatiana Surkova, Dinara Yessimova, Zhazira Baltabekova, Yerkezhan Abikak, Bekzat Abdikerim, and Zamzagul Dosymbayeva. 2023. "Extraction of Noble Metals from Pyrite Cinders" ChemEngineering 7, no. 1: 14. https://doi.org/10.3390/chemengineering7010014
APA StyleKenzhaliyev, B., Surkova, T., Yessimova, D., Baltabekova, Z., Abikak, Y., Abdikerim, B., & Dosymbayeva, Z. (2023). Extraction of Noble Metals from Pyrite Cinders. ChemEngineering, 7(1), 14. https://doi.org/10.3390/chemengineering7010014








