Enhancing the Photocatalytic Activity of TiO2/Na2Ti6O13 Composites by Gold for the Photodegradation of Phenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2/Na2Ti6O13 Composite
2.3. Synthesis of Au/TiO2/Na2Ti6O13 Composites
2.4. Characterization of Catalyst
2.5. Photocatalysis of Phenol Degradation
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Characterization
3.2. Scanning Electron Microscope (SEM) Analysis
3.3. Transmission Electron Microscope (TEM) Analysis
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5. UV-Vis Spectroscopy Analysis
3.6. Photocatalytic Activity Test on Phenol Degradation
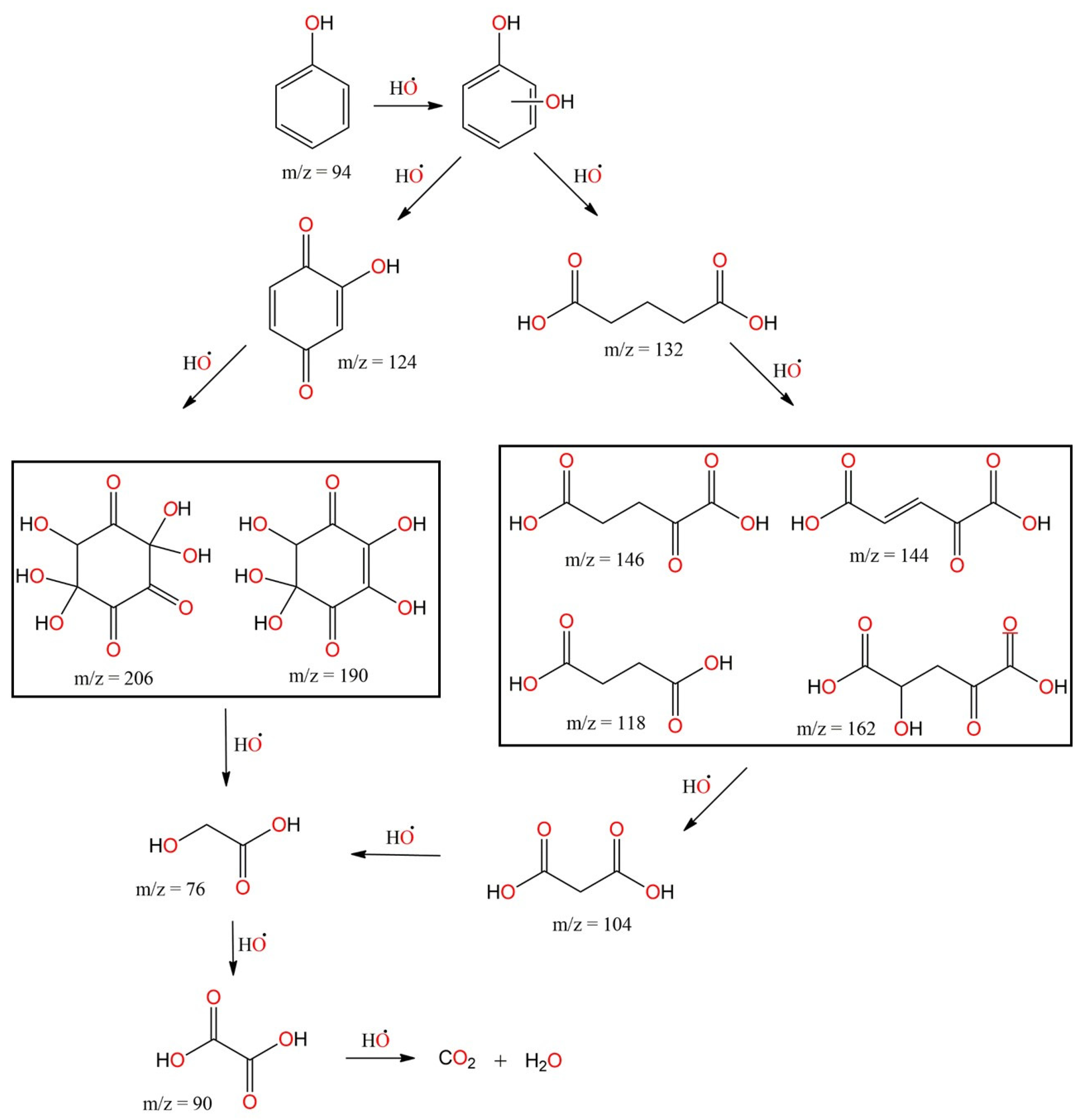
3.7. Degradation Pathway and Identification of the Intermediates
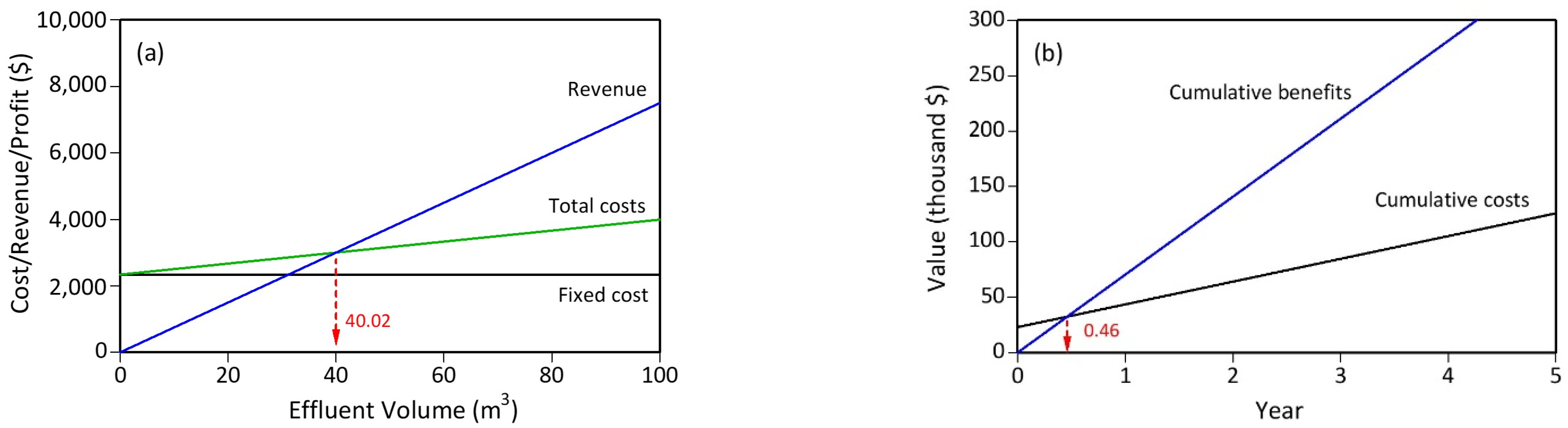
3.8. Large-Scale Economic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2-and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Dutta, K.; Rana, D. Polythiophenes: An emerging class of promising water purifying materials. Eur. Polym. J. 2019, 116, 370–385. [Google Scholar] [CrossRef]
- Babich, H.; Davis, D.L. Phenol: A review of environmental and health risks. Regul. Toxicol. Pharmacol. 1981, 1, 90–109. [Google Scholar] [CrossRef]
- Norouzi, M.; Fazeli, A.; Tavakoli, O. Phenol contaminated water treatment by photocatalytic degradation on electrospun Ag/TiO2 nanofibers: Optimization by the response surface method. J. Water Process Eng. 2020, 37, 101489. [Google Scholar] [CrossRef]
- Peiró, A.M.; Ayllón, J.A.; Peral, J.; Doménech, X. TiO2-photocatalyzed degradation of phenol and ortho-substituted phenolic compounds. Appl. Catal. B 2001, 30, 359–373. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernández, M., Tenango, M.P., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: London, UK, 2017; pp. 419–443. [Google Scholar]
- Lestari, P.R.; Takei, T.; Kumada, N. Novel ZnTi/C3N4/Ag LDH heterojunction composite for efficient photocatalytic phenol degradation. J. Solid State Chem. 2021, 294, 121858. [Google Scholar] [CrossRef]
- Permana, M.D.; Noviyanti, A.R.; Lestari, P.R.; Kumada, N.; Eddy, D.R.; Rahayu, I. Synthesis and Photocatalytic Activity of TiO2 on Phenol Degradation. Kuwait J. Sci. 2021, 49, 3. [Google Scholar] [CrossRef]
- Ameta, R.; Ameta, S.C. Photocatalysis: Principles and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; El Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500. [Google Scholar] [CrossRef]
- Shahzad, W.; Badawi, A.K.; Rehan, Z.A.; Khan, A.M.; Khan, R.A.; Shah, F.; Ali, S.; Ismail, B. Enhanced visible light photocatalytic performance of Sr0.3(Ba, Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 2022, 48, 24979. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Lutfiah, A.; Noviyanti, A.R.; Deawati, Y.; Rahayu, I. Challenges of TiO2-composite, as a visible active photocatalyst material for SARS-CoV-2 antiviral compared with the other viruses. Quím. Nova 2022, 45, 74–82. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Eddy, D.R.; Rahayu, I.; Wyantuti, S.; Hartati, Y.W.; Firdaus, M.L.; Bahti, H.H. Photocatalytic activity of gadolinium doped TiO2 particles for decreasing heavy metal chromium (VI) concentration. J. Phys. Conf. Ser. 2018, 1080, 012013. [Google Scholar] [CrossRef]
- Park, S.M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.I. Hybrid CuxO–TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef]
- Lee, M.S.; Hong, M.S.-S.; Mohseni, M. Synthesis of photocatalytic nanosized TiO2–Ag particles with sol–gel method using reduction agent. J. Mol. Catal. A Chem. 2005, 242, 135–140. [Google Scholar] [CrossRef]
- Roy, P.; Ho, L.; Periasamy, A.P.; Lin, Y.; Huang, M.; Chang, H. Graphene-ZnO-Au nanocomposites based photocatalytic oxidation of benzoic acid. Sciencejet 2015, 4, 1–7. [Google Scholar]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Papp, S.; Kõrösi, L.; Meynen, V.; Cool, P.; Vansant, E.F.; Dékány, I. The influence of temperature on the structural behaviour of sodium tri-and hexa-titanates and their protonated forms. J. Solid State Chem. 2005, 178, 1614–1619. [Google Scholar] [CrossRef]
- Jian, Z.; Huang, S.; Zhang, Y. Photocatalytic degradation of 2,4-dichlorophenol using nanosized Na2Ti6O13/TiO2 heterostructure particles. Int. J. Photoenergy 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.K.; Yuan, J.J.; Yu, H.J.; Zhu, X.R.; Yin, Z.; Shen, H.; Xie, Y.M. Synthesis, conversion, and comparison of the photocatalytic and electrochemical properties of Na2Ti6O13 and Li2Ti6O13 nanobelts. J. Alloys Compd. 2015, 631, 171–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Fu, B.; Li, W.; Guo, W.; Ruan, S.; Liu, D.; Chen, Y. Sodium Titanate Nanorod Moisture Sensor and Its Sensing Mechanism. IEEE Electron Device Lett. 2013, 34, 424–1426. [Google Scholar] [CrossRef]
- Wang, B.L.; Chen, Q.; Wang, L.H.; Peng, L.M. Synthesis and characterization of K2Ti6O13 nanowires. Chem. Phys. Lett. 2003, 376, 726–731. [Google Scholar] [CrossRef]
- Alam, U.; Ali, D.; Bahnemann, D.; Muneer, M. Surface modification of Na-K2Ti6O13 photocatalyst with Cu (II)-nanocluster for efficient visible-light-driven photocatalytic activity. Mater. Lett. 2018, 220, 50–53. [Google Scholar]
- Štengl, V.; Bakardjieva, S.; Šubrt, J.; Večerníková, E.; Szatmary, L.; Klementová, M.; Balek, V. Sodium titanate nanorods: Preparation, microstructure characterization and photocatalytic activity. Appl. Catal. B 2006, 63, 20–30. [Google Scholar] [CrossRef]
- Torres-Martínez, L.M.; Sánchez-Trinidad, C.; Rodríguez-González, V.; Lee, S.W.; Gómez, R. Synthesis, characterization, and 2,4-dichlorophenoxyacetic acid degradation on In-Na2Ti6O13 sol–gel prepared photocatalysts. Res. Chem. Intermed. 2010, 36, 5–15. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Zhao, X. A visible-light-active Au-Cu(I)@Na2Ti6O13 nanostructured hybrid pasmonic photocatalytic membrane for acetaldehyde elimination. Chin. J. Catal. 2017, 38, 2048–2055. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, L.I.; Huerta-Flores, A.M.; Torres-Martínez, L.M. Development of Na2Ti6O13/CuO/Cu2O heterostructures for solar photocatalytic production of low-carbon fuels. Mater. Res. Bull. 2020, 122, 110679. [Google Scholar] [CrossRef]
- Zhu, X.; Anzai, A.; Yamamoto, A.; Yoshida, H. Silver-loaded sodium titanate photocatalysts for selective reduction of carbon dioxide to carbon monoxide with water. Appl. Catal. B 2019, 243, 47–56. [Google Scholar] [CrossRef]
- Grover, I.S.; Singh, S.; Pal, B. A comprehensive systematic review of photocatalytic degradation of pesticides using nano TiO2. RSC Adv. 2014, 4, 51342–51348. [Google Scholar] [CrossRef]
- da Silva, J.P.; Biondo, M.M.; Nobre, F.X.; Anglada-Rivera, J.; Almeida, A.; Agostinho-Moreira, J.; Sanches, E.A.; Paula, M.D.S.; Aguilera, L.; Leyet, Y. Structure and electrical properties of the composite Na2Ti3O7/Na2Ti6O13/POMA: A study of the effect of adding POMA. J. Alloys Compd. 2021, 867, 159025. [Google Scholar] [CrossRef]
- Vázquez-Cuchillo, O.; Gómez, R.; Cruz-López, A.; Torres-Martínez, L.M.; Zanella, R.; Sandoval, F.A.; Del Ángel-Sánchez, K. Improving water splitting using RuO2-Zr/Na2Ti6O13 as a photocatalyst. J. Photochem. Photobiol. A 2013, 266, 6–11. [Google Scholar] [CrossRef]
- Zheng, H.; Svengren, H.; Huang, Z.; Yang, Z.; Zou, X.; Johnsson. Hollow titania spheres loaded with noble metal nanoparticles for photocatalytic water oxidation. Microporous Mesoporous Mater. 2018, 264, 147–150. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Roy, M.; Kundu, S.; Naskar, M.K. TiO2 hollow microspheres impregnated with biogenic gold nanoparticles for the efficient visible light-induced photodegradation of phenol. J. Phys. Chem. Solids 2019, 129, 329–339. [Google Scholar] [CrossRef]
- Stevenson, K.J. Review of originpro 8.5. J. Am. Chem. Soc. 2011, 133, 5621. [Google Scholar] [CrossRef]
- Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L. Synthesis of titanium dioxide/silicon dioxide from beach sand as photocatalyst for Cr and Pb remediation. Catalysts 2020, 10, 1248. [Google Scholar] [CrossRef]
- Lestari, P.R.; Takei, T.; Yanagida, S.; Kumada, N. Facile and controllable synthesis of Zn-Al layered double hydroxide/silver hybrid by exfoliation process and its plasmonic photocatalytic activity of phenol degradation. Mater. Chem. Phys. 2020, 250, 122988. [Google Scholar] [CrossRef]
- Al-Owaisi, M.; Al-Hadiwi, N.; Khan, S.A. GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 2014, 4, 964–970. [Google Scholar] [CrossRef]
- Murugesan, S.; Kuppusami, P.; Mohandas, E. Rietveld X-ray diffraction analysis of nanostructured rutile films of titania prepared by pulsed laser deposition. Mater. Res. Bull. 2010, 45, 6–9. [Google Scholar] [CrossRef]
- Torres-Martínez, L.M.; Juárez-Ramírez, I.; Del Ángel-Sánchez, K.; Garza-Tovar, L.; Cruz-López, A.; Del Ángel, G. Rietveld refinement of sol–gel Na2Ti6O13 and its photocatalytic performance on the degradation of methylene blue. J. Sol-Gel Sci. Technol. 2008, 47, 158–164. [Google Scholar] [CrossRef]
- Tatge, E.; Swanson, H.E. Zeitschrift fuer Kristallographie, Kristallgeometrie, Kristallphysik. Kristallchemie 1956, 107, 357–361. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The highscore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Yang, W.; Shen, H.; Min, H.; Ge, J. Enhanced visible light-driven photodegradation of rhodamine B by Ti3+ self-doped TiO2@Ag nanoparticles prepared using Ti vapor annealing. J. Mater. Sci. 2020, 55, 701–712. [Google Scholar] [CrossRef]
- Bargui, M.; Messaoud, M.; Elleuch, K. Electrophoretic impregnation of porous anodizing layer by synthesized TiO2 nanoparticles. Surf. Eng. Appl. Electrochem. 2017, 53, 467–474. [Google Scholar] [CrossRef]
- Alam, N.; Khatoon, T.; Chandel, V.S.; Azam, A. Comparative Analysis of Sodium Hexa-titanate (Na2Ti6O13) & Sodium-Potassium Hexa-titanate (Na1.5K0.5Ti6O13). J. Phys. Conf. Ser. 2020, 1495, 012034. [Google Scholar]
- Shirpour, M.; Cabana, J.; Doeff, M. New materials based on a layered sodium titanate for dual electrochemical Na and Li intercalation systems. Energy Environ. Sci. 2013, 6, 2538–2547. [Google Scholar] [CrossRef]
- Bobrova, A.M.; Zhigun, I.G.; Bragina, M.I.; Fotiev, A.A. Infrared absorption spectra of various titanium compounds. Appl. Spectrosc. 1968, 8, 59–63. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S.; Ohtani, B. Plasmonic titania photocatalysts active under UV and visible-light irradiation: Influence of gold amount, size, and shape. J. Nanotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Ribeiro, M.R.; Pontes, M.J.; Nogueira, B.V.; Guimaraes, M.C. A helpful method for controlled synthesis of monodisperse gold nanoparticles through response surface modeling. Arab. J. Chem. 2020, 13, 216–226. [Google Scholar] [CrossRef]
- Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L.; Rahayu, I.; El-Badry, Y.A.; Hussein, E.E.; El-Bahy, Z.M. Photocatalytic Phenol Degradation by Silica-Modified Titanium Dioxide. Appl. Sci. 2021, 11, 9033. [Google Scholar] [CrossRef]
- Kolaei, M.; Tayebi, M.; Lee, B.K. The synergistic effects of acid treatment and silver (Ag) loading for substantial improvement of photoelectrochemical and photocatalytic activity of Na2Ti3O7/TiO2 nanocomposite. Appl. Surf. Sci. 2021, 540, 148359. [Google Scholar] [CrossRef]
- Swamy, N.K.; Singh, P.; Sarethy, I.P. Precipitation of phenols from paper industry wastewater using ferric chloride. Rasayan J. Chem. 2011, 4, 452–456. [Google Scholar]
- Massoudinejad, M.; Mehdipour-Rabori, M.; Dehghani, M.H. Treatment of natural rubber industry wastewater through a combination of physicochemical and ozonation processes. J. Adv. Environ. Health Res. 2015, 3, 242–249. [Google Scholar]
- Yaseen, D.A.; Scholz, M. extile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, S.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Novel highly active anatase/rutile TiO2 photocatalyst with hydrogenated heterophase interface structures for photoelectrochemical water splitting into hydrogen. ACS Sustain. Chem. Eng. 2018, 6, 10823–10832. [Google Scholar] [CrossRef]
- Ding, L.; Yang, S.; Liang, Z.; Qian, X.; Chen, X.; Cui, H.; Tian, J. TiO2 nanobelts with anatase/rutile heterophase junctions for highly efficient photocatalytic overall water splitting. J. Colloid Interface Sci. 2020, 567, 181–189. [Google Scholar] [CrossRef]
- Pu, S.; Hou, Y.; Chen, H.; Deng, D.; Yang, Z.; Xue, S.; Zhu, R.; Diao, Z.; Chu, W. An efficient photocatalyst for fast reduction of Cr (VI) by ultra-trace silver enhanced titania in aqueous solution. Catalysts 2018, 8, 251. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; El-Bahy, Z.M. Effect of microwave heating on the structure, morphology and photocatalytic activity of hydrogen titanate nanotubes. J. Environ. Chem. Eng. 2015, 3, 744–751. [Google Scholar] [CrossRef]
- Salama, T.M.; El-Bahy, Z.M.; Zidan, F.I. Aqueous H2O2 as an oxidant for CO over Pt–and Au–NaY catalysts. J. Mol. Catal. A Chem. 2007, 264, 128–134. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 141253. [Google Scholar] [CrossRef] [PubMed]
- Garay-Rodríguez, L.F.; Torres-Martínez, L.M. Photocatalytic CO2 reduction over A2Ti6O13 (A= Na and K) titanates synthesized by different pH-catalyzed sol–gel. J. Sol-Gel Sci. Technol. 2020, 93, 428–437. [Google Scholar] [CrossRef]
- Chenchana, A.; Nemamcha, A.; Moumeni, H.; Rodríguez, J.D.; Araña, J.; Navío, J.A.; Díaz, O.G.; Melián, E.P. Photodegradation of 2,4-dichlorophenoxyacetic acid over TiO2 (B)/anatase nanobelts and Au-TiO2 (B)/anatase nanobelts. Appl. Surf. Sci. 2019, 467, 1076–1087. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Zeng, H.; Lin, H.; Li, H.; Tham, K.O.; Mohamed, A.R.; Lim, J.W.; Qin, Z. Magnetic NiFe2O4 nanoparticles decorated on N-doped BiOBr nanosheets for expeditious visible light photocatalytic phenol degradation and hexavalent chromium reduction via a Z-scheme heterojunction mechanism. Appl. Surf. Sci. 2021, 559, 149966. [Google Scholar] [CrossRef]
- Ren, T.; Jin, Z.; Yang, J.; Hu, R.; Zhao, F.; Gao, X.; Zhao, C. Highly efficient and stable p-LaFeO3/n-ZnO heterojunction photocatalyst for phenol degradation under visible light irradiation. J. Hazard. Mater. 2019, 377, 195–205. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nag, S.; Ray, A.K. Degradation of phenolic compounds through UV and visible-light-driven photocatalysis: Technical and economic aspects. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.R., Eds.; IntechOpen: London, UK, 2017; Volume 16, pp. 395–417. [Google Scholar]
- Bockenstedt, J.; Vidwans, N.A.; Gentry, T.; Vaddiraju, S. Catalyst recovery, regeneration and reuse during large-scale disinfection of water using photocatalysis. Water 2021, 13, 2623. [Google Scholar] [CrossRef]
- Ray, A.K. Design, modelling and experimentation of a new large-scale photocatalytic reactor for water treatment. Chem. Eng. Sci. 1999, 54, 3113–3125. [Google Scholar] [CrossRef]
- Mueses, M.A.; Colina-Márquez, J.; Machuca-Martínez, F.; Puma, G.L. Recent advances on modeling of solar heterogeneous photocatalytic reactors applied for degradation of pharmaceuticals and emerging organic contaminants in water. Curr. Opin. Green Sustain. Chem. 2021, 30, 100486. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; San Martín, I. Photocatalytic treatment of an industrial effluent using artificial and solar UV radiation: An operational cost study on a pilot plant scale. J. Environ. Manag. 2012, 98, 1–4. [Google Scholar] [CrossRef]
- Feitz, A.J.; Boyden, B.H.; Waite, T.D. Evaluation of two solar pilot scale fixed-bed photocatalytic reactors. Water Res. 2000, 34, 3927–3932. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward scaling-up photocatalytic process for multiphase environmental applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Baaloudj, O.; Badawi, A.K.; Kenfoud, H.; Benrighi, Y.; Hassan, R.; Nasrallah, N.; Assadi, A.A. Techno-economic studies for a pilot-scale Bi12TiO20 based photocatalytic system for pharmaceutical wastewater treatment: From laboratory studies to commercial-scale applications. J. Water Process Eng. 2022, 48, 102847. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Sponza, D.T.; Güney, G. Photodegradation of some brominated and phenolic micropollutants in raw hospital wastewater with CeO2 and TiO2 nanoparticles. Water Sci. Technol. 2017, 76, 2603–2622. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Gopalram, K.; Appunni, S. Photocatalytic degradation of 2,4-dicholorophenoxyacetic acid by TiO2 modified catalyst: Kinetics and operating cost analysis. Environ. Sci. Pollut. Res. 2021, 28, 33331–33343. [Google Scholar] [CrossRef]
- Ragadhita, R.; Nandiyanto, A.B.D.; Maulana, A.C.; Oktiani, R.; Sukmafitri, A.; Machmud, A.; Surachman, E. Techno-economic analysis for the production of titanium dioxide nanoparticle produced by liquid-phase synthesis method. J. Eng. Sci. Technol. 2019, 14, 1639–1652. [Google Scholar]
- Nurdiana, A.; Astuti, L.; Dewi, R.P.; Ragadhita, R.; Nandiyanto, A.B.D.; Kurniawan, T. Techno-economic analysis on the production of zinc sulfide nanoparticles by microwave irradiation method. ASEAN J. Sci. Eng. 2022, 2, 143–156. [Google Scholar] [CrossRef]
- Eustis, S.; Hsu, H.Y.; El-Sayed, M.A. Gold nanoparticle formation from photochemical reduction of Au3+ by continuous excitation in colloidal solutions. A proposed molecular mechanism. J. Phys. Chem. B 2005, 109, 4811–4815. [Google Scholar] [CrossRef] [PubMed]

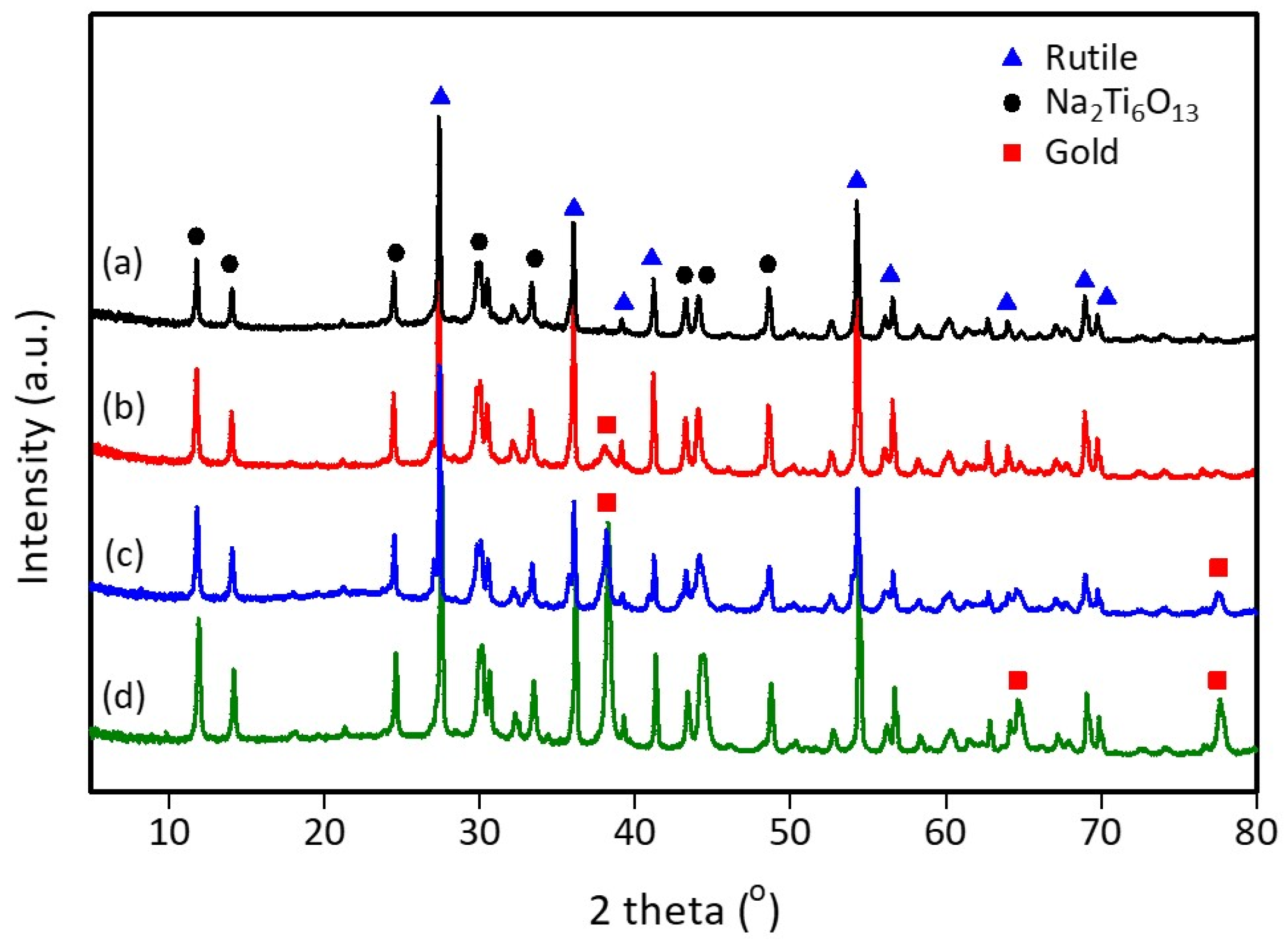
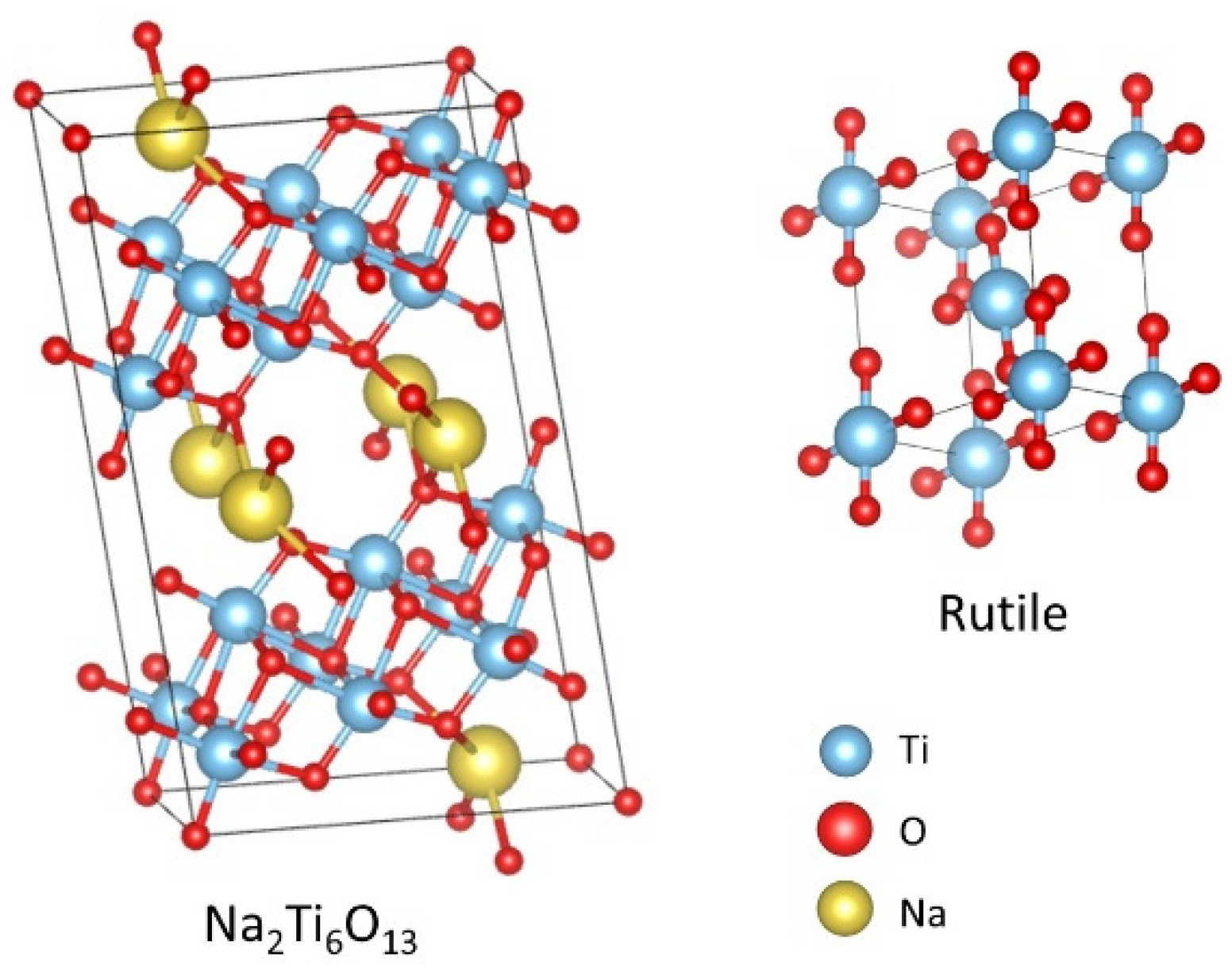
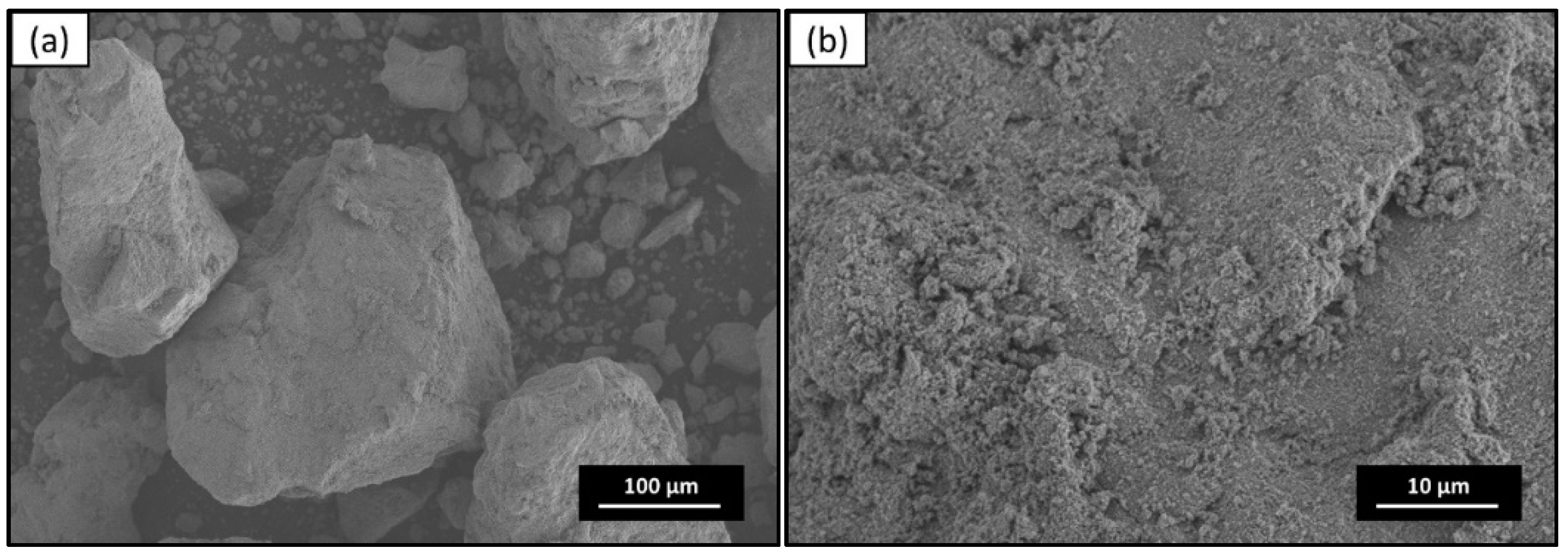






| Sample | Rietveld Refinement Parameters | ||
|---|---|---|---|
| Rexp | Rwp | GoF | |
| TiO2/NTO | 3.07 | 6.00 | 3.82 |
| 1% Au/TiO2/NTO | 2.77 | 5.73 | 4.28 |
| 2% Au/TiO2/NTO | 2.73 | 5.90 | 4.67 |
| 3% Au/TiO2/NTO | 2.57 | 5.02 | 3.81 |
| Sample | Crystal Phase (%) | Crystallite Size (nm) * | Crystallinity (%) | ||||
|---|---|---|---|---|---|---|---|
| Rutile | Na2Ti6O13 | Gold | Rutile | Na2Ti6O13 | Gold | ||
| TiO2/NTO | 43.4 | 56.6 | - | 38.08 ± 5.98 | 25.20 ± 4.35 | - | 76.05 |
| 1% Au/TiO2/NTO | 48.9 | 50.9 | 0.2 | 38.96 ± 4.77 | 25.60 ± 3.59 | 16.42 ± 4.72 | 83.32 |
| 2% Au/TiO2/NTO | 41.8 | 54.4 | 3.8 | 39.48 ± 4.25 | 25.39 ± 6.21 | 16.95 ± 4.07 | 83.00 |
| 3% Au/TiO2/NTO | 44.0 | 50.0 | 6.0 | 40.10 ± 4.90 | 25.08 ± 5.48 | 19.57 ± 4.90 | 84.48 |
| Sample | Rutile | Na2Ti6O13 | Gold | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a = b (Å) | c (Å) | V (Å3) | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) | a = b = c (Å) | V (Å3) | |
| ICSD 98-016-5920 | 4.600 | 2.960 | 62.630 | - | - | - | - | - | - | - |
| ICSD 98-016-3491 | - | - | - | 15.095 | 3.745 | 9.174 | 99.010 | 512.210 | - | - |
| ICSD 98-006-4701 | - | - | - | - | - | - | - | - | 4.079 | 67.870 |
| TiO2/NTO | 4.593 | 2.958 | 62.414 | 15.131 | 3.740 | 9.171 | 99.110 | 512.451 | - | - |
| 1% Au/TiO2/NTO | 4.592 | 2.958 | 62.371 | 15.123 | 3.739 | 9.173 | 99.081 | 512.183 | 4.079 | 67.867 |
| 2% Au/TiO2/NTO | 4.594 | 2.958 | 62.436 | 15.147 | 3.741 | 9.170 | 99.128 | 513.004 | 4.081 | 67.982 |
| 3% Au/TiO2/NTO | 4.593 | 2.958 | 62.416 | 15.131 | 3.740 | 9.173 | 99.087 | 512.581 | 4.079 | 67.865 |
| Composite | Application | Ref. |
|---|---|---|
| CuO/Cu2O/Na2Ti6O13 | Photocatalytic H2 evolution and CO2 reduction | [31] |
| In/Na2Ti6O13 | Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid | [29] |
| TiO2/Na2Ti6O13 | Photocatalytic degradation of 2,4-dichlorophenol | [23] |
| Ag/TiO2/Na2Ti3O7 | Photocatalytic degradation of RhB | [56] |
| Au/TiO2/Na2Ti6O13 | Photocatalytic degradation of phenol | This research |
| Sample | Phenol Adsorption (%) | Phenol Degradation (%) | Langmuir-Hinshelwood Kinetic | ||
|---|---|---|---|---|---|
| k1 (min−1) | r0 (mg/L·min) | R2 | |||
| TiO2 P25 | 1.41 | 73.13 | 0.0108 | 0.2160 | 0.8663 |
| TiO2/NTO | 1.68 | 57.17 | 0.0071 | 0.1420 | 0.8859 |
| 1% Au/TiO2/NTO | 1.81 | 58.40 | 0.0076 | 0.1520 | 0.9246 |
| 2% Au/TiO2/NTO | 2.88 | 60.40 | 0.0082 | 0.1640 | 0.9355 |
| 3% Au/TiO2/NTO | 3.39 | 82.94 | 0.0146 | 0.2920 | 0.9092 |
| Capitals Cost | |||
|---|---|---|---|
| Fabrication reactor cost (V = 0.36 m3) | 4000 USD/m3 | USD 1440 | |
| Mechanical and electrical equipment | 2500 USD/m3 | USD 900 | |
| Total | USD 2340 | ||
| Operating Costs | |||
| Catalytic material preparation cost Au/TiO2/NTO (for 0.15 g/L dose) | |||
| TTIP (4 g for 1 g TiO2/NTO) | 0.534 kg TTIP/m3 | RC = 1800 USD/ton | 0.961 USD/m3 |
| HPC (0.4 g for 1 g TiO2/NTO) | 0.057 kg HPC/m3 | RC = 6500 USD/ton | 0.370 USD/m3 |
| NaOH (0.8 g for 1 g TiO2/NTO) | 0.114 kg NaOH/m3 | RC = 500 USD/ton | 0.057 USD/m3 |
| HCl (0.38 g for 1 g TiO2/NTO) | 0.055 kg HCl/m3 | RC = 200 USD/ton | 0.011 USD/m3 |
| NaBH4 (0.011 g for 1 g TiO2/NTO) | 3.246 g NaBH4/m3 | RC = 30 USD/kg | 0.097 USD/m3 |
| HauCl4 (0.047 g for 1 g TiO2/NTO) | 13.47 g HauCl4/m3 | RC = 20,000 USD/kg | 269.400 USD/m3 |
| Total | 270.896 USD/m3 | ||
| Energy Consumption Cost | |||
| UV lamps (2 × 400 kWh) | 4.44 kWh/m3 | 0.074 USD/kWh | 0.329 USD/m3 |
| Pump and stirrer (400 kWh) | 2.22 kWh/m3 | 0.074 USD/kWh | 0.164 USD/m3 |
| Total | 0.493 USD/m3 | ||
| Material Treatment Cost | 13.545 USD/m3 | ||
| Technicians Cost | 14.467 USD/m3 | ||
| Total | 28.012 USD/m3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permana, M.D.; Noviyanti, A.R.; Lestari, P.R.; Kumada, N.; Eddy, D.R.; Rahayu, I. Enhancing the Photocatalytic Activity of TiO2/Na2Ti6O13 Composites by Gold for the Photodegradation of Phenol. ChemEngineering 2022, 6, 69. https://doi.org/10.3390/chemengineering6050069
Permana MD, Noviyanti AR, Lestari PR, Kumada N, Eddy DR, Rahayu I. Enhancing the Photocatalytic Activity of TiO2/Na2Ti6O13 Composites by Gold for the Photodegradation of Phenol. ChemEngineering. 2022; 6(5):69. https://doi.org/10.3390/chemengineering6050069
Chicago/Turabian StylePermana, Muhamad Diki, Atiek Rostika Noviyanti, Putri Rizka Lestari, Nobuhiro Kumada, Diana Rakhmawaty Eddy, and Iman Rahayu. 2022. "Enhancing the Photocatalytic Activity of TiO2/Na2Ti6O13 Composites by Gold for the Photodegradation of Phenol" ChemEngineering 6, no. 5: 69. https://doi.org/10.3390/chemengineering6050069
APA StylePermana, M. D., Noviyanti, A. R., Lestari, P. R., Kumada, N., Eddy, D. R., & Rahayu, I. (2022). Enhancing the Photocatalytic Activity of TiO2/Na2Ti6O13 Composites by Gold for the Photodegradation of Phenol. ChemEngineering, 6(5), 69. https://doi.org/10.3390/chemengineering6050069








