Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen
Abstract
:1. Introduction
2. Materials and Methods
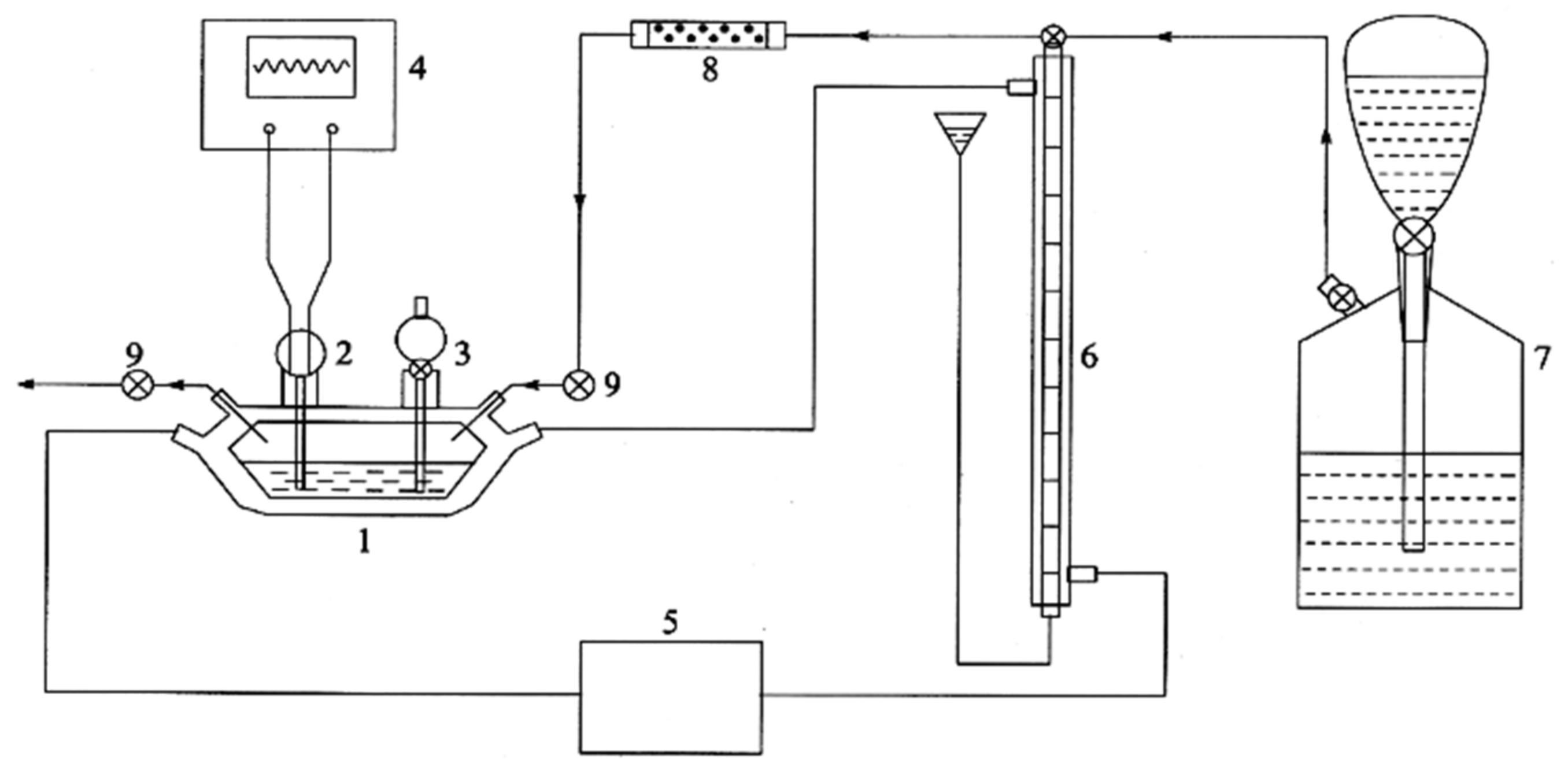
2.1. Preparation of Magnetic Nanocomposites
2.2. Tests of Synthesized Catalysts in the Process of Oxidation of Phenol with Oxygen
2.3. Conducting an Analysis
3. Results
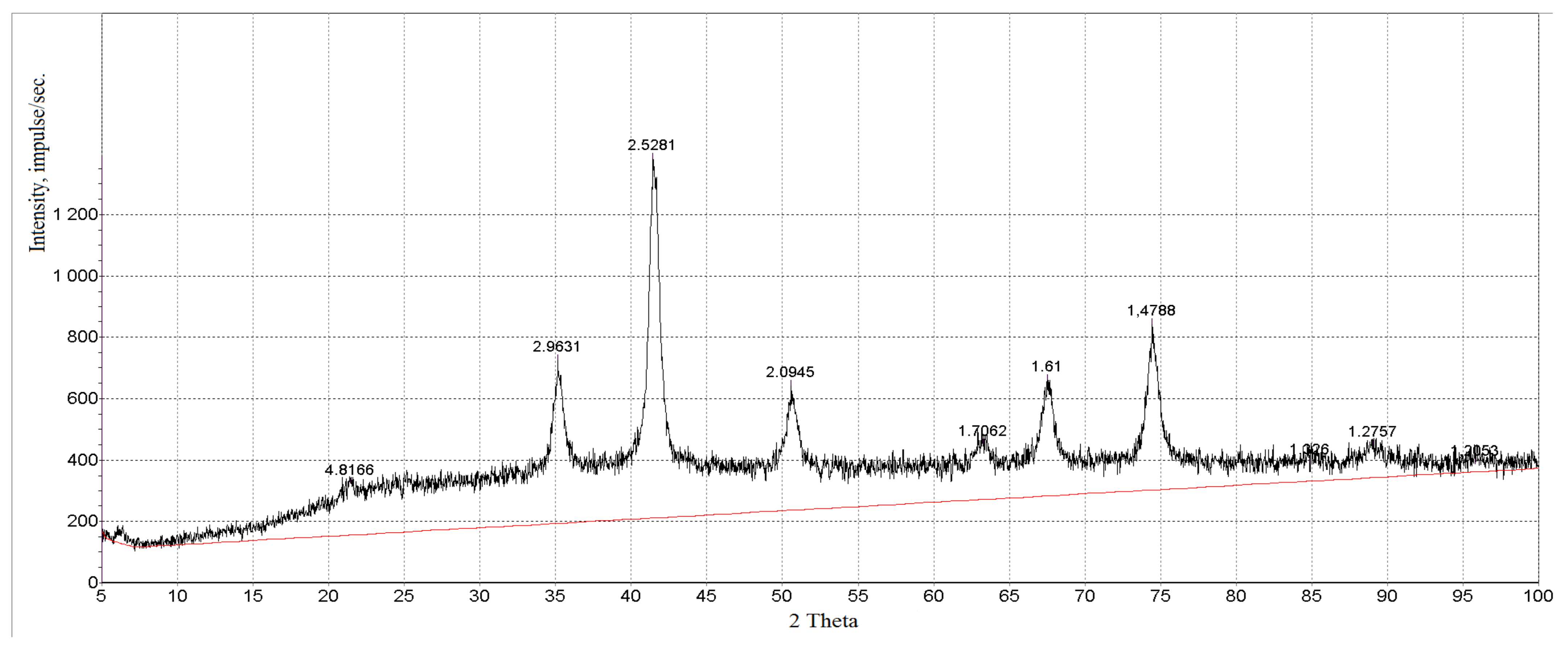
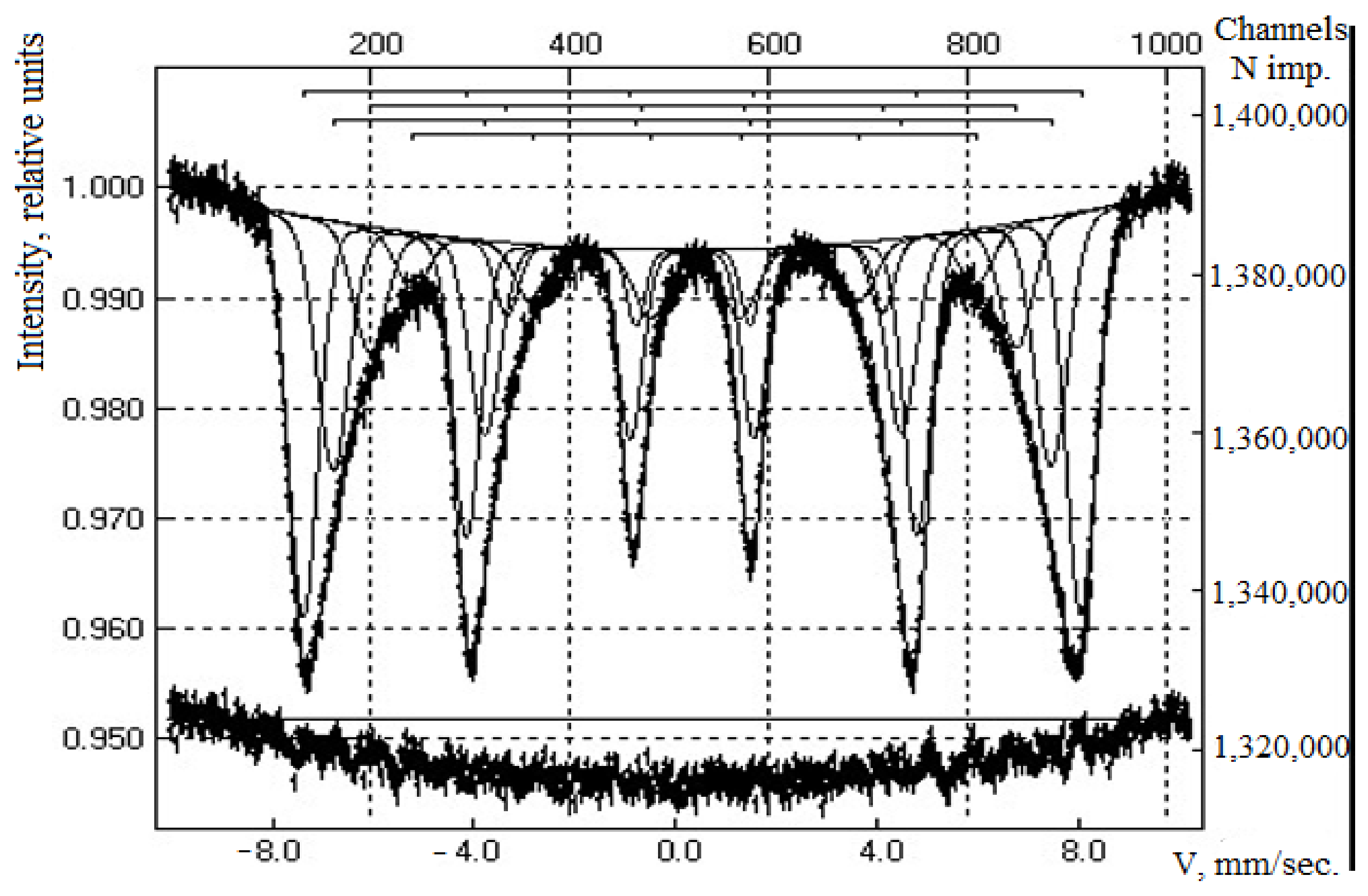
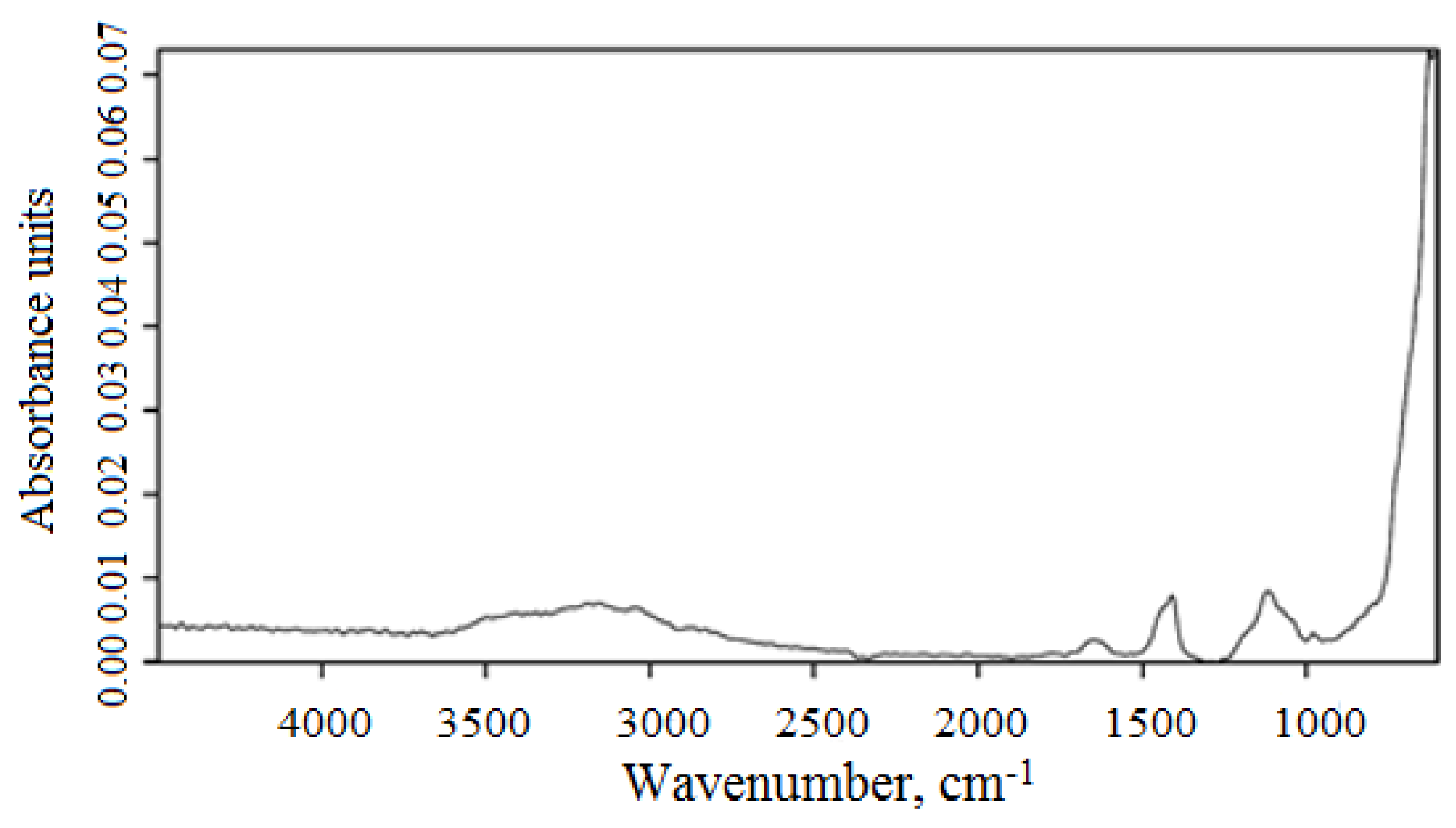
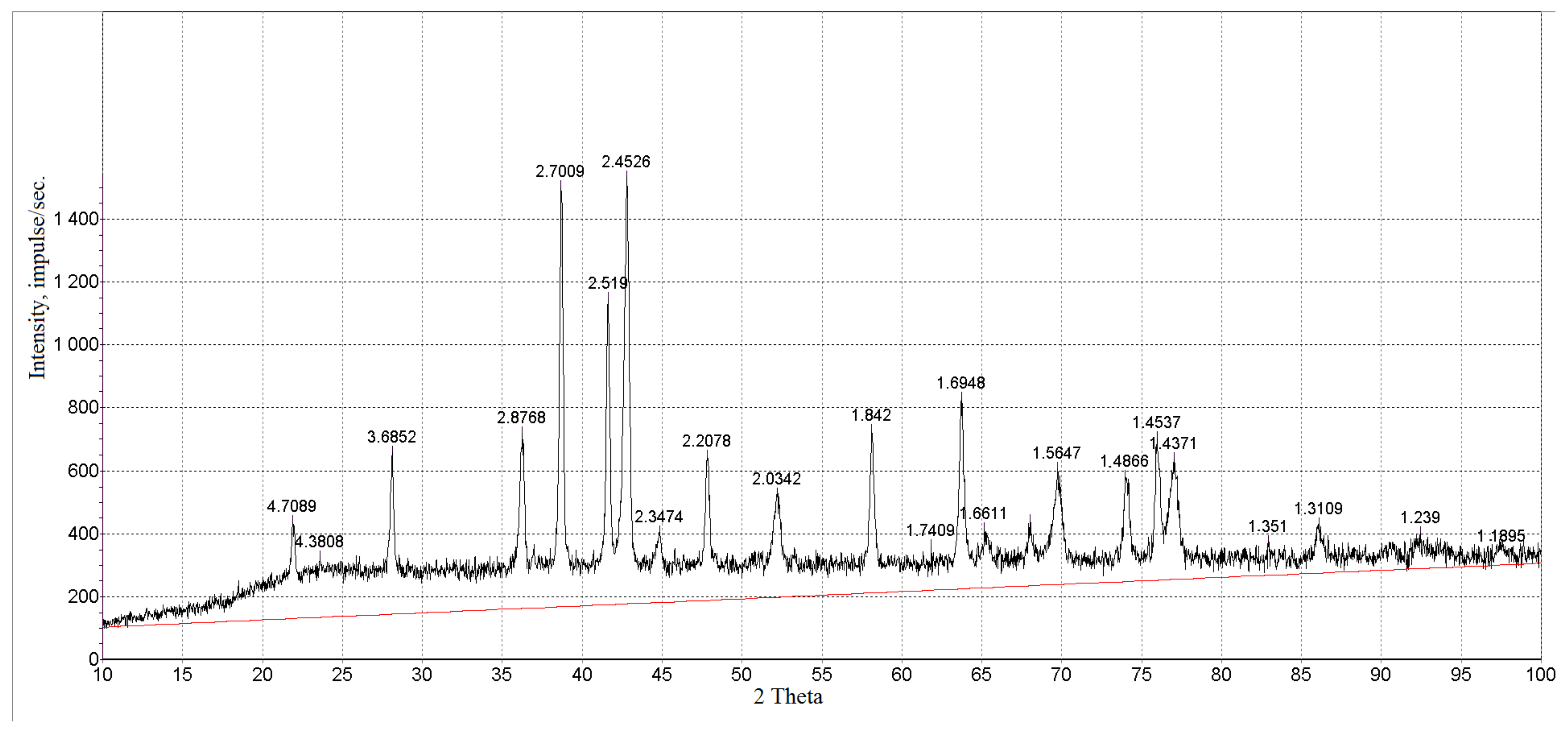
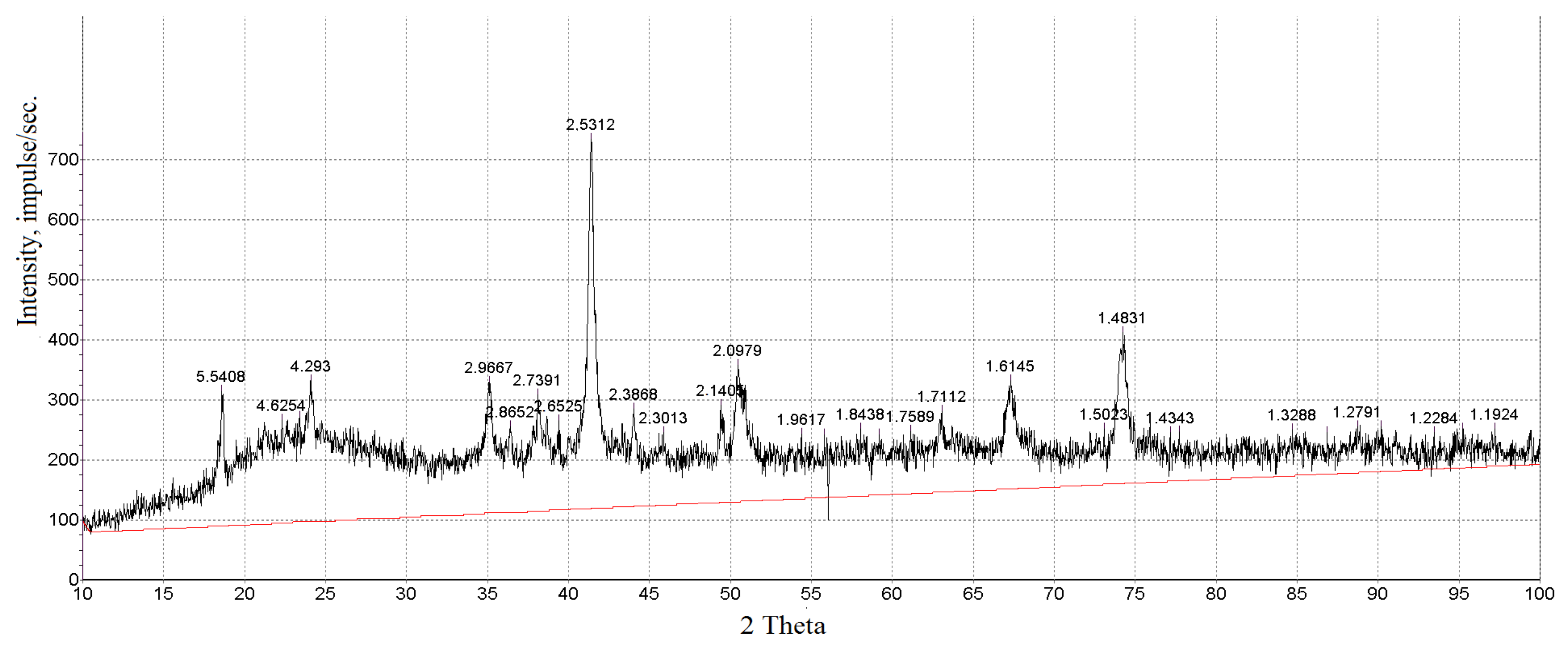
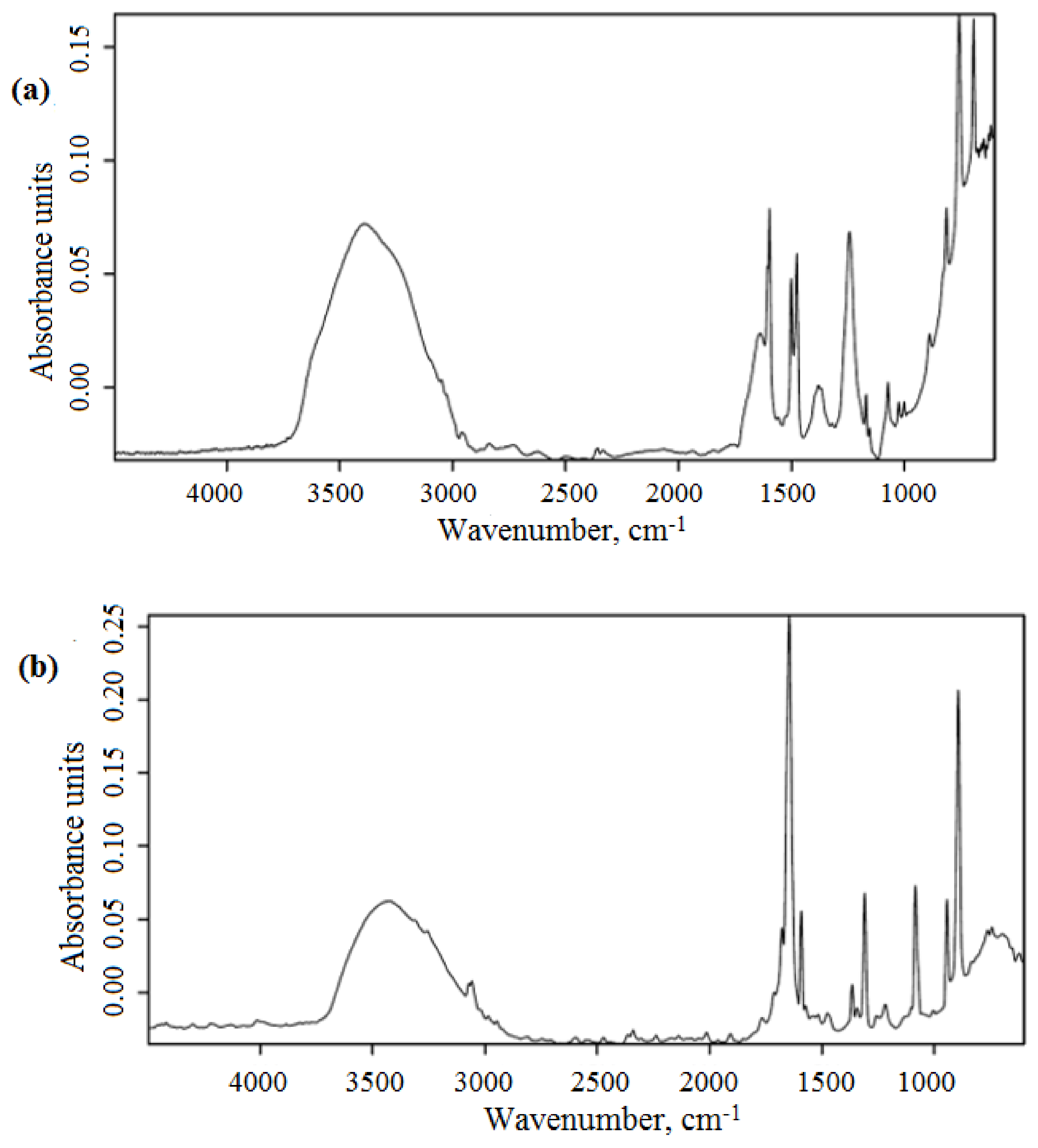
3.1. Characterization of Magnetic Composites
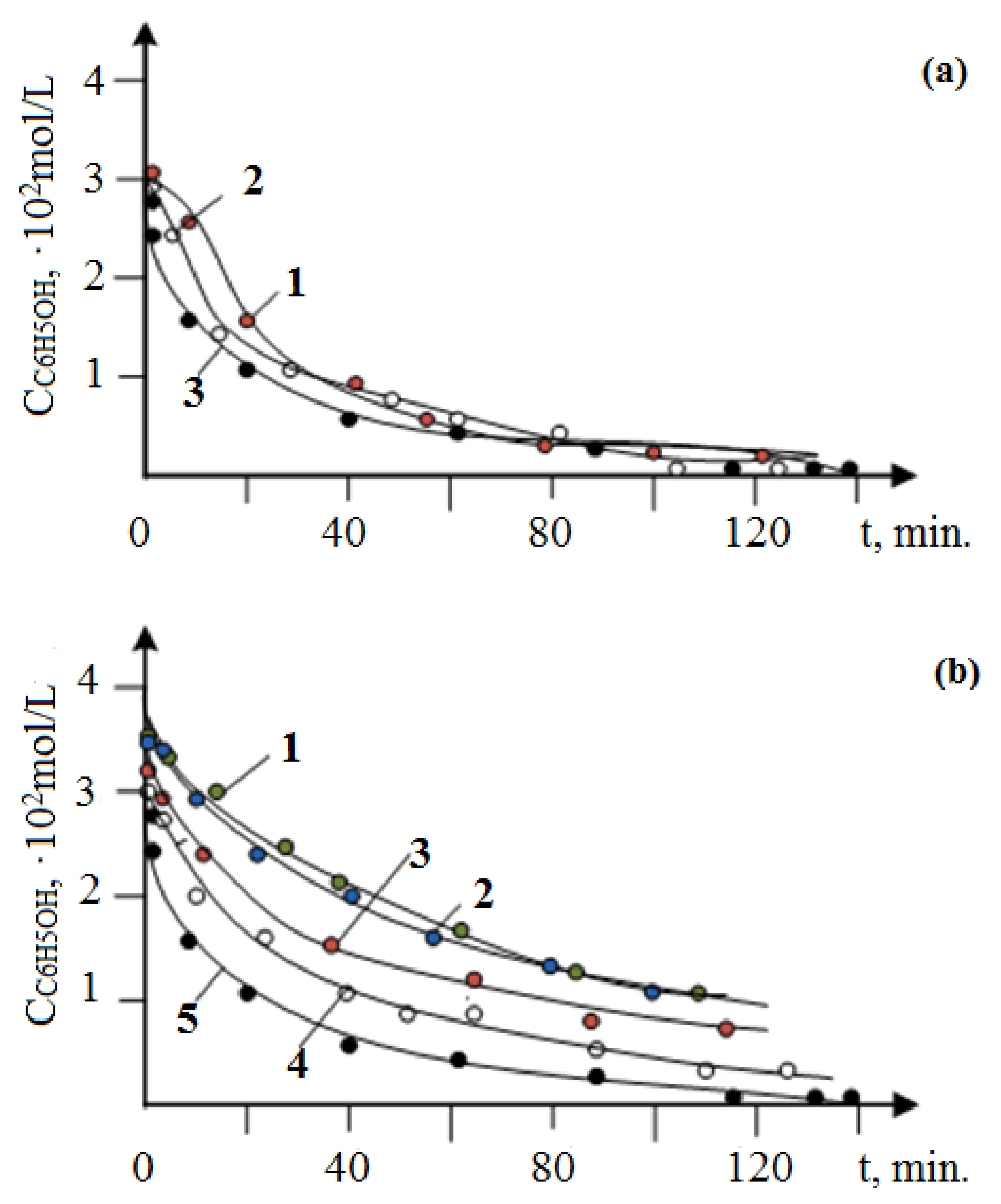
3.2. Results of Testing the Synthesized Catalysts in the Oxidation of Phenol with Oxygen
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maurya, M.R.; Titinchi, S.J.J.; Chand, S. Oxidation of phenol with H2O2 catalysed by Cr(III), Fe(III) or Bi(III) N,N′-bis(salicylidene)diethylenetriamine (H2saldien) complexes encapsulated in zeolite-Y. J. Mol. Catal. A Chem. 2003, 193, 165–176. [Google Scholar] [CrossRef]
- Barrault, J. Catalytic wet peroxide oxidation over mixed (Al–Fe) pillared clays. Appl. Catal. B 2000, 27, L225–L230. [Google Scholar] [CrossRef]
- Guélou, E.; Barrault, J.; Fournier, J.; Tatibouët, J.M. Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl. Catal. B 2003, 44, 1–8. [Google Scholar] [CrossRef]
- Carriazo, J.; Guélou, E.; Barrault, J.; Tatibouët, J.M.; Molina, R.; Moreno, S. Catalytic wet peroxide oxidation of phenol by pillared clays containing Al–Ce–Fe. Water Res. 2005, 39, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical Pathway and Kinetics of Phenol Oxidation by Fenton’s Reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Al-Dahhan, M. Catalytic wet air oxidation of phenol in concurrent downflow and upflow packed-bed reactors over pillared clay catalyst. Chem. Eng. Sci. 2005, 60, 735–746. [Google Scholar] [CrossRef]
- Krajnc, M.; Levec, J. Oxidation of Phenol over a Transition-Metal Oxide Catalyst in Supercritical Water. Ind. Eng. Chem. Res. 1997, 36, 3439–3445. [Google Scholar] [CrossRef]
- Tashmukhambetova, Z.K.; Sassykova, L.R.; Aubakirov, Y.A.; Dangaliyeva, A.K.; Kanatbayeva, M.A.; Rustem, A.E. New catalysts for toluene oxidation technology in the liquid phase. Mater. Today Proc. 2020, 31, 529–531. [Google Scholar] [CrossRef]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Guo, J.; Al-Dahhan, M. Kinetics of Wet Air Oxidation of Phenol over a Novel Catalyst. Ind. Eng. Chem. Res. 2003, 42, 5473–5481. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Vasilyeva, M.S.; Kuryavy, V.G.; Ustinov, A.Y.; Zemnukhova, L.A.; Gushchina, D.D. Oxidative destruction of phenol on Fe/SiO2 catalysts. Water Sci. Technol. Water Supply 2020, 81, 2189–2201. [Google Scholar] [CrossRef]
- Guo, J.; Al-Dahhan, M. Catalytic Wet Oxidation of Phenol by Hydrogen Peroxide over Pillared Clay Catalyst. Ind. Eng. Chem. Res. 2003, 42, 2450–2460. [Google Scholar] [CrossRef]
- Tomita, K.; Oshima, Y. Stability of Manganese Oxide in Catalytic Supercritical Water Oxidation of Phenol. Ind. Eng. Chem. Res. 2004, 43, 7740–7743. [Google Scholar] [CrossRef]
- Álvarez, P.M.; McLurgh, D.; Plucinski, P. Copper Oxide Mounted on Activated Carbon as Catalyst for Wet Air Oxidation of Aqueous Phenol. 2. Catalyst Stability. Ind. Eng. Chem. Res. 2002, 41, 2153–2158. [Google Scholar] [CrossRef]
- Atoguchi, T.; Yao, S. Phenol oxidation over titanosilicalite-1: Experimental and DFT study of solvent. J. Mol. Catal. A Chem. 2001, 176, 173–178. [Google Scholar] [CrossRef]
- Guerra, R. Ecotoxicological and chemical evaluation of phenolic compounds in industrial effluents. Chemosphere 2001, 44, 1737–1747. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.; Mohedano, A.; Rodriguez, J. Reaction pathway of the catalytic wet air oxidation of phenol with a Fe/activated carbon catalyst. Appl. Catal. B 2006, 67, 206–216. [Google Scholar] [CrossRef]
- Rey, A.; Faraldos, M.; Casas, J.A.; Zazo, J.A.; Bahamonde, A.; Rodríguez, J.J. Catalytic wet peroxide oxidation of phenol over Fe/AC catalysts: Influence of iron precursor and activated carbon surface. Appl. Catal. B 2009, 86, 69–77. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, X.; Yue, P. Kinetics study on catalytic wet air oxidation of phenol. Chem. Eng. Sci. 2003, 58, 923–928. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Quintanilla, A.; García-Ochoa, F. Influence of pH on the wet oxidation of phenol with copper catalyst. Top Catal. 2005, 33, 181–192. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Quintanilla, A.; Garcia-Ochoa, F. Kinetic model of wet oxidation of phenol at basic pH using a copper catalyst. Chem. Eng. Sci. 2005, 60, 4866–4878. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Cordero, T.; Gomis, S.; Rodríguez, S.; García-Ochoa, F. Catalytic wet oxidation of phenol on active carbon: Stability, phenol conversion and mineralization. Catal. Today 2005, 102–103, 213–218. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Mirasol, J.; Bedia, J.; Gomis, S.; Yustos, P.; García-Ochoa, F.; Santos, A. Activated carbon as catalyst in wet oxidation of phenol: Effect of the oxidation reaction on the catalyst properties and stability. Appl. Catal. B 2008, 81, 122–131. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Quintanilla, A.; Rodríguez, S.; García-Ochoa, F. Route of the catalytic oxidation of phenol in aqueous phase. Appl. Catal. B 2002, 39, 97–113. [Google Scholar] [CrossRef]
- Dosumov, K.; Ergazieva, G.E. Morphology and activity of vanadium-containing catalysts for the selective oxidation of benzene to maleic anhydride. Russ. J. Phys. Chem.A 2012, 86, 1766–1768. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Durbán, B.; García-Ochoa, F. Oxidation of phenol in aqueous solution with copper catalysts. Catal. Today 2001, 66, 511–517. [Google Scholar] [CrossRef]
- Stüber, F.; Polaert, I.; Delmas, H.; Font, J.; Fortuny, A.; Fabregat, A. Catalytic wet air oxidation of phenol using active carbon: Performance of discontinuous and continuous reactors. J. Chem. Technol. Biotechnol. 2001, 76, 743–751. [Google Scholar] [CrossRef]
- Cao, S.; Chen, G.; Hu, X.; Yue, P.L. Catalytic wet air oxidation of wastewater containing ammonia and phenol over activated carbon supported Pt catalysts. Catal. Today 2003, 88, 37–47. [Google Scholar] [CrossRef]
- Rey, A.; Hungria, A.B.; Duran-Valle, C.J.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Rodriguez, J.J. On the optimization of activated carbon-supported iron catalysts in catalytic wet peroxide oxidation process. Appl. Catal. B 2016, 181, 249–259. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Zazo, J.A.; Rodriguez, J.J.; Casas, J.A. Microwave-assisted catalytic wet peroxide oxidation. Comparison of Fe catalysts supported on activated carbon and γ-alumina. Appl. Catal. B 2017, 218, 637–642. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Catalytic wet peroxide oxidation of phenol with a Fe/active carbon catalyst. Appl. Catal. B 2006, 65, 261–268. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic nanostructures: Rational design and fabrication strategies toward diverse applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, X.; Li, T.; Mao, Y.; Liu, C.; Chen, Y.; Wang, W. Mo-doped cobalt hydroxide nanosheets coupled with cobalt phosphide nanoarrays as bifunctional catalyst for efficient and high-stability overall water splitting. Int. J. Hydrogen Energy 2022, 47, 9915–9924. [Google Scholar] [CrossRef]
- Fernández, S.D.S.; Odio, O.F.; Crespo, P.M.; Pérez, E.O.; Salas, G.; Gutiérrez, L.; Morales, M.D.P.; Reguera, E. Tunable control of the structural features and related physical properties of MnxFe3–xO4 nanoparticles: Implication on their heating performance by magnetic hyperthermia. J. Phys. Chem. C 2022, 126, 10110–10128. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Bongiovanni, R. Synthesis and Organic Functionalization Approaches for Magnetite (Fe3O4) Nanoparticles. Adv. Mater. Lett. 2012, 3, 356–361. [Google Scholar] [CrossRef]
- Amrousse, R.; Tsutsumi, A.; Bachar, A.; Lahcene, D. N2O catalytic decomposition over nano-sized particles of Co-substituted Fe3O4 substrates. Appl. Catal. A Gen. 2013, 450, 253–260. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Sassykova, A.R.; Kubekova, S.N.; Batyrbayeva, A.A.; Azhigulova, R.N.; Zhaxibayeva, Z.M.; Kozhaisakova, M.A.; Zhusupova, L.A.; Sendilvelan, S.; Ponomarenko, O.I. Hydrogenation of aromatic nitro compounds to amines on nickel and iron-containing catalysts. Rasayan J. Chem. 2021, 14, 1223–1229. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, F.; Hua, W.; Zhao, F. Fe3O4 nanoparticles: Microwave-assisted Synthesis and Mechanism. Mater. Lett. 2012, 67, 358–361. [Google Scholar] [CrossRef]
- Subash, M.; Chandrasekar, M.; Panimalar, S.; Inmozhi, C.; Uthrakumar, R. Synthesis, characterizations of pure and Co2+ doped iron oxide nanoparticles for magnetic applications. Mater. Today Proc. 2022, 56, 3413–3417. [Google Scholar] [CrossRef]
- Tulepov, M.; Mansurov, Z.; Sassykova, L.; Baiseitov, D.; Dalelhanuly, O.; Ualiev, Z.; Gabdrashova, S.; Kudyarova, Z. Research of iron-containing concentrates of Balkhash deposit (Kazakhstan) for processing of low-grade coal. J. Chem. Technol. Metall. 2019, 54, 531–538. [Google Scholar]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Wei, H.; Liu, X.; Wang, A.; Zhang, L.; Qiao, B.; Yang, X.; Huang, Y.; Miao, S.; Liu, J.; Zhang, T. FeO x-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634. [Google Scholar] [CrossRef]
- Macpherson, H.A.; Stoldt, C.R. Iron pyrite nanocubes: Size and shape considerations for photovoltaic application. ACS Nano 2012, 6, 8940–8949. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Harris, J.; Korgel, B.A. Pyrite nanocrystal solar cells: Promising, or fool’s gold? J. Phys. Chem. Lett. 2012, 3, 2352–2356. [Google Scholar] [CrossRef]
- Douglas, A.; Carter, R.; Oakes, L.; Share, K.; Cohn, A.P.; Pint, C.L. Ultrafine iron pyrite (FeS2) nanocrystals improve sodium−sulfur and lithium−sulfur conversion reactions for efficient batteries. ACS Nano 2015, 9, 11156–11165. [Google Scholar] [CrossRef]
- Li, L.; Cabán-Acevedo, M.; Girard, S.N.; Jin, S. High-purity iron pyrite (FeS2) nanowires as high-capacity nanostructured cathodes for lithium-ion batteries. Nanoscale 2014, 6, 2112–2118. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, M.; Zhou, Y.; Zhang, D.; Wang, H.; Liu, X.; Wang, D.; Wang, W. Pyrite FeS2/C nanoparticles as an efficient bifunctional catalyst for overall water splitting. Dalton Trans. 2018, 47, 14917–14923. [Google Scholar] [CrossRef]
- Gao, M.-R.; Zheng, Y.-R.; Jiang, J.; Yu, S.-H. Pyrite-type Nanomaterials for Advanced Electrocatalysis. Acc. Chem. Res. 2017, 50, 2194–2204. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.M.; Tuan, C.-C.; Lounis, S.D.; Britt, D.K.; Qiao, R.; Yang, W.; Lanzara, A.; Alivisatos, A.P. Ligand-Controlled Colloidal Synthesis and Electronic Structure Characterization of Cubic Iron Pyrite (FeS2). Nanocryst. Chem. Mater. 2013, 25, 1615–1620. [Google Scholar]
- Wadia, C.; Wu, Y.; Gul, S.; Volkman, S.K.; Guo, J.; Alivisatos, A.P. Surfactant-Assisted Hydrothermal Synthesis of Single Phase Pyrite FeS2 Nanocrystals. Chem. Mater. 2009, 21, 2568–2570. [Google Scholar] [CrossRef]
- Jamei, M.R.; Khosravi, M.R. Anvaripour, B. Investigation of ultrasonic effect on synthesis of nano zero valent iron particles and comparison with conventional method. Asia Pac. J. Chem. Eng. 2013, 8, 767–774. [Google Scholar] [CrossRef]
- Trinh, T.K.; Pham, V.T.H.; Truong, N.T.N.; Kim, C.D.; Park, C. Iron pyrite: Phase and shape control by facile hot injection method. J. Cryst. Growth 2017, 461, 53–59. [Google Scholar] [CrossRef]
- Thomson, J.W.; Nagashima, K.; Macdonald, P.M.; Ozin, G.A. From sulfur−amine solutions to metal sulfide nanocrystals: Peering into the oleylamine−sulfur black box. J. Am. Chem. Soc. 2011, 133, 5036–5041. [Google Scholar] [CrossRef]
- Raabe, T.; Mehne, M.; Rasser, H.; Krause, H.; Kureti, S. Study on iron-based adsorbents for alternating removal of H2S and O2 from natural gas and biogas. Chem. Eng. J. 2019, 371, 738–749. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Zhong, M.; Wei, Z.; Li, J. Electrical and magnetic properties of FeS2 and CuFeS2 nanoplates. RSC Adv. 2015, 5, 91103–91107. [Google Scholar] [CrossRef]
- Von Oertzen, G.U.; Skinner, W.M.; Nesbitt, H.W. Ab initio and XPS studies of pyrite (100) surface states. Radiat. Phys. Chem. 2006, 75, 1855–1860. [Google Scholar] [CrossRef]
- Jiang, F.; Peckler, L.T.; Muscat, A.J. Phase pure pyrite FeS2 nanocubes synthesized using oleylamine as ligand, solvent, and reductant. Cryst. Growth Des. 2015, 15, 3565–3572. [Google Scholar] [CrossRef]
- Dosumov, K.; Ergazieva, G.E.; Churina, D.K.; Tel’Baeva, M.M. Cerium-containing catalysts for converting ethanol into ethylene. Russ. J. Phys. Chem. A 2014, 88, 1806–1808. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; 408p, ISBN 978-0-471-74339-2. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; 427p, ISBN 978-0-471-74339-2. [Google Scholar]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2003; 690p, ISBN 978-0-471-98731-4. [Google Scholar]
- Jung, H.; Kim, J.-W.; Choi, H.; Lee, J.-H.; Hur, H.-G. Synthesis of nanosized biogenic magnetite and comparison of its catalytic activity in ozonation. Appl. Catal. B Environ. 2008, 83, 208–213. [Google Scholar] [CrossRef]
- Carriazo, J.; Guélou, E.; Barrault, J.; Tatibouët, J.M.; Molina, R.; Moreno, S. Synthesis of pillared clays containing Al, Al-Fe or Al-Ce-Fe from a bentonite: Characterization and catalytic activity. Catal. Today 2005, 107–108, 126–132. [Google Scholar] [CrossRef]
- Nevmyvaka, A.A.; Magaeva, A.A.; Pershina, A.G.; Itin, V.I. Nanosized magnetic powders based on oxides. J. Phys. Conf. Ser. 2018, 1115, 042054. [Google Scholar] [CrossRef]
- Altunina, L.K.; Svarovskaya, L.I.; Tepexova, O.G.; Magaeva, A.A.; Itin, V.I. Sorption activity of nanosized SnO2 and CoFe2O4 powders. Chem. Sustain. Dev. 2011, 19, 237–242. [Google Scholar]
- Naĭden, E.P.; Zhuravlev, V.A.; Itin, V.I.; Terekhova, O.G.; Magaeva, A.A.; Ivanov, Y.F. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys. Solid State 2008, 50, 894–900. [Google Scholar] [CrossRef]
- Yergaziyeva, G.Y.; Dossumov, K.; Mambetova, M.M.; Kurokawa, H.; Baizhomartov, B. Effect of Ni, La, and Ce Oxides on a Cu/Al2O3 Catalyst with Low Copper Loading for Ethanol Non-oxidative Dehydrogenation. Chem. Eng. Technol. 2021, 44, 1890–1899. [Google Scholar] [CrossRef]
- Shomanova, Z.; Safarov, R.; Tashmukhambetova, Z.; Sassykova, L.; Nosenko, Y.; Mukanova, R. Complex Research of Ferroalloys Production Wastes by Physical and Chemical Methods. J. Chem. Technol. Metall. 2021, 56, 629–636. [Google Scholar]
- Dehmani, Y.; Lgaz, H.; Alrashdi, A.A.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.-M. Phenol adsorption mechanism on the zinc oxide surface: Experimental, cluster DFT calculations, and molecular dynamics simulations. J. Mol. Liq. 2021, 324, 114993. [Google Scholar] [CrossRef]
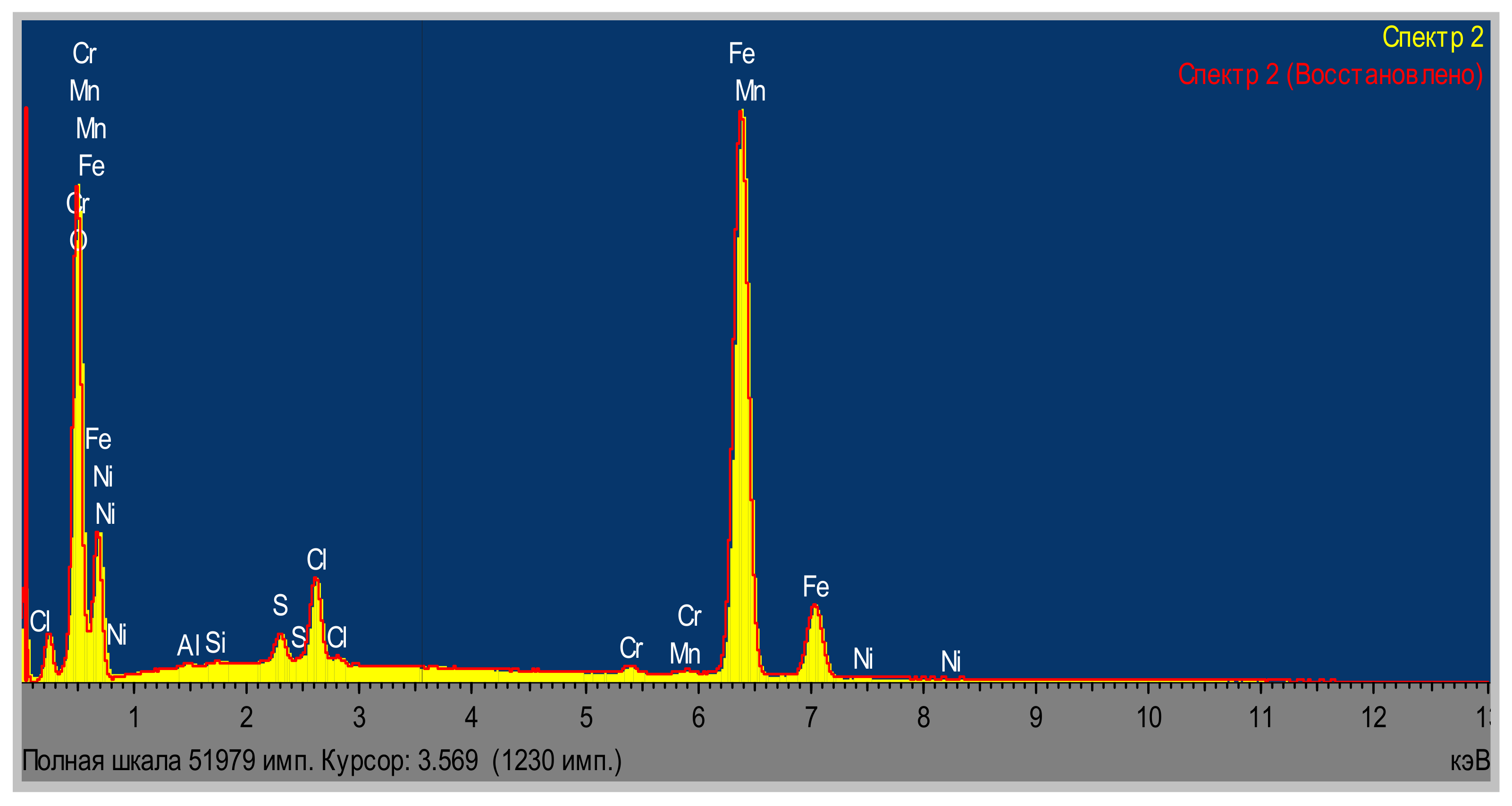
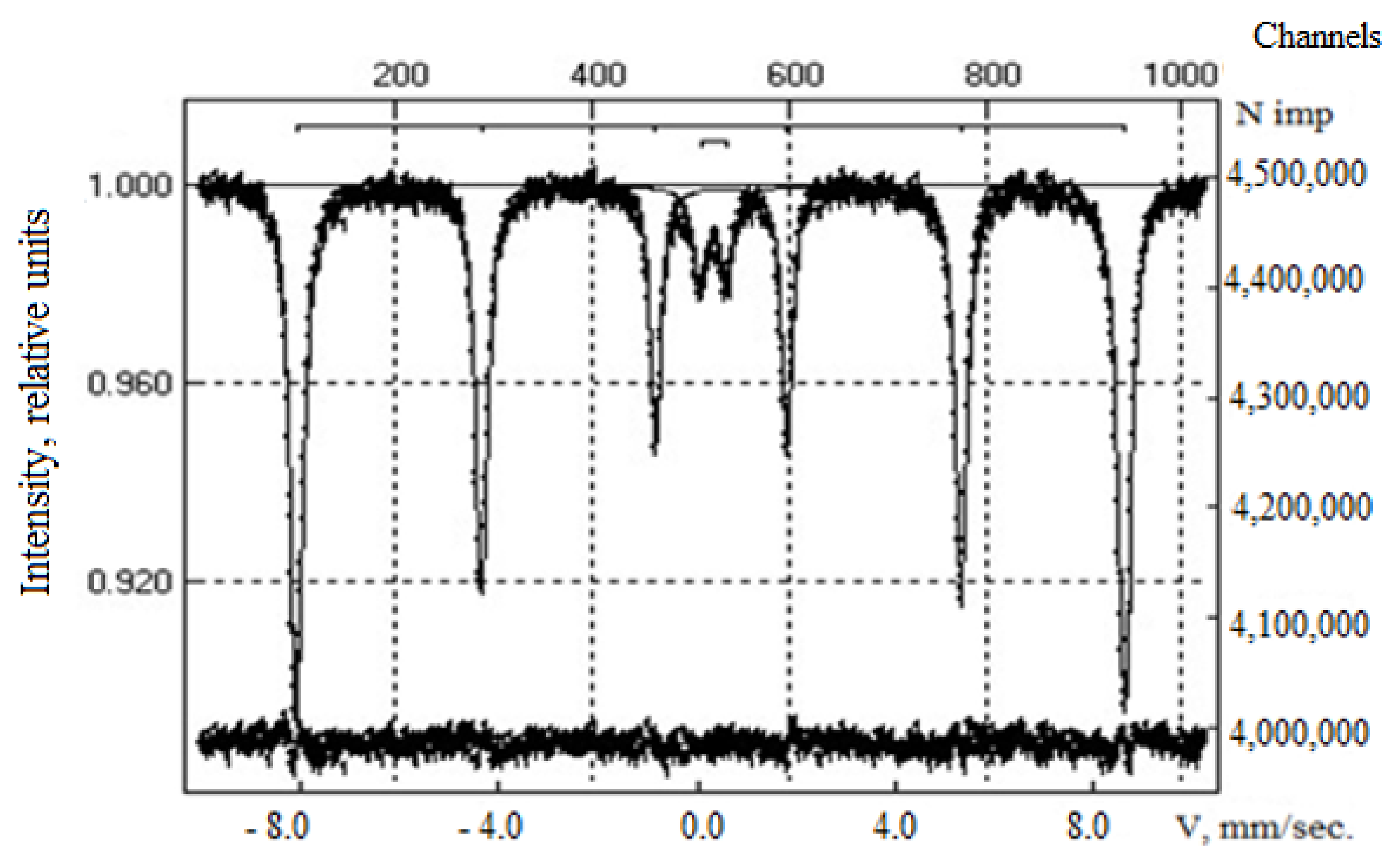
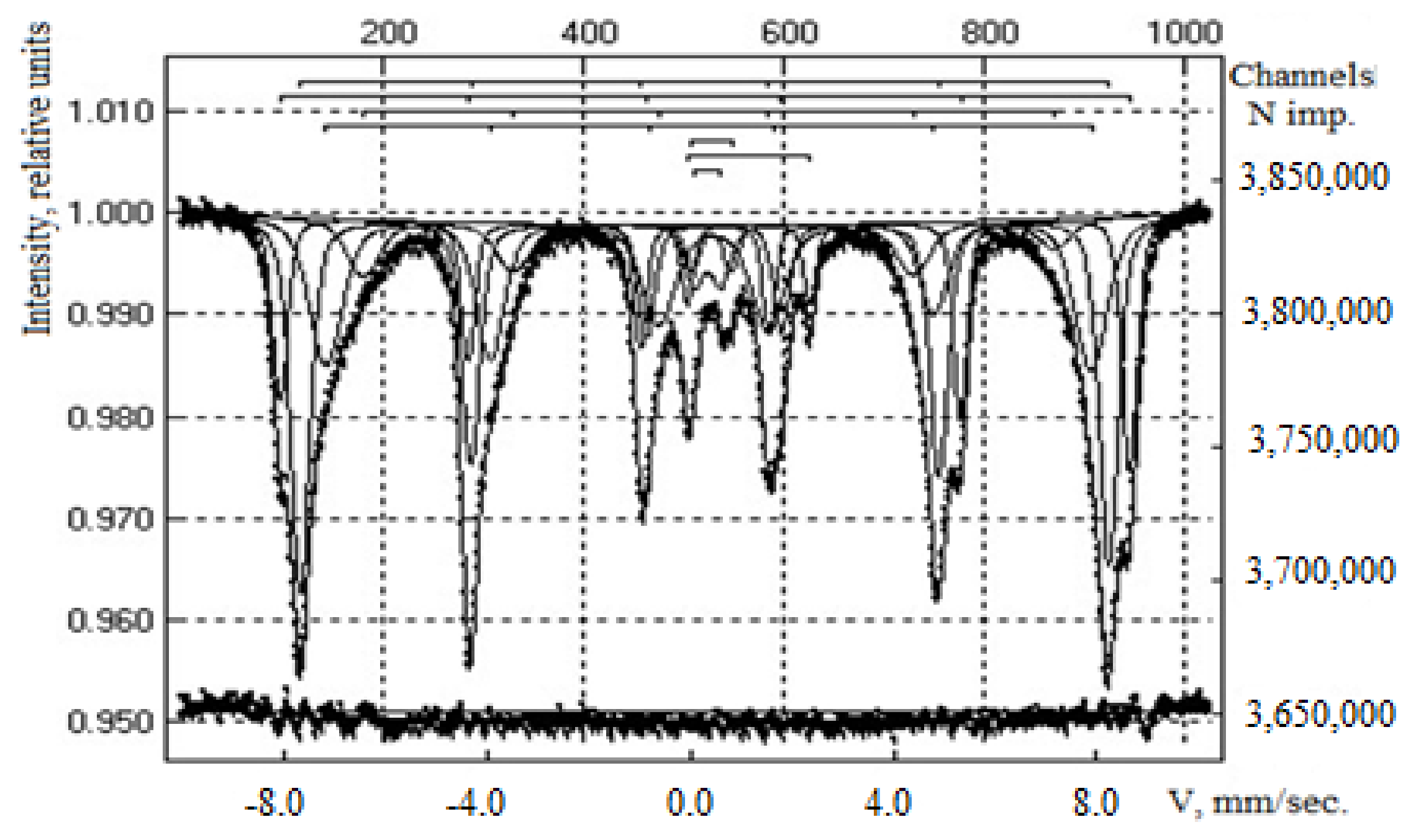
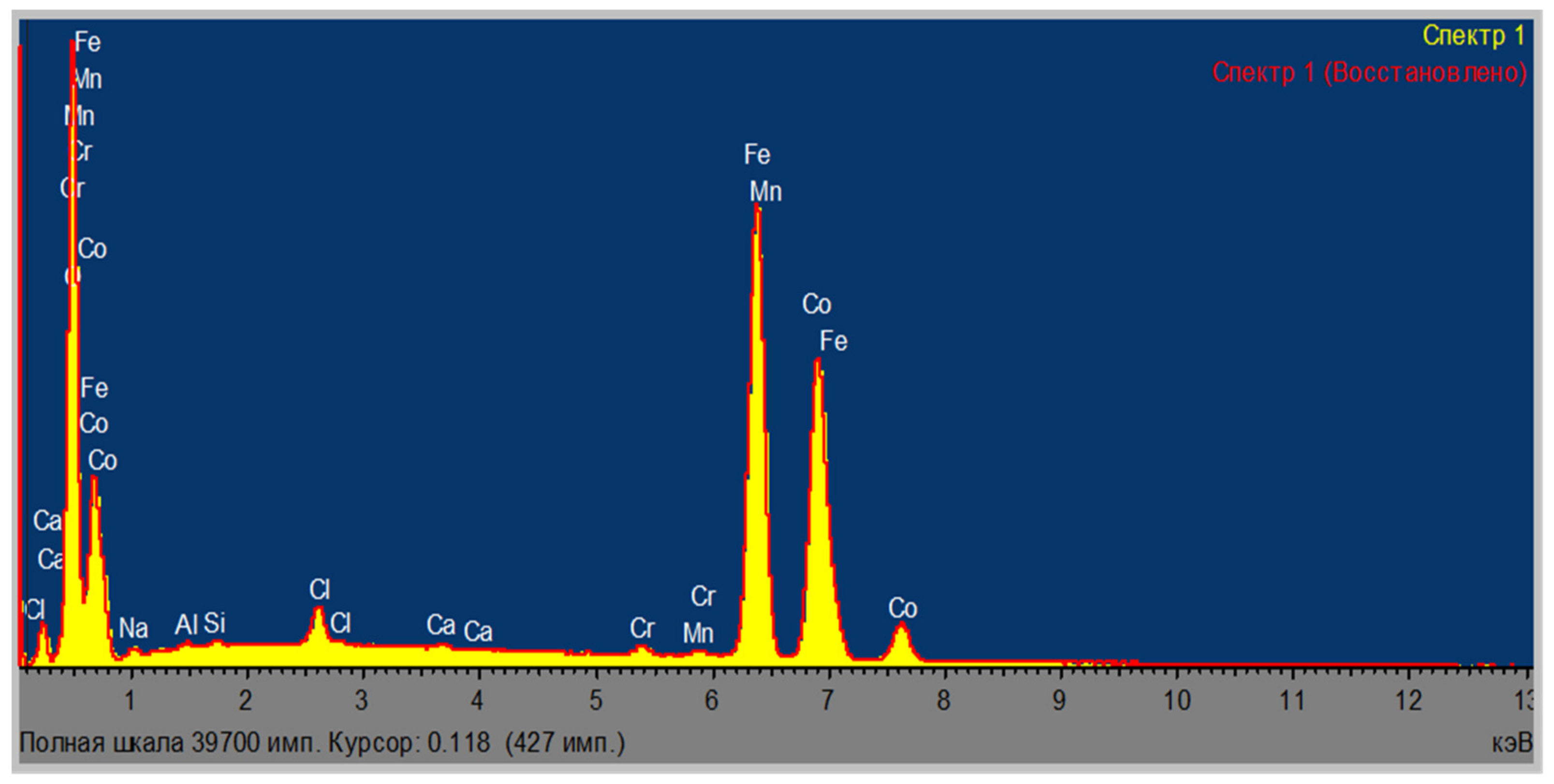


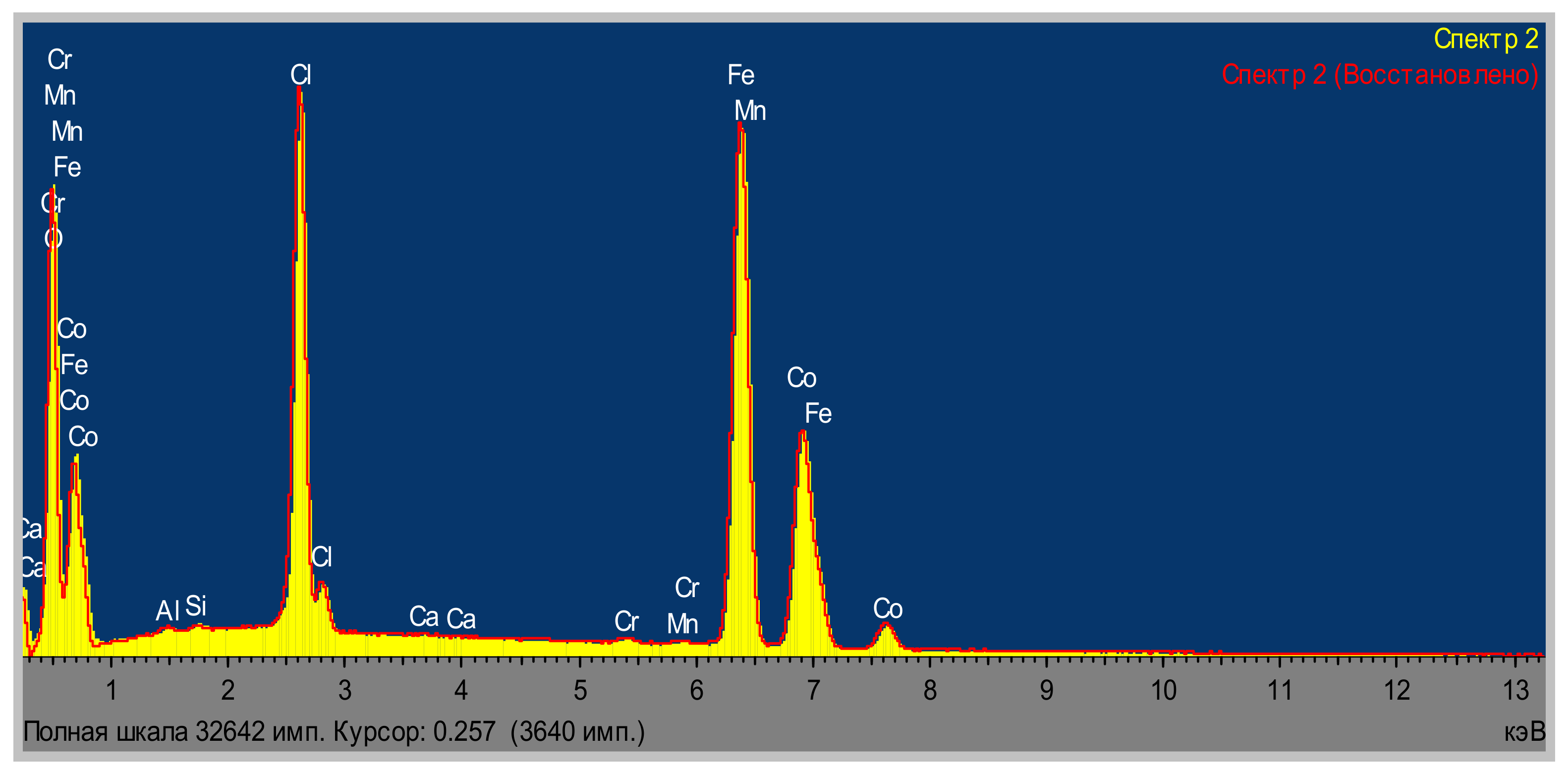

| Area | Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Al | Si | S | Cl | Cr | Mn | Fe | Total | |
| Area 1 | 26.51 | 0.15 | 0.13 | 1.13 | 1.27 | 0.47 | 0.40 | 69.94 | 100.00 |
| Area 2 | 26.52 | 0.12 | 0.13 | 0.87 | 1.34 | 0.50 | 0.30 | 70.22 | 100.00 |
| Area 3 | 26.21 | 0.15 | 0.11 | 1.00 | 1.33 | 0.47 | 0.33 | 70.38 | 100.00 |
| Average | 26.42 | 0.14 | 0.12 | 1.00 | 1.31 | 0.48 | 0.34 | 70.18 | 100.00 |
| Sample | IS (mm·s−1) | QS (mm·s−1) | Heffective (keV) | S (%) |
|---|---|---|---|---|
| Composite CoFe2O4 | 0.37 | −0.22 | 518 | 92 |
| 0.31 | 0.54 | - | 8.0 | |
| Composite CoFe2O4/PEI | 0.37 | −0.18 | 520 | 20.0 |
| 0.28 | −0.01 | 495 | 34.0 | |
| 0.41 | −0.09 | 423 | 17.0 | |
| 0.39 | −0.07 | 469 | 21.0 | |
| 0.42 | 0.76 | - | 2.0 | |
| 1.15 | 2.42 | - | 3.0 | |
| 0.34 | 0.53 | - | 3.0 |
| Area | Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Si | Cl | Ca | Cr | Mn | Fe | Co | Total | |
| Area 1 | 21.16 | 0.17 | 0.12 | 14.68 | 0.03 | 0.19 | 0.22 | 43.28 | 20.15 | 100.00 |
| Area 2 | 21.29 | 0.14 | 0.11 | 13.76 | 0.05 | 0.22 | 0.14 | 42.48 | 21.80 | 100.00 |
| Area 3 | 20.69 | 0.15 | 0.13 | 14.81 | 0.18 | 0.27 | 0.24 | 42.56 | 20.98 | 100.00 |
| Average | 21.05 | 0.15 | 0.12 | 14.42 | 0.09 | 0.23 | 0.20 | 42.77 | 20.98 | 100.00 |
| Area | Elements | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Na | Si | Cl | Ca | Cr | Mn | Fe | Co | Total | |
| Area 1 | 23.08 | 0.18 | 0.71 | 0.13 | 1.06 | 0.14 | 0.41 | 0.33 | 40.32 | 33.63 | 100.00 |
| Area 2 | 24.45 | 0.20 | 0.72 | 0.16 | 1.14 | 0.12 | 0.62 | 0.32 | 47.38 | 24.89 | 100.00 |
| Area 3 | 23.61 | 0.18 | 0.77 | 0.29 | 1.17 | 0.12 | 0.52 | 0.36 | 40.90 | 32.06 | 100.00 |
| Average | 23.71 | 0.19 | 0.73 | 0.19 | 1.12 | 0.13 | 0.52 | 0.34 | 42.87 | 30.19 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dossumova, B.T.; Shakiyeva, T.V.; Muktaly, D.; Sassykova, L.R.; Baizhomartov, B.B.; Subramanian, S. Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering 2022, 6, 68. https://doi.org/10.3390/chemengineering6050068
Dossumova BT, Shakiyeva TV, Muktaly D, Sassykova LR, Baizhomartov BB, Subramanian S. Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering. 2022; 6(5):68. https://doi.org/10.3390/chemengineering6050068
Chicago/Turabian StyleDossumova, Binara T., Tatyana V. Shakiyeva, Dinara Muktaly, Larissa R. Sassykova, Bedelzhan B. Baizhomartov, and Sendilvelan Subramanian. 2022. "Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen" ChemEngineering 6, no. 5: 68. https://doi.org/10.3390/chemengineering6050068
APA StyleDossumova, B. T., Shakiyeva, T. V., Muktaly, D., Sassykova, L. R., Baizhomartov, B. B., & Subramanian, S. (2022). Synthesis, Characterization of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering, 6(5), 68. https://doi.org/10.3390/chemengineering6050068








