Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of WO3 Powders
2.2. Characterization of WO3 Powders
2.3. Photoelectrochemical Measurements
3. Results and Discussion
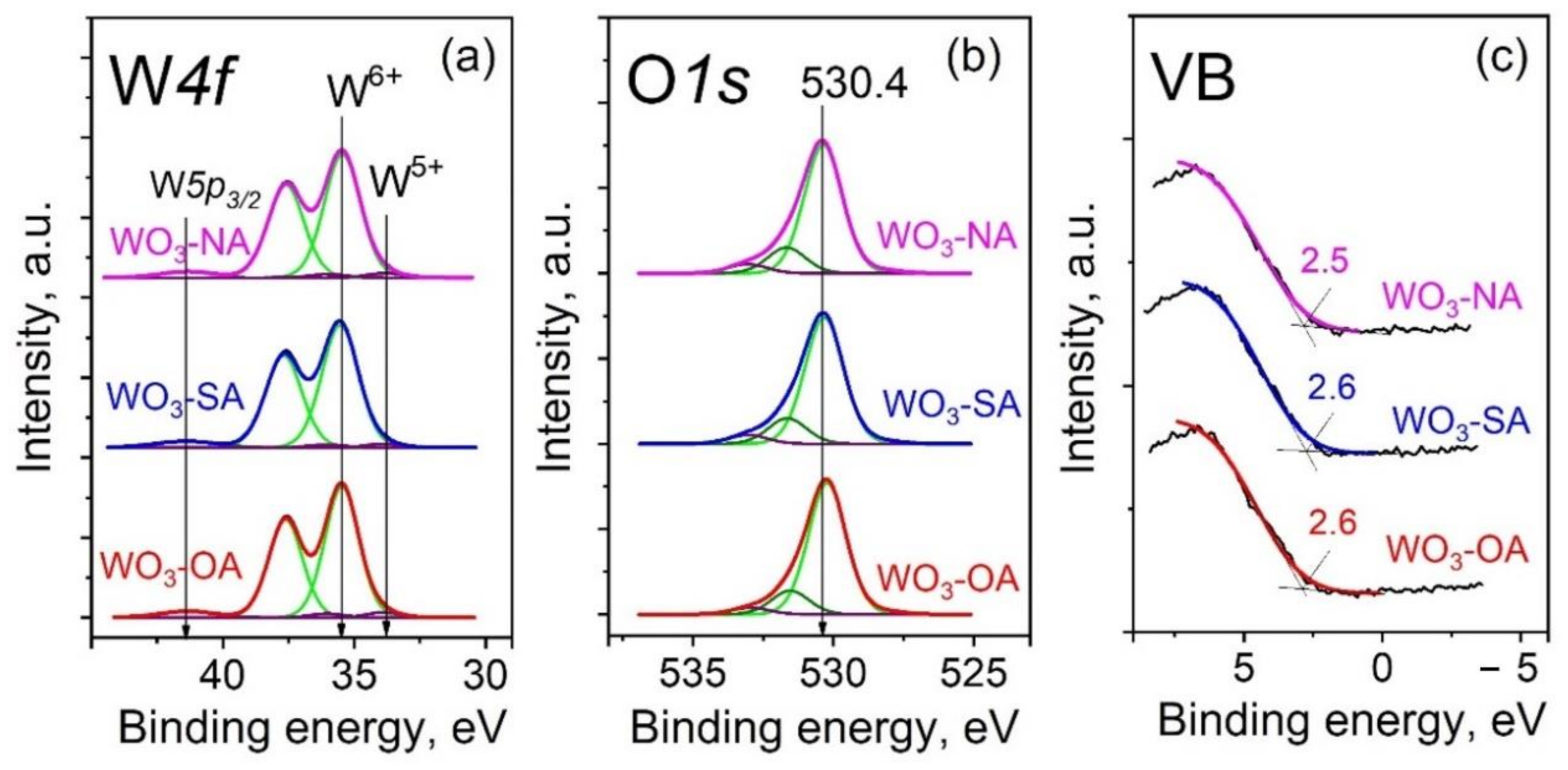
3.1. Characterization of the Powders
3.2. Possible Formation Mechanism
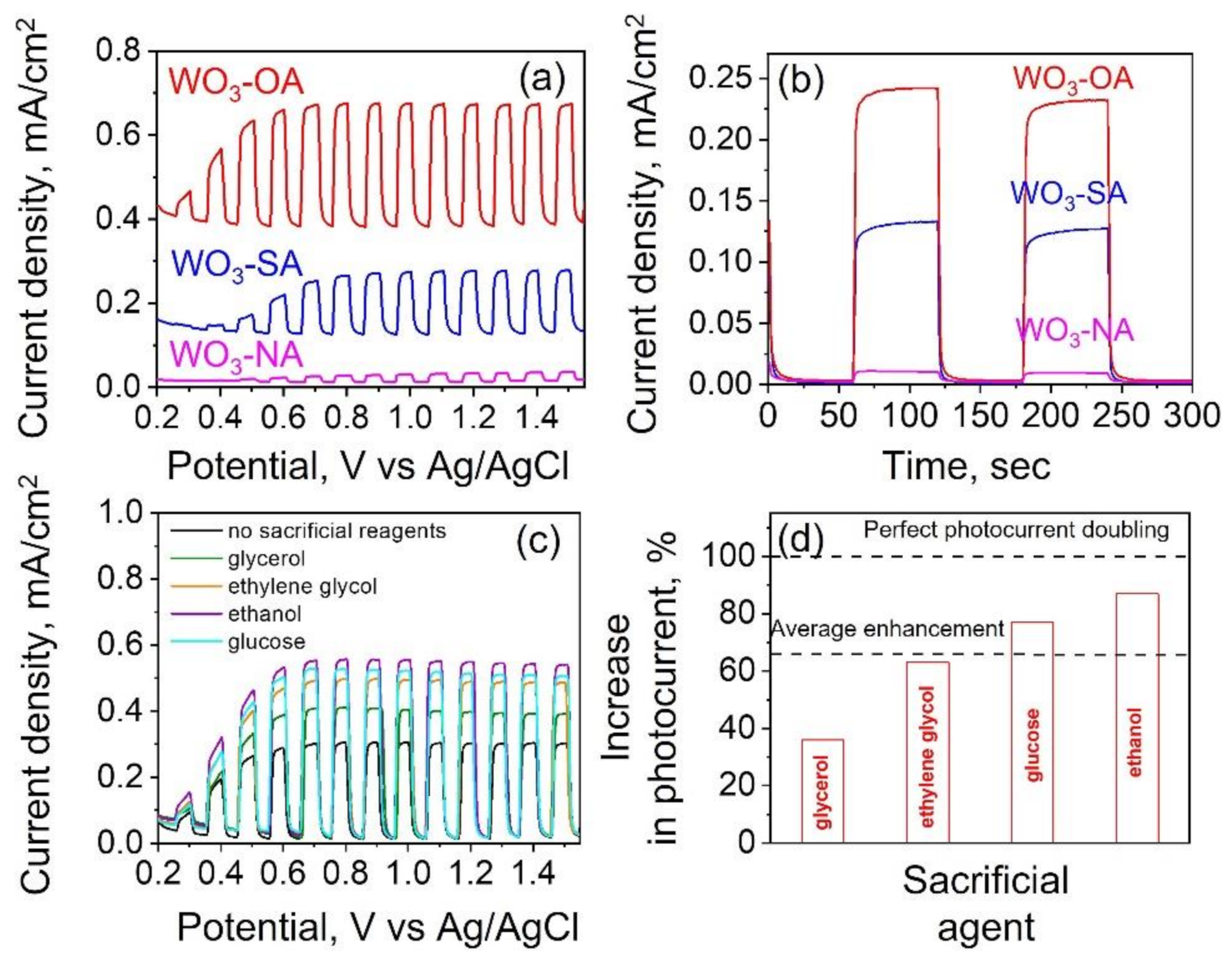
3.3. Photoelectrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momeni, M.M.; Akbarnia, M.; Ghayeb, Y. Preparation of S–W-codoped TiO2 nanotubes and effect of various hole scavengers on their photoelectrochemical activity: Alcohol series. Int. J. Hydrogen Energy 2020, 45, 33552–33562. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Markovskaya, D.V.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlova, E.A. New insights into the mechanism of photocatalytic hydrogen evolution from aqueous solutions of saccharides over CdS-based photocatalysts under visible light. Int. J. Hydrogen Energy 2020, 45, 30165–30177. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Zhurenok, A.V.; Kurenkova, A.Y.; Kremneva, A.M.; Saraev, A.A.; Zharkov, S.M.; Kozlova, E.A.; Kaichev, V.V. New titania-based photocatalysts for hydrogen production from aqueous-alcoholic solutions of methylene blue. RSC Adv. 2020, 10, 34137–34148. [Google Scholar] [CrossRef]
- López, C.R.; Melián, E.P.; Ortega Méndez, J.A.; Santiago, D.E.; Doña Rodríguez, J.M.; González Díaz, O. Comparative study of alcohols as sacrificial agents in H2 production by heterogeneous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.; Ni, B.-J. Photocatalytic and Photoelectrochemical Reforming of Biomass. In Solar-to-Chemical Conversion; WILEY-VCH: Weinheim, Germany, 2021; pp. 389–417. [Google Scholar]
- Raptis, D.; Dracopoulos, V.; Lianos, P. Renewable energy production by photoelectrochemical oxidation of organic wastes using WO3 photoanodes. J. Hazard. Mater. 2017, 333, 259–264. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.S.; Tong, Y.; Huang, Y. Oxygen Vacancy-Based Metal Oxides Photoanodes in Photoelectrochemical Water Splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Zhang, J.; Salles, I.; Pering, S.; Cameron, P.J.; Mattia, D.; Eslava, S. Nanostructured WO3 photoanodes for efficient water splitting via anodisation in citric acid. RSC Adv. 2017, 7, 35221–35227. [Google Scholar] [CrossRef] [Green Version]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Izzi, M.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Innocenti, M.; Sabbatini, L.; Cioffi, N. Pros and Cons of Sacrificial Anode Electrolysis for the Preparation of Transition Metal Colloids: A Review. ChemElectroChem 2020, 7, 386–394. [Google Scholar] [CrossRef]
- Gao, D.; Li, H.; Wei, P.; Wang, Y.; Wang, G.; Bao, X. Electrochemical synthesis of catalytic materials for energy catalysis. Chin. J. Catal. 2022, 43, 1001–1016. [Google Scholar] [CrossRef]
- Zhang, T.; Paulose, M.; Neupane, R.; Schaffer, L.A.; Rana, D.B.; Su, J.; Guo, L.; Varghese, O.K. Nanoporous WO3 films synthesized by tuning anodization conditions for photoelectrochemical water oxidation. Sol. Energy Mater. Sol. Cells 2020, 209, 110472. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Roselló-Márquez, G.; García-Antón, J. A simple method to fabricate high-performance nanostructured WO3 photocatalysts with adjusted morphology in the presence of complexing agents. Mater. Des. 2017, 116, 160–170. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Chen, X.; Liu, J.; Gao, J.; Li, G. Fabrication of WO3·2H2O nanoplatelet powder by breakdown anodization. Electrochem. Commun. 2019, 104, 106479. [Google Scholar] [CrossRef]
- Ulyankina, A.; Molodtsova, T.; Gorshenkov, M.; Leontyev, I.; Zhigunov, D.; Konstantinova, E.; Lastovina, T.; Tolasz, J.; Henych, J.; Licciardello, N.; et al. Photocatalytic degradation of ciprofloxacin in water at nano-ZnO prepared by pulse alternating current electrochemical synthesis. J. Water Process Eng. 2021, 40, 101809. [Google Scholar] [CrossRef]
- Ulyankina, A.; Leontyev, I.; Maslova, O.; Allix, M.; Rakhmatullin, A.; Nevzorova, N.; Valeev, R.; Yalovega, G.; Smirnova, N. Copper oxides for energy storage application: Novel pulse alternating current synthesis. Mater. Sci. Semicond. Process. 2018, 73, 111–116. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Zheng, H.; Breedon, M.; Bansal, V.; Bhargava, S.K.; Latham, K.; Zhu, J.; Yu, L.; Hu, Z.; Spizzirri, P.G.; et al. High-Temperature Anodized WO3 Nanoplatelet Films for Photosensitive Devices. Langmuir 2009, 25, 9545–9551. [Google Scholar] [CrossRef]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.-M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746–6751. [Google Scholar] [CrossRef]
- Pokhrel, S.; Birkenstock, J.; Dianat, A.; Zimmermann, J.; Schowalter, M.; Rosenauer, A.; Ciacchi, L.C.; Mädler, L. In situ high temperature X-ray diffraction, transmission electron microscopy and theoretical modeling for the formation of WO3 crystallites. CrystEngComm 2015, 17, 6985–6998. [Google Scholar] [CrossRef]
- Desseigne, M.; Dirany, N.; Chevallier, V.; Arab, M. Shape dependence of photosensitive properties of WO3 oxide for photocatalysis under solar light irradiation. Appl. Surf. Sci. 2019, 483, 313–323. [Google Scholar] [CrossRef]
- Efkere, H.İ.; Gümrükçü, A.E.; Özen, Y.; Kınacı, B.; Aydın, S.Ş.; Ates, H.; Özçelik, S. Investigation of the effect of annealing on the structural, morphological and optical properties of RF sputtered WO3 nanostructure. Phys. B Condens. Matter 2021, 622, 413350. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Zhang, J.; Zhang, Z. Defect Engineering of Air-Treated WO3 and Its Enhanced Visible-Light-Driven Photocatalytic and Electrochemical Performance. J. Phys. Chem. C 2016, 120, 9750–9763. [Google Scholar] [CrossRef]
- Jin, B.; Wang, J.; Xu, F.; Li, D.; Men, Y. Hierarchical hollow WO3 microspheres with tailored surface oxygen vacancies for boosting photocatalytic selective conversion of biomass-derived alcohols. Appl. Surf. Sci. 2021, 547, 149239. [Google Scholar] [CrossRef]
- Vargas-Consuelos, C.I.; Seo, K.; Camacho-López, M.; Graeve, O.A. Correlation between Particle Size and Raman Vibrations in WO3 Powders. J. Phys. Chem. C 2014, 118, 9531–9537. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Commun. 2015, 6, 8064. [Google Scholar] [CrossRef] [Green Version]
- Abbaspoor, M.; Aliannezhadi, M.; Tehrani, F.S. Effect of solution pH on as-synthesized and calcined WO3 nanoparticles synthesized using sol-gel method. Opt. Mater. 2021, 121, 111552. [Google Scholar] [CrossRef]
- Colton, R.J.; Rabalais, J.W. Electronic structure to tungsten and some of its borides, carbides, nitrides, and oxides by x-ray electron spectroscopy. Inorg. Chem. 1976, 15, 236–238. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Mains, G.J. An XPS study of the UV reduction and photochromism of MoO3 and WO3. J. Chem. Phys. 1982, 76, 780–786. [Google Scholar] [CrossRef]
- Ramana, C.V.; Vemuri, R.S.; Kaichev, V.V.; Kochubey, V.A.; Saraev, A.A.; Atuchin, V.V. X-ray Photoelectron Spectroscopy Depth Profiling of La2O3/Si Thin Films Deposited by Reactive Magnetron Sputtering. ACS Appl. Mater. Interfaces 2011, 3, 4370–4373. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Al-Kharafi, F.; Al-Awadi, N.; El-Dusouqui, O.M.; Katrib, A. Surface electronic structure–catalytic activity relationship of partially reduced WO3 bulk or deposited on TiO2. J. Electron Spectrosc. Relat. Phenom. 2006, 151, 128–134. [Google Scholar] [CrossRef]
- Cheshme Khavar, A.H.; Moussavi, G.; Yaghmaeian, K.; Mahjoub, A.R.; Khedri, N.; Dusek, M.; Vaclavu, T.; Hosseini, M. A new Ru(ii) polypyridyl complex as an efficient photosensitizer for enhancing the visible-light-driven photocatalytic activity of a TiO2/reduced graphene oxide nanocomposite for the degradation of atrazine: DFT and mechanism insights. RSC Adv. 2020, 10, 22500–22514. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Lin, H.; Xie, Y.; Tong, N.; Lin, J.; Zhang, X.; Zhang, Z.; Wang, X. Surface oxygen vacancy and defect engineering of WO3 for improved visible light photocatalytic performance. Catal. Sci. Technol. 2018, 8, 4399–4406. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Cheng, Y.; Liu, Z.; Guo, Q.; Ha, M.N.; Zhao, Z. Hydrogen-treated mesoporous WO3 as a reducing agent of CO2 to fuels (CH4 and CH3OH) with enhanced photothermal catalytic performance. J. Mater. Chem. A 2016, 4, 5314–5322. [Google Scholar] [CrossRef]
- Anik, M.; Cansizoglu, T. Dissolution kinetics of WO3 in acidic solutions. J. Appl. Electrochem. 2006, 36, 603–608. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Segura-Sanchís, E.; García-Antón, J. Novel tree-like WO3 nanoplatelets with very high surface area synthesized by anodization under controlled hydrodynamic conditions. Chem. Eng. J. 2016, 286, 59–67. [Google Scholar] [CrossRef]
- Seifollahi Bazarjani, M.; Hojamberdiev, M.; Morita, K.; Zhu, G.; Cherkashinin, G.; Fasel, C.; Herrmann, T.; Breitzke, H.; Gurlo, A.; Riedel, R. Visible Light Photocatalysis with c-WO3–x/WO3 × H2O Nanoheterostructures In Situ Formed in Mesoporous Polycarbosilane-Siloxane Polymer. J. Am. Chem. Soc. 2013, 135, 4467–4475. [Google Scholar] [CrossRef]
- Pham, N.L.; Luu, T.L.A.; Nguyen, H.L.; Nguyen, C.T. Effects of acidity on the formation and adsorption activity of tungsten oxide nanostructures prepared via the acid precipitation method. Mater. Chem. Phys. 2021, 272, 125014. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Chung, C.-H. Pulse-reverse electrodeposition for mesoporous metal films: Combination of hydrogen evolution assisted deposition and electrochemical dealloying. Nanoscale 2012, 4, 568–575. [Google Scholar] [CrossRef]
- Tang, R.; Wang, L.; Zhang, Z.; Yang, W.; Xu, H.; Kheradmand, A.; Jiang, Y.; Zheng, R.; Huang, J. Fabrication of MOFs’ derivatives assisted perovskite nanocrystal on TiO2 photoanode for photoelectrochemical glycerol oxidation with simultaneous hydrogen production. Appl. Catal. B Environ. 2021, 296, 120382. [Google Scholar] [CrossRef]
- Esposito, D.V.; Forest, R.V.; Chang, Y.; Gaillard, N.; McCandless, B.E.; Hou, S.; Lee, K.H.; Birkmire, R.W.; Chen, J.G. Photoelectrochemical reforming of glucose for hydrogen production using a WO3-based tandem cell device. Energy Environ. Sci. 2012, 5, 9091–9099. [Google Scholar] [CrossRef]
- Kalamaras, E.; Lianos, P. Current Doubling effect revisited: Current multiplication in a PhotoFuelCell. J. Electroanal. Chem. 2015, 751, 37–42. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Verma, M.; Sohn, Y.; Deshpande, P.A.; Pradhan, D. Highly Active Tungsten Oxide Nanoplate Electrocatalysts for the Hydrogen Evolution Reaction in Acidic and Near Neutral Electrolytes. ACS Omega 2017, 2, 7039–7047. [Google Scholar] [CrossRef]
- Yoo, S.J.; Lim, J.W.; Sung, Y.-E.; Jung, Y.H.; Choi, H.G.; Kim, D.K. Fast switchable electrochromic properties of tungsten oxide nanowire bundles. Appl. Phys. Lett. 2007, 90, 173126. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsarenko, A.; Gorshenkov, M.; Yatsenko, A.; Zhigunov, D.; Butova, V.; Kaichev, V.; Ulyankina, A. Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders. ChemEngineering 2022, 6, 31. https://doi.org/10.3390/chemengineering6020031
Tsarenko A, Gorshenkov M, Yatsenko A, Zhigunov D, Butova V, Kaichev V, Ulyankina A. Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders. ChemEngineering. 2022; 6(2):31. https://doi.org/10.3390/chemengineering6020031
Chicago/Turabian StyleTsarenko, Anastasia, Mikhail Gorshenkov, Aleksey Yatsenko, Denis Zhigunov, Vera Butova, Vasily Kaichev, and Anna Ulyankina. 2022. "Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders" ChemEngineering 6, no. 2: 31. https://doi.org/10.3390/chemengineering6020031
APA StyleTsarenko, A., Gorshenkov, M., Yatsenko, A., Zhigunov, D., Butova, V., Kaichev, V., & Ulyankina, A. (2022). Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders. ChemEngineering, 6(2), 31. https://doi.org/10.3390/chemengineering6020031






