Recent Advances in the Preparation of Barium Sulfate Nanoparticles: A Mini-Review
Abstract
:1. Introduction
- Direct precipitation;
- Microemulsions;
- Microreactors;
- Membrane dispersion;
- Spinning disc reactors.
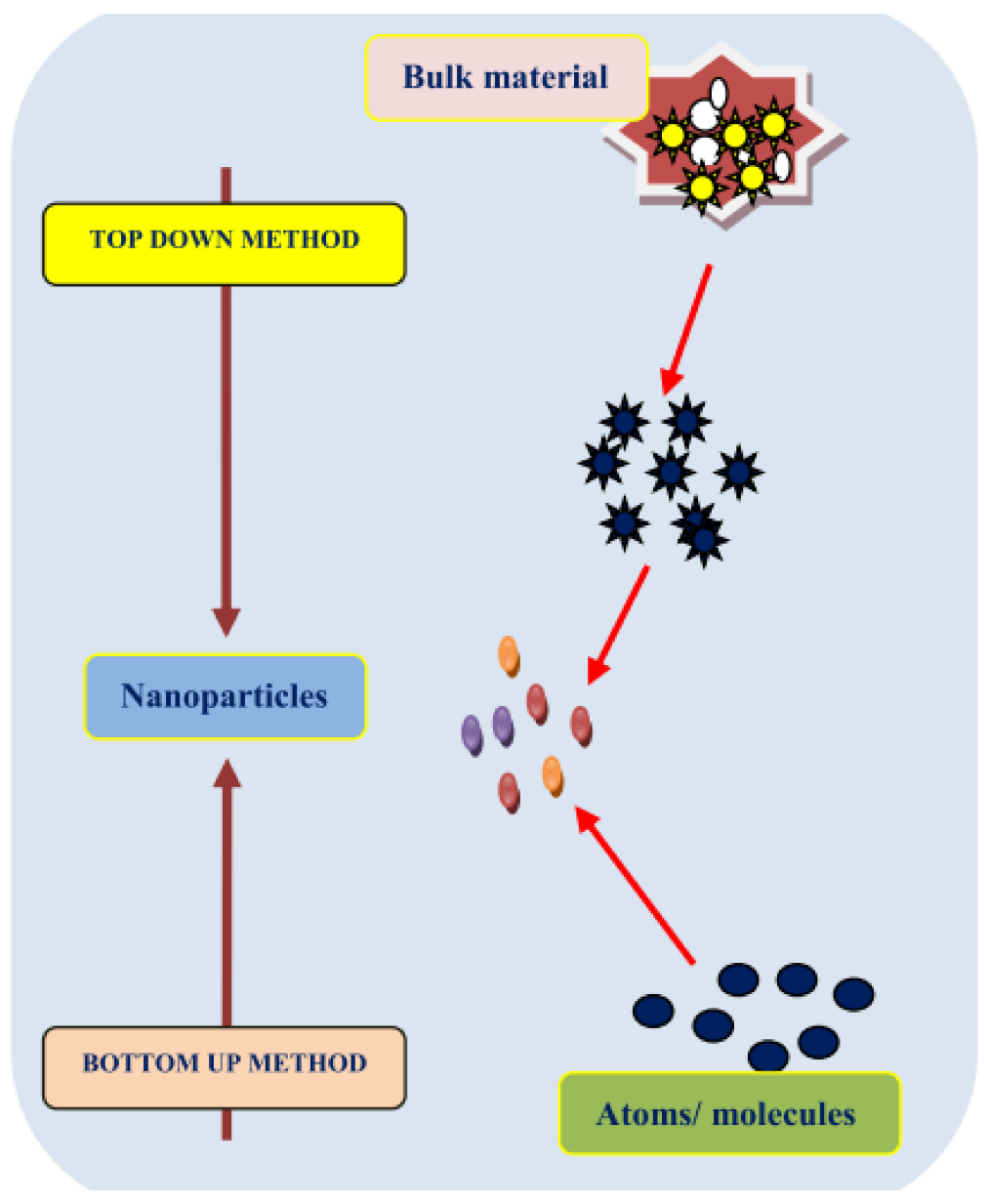
2. Top-Down Approach
3. Bottom-Up Strategy
3.1. Thermodynamic Origin
3.2. Direct Precipitation
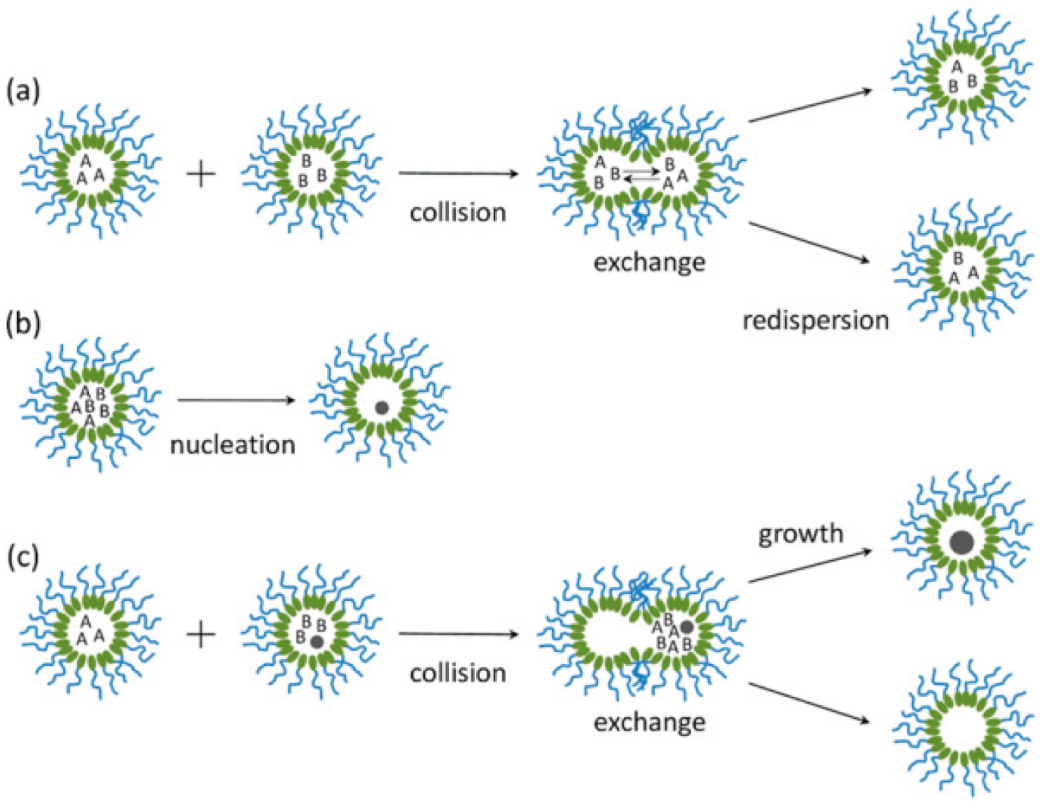
3.3. Microemulsion Method
3.4. Obtaining BaSO4 NPs in Microreactors
3.5. Membrane Dispersion
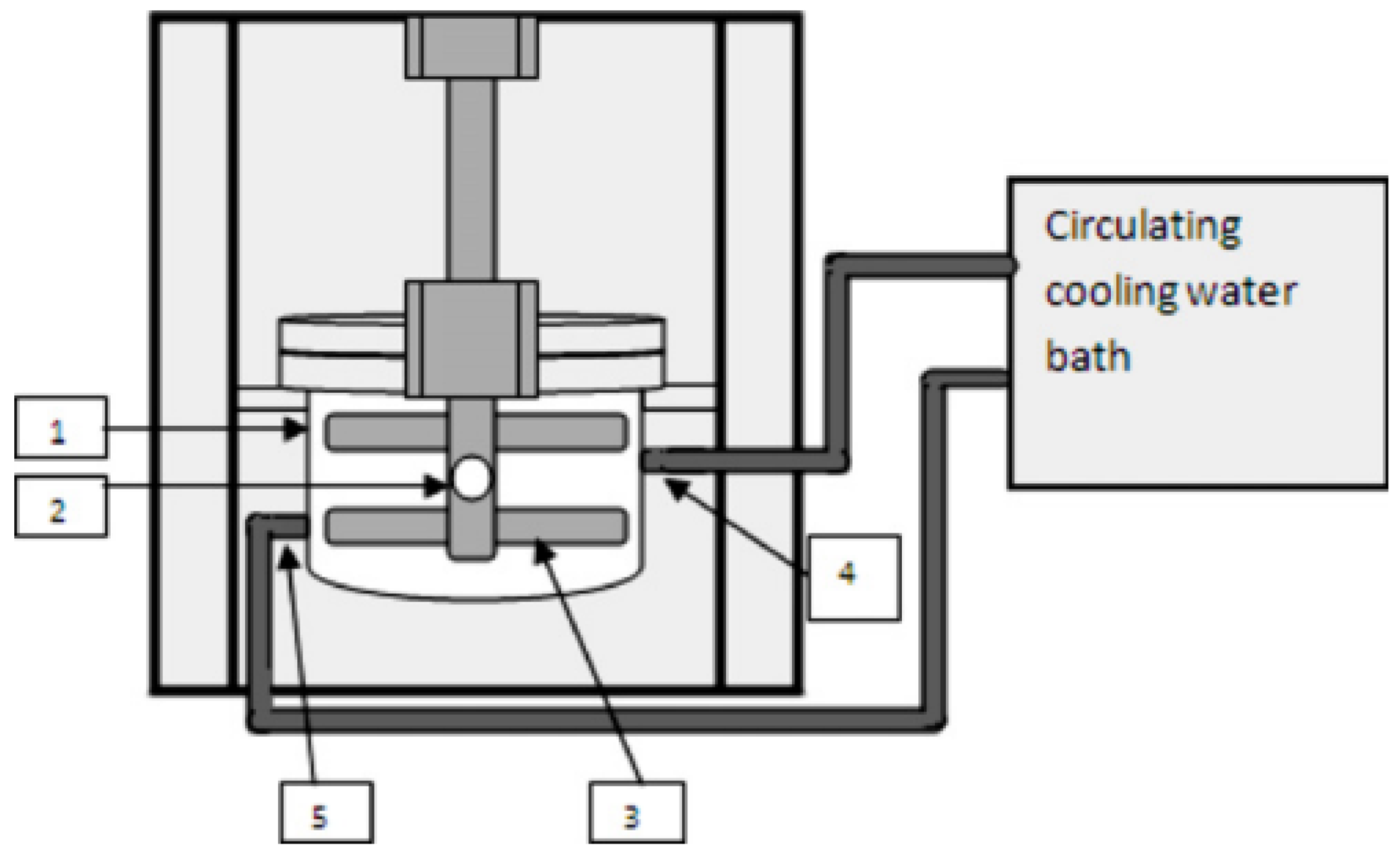
3.6. Obtaining BaSO4 NPs in Spinning Disc Reactors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Urakaev, F.K.; Khan, N.V.; Shalabaev, Z.S.; Tatykaev, B.B.; Nadirov, R.K.; Burkitbaev, M.M. Synthesis and photocatalytic properties of silver chloride/silver composite colloidal particles. Colloid J. 2020, 82, 76–80. [Google Scholar] [CrossRef]
- Sadeghi-Aghbash, M.; Rahimnejad, M. Zinc Phosphate Nanoparticles: A Review on Physical, Chemical, and Biological Synthesis and their Applications. Curr. Pharm. Biotechnol. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef] [Green Version]
- Tighe-Neira, R.; Carmora, E.; Recio, G.; Nunes-Nesi, A.; Reyes-Diaz, M.; Alberdi, M.; Rengel, Z.; Inostroza-Blancheteau, C. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiol. Biochem. 2018, 130, 408–417. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef]
- Roy, S.D.; Das, K.C.; Dhar, S.S. Conventional to green synthesis of magnetic iron oxide nanoparticles; its application as catalyst, photocatalyst and toxicity: A short review. Inorg. Chem. Commun. 2021, 134, 109050. [Google Scholar] [CrossRef]
- Yang, B.; Peng, S.; Choy, W.C. Inorganic top electron transport layer for high performance inverted perovskite solar cells. EcoMat 2021, 3, e12127. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wang, H.; Zhang, C.; Gong, C.; Li, H.; Chen, J.; Zang, Z. Ion diffusion-induced double layer doping toward stable and efficient perovskite solar cells. Nano Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Sooch, B.S.; Mann, M.K.; Sharma, M. Metal-doped barium sulphate nanoparticles decorated with gelatin as antibacterial agents. J. Clust. Sci. 2021, 32, 1141–1154. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Hübner, R.; Pietzsch, H.J.; Kopka, K.; Mamat, C. Sub-10 nm Radiolabeled Barium Sulfate Nanoparticles as Carriers for Theranostic Applications and Targeted Alpha Therapy. Chemistryopen. 2020, 9, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; Cedimagar, M.A. Size and morphology controlled synthesis of barium sulfate. Cryst. Res. Technol. 2016, 51, 393–399. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.J.; Lu, Y.C.; Luo, G.S. Process intensification of BaSO4 nanoparticle preparation with agitation of microbubbles. Powder Technol. 2013, 247, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Konduru, N.; Keller, J.; Ma-Hock, L.; Gröters, S.; Landsiedel, R.; Donaghey, T.C.; Molina, R.M. Biokinetics and effects of barium sulfate nanoparticles. Part. Fibre Toxicol. 2014, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Schwotzer, D.; Ernst, H.; Schaudien, D.; Kock, H.; Pohlmann, G.; Dasenbrock, C.; Creutzenberg, O. Effects from a 90-day inhalation toxicity study with cerium oxide and barium sulfate nanoparticles in rats. Part. Fibre Toxicol. 2017, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Mohn, D.; Zehnder, M.; Imfeld, T.; Stark, W.J. Radio-opaque nanosized bioactive glass for potential root canal application: Evaluation of radiopacity, bioactivity and alkaline capacity. Int. Endod. J. 2010, 43, 210–217. [Google Scholar] [CrossRef]
- Molina, R.M.; Konduru, N.V.; Queiroz, P.M.; Figueroa, B.; Fu, D.; Ma-Hock, L.; Groeters, S.; Schaudien, D.; Brain, J.D. Fate of barium sulfate nanoparticles deposited in the lungs of rats. Sci. Rep. 2019, 9, 8163. [Google Scholar] [CrossRef]
- Aninwene, G.E.; Stout, D., II; Yang, Z.; Webster, T.J. Nano-BaSO4: A novel antimicrobial additive to pellethane. Int. J. Nanomed. 2013, 8, 1197. [Google Scholar]
- Meagher, M.J.; Leone, B.; Turnbull, T.L.; Ross, R.D.; Zhang, Z.; Roeder, R.K. Dextran-encapsulated barium sulfate nanoparticles prepared for aqueous dispersion as an X-ray contrast agent. J. Nanoparticle Res. 2013, 15, 2146. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Granozzi, G.; Agnoli, S.; Polizzi, S.; Riello, P.; Sangregorio, C. Top-down synthesis of multifunctional iron oxide nanoparticles for macrophage labelling and manipulation. J. Mater. Chem. 2011, 21, 3803–3813. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.; Kannangara, Y.Y.; Lin, Y.; Amaratunga, G.A.; de Silva, K.N. A method for top down preparation of chitosan nanoparticles and nanofibers. Carbohydr. Polym. 2015, 117, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ling, T.; Wu, W.T.; Liu, H.; Gao, M.R.; Ling, C.; Du, X.W. A top–down strategy towards monodisperse colloidal lead sulphide quantum dots. Nat. Commun. 2013, 4, 1695. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Chan, H.K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [Green Version]
- Pini, M.; Rosa, R.; Neri, P.; Bondioli, F.; Ferrari, A.M. Environmental assessment of a bottom-up hydrolytic synthesis of TiO2 nanoparticles. Green Chem. 2015, 17, 518–531. [Google Scholar] [CrossRef]
- Sierra-Pallares, J.; Huddle, T.; García-Serna, J.; Alonso, E.; Mato, F.; Shvets, I.; Lester, E. Understanding bottom-up continuous hydrothermal synthesis of nanoparticles using empirical measurement and computational simulation. Nano Res. 2016, 9, 3377–3387. [Google Scholar] [CrossRef] [Green Version]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- Baláž, M.; Daneu, N.; Balážová, Ľ.; Dutková, E.; Tkáčiková, Ľ.; Briančin, J.; Baláž, P. Bio-mechanochemical synthesis of silver nanoparticles with antibacterial activity. Adv. Powder Technol. 2017, 28, 3307–3312. [Google Scholar] [CrossRef]
- Shalabayev, Z.; Baláz, M.; Daneu, N.; Dutková, E.; Bujnáková, Z.; Kanuchová, M.; Burkitbayev, M. Sulfur-mediated mechanochemical synthesis of spherical and needle-like copper sulfide nanocrystals with antibacterial activity. ACS Sustain. Chem. Eng. 2019, 7, 12897–12909. [Google Scholar] [CrossRef]
- Baláž, M.; Zorkovská, A.; Urakaev, F.; Baláž, P.; Briančin, J.; Bujňáková, Z.; Gock, E. Ultrafast mechanochemical synthesis of copper sulfides. RSC Adv. 2016, 6, 87836–87842. [Google Scholar] [CrossRef]
- Baláž, M.; Dobrozhan, O.; Tešinský, M.; Zhang, R.Z.; Džunda, R.; Dutková, E.; Baláž, P. Scalable and environmentally friendly mechanochemical synthesis of nanocrystalline rhodostannite (Cu2FeSn3S8). Powder Technol. 2021, 388, 192–200. [Google Scholar] [CrossRef]
- Dutková, E.; Čaplovičová, M.; Škorvánek, I.; Baláž, M.; Zorkovská, A.; Baláž, P.; Čaplovič, Ľ. Structural, surface and magnetic properties of chalcogenide Co9S8 nanoparticles prepared by mechanochemical synthesis. J. Alloy. Compd. 2018, 745, 863–867. [Google Scholar] [CrossRef]
- Patel, C.M.; Murthy, Z.V.P.; Chakraborty, M. Effects of operating parameters on the production of barium sulfate nanoparticles in stirred media mill. J. Ind. Eng. Chem. 2012, 18, 1450–1457. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z.V.P. Study on the stability and microstructural properties of barium sulfate nanoparticles produced by nanomilling. Adv. Powder Technol. 2014, 25, 226–235. [Google Scholar] [CrossRef]
- Vicum, L.; Mazzotti, M.; Baldyga, J. Applying a thermodynamic model to the non-stoichiometric precipitation of barium sulfate. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2003, 26, 325–333. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Kibalczyc, W. Light scattering study of KH2PO4 and BaSO4 nucleation process. J. Cryst. Growth 1986, 76, 379–382. [Google Scholar] [CrossRef]
- Bromley, L.A. Thermodynamic properties of strong electrolytes in aqueous solutions. AIChE J. 1973, 19, 313–320. [Google Scholar] [CrossRef]
- Monnin, C. A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200 °C and to 1 kbar. Chem. Geol. 1999, 153, 187–209. [Google Scholar] [CrossRef]
- Leng, H.; Wang, X.; Niebur, G.L.; Roeder, R.K. Synthesis of a barium sulfate nanoparticle contrast agent for micro-computed tomography of bone microstructure. Ceram. Nanomater. Nanotechnol. III 2005, 159, 217–229. [Google Scholar]
- Bala, H.; Fu, W.; Zhao, J.; Ding, X.; Jiang, Y.; Yu, K.; Wang, Z. Preparation of BaSO4 nanoparticles with self-dispersing properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 129–134. [Google Scholar] [CrossRef]
- Sifontes, Á.B.; Cañizales, E.; Toro-Mendoza, J.; Ávila, E.; Hernández, P.; Delgado, B.A.; Cruz-Barrios, E. Obtaining highly crystalline barium sulphate nanoparticles via chemical precipitation and quenching in absence of polymer stabilizers. J. Nanomater. 2015, 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Hou, R.; Zhou, K.; Hua, F.; Cong, Y.; Zhang, J.; Cheng, Y.J. Surface functionalized barium sulfate nanoparticles: Controlled in situ synthesis and application in bone cement. J. Mater. Chem. B 2014, 2, 1264–1274. [Google Scholar] [CrossRef]
- Bala, H.; Fu, W.; Guo, Y.; Zhao, J.; Jiang, Y.; Ding, X.; Wang, Z. In situ preparation and surface modification of barium sulfate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 71–76. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, P.; Shivakumara, C. Synthesis of BaSO4 nanoparticles by precipitation method using sodium hexa metaphosphate as a stabilizer. Solid State Commun. 2010, 150, 386–388. [Google Scholar] [CrossRef]
- Li, S.; Zheng, L.; Yu, L. Preparation of Flower-Shaped Barium Sulfate Nanoparticles in the Presence of DTAB. J. Dispers. Sci. Technol. 2011, 32, 601–603. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Cui, Y.; Ma, W.; Guo, H. High dispersion barium sulfate nanoparticles prepared with dodecyl benzene sulfonic acid. Int. J. Nanosci. 2012, 11, 1240040. [Google Scholar] [CrossRef]
- Ramaswamya, V.; Vimalathithan, R.M.; Ponnusamy, V. Synthesis of monodispersed barium sulphate nanoparticles using water-benzene mixed solvent. Adv Mater Lett 2012, 3, 29–33. [Google Scholar] [CrossRef]
- Sokolova, V.; Loza, K.; Knuschke, T.; Heinen-Weiler, J.; Jastrow, H.; Hasenberg, M.; Epple, M. A systematic electron microscopic study on the uptake of barium sulphate nano-, submicro-, microparticles by bone marrow-derived phagocytosing cells. Acta Biomater. 2018, 80, 352–363. [Google Scholar] [CrossRef]
- Prutviraj, K.; Ramesh, T.N. Surfactant mediated synthesis of barium sulfate, strontium sulfate and barium-strontium sulfate nanoparticles. Inorg. Nano-Met. Chem. 2019, 49, 93–99. [Google Scholar]
- Shimpi, N.G.; Mishra, S. Ultrasonic-assisted synthesis of nano-BaSO4 and its effect on thermal and cross-linking density of epoxy nanocomposites. J. Reinf. Plast. Compos. 2013, 32, 947–954. [Google Scholar] [CrossRef]
- Jha, M.; Ansari, S.; Shimpi, N.G. Novel sonochemical green approach for synthesis of highly crystalline and thermally stable barium sulphate nanoparticles using Azadirachta indica leaf extract. Bull. Mater. Sci. 2019, 42, 22. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M.A. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Solanki, J.N.; Sengupta, R.; Murthy, Z.V.P. Synthesis of copper sulphide and copper nanoparticles with microemulsion method. Solid State Sci. 2010, 12, 1560–1566. [Google Scholar] [CrossRef]
- Yıldırım, Ö.A.; Durucan, C. Synthesis of zinc oxide nanoparticles elaborated by microemulsion method. J. Alloy. Compd. 2010, 506, 944–949. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Yang, C.; Hilliard, L.R.; Tan, W. Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 2004, 20, 8336–8342. [Google Scholar] [CrossRef] [PubMed]
- Salabat, A.; Mirhoseini, F. A novel and simple microemulsion method for synthesis of biocompatible functionalized gold nanoparticles. J. Mol. Liq. 2018, 268, 849–853. [Google Scholar] [CrossRef]
- Vafa, E.; Shahrokhi, M.; Molaei Dehkordi, A. Population Balance Modeling of Barium Sulfate Nanoparticle Synthesis via Inverse Microemulsion Including Coagulation Effect. Ind. Eng. Chem. Res. 2014, 53, 12705–12719. [Google Scholar] [CrossRef]
- Gan, L.M.; Zhang, L.H.; Chan, H.S.O.; Chew, C.H. Preparation of conducting polyaniline-coated barium sulfate nanoparticles in inverse microemulsions. Mater. Chem. Phys. 1995, 40, 94–98. [Google Scholar] [CrossRef]
- Qi, L.; Ma, J.; Cheng, H.; Zhao, Z. Preparation of BaSO4 nanoparticles in non-ionic w/o microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 1996, 108, 117–126. [Google Scholar] [CrossRef]
- Hopwood, J.D.; Mann, S. Synthesis of barium sulfate nanoparticles and nanofilaments in reverse micelles and microemulsions. Chem. Mater. 1997, 9, 1819–1828. [Google Scholar] [CrossRef]
- Ivanova, N.I.; Rudelev, D.S.; Summ, B.D.; Chalykh, A.A. Synthesis of barium sulfate nanoparticles in water-in-oil microemulsion systems. Colloid J. 2001, 63, 714–717. [Google Scholar] [CrossRef]
- Adityawarman, D.; Voigt, A.; Veit, P.; Sundmacher, K. Precipitation of BaSO4 nanoparticles in a non-ionic microemulsion: Identification of suitable control parameters. Chem. Eng. Sci. 2005, 60, 3373–3381. [Google Scholar] [CrossRef]
- Ethayaraja, M.; Bandyopadhyaya, R. Population balance models and Monte Carlo simulation for nanoparticle formation in water-in-oil microemulsions: Implications for CdS synthesis. J. Am. Chem. Soc. 2006, 128, 17102–17113. [Google Scholar] [CrossRef]
- Nourafkan, E.; Alamdari, A. Modeling of Silver Nanoparticle Synthesis in Ternary Reverse Microemulsion of Cyclohexane/Water/SDS. Part. Sci. Technol. 2014, 32, 215–223. [Google Scholar] [CrossRef]
- Muralidharan, G.; Subramanian, L.; Nallamuthu, S.K.; Santhanam, V.; Kumar, S. Effect of reagent addition rate and temperature on synthesis of gold nanoparticles in microemulsion route. Ind. Eng. Chem. Res. 2011, 50, 8786–8791. [Google Scholar] [CrossRef]
- Ramkrishna, D.; Mahoney, A.W. Population balance modeling. Promise for the future. Chem. Eng. Sci. 2002, 57, 595–606. [Google Scholar] [CrossRef]
- Viswanadh, B.; Tikku, S.; Khilar, K.C. Modeling core–shell nanoparticle formation using three reactive microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 149–157. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Sun, D.; Odoom-Wubah, T.; Huang, J.; Li, Q. Modeling of silver nanoparticle formation in a microreactor: Reaction kinetics coupled with population balance model and fluid dynamics. Ind. Eng. Chem. Res. 2014, 53, 4263–4270. [Google Scholar] [CrossRef]
- Niemann, B.; Rauscher, F.; Adityawarman, D.; Voigt, A.; Sundmacher, K. Microemulsion-assisted precipitation of particles: Experimental and model-based process analysis. Chem. Eng. Processing Process Intensif. 2006, 45, 917–935. [Google Scholar] [CrossRef]
- Costa, C.B.B.; Maciel Filho, R. Nanoparticle processes modelling: The role of key parameters for population balances for on-line crystallization processes applications. Powder Technol. 2010, 202, 89–94. [Google Scholar] [CrossRef]
- Niemann, B.; Sundmacher, K. Reduced discrete population balance model for precipitation of barium sulfate nanoparticles in non-ionic microemulsions. Chem. Eng. J. 2008, 143, 314–325. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Kumar, R.; Gandhi, K.S. Modelling of CaCO3 nanoparticle formation during overbasing of lubricating oil additives. Langmuir 2001, 17, 1015–1029. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Kumar, R.; Gandhi, K.S. Simulation of precipitation reactions in reverse micelles. Langmuir 2000, 16, 7139–7149. [Google Scholar] [CrossRef]
- Öncül, A.A.; Niemann, B.; Sundmacher, K.; Thévenin, D. CFD modelling of BaSO4 precipitation inside microemulsion droplets in a semi-batch reactor. Chem. Eng. J. 2008, 138, 498–509. [Google Scholar] [CrossRef]
- Niemann, B.; Veit, P.; Sundmacher, K. Nanoparticle precipitation in reverse microemulsions: Particle formation dynamics and tailoring of particle size distributions. Langmuir 2008, 24, 4320–4328. [Google Scholar] [CrossRef]
- Köhler, J.M.; Abahmane, L.; Wagner, J.; Albert, J.; Mayer, G. Preparation of metal nanoparticles with varied composition for catalytical applications in microreactors. Chem. Eng. Sci. 2008, 63, 5048–5055. [Google Scholar] [CrossRef]
- Rahman, M.; Rebrov, E.V. Microreactors for gold nanoparticles synthesis: From Faraday to flow. Processes 2014, 2, 466–493. [Google Scholar] [CrossRef] [Green Version]
- Toyota, A.; Nakamura, H.; Ozono, H.; Yamashita, K.; Uehara, M.; Maeda, H. Combinatorial synthesis of CdSe nanoparticles using microreactors. J. Phys. Chem. C 2010, 114, 7527–7534. [Google Scholar] [CrossRef]
- Simmons, M.; Wiles, C.; Rocher, V.; Francesconi, M.G.; Watts, P. The preparation of magnetic iron oxide nanoparticles in microreactors. J. Flow Chem. 2013, 3, 7–10. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Neveu, S.; Dupuis, V.; Cabuil, V. Synthesis of cobalt ferrite nanoparticles in continuous-flow microreactors. RSC Adv. 2012, 2, 11263–11266. [Google Scholar] [CrossRef]
- Wu, H.; Wang, C.; Zeng, C.; Zhang, L. Preparation of barium sulfate nanoparticles in an interdigital channel configuration micromixer SIMM-V2. Ind. Eng. Chem. Res. 2013, 52, 5313–5320. [Google Scholar] [CrossRef]
- Kockmann, N.; Kastner, J.; Woias, P. Reactive particle precipitation in liquid microchannel flow. Chem. Eng. J. 2008, 135, S110–S116. [Google Scholar] [CrossRef]
- Jeevarathinam, D.; Gupta, A.K.; Pitchumani, B.; Mohan, R. Effect of gas and liquid flowrates on the size distribution of barium sulfate nanoparticles precipitated in a two phase flow capillary microreactor. Chem. Eng. J. 2011, 173, 607–611. [Google Scholar] [CrossRef]
- Su, Y.F.; Kim, H.; Kovenklioglu, S.; Lee, W.Y. Continuous nanoparticle production by microfluidic-based emulsion, mixing and crystallization. J. Solid State Chem. 2007, 180, 2625–2629. [Google Scholar] [CrossRef]
- Schwarzer, H.C.; Peukert, W. Combined experimental/numerical study on the precipitation of nanoparticles. AIChE J. 2004, 50, 3234–3247. [Google Scholar] [CrossRef]
- McCarthy, E.D.; Dunk, W.A.E.; Boodhoo, K.V.K. Application of an intensified narrow channel reactor to the aqueous phase precipitation of barium sulphate. J. Colloid Interface Sci. 2007, 305, 72–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucher, M.; Babic, D.; Kind, M. Precipitation of barium sulfate: Experimental investigation about the influence of supersaturation and free lattice ion ratio on particle formation. Chem. Eng. Processing Process Intensif. 2006, 45, 900–907. [Google Scholar] [CrossRef]
- Wang, Q.A.; Wang, J.X.; Li, M.; Shao, L.; Chen, J.F.; Gu, L.; An, Y.T. Large-scale preparation of barium sulphate nanoparticles in a high-throughput tube-in-tube microchannel reactor. Chem. Eng. J. 2009, 149, 473–478. [Google Scholar] [CrossRef]
- Sen, N.; Koli, V.; Singh, K.K.; Panicker, L.; Sirsam, R.; Mukhopadhyay, S.; Shenoy, K.T. Segmented microfluidics for synthesis of BaSO4 nanoparticles. Chem. Eng. Processing-Process Intensif. 2018, 125, 197–206. [Google Scholar] [CrossRef]
- Pohl, B.; Jamshidi, R.; Brenner, G.; Peuker, U.A. Experimental study of continuous ultrasonic reactors for mixing and precipitation of nanoparticles. Chem. Eng. Sci. 2012, 69, 365–372. [Google Scholar] [CrossRef]
- Hakke, V.; Sonawane, S.; Anandan, S.; Sonawane, S.; Ashokkumar, M. Process intensification approach using microreactors for synthesizing nanomaterials—A critical review. Nanomaterials 2021, 11, 98. [Google Scholar] [CrossRef]
- Potapov, V.; Fediuk, R.; Gorev, D. Membrane concentration of hydrothermal SiO2 nanoparticles. Sep. Purif. Technol. 2020, 251, 117290. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Bi, S.; Luo, G. Preparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactor. Powder Technol. 2010, 202, 130–136. [Google Scholar] [CrossRef]
- Yang, L.; Guo, M.; Zhang, F.; Jing, Y.; Wang, Y.; Luo, G. Controllable preparation of γ-alumina nanoparticles with bimodal pore size distribution in membrane dispersion microreactor. Particuology 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Piacentini, E.; Poerio, T.; Bazzarelli, F.; Giorno, L. Continuous production of PVA-based hydrogel nanoparticles by membrane nanoprecipitation. J. Membr. Sci. 2021, 637, 119649. [Google Scholar] [CrossRef]
- Chen, G.; Luo, G.; Xu, J.; Wang, J. Preparing ultrafine barium sulfate particles using membrane-dispersion-precipitation technology. J.-Tsinghua Univ. 2004, 44, 315–318. [Google Scholar]
- Zhiqian, J.; Zhongzhou, L. Synthesis of nanosized BaSO4 particles with a membrane reactor: Effects of operating parameters on particles. J. Membr. Sci. 2002, 209, 153–161. [Google Scholar] [CrossRef]
- Chen, G.G.; Luo, G.S.; Xu, J.H.; Wang, J.D. Membrane dispersion precipitation method to prepare nanopartials. Powder Technol. 2004, 139, 180–185. [Google Scholar] [CrossRef]
- Baker, R.W. Overview of membrane science and technology. Membr. Technol. Appl. 2004, 3, 1–14. [Google Scholar]
- Chiavola, A.; Amato, E.D.; Stoller, M.; Chianese, A.; Boni, M.R. Application of iron based nanoparticles as adsorbents for arsenic removal from water. Chem. Eng. Trans. 2016, 47, 325–330. [Google Scholar]
- Pask, S.D.; Nuyken, O.; Cai, Z. The spinning disk reactor: An example of a process intensification technology for polymers and particles. Polym. Chem. 2012, 3, 2698–2707. [Google Scholar] [CrossRef]
- Tai, C.Y.; Wang, Y.H.; Liu, H.S. A green process for preparing silver nanoparticles using spinning disk reactor. AIChE J. 2008, 54, 445–452. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Raston, C.L.; Saunders, M. Controlled scalable synthesis of ZnO nanoparticles. Chem. Mater. 2007, 19, 5453–5459. [Google Scholar] [CrossRef]
- Chang, M.H.; Liu, H.S.; Tai, C.Y. Preparation of copper oxide nanoparticles and its application in nanofluid. Powder Technol. 2011, 207, 378–386. [Google Scholar] [CrossRef]
- Mohammadi, S.; Harvey, A.; Boodhoo, K.V. Synthesis of TiO2 nanoparticles in a spinning disc reactor. Chem. Eng. J. 2014, 258, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Cafiero, L.M.; Baffi, G.; Chianese, A.; Jachuck, R.J.J. Process intensification: Precipitation of barium sulfate using a spinning disk reactor. Ind. Eng. Chem. Res. 2002, 41, 5240–5246. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Vafaeimanesh, A. Synthesis of barium sulfate nanoparticles using a spinning disk reactor: Effects of supersaturation, disk rotation speed, free ion ratio, and disk diameter. Ind. Eng. Chem. Res. 2009, 48, 7574–7580. [Google Scholar] [CrossRef]
- Jacobsen, N.C.; Hinrichsen, O. Micromixing efficiency of a spinning disk reactor. Ind. Eng. Chem. Res. 2012, 51, 11643–11652. [Google Scholar] [CrossRef]
- Farahani, H.B.; Shahrokhi, M.; Dehkordi, A.M. Experimental investigation and process intensification of barium sulfate nanoparticles synthesis via a new double coaxial spinning disks reactor. Chem. Eng. Processing Process Intensif. 2017, 115, 11–22. [Google Scholar] [CrossRef]
- Jahanshahi-Anboohi, J.; Molaei Dehkordi, A. Continuous Synthesis of Barium Sulfate Nanoparticles in a New High-Speed Spinning Disk Reactor. Ind. Eng. Chem. Res. 2019, 58, 16597–16609. [Google Scholar] [CrossRef]
- Meeuwse, M.; van der Schaaf, J.; Schouten, J.C. Multistage rotor-stator spinning disc reactor. AIChE J. 2012, 58, 247–255. [Google Scholar] [CrossRef]
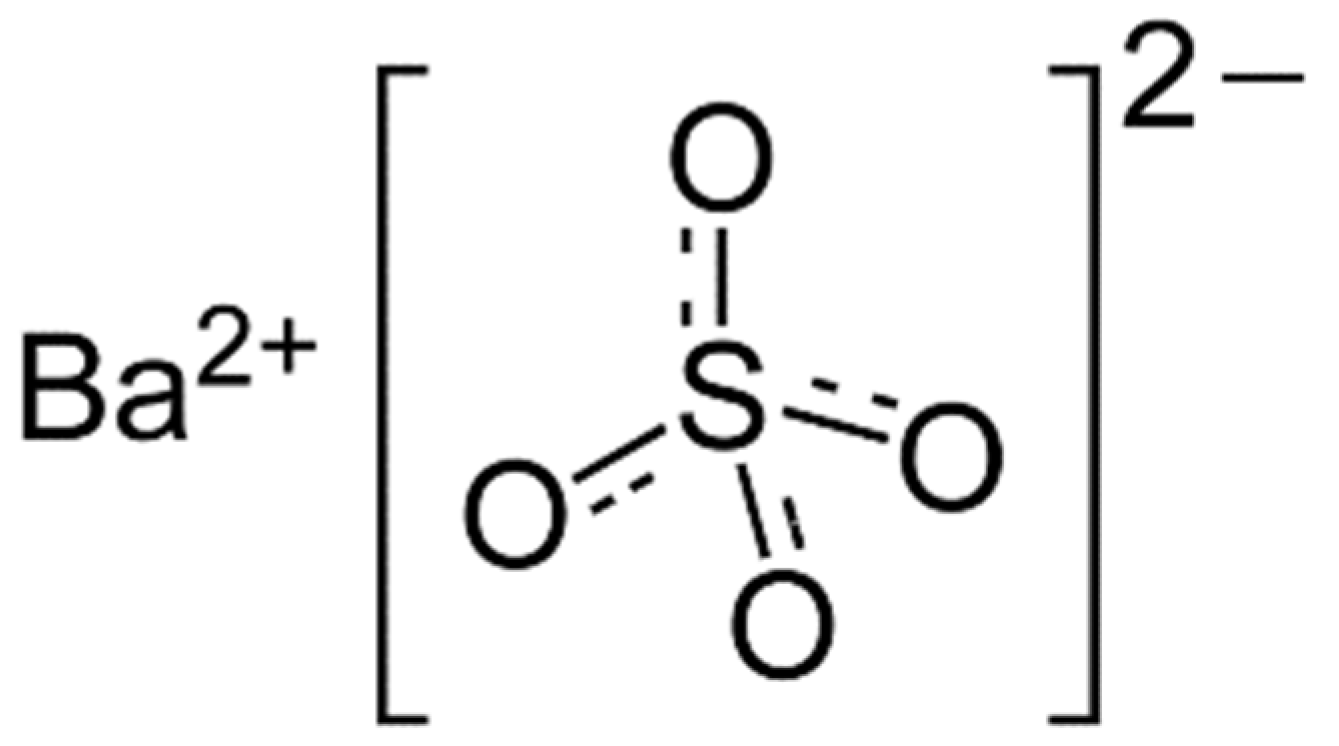
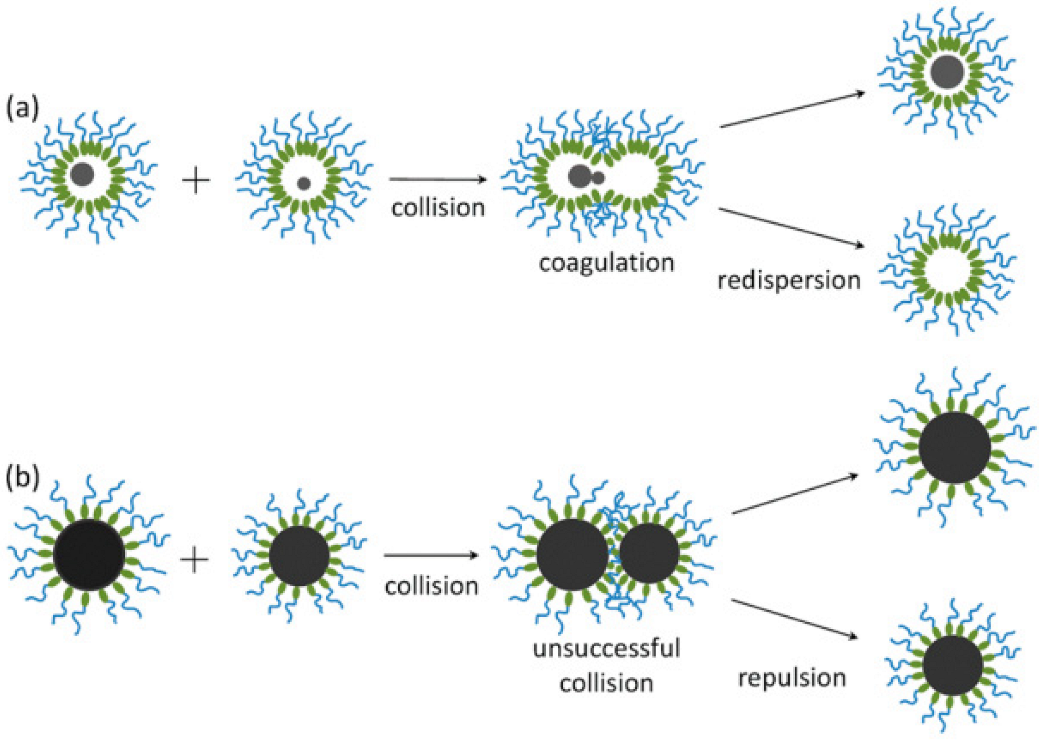
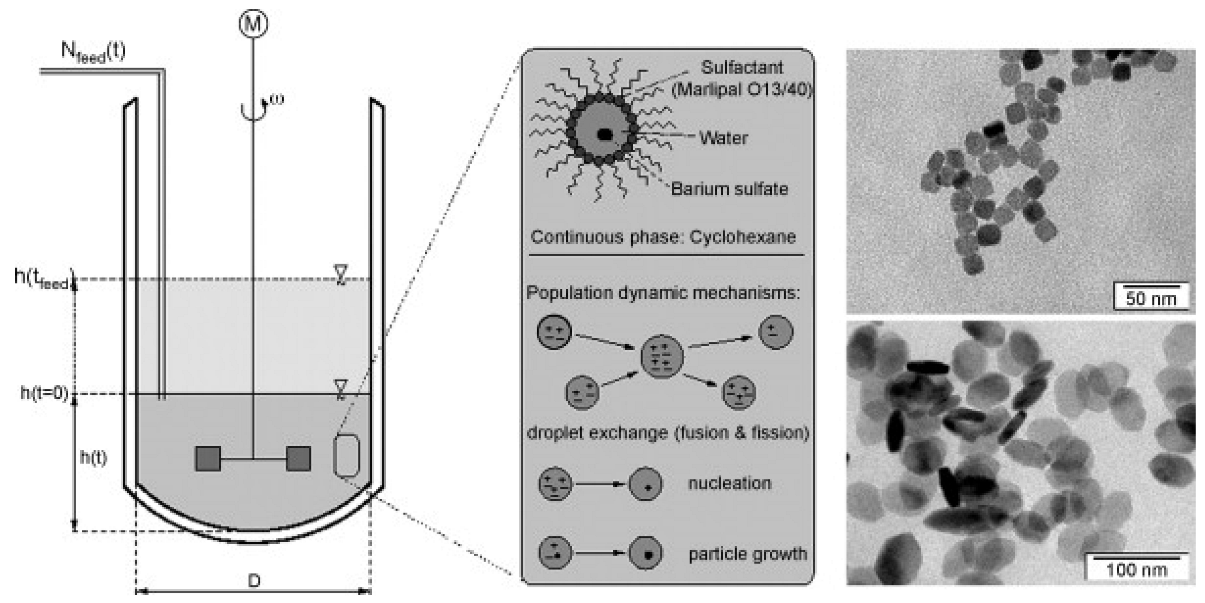
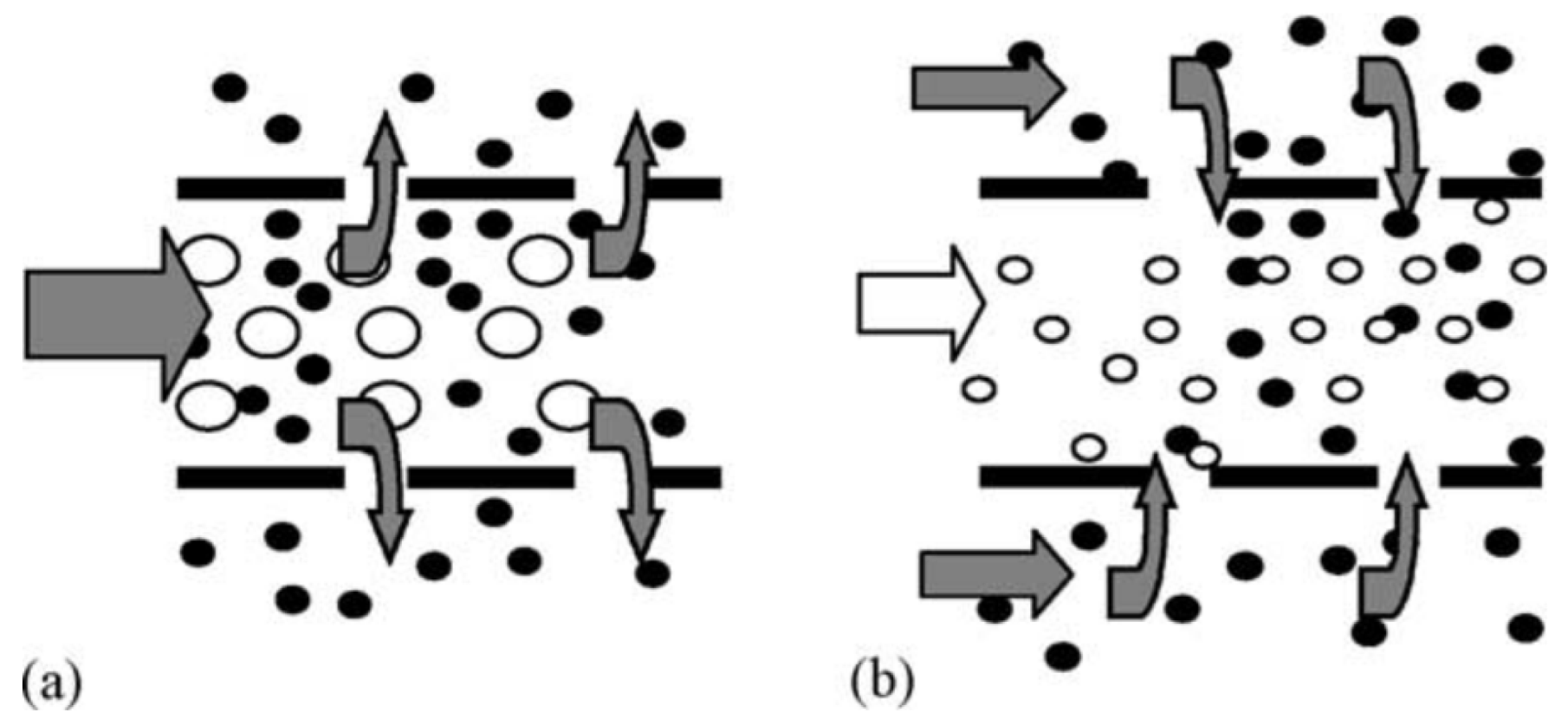
| Precursors | Medium | Stabilizer | Mean Particles Size, nm | Ref. |
|---|---|---|---|---|
| Na2SO4, BaCl2 | H2O | gelatin | 50.80 ± 0.02 | [12] |
| Na2SO4, BaCl2 | H2O | No | <100 | [46] |
| (NH4)2SO4, BaCl2 | H2O − C2H5OH | No | 24.3 | [47] |
| H2SO4, BaCl2, Ba(OH)2 | H2O | No | 4–92 | [48] |
| K2S2O8, BaCl2 | H2O | (2-(methacryloyloxy)ethyldimethyl-(3-sulfopropyl)ammoniumhydroxide; 3-sulfopropylmethacrylate potassium salt; 2-acrylamido-2- methylpropane sulfonic acid. | 10–20 | [49] |
| (NH4)2SO4, H2SO4, BaCl2 | H2O | octadecyl dihydrogen phosphate | 76 | [50] |
| Na2SO4, Ba(NO3)2 | H2O | sodium hexametaphosphate | 30–55 | [51] |
| Na2SO4, BaCl2 | H2O | dodecyl trimethyl ammonium bromide | 180–190 | [52] |
| (NH4)2SO4, BaCl2 | H2O − C2H5OH | dodecyl benzene sulfonic acid | 46 | [53] |
| Na2SO4, BaCl2 | H2O | No | 30.4 | [54] |
| Na2SO4, BaCl2 | H2O − C6H6 | No | 35.9 | [54] |
| Na2SO4, BaCl2 | H2O | carboxymethylcellulose | 45 ± 12 | [55] |
| Na2SO4, BaCl2 | H2O | sodium dodecyl sulfate | 80–150 | [56] |
| * (NH4)2SO4, BaCl2 | H2O | sodium lauryl sulfate | 9 | [57] |
| * Na2SO4, BaCl2 | H2O | cetyltrimethylammonium bromide | 55.6 | [58] |
| Precursors | Organic Parts of Microemulsion | Mean Particles Size, nm | Ref. |
|---|---|---|---|
| (NH4)2SO4, BaCl2 | cyclohexane; benzene; Triton X-100; t-Oct-C6H4-(OCH2CH2)xOH (x = 9–10); 1-hexanol. | 13 | [21] |
| K2S2O8, H2SO4, BaCl2 | poly(oxyethylene)5 phenol ether; poly(oxyethylene)9 phenol ether. | 10–20 | [65] |
| (NH4)2SO4, Ba(OAc)2 | Triton X-100; n-hexanol; cyclohexane. | 10 | [66] |
| Na2SO4, BaCl2 | sodium bis(2-ethylhexylsulfosuccinate; poly(oxyethylene-4-dodecyl ether); didodecyldimethylammonium bromide; decane; dodecane; isooctane. | 2–4 | [67] |
| (NH4)2SO4, H2SO4, BaCl2 | poly(ethylene glycol) octylphenyl ether; Triton X-100; n-hexyl alcohol; cyclohexane. | 5–10 | [68] |
| K2SO4, BaCl2 | Marlipal O13/40; cyclohexane. | 6 ± 2 | [69] |
| Type of Microreactor | Reaction System | Channel Size, μm | Ba2+/SO42− Molar Ratio | Ba2+ Solution Concentration, mol/L | Flow Rate, mL/min | Mean Particle Size, nm | Ref. |
|---|---|---|---|---|---|---|---|
| T | Microemulsion | 500 | 1 | 0.1 | 0.8 | 15 | [89] |
| T | Liquid-liquid | 1000 | 3 | 0.7 | 180 | 35 | [90] |
| T | Liquid-liquid | 600 × 300 | 1.5 | 0.5 | 24 | 91 | [91] |
| T | Two-phase | 830 × 1000 | 5 | 0.5 | 6.5 | 300 | [92] |
| Y | - | 500 | 1 | 0.1 | 30 | 200 | [93] |
| Y | - | 500 | 5 | ≤0.5 | 600 | 40 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketegenov, T.; Kamunur, K.; Batkal, A.; Gani, D.; Nadirov, R. Recent Advances in the Preparation of Barium Sulfate Nanoparticles: A Mini-Review. ChemEngineering 2022, 6, 30. https://doi.org/10.3390/chemengineering6020030
Ketegenov T, Kamunur K, Batkal A, Gani D, Nadirov R. Recent Advances in the Preparation of Barium Sulfate Nanoparticles: A Mini-Review. ChemEngineering. 2022; 6(2):30. https://doi.org/10.3390/chemengineering6020030
Chicago/Turabian StyleKetegenov, Tlek, Kaster Kamunur, Aisulu Batkal, Diana Gani, and Rashid Nadirov. 2022. "Recent Advances in the Preparation of Barium Sulfate Nanoparticles: A Mini-Review" ChemEngineering 6, no. 2: 30. https://doi.org/10.3390/chemengineering6020030
APA StyleKetegenov, T., Kamunur, K., Batkal, A., Gani, D., & Nadirov, R. (2022). Recent Advances in the Preparation of Barium Sulfate Nanoparticles: A Mini-Review. ChemEngineering, 6(2), 30. https://doi.org/10.3390/chemengineering6020030






