Abstract
The oxygen reduction reaction has been the object of intensive research in an attempt to improve the sluggish kinetics that limit the performance of renewable energy storage and utilization systems. Platinum or platinum bimetallic alloys are common choices as the electrode material, but prohibitive costs hamper their use. Complex alloy materials, such as high-entropy alloys (HEAs), or more generally, multiple principal component alloys (MPCAs), have emerged as a material capable of overcoming the limitations of platinum and platinum-based materials. Theoretically, due to the large variety of active sites, this new kind of material offers the opportunity to identify experimentally the optimal binding site on the catalyst surface. This review discusses recent advances in the application of such alloys for the oxygen reduction reaction and existing experimental challenges in the benchmarking of the electrocatalytic properties of these materials.
1. Introduction
The need for the proliferation of renewable energy sources, motivated by the demand for the mitigation of climate change, has triggered a widespread interest in the research of electrochemical devices for energy conversion and storage. Along with Li-ion batteries, proton exchange membrane fuel cells (PEMFC) have been a prime candidate for mid- and long-term energy storage and utilization devices [1,2,3,4,5,6]. It is expected that PEMFC technology plays an important role in mass transport systems, a recent example being the Coradia iLint, the world’s first passenger train powered by a hydrogen fuel cell [7]. In a PEMFC, green hydrogen expected to be gained from water electrolysis, is oxidized at the anode and oxygen is reduced at the cathode. In the development of PEMFCs and other related technologies the high overpotential for the oxygen reduction reaction (ORR) is the bottleneck for the improvement of the efficiency and cost-effectiveness and, in turn, the widespread use of these technologies [8].
Few reactions have received as much attention in the last 50 years in both fundamental and applied research as the ORR. The ORR also has a key role in other important processes such as corrosion, enzymatic reactions, and metal–air batteries, among others [9,10,11,12]. In acidic media, the best know catalyst for the ORR is platinum (Pt), but even on Pt, the overpotential for the ORR is around 0.3 V [8,13,14]. Pt is also one of the few materials displaying sufficient stability under harsh ORR reaction conditions [15]. However, platinum is scarce, expensive, and 77% of its production is concentrated in only one country [16].
In fuel cells the noble metal (generally Pt) loading of the cathode, for the catalysis of ORR, is considerably higher than of the anode (0.2 vs. 0.05 mg∙cm−2) [17,18]. This contributes significantly to the cost of fuel cell stack and inhibits their proliferation [18], since the platinum price is around 35$/g (November 2021), the price of the platinum catalyst is about 20% of the total stack cost [19,20]. Therefore, to accelerate the application of this technology, it is necessary to develop both more active and more durable catalysts for the ORR.
One of the most used strategies to reduce the necessary platinum loading is to alloy the platinum with other elements. This can produce catalysts of similar or even higher activity than Pt, and at the same time maintaining or increasing the stability.
Pt-alloy catalysts have been used in electrocatalysts widely since the 1970s [21] and have been considered as the next generation fuel cell catalyst since then. Pt alloying is a well-known strategy to modify the electrocatalytic properties of materials [22] and has been used to make various Pt-alloys with chromium [23], tin [24], ruthenium [25], gold [26], copper [27,28,29,30], nickel [31], cobalt [31], iron [32], and more recently lanthanides [33,34] and other rare earths [34,35]. The list of chemical elements that have been used in combination with platinum for ORR and in general for electrocatalytic applications is large and could be extended to include almost every element suitable to be alloyed with platinum. However, few of the materials reported until now have been used in practical applications. Fuel cells in real-world applications still often use Pt, and Pt-alloys have been introduced only relatively recently (e.g., the electrical vehicle model Mirai from Toyota uses Pt-Co catalysts [36]).
In recent years, advances in nanotechnology have facilitated the synthesis of nanomaterials with a precise control of the size, composition, shape, structure, and the chemical state of different platinum alloys [37,38,39,40]. In addition, advances in in situ and in operando techniques have allowed the establishment of relationships between parameters that can be adjusted in the synthesis process for tuning of the catalytic activity, complemented with theoretical calculations [41,42]. This rational design strategy contributed to overcoming the trial-and-error methodology used for example by Motoo and Furuya [28,29,30] in the 1970s to investigate the effect of the combinations of different electrodes and adatoms on the catalysis of multiple reactions.
Despite the progress made, there have only been modest developments in improving the activity of ORR catalysts in recent years. This is mostly due to the scaling relations, which limit the degrees of freedom for the adjustment of intermediate binding energies [43,44]. This highlights the need for the creation of a new type of catalyst, beyond the well-known and widely investigated binary alloys. Multiple principal element alloys (MPCAs) and high-entropy alloys (HEAs), while they might have been investigated by Franz Karl Achard as early as 1788 [45], have recently received attention for their unique properties and the fact that the potential designs are abundant, also sparking their investigation as catalysts. The number of possible alloy combinations N, for the number of components C, with varying the composition by X percent, is given as [45]. If we take into account transition and post-transition metals, excluding Hg, Cd, As, Tl, Os, and Pb, because of their toxicity, Tc due to its radioactivity, and Ga and Se as potentially decreasing conductivity, varying their composition by 1% gives 1062 potential materials. Including lanthanides (excluding Pr), that number grows to 1088. In this work, we review recent progress in bimetallic Pt-alloys, multiple principal component alloys (MPCAs), and high-entropy alloys (HEAs), e.g., NiCoCuFePt, for the ORR in acidic aqueous media with special emphasis on experimental aspects. To do this, we start briefly by reviewing the state-of-the-art on bimetallic alloys and comparing it with earlier literature results. Further, we analyze the progress in the characterization of new Pt-based multiple principal component catalysts over the recent years in terms of activity, stability, and selectivity, and the understanding regarding the control of these parameters in catalyst design. Finally, we present some experimental challenges in the ORR study with multicomponent materials or high-entropy alloys and some concluding remarks.
2. Bimetallic Alloys
The ORR is a complex reaction that involves a four-electron transfer process to produce water as the end product. In acidic media, three pathways are considered to explain the experimental results: the O2 dissociation, *OOH dissociation, and the H2O2 dissociation pathway [46,47,48,49]. In basic media, the ORR takes place through the formation of superoxide anion (O2−) [50]. The different intermediates (*OH, *O, *OOH, etc.) involved in the proposed mechanism have different adsorption energies. According to the scaling relations, it is impossible to carry out the optimization for all the different intermediates on a unique active site [42,51,52,53]. This holds for approximately all close-packed transition-metal facets electrocatalysts [22]. While differences between individual metals can be up to 1 eV, alloying allows us to tune these energies much more finely [42,51,54,55]. This fact has driven the study of platinum alloys to overcome the limitations associated with the use of a single metal catalyst.
The low price and high abundance of transition metals such as copper, nickel, iron, and cobalt along with excellent catalytic activity observed in PtnM alloys (where M is the non-platinum group (non-PGM) metal) gave impulse to the research of these materials [56,57,58,59,60,61]; however, as the demand for these materials increases it is likely that the price will also increase, e.g., the cobalt price has almost doubled since the beginning of 2021. Moreover, the miscibility of M with platinum and the inability of the less noble metal to segregate into the bulk of the PtnM make these kinds of alloys an excellent option to address the limitations associated with pure platinum electrodes [62].
It is known that under ORR conditions polycrystalline bimetallic catalysts dealloy [55,63,64,65] and form a core-shell structure [65,66,67,68,69], meaning that the alloy’s core is covered by a layer of Pt several atomic layers thick [68,69,70,71]. Considering that the ligand effects can be significant only through 2–3 atomic layers, the electronic properties of the surface are determined by the strain effects [56], which have a longer range [72]. The process is less prominent on single crystal surfaces (both low-index and stepped/kinked), as the dealloying process is faster at defect sites [63,73,74]. The strain effects lead to the weakening of the binding of ORR intermediates [70,75,76].
This means that the adsorption energies, and consequentially the activities, of these catalysts can be tuned by tuning the strain in the Pt overlayer. At the same time, the Pt overlayer acts as a barrier preventing further dealloying in the bulk. However, this also means that the active site is always on the Pt atoms on the surface layer, and the electronic structure is modified mainly through lattice strain. Since the binding energies of the intermediates are related to each other through the scaling relations, this presents a fundamental limitation to the degrees of freedom available for the optimization of these catalysts.
Figure 1 shows the specific (SA) and mass activity (MA) for some selected Pt alloys reported mostly in the last two years for the ORR in acid media, as well as some extended surfaces and single crystals surfaces, shown for reference [14,54,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]. The mass and specific activity of these electrocatalysts is excellent compared to commercial Pt/C catalysts and the variety of nanostructured that can be synthesized is wide (core-shell, nanoplates, nanowires, nanoframes, etc.). Joo et al. prepared Pt-Cu nanoframes with an excellent mass and specific catalytic activity (2.47 A mgPt−1 and 4.69 mA∙m−2) but also with a high stability [94]. For instance, Xia et al. synthesized using a colloidal route and afterwards etched with nitric acid platinum nickel nanospheres connected with a Pt-skin structure with mass and specific activities that are 17 and 14 times higher than commercial catalysts [95]. Shen et al. used a self-etched engineering to prepare a Pt-Co nanodendrite in an external nanoframe with Pt-skin with a mass activity of 0.939 A∙mgPt−1 [87]. Peng et al. prepared Fe-Pt supported in N-doped mesoporous carbon using a thermal treatment with a mass activity of 0.433 A∙mgPt−1 and only a small 16 mV decrease in the E1/2 after 5000 cycles [100]. In Pt-lanthanide alloys, the lanthanide contraction could be used to control the electrocatalytic activity and stability [64]. Figure 1 shows some specific and mass activities for selected Pt-lanthanides alloys. One of the most active is the PtxY alloy with a specific activity of around 12 mA∙cm−2. The superior performance is rationalized, taking into account a compressive strain exerted by the catalyst core onto the surface [69]. Recently, Bandarenka reported a PtxPr/C electrocatalysts with a specific and mass activity of 1.96 mA∙cm−2 and 0.7 A mgPt−1, respectively, with an affordable and scalable synthesis procedure, solving one of the bottlenecks for the wide use of Pt-lanthanides alloys (e.g., Y, Pr, Tb, Gd, Sm, etc.) [98]. The examples presented here show how the introduction of a non-noble material can be used to tune the electrocatalytic activity in Pt-M alloys while retaining much of the stability.
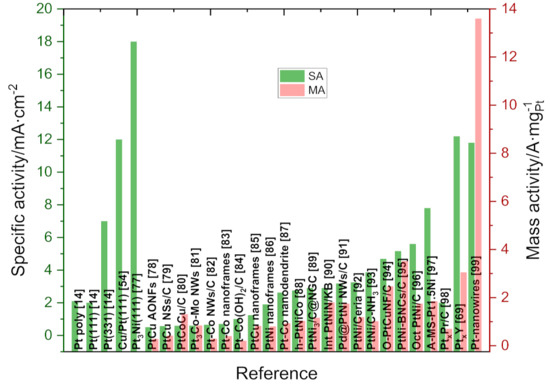
Figure 1.
Specific and mass activity of selected bi- and tri-metallic Pt-alloys for ORR in 0.1 M HClO4. Results obtained on Pt poly, Pt(111), Pt(331), Cu/Pt(111), and Pt3Ni(111) are shown as a reference for the specific activity, whereas PtxY, one of the highest SAs reported among lanthanide alloys and Pt-nanowires, displayed the highest MA to date.
Trimetallic alloys have been tested for the ORR in an attempt to benefit from the synergistic effect that can result from the introduction of three different elements [101,102,103,104,105]. Gan et al. synthesized a PtCoFe alloy catalyst at 600 °C with a mass activity around 0.65 A∙mgPt−1 in 0.1 M HClO4. The Co inhibits the agglomeration; meanwhile, the Fe promotes ordering at high temperatures [101]. Cruz–Martínez et al. produced, using the oleylamine-oleic acid method, NiPdPt nanoparticles with a specific and mass activity of 0.25 mA∙cm−2 and 0.2 A∙mgPt−1, respectively, in acidic media [102]. Xia et al. created PtIrPd trimetallic alloy nanocages with a porous structure and 100 facets, yielding an ECSA of around 63 m2∙g−1 with a specific and mass activity are 0.8 mA∙cm−2 and 0.5 A∙mgPt−1 respectively. Zhu et al. synthesized a PtCuNi/C intermetallic catalyst using an impregnation reduction method with a mass activity 9.2 times higher than the commercial Pt/C catalyst [106]. Chen et al. prepared a platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with an enhancement factor of 30.6 relative to commercial platinum nanoparticles in alkaline media [103]. In the case of these alloys, the state of the surface under reaction conditions is not completely clarified; however, apart from PGM alloying elements, it could be assumed that the non-noble elements dissolve. Generally, increasing the number of alloying elements from two to three did not lead to obvious advantages in terms of electrocatalytic activity. However, using a rational design, it was shown to be possible to obtain platinum-based catalysts with three constituent elements that have higher activities than observed in polycrystalline platinum [107], although not outperforming bimetallic alloys.
Several strategies for overcoming the scaling relations have been proposed in the literature [108]: 1. Multifunctionality [109]—separating the ORR into two 2-electron steps and provide optimized active sites for both steps on a multifunctional material; 2. Additional bonding of the OOH* intermediate [110] with a nearby functional group or an appropriate coordination of active sites; 3. Creating catalysts with a large variety of active sites [111]. Multiple principal component and high-entropy alloys, in particular, could satisfy such demands as they provide a high variety of different active sites, which could serve to explore new areas in the binding energies, provide optimal binding for different steps of the reaction and provide various “spectator species” coverages at different potentials.
3. Multicomponent and High-Entropy Alloys
Multiple principal component and high-entropy alloys have gained a lot of attention due to their unique properties that bring the possibility to move from a using the materials we have approach to engineering the materials we need approach [111,112,113,114,115]. They have been investigated in electrocatalysis, as catalysts for ORR [116], carbon dioxide reduction reaction (CO2RR) [117,118], methanol oxidation [119], and hydrogen evolution reaction (HER) [120]. Figure 2 shows the number of publications on the database Scopus when the keywords “oxygen reduction” are used together with the listed phrases: “platinum catalyst”, “platinum catalyst alloy”, “high-entropy alloy”, or “multicomponent alloy” from 1995 to 2021. In the last 5 five years, “high-entropy alloy” experienced a similar exponential growth observed for “platinum catalyst” and “platinum catalyst alloy” around 2008.
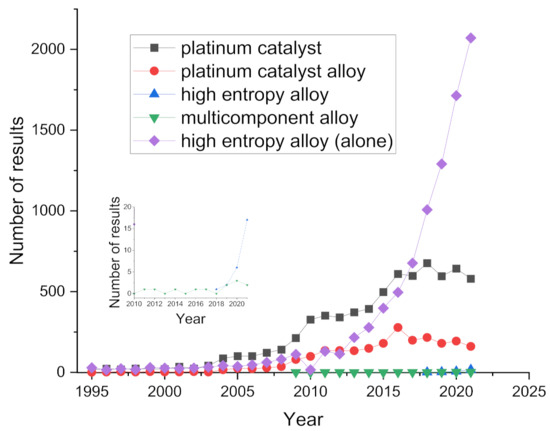
Figure 2.
Time evolution of the overall number of papers that appear on Scopus using keywords “oxygen reduction reaction together with “platinum catalyst”, “platinum catalyst alloy”, “high-entropy alloy”, or “multicomponent alloy”, as well as “high-entropy alloy” alone. The inset shows the details for last years for HEA and multicomponent alloy.
The promising property of multicomponent alloys, especially high-entropy alloys, is that they might not dealloy the same way as bi-metallic alloys, i.e., they could keep dissimilar atoms on the surface, and, therefore have different atoms as active sites present [117]. This would mean that besides the well-investigated strain effects, ligand and ensemble effects would also be pronounced [117]. The abundance of different active sites with different binding energies could, on one side, allow the identification of sites with optimal binding energies and furthermore, it could provide different optimal sites for multistep reactions, thus enabling overcoming the issues with the scaling relations [121] (scaling relations are estimated to lower the activity about several/six orders of magnitude [122]). Some surface segregation is certain to occur, as predicted by theory [123], but there might be stable phases on the surface, in contrast to PtnM-type alloys, which develop a Pt-skin where only the strain effects will be present. According to theoretical considerations, HEAs should have a continuous distribution of energies [111]. Considering the number of degrees of freedom in multicomponent alloys is very high [124,125], possibilities for the tuning of binding energies are plentiful, even though the number of different crystal structures complex alloys can form is limited [126].
Generally, the unique properties of HEAs and MPCAs are explained through 4 proposed “core effects” [127]: 1. the high entropy effect; 2. the lattice distortion effect; 3. sluggish diffusion; and 4. the “cocktail” effect, describing the already discussed property of MPCAs that their properties are difficult to predict based on the properties of the components themselves [124].
For MPCAs, it is considered that their structure may become stabilized by the so-called high-entropy effect [125,127,128,129]. However, the entropy as the thermodynamic cause is not the only effect contributing to the stability of such phases, and there is also a kinetic contribution from sluggish diffusion in such alloys [45]. The lattice distortion, which can be quite severe in complex alloys, is dependent on the nature of the atom and the nature of the atoms in the immediate surrounding, and can significantly affect the properties of surface atoms. Although it has been asserted that the resulting HEA will be as stable as its most stable element, tentatively speaking, nonetheless, segregation processes were observed (e.g., [130]) under temperature changes and oxidation at elevated temperatures [131].
HEAs often have FCC structure [45] (and it has been shown that the FCC microstructure is retained in equimolar alloys with as many as 6 elements, and up to 7 elements in non-equimolar concentrations), but BCC and HCP phases have also been identified [127]. However, more electronegative elements, for instance, are less stable in FCC phases and are often expelled to interdendritic regions during the dendritic growth of the FCC phase [132].
Theory shows that the presence of adsorbed oxygen on the surface leads to increased surface segregation in HEAs compared to vacuum [123]; however, multi-element oxide materials can show good stability [133]), which can make MPCA catalysts sensitive to oxidation, both during synthesis and under operational conditions. Thermodynamic phase calculations also indicate that configurational entropy might not be able to prevent the formation of compounds with large heats of formation like oxides [134].
Figure 3 shows the mass activity for some selected HEAs for the ORR in acid and alkaline media, reported recently. Sun et al. developed, using a cooling-dealloying strategy, a senary high-entropy alloy for the ORR in acid media. The SA and MA (only considering the platinum mass) are 3.2 mA∙cm−2 and 2.24 A∙mg−1, respectively, and these values are 11 and 10 times higher than that of Pt/C. Moreover, the material retained 92.5% of his activity after 100,000 cycles [135]. In alkaline media, Li et al., using an alloying–dealloying strategy, produced an np-AlCuNiPtMn electrocatalyst which showed around 16-fold mass activity of the commercial Pt/C catalyst [136]. Other recently published works are included in the figure [137,138,139,140,141].
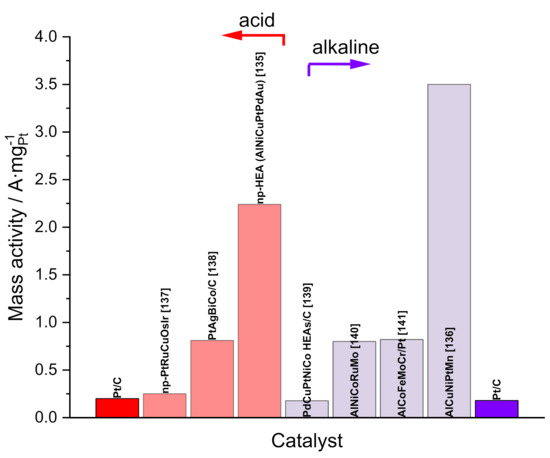
Figure 3.
The mass activity of multicomponent alloys for ORR in 0.1 M HClO4 or 0.1 M KOH. Mass activity for Pt/C commercial is shown in both media to facilitate comparison.
However, the experimental investigation of the long-term behavior of these alloys is lacking and the understanding of the operando behavior and surface structure under reaction conditions is still not well understood, in stark contrast to the well-researched bimetallic counterparts. Considering the complexity of their structure, understanding the behavior of these alloys under reaction conditions is crucial to understanding their activity, selectivity, and stability. For this, it would be essential that these types of alloys are characterized (see Scheme 1) not only as-synthesized, but also in situ, and after the reaction or, optimally, in operando conditions.
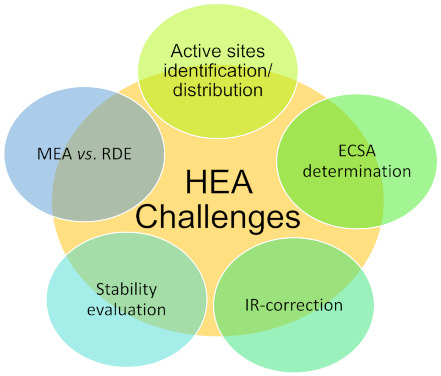
Scheme 1.
Challenges in the electrochemical evaluation of high-entropy alloys.
4. Experimental Challenges in the Benchmarking of HEA and MPCA Activity for ORR
4.1. Active sites Distribution and Identification
It has been inferred that the behavior of multinary alloys cannot be predicted solely based on volcano plots [116]; thus, with the formation of the high-entropy phase it is possible to produce new properties which do not arise directly from the properties of the constituent elements [57,116,142], opening the possibility to potentially replace scarce materials. It has been indicated that the synergistic influence of all the elements present is crucial, and leaving out a single element leads to an activity drop [116], which is justifiable, considering it is known that specific elements can influence the alloy microstructure in specific ways [127]. Additionally, MPCAs can provide additional possibilities for the control of selectivity (2- vs. 4-electron ORR), as it is known that alloying Pt with certain elements can switch the selectivity to the 2-electron process [143]. However, the overall number of desired active sites may be decreased [122] as the high compositional complexity also necessarily leads to the fact that the number of optimal active sites may, statistically, be low. This raises the question of how to identify the optimal active site and distinguish it from a very large number of possible different contributions. One approach developed by Schuhmann et al. is the concept of inflection points (Figure 4) in the voltammetric curves as activity descriptors for the ORR in HEA [144]. The different inflection points are assigned to active sites on the electrode surface, not to different processes (e.g., 2e- or 4e- process) [144].
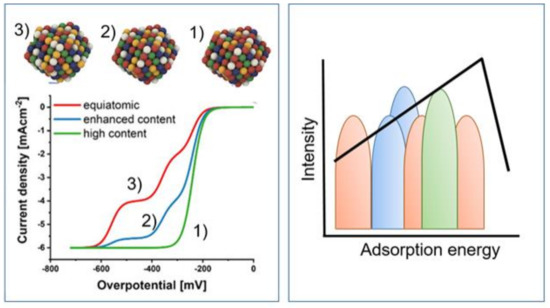
Figure 4.
Left: Model representation of three different HEA with a high content of one element (1), enhanced content of one element (2), and equiatomic (3) compositions along with the polarization curves. Right: Adsorption energy distribution patterns for the three different materials (1), (2), and (3), respectively. Adapted with permission from [144]. Copyright © 2021, American Chemical Society.
For nanoparticulate catalysts, it is also important to keep in mind that the surface binding energies also depend on the number of coordinating atoms of the surface atoms. The number of these, in turn, decreases with the decrease of nanoparticle (NP) size, which leads to a general increase in surface binding energies. This may lead to different specific activity vs. size functions for different types of active sites (e.g., [69,145,146]) depending on the relation of their intermediate binding energy to the optimal one. With the characterization of nanoparticulate MPCAs, one must keep in mind that the huge variety of active sites means that no single or a small number of nanoparticles will contain all the active sites theoretically achievable for an MPCA. This is particularly important to consider when characterizing individual NPs with techniques such as HR-TEM, EDX, or SEM. Additionally, the roughness of the created MPCA surface will need to be taken into account, as undercoordinated sites will bind the intermediates more strongly; thus, the structuring or roughness of the surface introduces even more complexity to the binding energy distribution.
Theory shows that, unlike for single-element surfaces, the binding energies of on-top and hollow sites on FCC phases do not scale, and, in addition, there might be complex interactions between the adsorbed intermediates and the resulting site blocking that defines the electrocatalytic properties of the material [147]. It is, thus, extremely important to apply and develop methods of surface characterization with atomic resolution to understand their structure [148].
Atomic probe tomography (APT) is commonly used for the analysis of complex metallic materials [149,150,151] as it offers both high special resolution and chemical sensitivity. Since the method requires UHV and is destructive, it can be applied before and after the electrochemical measurement on different samples. Nonetheless, during APT measurement the sample is far from the conditions of the operando catalyst and certain 3D distortions might be introduced; thus, the results must be interpreted accordingly. The sample also needs to be of a particular shape, which might impose limitations.
XPS and XRD of the as synthesized and postmortem catalyst can also offer insights into the changes the catalyst undergoes; however, near operando and in situ versions of these techniques will likely be able to shed more light on to the ongoing processes under reaction conditions. Figure 5 shows XPS after a stability test carried out in an AlNiCoRuMo HEA [140]. Because of the extreme potentials, the surface Ru0 changed to Ru4+; meanwhile, the Mo signals were less visible and moved to higher binding energies [140]. This points out the importance of performing physicochemical characterization not only on as-prepared materials but also aged electrodes or in an ideal situation in situ or in operando conditions. Synchrotron-based techniques developed in conjunction with the availability of in situ and in operando cells have been used to study electrocatalysts under near-real conditions, and to determine the active sites in Pt-based materials [152]. For HEA studies, the use of synchrotron-based techniques, especially spectroscopic, imaging, and scattering would allow determining details about structure and composition and their links to catalytic properties, thus allowing a more rationalized material’s design. In addition, scanning tunneling microscopy (STM) could provide valuable information on HEA/MPCA behavior as it has done for platinum-based electrocatalysts [111,153], especially regarding material dissolution.
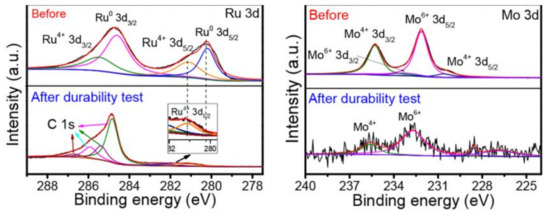
Figure 5.
XPS spectra of Ru 3d and Mo 3d of the AlNiCoRuMo before and after durability test. Adapted with permission from [140]. Copyright © 2021, American Chemical Society.
Aside from the experimental challenges discussed previously, computational approaches, mainly based on density functional theory (DFT) can be used to study the properties of HEA/MPCA materials. Norskov et al. used DFT to explain the volcano-type trend for the ORR over noble and transition metals [8]. Furthermore, was established that oxygen and hydroxyl adsorption at potentials close to the equilibrium is the cause of the overpotential for the ORR [8]. The use of DFT methods in the study of HEA/MPCAs is a relatively recent field. The large number of possible configurations on the surface and the consequent computational issues pose a challenge to the extensive use of DFT in the evaluation of the electrocatalytic activity of HEA/MPCAs materials. Nonetheless, recently, Rossmeisl et al. developed a theoretical approach using DFT to calculate the *OH and O* adsorption energies on an IrPdPtRhRu high-entropy alloy [111]. This approach is capable of determining the ranges of binding energies present in complex solid solutions/MPCs/HEAs [111,122,147].
4.2. Electrochemical Active Surface Area Determination
The electrochemically active surface area (ECSA) determination is vital for the comparison of the intrinsic activities of different electrocatalysts and determining the activity per surface site (turnover frequency). Furthermore, the change of the ECSA before and after the reaction can help to rationalize the changes observed in the catalytic activity after continuous cycling. CO-stripping and hydrogen underpotential deposition (Hupd) have been used extensively to determinate the ECSA in pure Pt and Pt alloys materials. No general agreement exists on the most suitable method to correctly determine the ECSA of Pt alloys since the introduction of a non-noble second metal to form the Pt alloy could change the nature of hydrogen adsorption [154]. CO-stripping could be problematic as well, as the Pt-CO bonding will not necessarily be mirrored in the interaction with other elements at the surface, i.e., the common 420 μC∙cm−2 value used to find the ECSA is obtained considering only CO bound at the on-top position [155,156,157], which might not be mirrored in MPCAs and HEAs since the CO coverage and adsorption configuration depend on the potential, composition, and surface structure [155,156]. Moreover, the non-noble metal could dissolve from the surface during the reaction, complicating even more the analysis [158]. Copper underpotential deposition (CuUPD) is another common choice to determinate the ECSA [159,160]. Nevertheless, as discussed previously the introduction of non-noble metals makes the analysis more difficult since the CuUPD process is not well understood on electrodes containing non-noble metals. The determination of the ECSA in MPCAs or HEA could be divided into two main groups. In materials that contain platinum, the ECSA could be determined from Hupd or CO stripping, but only after the bonding of these species has been understood on a particular surface and standardized. This would require performing measurements on a surface with a known ECSA (determined with the help of, e.g., AFM), which often is not a trivial task. In the second group, for materials that do not contain platinum, the active area is determined from the double layer capacitance; the Cdl is determined from cyclic voltammetric experiments at different scan rates or from electrochemical impedance spectroscopy (EIS) [161,162,163]. The main concern about the ECSA determination from double layer capacitance measurements is the value of specific capacitance (Cs) such the error in the accuracy could be as large as 70% [161]. Problems with the determination of the electrochemical active surface area (ECSA) should not be underestimated since they can cause serious issues, i.e., an underestimation of 10% causes an increase of the SA nearly 11%, but an error of 30% in the ECSA yields a false increase of the SA of around 45%.
4.3. Ohmic Drop Correction
The significance of the iR-drop correction and the methods for the compensation has been discussed at length in numerous works [164,165,166]. For the ORR, since the activities of the catalyst are benchmarked on the level of mV or tens of mV, it is of great importance to conduct the potential correction carefully. The most widely applied technique to find the uncompensated resistance (Ru) is the electrochemical impedance spectroscopy (EIS). After fitting the acquired spectra to a suitable equivalent electrical circuit [165] and subtracting the iRu product from the applied potential the correct potential is obtained. The best strategy to avoid or mitigate the iR-drop is using ultramicroelectrodes, positive feedback, or a Luggin capillary since a posteriori correction for a non-steady-state system could be problematic [165]. In cases where the iR-drop cannot be avoided the use of a shunt capacitance [164] during EIS measurements may be necessary to overcome the artifacts arising from the limitations of the potentiostat’s control amplifier at high frequencies. The use of certain HEA or MPCAs materials might represents new challenges to ohmic compensation. They could form different phases and a (partial) passive layer under reaction conditions that increase the electrode resistance and complicate the compensation since the Ru value will change during the experiment [166,167].
4.4. Stability Testing
Besides the electrocatalytic activity, the stability of electrocatalysts is also of paramount importance for the complete evaluation of electrocatalysts. The most common experimental protocol to evaluate the stability is so-called the accelerated stress test (AST) recommended by the U.S. Department of Energy (DOE). The AST consist in performing a number of cycles (10, 100, 1 k, 3 k, 10 k, 20 k, and 30 k) between 0.6 and 1.0 V vs. RHE in 0.1 or 1 M of HClO4 or 0.5 M of H2SO4 [168]. The ECSA and the ORR activity after cycling should be compared to non-aged electrodes to determine the stability of the electrocatalysts. Most Pt alloys show a decrease in the ECSA and the ORR activity after the AST. The reasons for this are corrosion of the carbon used as support material, particle migration, coalescence, loss of faceted edges, among others. Since the nature and extent of phase transformations, dissolution, and migration on the surface of complex alloys are not clear and might be different for different types of material, it is of crucial importance to assess the state of catalysts in situ or in operando conditions, or at least after the reaction. This will allow a more correct interpretation of the CVs, as it will exclude potential parasitic currents from oxidation, dissolution, or other non-desirable processes. In addition, it will aid the correct identification of active sites, as it will indicate the state of the catalyst surface under reaction conditions, which is not necessarily the same as in the as-synthesized catalyst (as it was proven extensively for bimetallic Pt-catalysts).
4.5. Membrane Electrode Assembly vs. Rotating Disc Electrode Studies
The rotating disc electrode (RDE) is the most used technique to evaluate the intrinsic activity of an electrocatalysts for the ORR [169]. A variation of the RDE is the rotating ring disc electrode (RRDE) which in addition to the disc incorporates a ring that serves to detect reaction products that come from the disc reaction [170]. RDE allows quick evaluation of SA, MA, and ECSA. A common procedure for depositing the electrocatalysts in the disc surface of the RDE is the drop-casting method. Their simplicity as well the speed of implementation contribute to their high popularity [171]. The lack of homogeneity in the thin-film caused by the drop-casting method might cause problems, also the loading (grams of catalyst/cm2) could be a critical factor. For Pt and Pt alloys guidelines for assessing the activity using RDE are available and used by the majority of the research community [171]. However, for HEA or MPCAs studies, a wide consensus in the guidelines that should be followed do not exist yet. The use of common guidelines for all researchers will contribute finally to boosting the understanding of the HEA/MPCAs electrocatalysts for the ORR. In addition, special attention is required to overcome the gap that exists between RDE and electrode layers of the membrane electrode assembly (MEA), as have been observed in platinum alloys. The promising results obtained in RDE could not necessarily be replicated in MEA studies [172]. The high local oxygen pressures contribute to obtaining high current densities (at least 3-4 orders of magnitude larger than in RDE) could drive the degradation of HEA-MPCAs materials, similarly observed in platinum/platinum alloys [173].
5. Concluding Remarks and Outlook
The application of MPCAs and HEAs in electrocatalysis has many exciting prospects. The high complexity of the surface of such alloys (high-entropy alloys, multiple principal components alloy, complex solid solutions) offers the possibility of identifying active sites previously not achievable by the manufacturing of “classical” alloy catalysts. Additionally, the presence of a multitude of active sites on the surface also offers new possibilities for breaking scaling relations, which have been the limiting factor for the development of improved ORR catalysts, as well as the catalysis of multi-step reactions. However, the high complexity of these materials offers significant challenges as well, particularly in surface site identification, selectivity control, the understanding of stability dependence and their in operando behavior. Additionally, the fact that the properties of such complex materials are difficult to predict based on the properties of components will offer new challenges to computational chemistry and high-throughput screening of materials, enabling researchers to navigate the multidimensional, highly complex compositional and structural space with an unprecedented number of degrees of freedom for the modification of their properties.
Author Contributions
V.Č.: conceptualization, data curation, formal analysis and writing—review and editing; R.M.-H.: writing—original draft preparation, data curation, visualization, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external founding.
Acknowledgments
We would like to acknowledge the contribution from and the financing received by the Max-Planck-Institute for Chemical Energy Conversion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gottesfeld, S. Electrocatalysis of Oxygen Reduction in Polymer Electrolyte Fuel Cells: A Brief History and a Critical Examination of Present Theory and Diagnostics. Fuel Cell Catal. Surf. Sci. Approach 2008, 1–30. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Mathias, M.F.; Makharia, R.; Gasteiger, H.A. Conley, J.J.; Fuller, T.J.; Gittleman, C.J.; Kocha, S.S.; Miller, D.P.; Mittelsteadt, C.K.; Xie, T.; Van, S.G.; Yu, P.T. Two fuel cell cars in every garage? Electrochem. Soc. Interface 2005, 14, 24–35. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells. In Fuel Cell Technology; Springer: London, UK, 2006; pp. 27–51. [Google Scholar]
- ALSTO. Successful year and a half of trial operation of the world’s first two hydrogen trains, next project phase begins. Available online: https://www.partners.alstom.com/Assets/View/92a183b6-b12a-4561-b356-76a587d0de4e (accessed on 24 November 2021).
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, B.; Ocon, J.D. Oxygen electrocatalysis in chemical energy conversion and storage technologies. Curr. Appl. Phys. 2012, 13, 309–321. [Google Scholar] [CrossRef]
- Wang, C.; Mei, D.; Wiese, G.; Wang, L.; Deng, M.; Lamaka, S.V.; Zheludkevich, M.L. High rate oxygen reduction reaction during corrosion of ultra-high-purity magnesium. npj Mater. Degrad. 2020, 4, 42. [Google Scholar] [CrossRef]
- Chung, Y.; Ji, J.; Kwon, Y. Performance evaluation of enzymatic biofuel cells using a new cathodic catalyst containing hemin and poly acrylic acid promoting the oxygen reduction reaction. J. Mater. Chem. C 2019, 7, 11597–11605. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Zhang, J., Ed.; Springer: London, UK, 2008; pp. 89–134. [Google Scholar]
- Gómez-Marín, A.M.; Rizo, R.; Feliu, J.M. Oxygen reduction reaction at Pt single crystals: A critical overview. Catal. Sci. Technol. 2014, 4, 1685–1698. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Feliu, J. Oxygen Reduction on Platinum Single Crystal Electrodes. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 820–830. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Bretzler, P.; Cleemann, L.N.; Yu, J.; Li, Q. Revealing the genuine stability of the reference Pt/C electrocatalyst toward the ORR. Electrochimica Acta 2021, 391, 138963. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947. [Google Scholar] [CrossRef]
- Shao, M. Electrocatalysis in Fuel Cells. Catalysts 2015, 5, 2115–2121. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Sun, Y.; Delucchi, M.; Ogden, J. The impact of widespread deployment of fuel cell vehicles on platinum demand and price. Int. J. Hydrogen Energy 2011, 36, 11116–11127. [Google Scholar] [CrossRef]
- James, B.D.; Kalinoski, J.A. Mass Production Cost Estimation for Direct H2 PEM Fuel Cell System for Automotive Applications. DOE Hydrog. Program Rev. 2007. Available online: https://www.energy.gov/sites/prod/files/2019/12/f70/fcto-sa-2018-transportation-fuel-cell-cost-analysis.pdf (accessed on 24 November 2021).
- Appleby, A.J. Electrocatalysis and fuel cells. Catal. Rev. 1971, 4, 221–244. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Paffett, M.T.; Beery, J.G.; Gottesfeld, S. Oxygen reduction at Pt0.65Cr0.35, Pt0.2Cr0.8 and roughened platinum. J. Electrochem. Soc. 1988, 135, 1431–1436. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, S.M.; Nam, S.H.; Seo, M.H.; Choi, S.H.; Kim, W.B. Influence of Sn content on PtSn/C catalysts for electrooxidation of C1-C3 alcohols: Synthesis, characterization, and electrocatalytic activity. Appl. Catal. B Environ. 2008, 82, 89–102. [Google Scholar] [CrossRef]
- Alayoglu, S.; Nilekar, A.U.; Mavrikakis, M.; Eichhorn, B.W. Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat. Mater. 2008, 7, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.M.P.; Moolhuysen, J. Platinum—Tin catalysts for methanol fuel cells prepared by a novel immersion technique, by electrocodeposition and by alloying. Electrochim. Acta 1976, 21, 861–868. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms Part I. Enhancement of the oxidation of methanol on platinum and palladium by gold ad-atoms. J. Electroanal. Chem. 1975, 60, 259–266. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms part II. Enhancement of the oxidation of methanol on platinum by ruthenium ad-atoms. J. Electroanal. Chem. 1975, 60, 267–273. [Google Scholar] [CrossRef]
- Watanabe, M.; Motoo, S. Electrocatalysis by ad-atoms Part III. Enhancement of the oxidation of carbon monoxide on platinum by ruthenium ad-atoms. J. Electroanal. Chem. 1975, 60, 275–283. [Google Scholar] [CrossRef]
- Stamenković, V.; Schmidt, T.J.; Ross, A.P.N.; Marković, N.M. Surface Composition Effects in Electrocatalysis: Kinetics of Oxygen Reduction on Well-Defined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. B 2002, 106, 11970–11979. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.-H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Ohm, V.; Raetz, S.; Sauer, M.; Merkens, M.; Schilder, H.; Lueken, H. Formation of lanthanide-platinum alloys by reaction of platinum with lanthanide iodides Part II. Structural and magnetochemical investigations into the systems with Ln ≜ Nd, Gd, Tb, Dy, Tm2. J. Alloy. Compd. 1996, 238, 95–101. [Google Scholar] [CrossRef]
- Roy, C.; Knudsen, B.P.; Pedersen, C.M.; Palenzuela, A.A.V.; Christensen, L.H.; Damsgaard, C.D.; Stephens, I.E.L.; Chorkendorff, I. Scalable Synthesis of Carbon-Supported Platinum–Lanthanide and—Rare-Earth Alloys for Oxygen Reduction. ACS Catal. 2018, 8, 2071–2080. [Google Scholar] [CrossRef]
- Chu, T.; Xie, M.; Yang, D.; Ming, P.; Li, B.; Zhang, C. Highly active and durable carbon support Pt-rare earth catalyst for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 27291–27298. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI Fuel Cell Vehicle and Progress Toward a Future Hydrogen Society. Electrochem. Soc. Interface 2015, 24, 45–49. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, H. Platinum-Based Oxygen Reduction Electrocatalysts. Accounts Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef]
- Timoshenko, J.; Cuenya, B.R. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2020, 121, 882–961. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Schuhmann, W. Breaking scaling relations in electrocatalysis. J. Solid State Electrochem. 2020, 24, 1–2. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; López, N. Strategies to break linear scaling relationships. Nat. Catal. 2019, 2, 971–976. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fantauzzi, D.; Zhu, T.; Mueller, J.E.; Filot, I.A.; Hensen, E.J.; Jacob, T. Microkinetic Modeling of the Oxygen Reduction Reaction at the Pt(111)/Gas Interface. Catal. Lett. 2015, 145, 451–457. [Google Scholar] [CrossRef][Green Version]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Universality in Oxygen Reduction Electrocatalysis on Metal Surfaces. ACS Catal. 2012, 2, 1654–1660. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Kodama, K.; Hatanaka, T.; Morimoto, Y. First principles based mean field model for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2011, 13, 21070–21083. [Google Scholar] [CrossRef] [PubMed]
- Tripković, V.; Skúlason, E.; Siahrostami, S.; Nørskov, J.K.; Rossmeisl, J. The oxygen reduction reaction mechanism on Pt(111) from density functional theory calculations. Electrochim. Acta 2010, 55, 7975–7981. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Koper, M.T.M.; Bandarenka, A.S. Tailoring the catalytic activity of electrodes with monolayer amounts of foreign metals. Chem. Soc. Rev. 2013, 42, 5210–5230. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Greeley, J.; Studt, F.; Rossmeisl, J.; Munter, T.R.; Moses, P.G.; Skúlason, E.; Bligaard, T.; Nørskov, J.K. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces. Phys. Rev. Lett. 2007, 99, 016105. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Bondarenko, A.S.; Perez-Alonso, F.J.; Calle-Vallejo, F.; Bech, L.; Johansson, T.P.; Jepsen, A.K.; Frydendal, R.; Knudsen, B.P.; Rossmeisl, J.; et al. Tuning the Activity of Pt(111) for Oxygen Electroreduction by Subsurface Alloying. J. Am. Chem. Soc. 2011, 133, 5485–5491. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef]
- Čolić, V.; Bandarenka, A.S. Pt Alloy Electrocatalysts for the Oxygen Reduction Reaction: From Model Surfaces to Nanostructured Systems. ACS Catal. 2016, 6, 5378–5385. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Ross, Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum Monolayer Electrocatalysts for O2 Reduction: Pt Monolayer on Pd(111) and on Carbon-Supported Pd Nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Zhang, J.; Lima, F.H.B.; Shao, M.H.; Sasaki, K.; Wang, J.X.; Hanson, J.; Adzic, R.R. Platinum Monolayer on Nonnoble Metal−Noble Metal Core−Shell Nanoparticle Electrocatalysts for O2 Reduction. J. Phys. Chem. B 2005, 109, 22701–22704. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Zhang, J.; Sasaki, K.; Nilekar, A.U.; Uribe, F.; Mavrikakis, M.; Adzic, R.R. Platinum monolayer electrocatalysts for oxygen reduction. Electrochim. Acta 2007, 52, 2257–2263. [Google Scholar] [CrossRef]
- Strasser, P. Dealloyed Core-Shell Fuel Cell Electrocatalysts. Rev. Chem. Eng. 2009, 25. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.V.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.; Lucas, C.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, V.; Schmidt, T.J.; Ross, P.; Marković, N. Surface segregation effects in electrocatalysis: Kinetics of oxygen reduction reaction on polycrystalline Pt3Ni alloy surfaces. J. Electroanal. Chem. 2003, 554-555, 191–199. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M. Effect of Surface Composition on Electronic Structure, Stability, and Electrocatalytic Properties of Pt-Transition Metal Alloys: Pt-Skin versus Pt-Skeleton Surfaces. J. Am. Chem. Soc. 2006, 128, 8813–8819. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.P.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Hernandez-Fernandez, P.; Masini, F.; McCarthy, D.N.; Strebel, C.E.; Friebel, D.; Deiana, D.; Malacrida, P.; Nierhoff, A.U.F.; Bodin, A.; Wise, A.M.; et al. Mass-selected nanoparticles of PtxY as model catalysts for oxygen electroreduction. Nat. Chem. 2014, 6, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, M.; Hammer, B.; Nørskov, J.K. Effect of Strain on the Reactivity of Metal Surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822. [Google Scholar] [CrossRef]
- Lischka, M.; Mosch, C.; Groß, A. Tuning catalytic properties of bimetallic surfaces: Oxygen adsorption on pseudomorphic Pt/Ru overlayers. Electrochimica Acta 2007, 52, 2219–2228. [Google Scholar] [CrossRef]
- Schlapka, A.; Lischka, M.; Groß, A.; Käsberger, U.; Jakob, P. Surface Strain versus Substrate Interaction in Heteroepitaxial Metal Layers: Pt on Ru(0001). Phys. Rev. Lett. 2003, 91, 016101. [Google Scholar] [CrossRef]
- Moffat, T.P.; Fan, F.F.; Bard, A.J. Electrochemical and Scanning Tunneling Microscopic Study of Dealloying of Cu3Au. J. Electrochem. Soc. 1991, 138, 3224–3235. [Google Scholar] [CrossRef]
- Jacobse, L.; Rost, M.J.; Koper, M.T.M. Atomic-Scale Identification of the Electrochemical Roughening of Platinum. ACS Central Sci. 2019, 5, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Role of Strain and Ligand Effects in the Modification of the Electronic and Chemical Properties of Bimetallic Surfaces. Phys. Rev. Lett. 2004, 93, 156801. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, J.; Li, S.; Zuo, Y.; Li, D.; Han, H. Nickel-Ion-Oriented Fabrication of Spiny PtCu Alloy Octahedral Nanoframes with Enhanced Electrocatalytic Performance. ACS Appl. Energy Mater. 2019, 2, 2862–2869. [Google Scholar] [CrossRef]
- Li, W.; Hu, Z.-Y.; Zhang, Z.; Wei, P.; Zhang, J.; Pu, Z.; Zhu, J.; He, D.; Mu, S.; Van Tendeloo, G. Nano-single crystal coalesced PtCu nanospheres as robust bifunctional catalyst for hydrogen evolution and oxygen reduction reactions. J. Catal. 2019, 375, 164–170. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Wang, X.; Fu, Y.; Zhu, H.; Liu, T. Chemically Ordered Pt–Co–Cu/C as Excellent Electrochemical Catalyst for Oxygen Reduction Reaction. J. Electrochem. Soc. 2020, 167, 024507. [Google Scholar] [CrossRef]
- Deng, Z.; Pang, W.; Gong, M.; Jin, Z.; Wang, X. Revealing the role of mo doping in promoting oxygen reduction reaction performance of Pt3Co nanowires. J. Energy Chem. 2021, 66, 16–23. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Y.; Yang, D.; Zhang, C.; Ming, P.; Li, B.; Yang, S. Efficient synthesis of Pt–Co nanowires as cathode catalysts for proton exchange membrane fuel cells. RSC Adv. 2020, 10, 6287–6296. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Gao, M.; Jin, J.; van Spronsen, M.; Salmeron, M.B.; Yang, P. High-Performance Pt–Co Nanoframes for Fuel-Cell Electrocatalysis. Nano Lett. 2020, 20, 1974–1979. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Guo, W.; Xiao, S.; Wang, L.; OuYang, Y.; Gao, P. The mechanism of Co oxyhydroxide nano-islands deposited on a Pt surface to promote the oxygen reduction reaction at the cathode of fuel cells. RSC Adv. 2020, 10, 44719–44727. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, D.; Deng, Z.; Zhang, R.; Xia, W.; Zhao, T.; Liu, X.; Shen, T.; Hu, Y.; Lu, Y.; et al. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction. Appl. Catal. B Environ. 2020, 282, 119617. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Wang, X.; Rosen, A.; Beatrez, W.; Sztaberek, L.; Tan, H.; Zhang, L.; Koenigsmann, C.; Zhao, J. Composition-Dependent Oxygen Reduction Reaction Activity of Pt-Surfaced PtNi Dodecahedral Nanoframes. ACS Appl. Energy Mater. 2020, 3, 768–776. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, L.; Wei, M.; Tsiakaras, P.; Shen, P.K. Highly stable Pt-Co nanodendrite in nanoframe with Pt skin structured catalyst for oxygen reduction electrocatalysis. Appl. Catal. B Environ. 2020, 281, 119460. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, Z.; Zhao, H.; Shen, C.; Chen, H.; Li, H.; Cao, Z.; Kuang, Q.; Lin, H.; Xie, Z. Trimetallic PtNiCo branched nanocages as efficient and durable bifunctional electrocatalysts towards oxygen reduction and methanol oxidation reactions. J. Mater. Chem. A 2021, 9, 23444–23450. [Google Scholar] [CrossRef]
- Sun, K.; Li, J.; Wang, F.; He, W.; Fei, M.; Lu, Z.; Zhang, H.; Liu, J.; Zou, Z. Highly enhanced durability of a graphitic carbon layer decorated PtNi3 alloy electrocatalyst toward the oxygen reduction reaction. Chem. Commun. 2019, 55, 5693–5696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xi, C.; Zhang, R.; Song, L.; Wang, C.; Spendelow, J.S.; Frenkel, A.I.; Yang, J.; Xin, H.L.; Sasaki, K. High-Performance Nitrogen-Doped Intermetallic PtNi Catalyst for the Oxygen Reduction Reaction. ACS Catalysis 2020, 10, 10637–10645. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, L.; Dang, W.; Wang, L.; Xia, M.; Wang, B.; Liu, M.; Gao, F.; Zhang, J.; Zhao, Y. High-Indexed PtNi Alloy Skin Spiraled on Pd Nanowires for Highly Efficient Oxygen Reduction Reaction Catalysis. Small 2019, 15, e1900288. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, Y.; Hong, J.W.; Whang, Y.; Kim, S.; Wi, D.H.; Byon, H.R.; Han, S.W. One-pot production of ceria nanosheet-supported PtNi alloy nanodendrites with high catalytic performance toward methanol oxidation and oxygen reduction. J. Mater. Chem. A 2020, 8, 25842–25849. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Chi, B.; Zhao, J.; Dang, D.; Tian, X.; Liao, S.; Li, G.-R. Nodal PtNi nanowires with Pt skin and controllable Near-Surface composition for enhanced oxygen reduction electrocatalysis in fuel cells. Chem. Eng. J. 2021, 418, 129322. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, T.; Ha, Y.; Jun, M.; Baik, H.; Jeong, H.Y.; Kim, H.; Lee, K.; Joo, S.H. Intermetallic PtCu Nanoframes as Efficient Oxygen Reduction Electrocatalysts. Nano Lett. 2020, 20, 7413–7421. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gao, X.; Qin, C.; Yang, D.; Lv, H.; Xiao, Q.; Zhang, C. High performance octahedral PtNi/C catalysts investigated from rotating disk electrode to membrane electrode assembly. Nano Res. 2018, 12, 281–287. [Google Scholar] [CrossRef]
- Kong, F.; Ren, Z.; Banis, M.N.; Du, L.; Zhou, X.; Chen, G.; Zhang, L.; Li, J.; Wang, S.; Li, M.; et al. Active and Stable Pt–Ni Alloy Octahedra Catalyst for Oxygen Reduction via Near-Surface Atomical Engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Fichtner, J.; Garlyyev, B.; Watzele, S.; El-Sayed, H.A.; Schwämmlein, J.N.; Li, W.-J.; Maillard, F.M.; Dubau, L.; Michalička, J.; Macak, J.M.; et al. Top-Down Synthesis of Nanostructured Platinum–Lanthanide Alloy Oxygen Reduction Reaction Catalysts: PtxPr/C as an Example. ACS Appl. Mater. Interfaces 2019, 11, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Z.; Zhou, Y.; Ma, X.; Lin, H.; Ying, W.; Peng, X. Fe3Pt intermetallic nanoparticles anchored on N-doped mesoporous carbon for the highly efficient oxygen reduction reaction. Chem. Commun. 2020, 56, 4898–4901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, X.; Kang, Y.; Xia, D.; Gan, L. Rational Development of Structurally Ordered Platinum Ternary Intermetallic Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2019, 9, 569. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Tellez-Cruz, M.; Rojas-Chávez, H.; Ramírez-Herrera, C.; Calaminici, P.; Solorza-Feria, O. NiPdPt trimetallic nanoparticles as efficient electrocatalysts towards the oxygen reduction reaction. Int. J. Hydrogen Energy 2018, 44, 12463–12469. [Google Scholar] [CrossRef]
- Dai, S.; Chou, J.-P.; Wang, K.-W.; Hsu, Y.-Y.; Hu, A.; Pan, X.; Chen, T.-Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xie, M.; Chen, Z.; Lyu, Z.; Chi, M.; Jin, W.; Xia, Y. Pt-Ir-Pd Trimetallic Nanocages as a Dual Catalyst for Efficient Oxygen Reduction and Evolution Reactions in Acidic Media. Adv. Energy Mater. 2020, 10, 4114. [Google Scholar] [CrossRef]
- Duan, J.-J.; Zheng, X.-X.; Niu, H.-J.; Feng, J.-J.; Zhang, Q.-L.; Huang, H.; Wang, A.-J. Porous dendritic PtRuPd nanospheres with enhanced catalytic activity and durability for ethylene glycol oxidation and oxygen reduction reactions. J. Colloid Interface Sci. 2019, 560, 467–474. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Wang, F.; Yu, J.; Zhu, H. Nickel-introduced structurally ordered PtCuNi/C as high performance electrocatalyst for oxygen reduction reaction. Prog. Nat. Sci. 2020, 30, 905–911. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Chi, M.; Pearson, J.; Rankin, R.B.; Greeley, J.; Duan, Z.; Wang, G.; van der Vliet, D.; More, K.L.; et al. Rational Development of Ternary Alloy Electrocatalysts. J. Phys. Chem. Lett. 2012, 3, 1668–1673. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Siahrostami, S.; Björketun, M.E.; Strasser, P.; Greeley, J.; Rossmeisl, J. Tandem cathode for proton exchange membrane fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 9326–9334. [Google Scholar] [CrossRef]
- Busch, M.; Halck, N.B.; Kramm, U.; Siahrostami, S.; Krtil, P.; Rossmeisl, J. Beyond the top of the volcano? A unified approach to electrocatalytic oxygen reduction and oxygen evolution. Nano Energy 2016, 29, 126–135. [Google Scholar] [CrossRef]
- Batchelor, T.; Pedersen, J.K.; Winther, S.H.; Castelli, I.E.; Jacobsen, K.W.; Rossmeisl, J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3, 834–845. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition, Metallurgical and Materials Transactions A: Physical. Metall. Mater. Sci. 2004, 35, 1465–1469. [Google Scholar]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.; Yan, D.; Skjegstad, L.E.J.; Rossmeisl, J. Surface electrocatalysis on high-entropy alloys. Curr. Opin. Electrochem. 2020, 26, 100651. [Google Scholar] [CrossRef]
- Löffler, T.; Meyer, H.; Savan, A.; Wilde, P.; Garzón Manjón, A.; Chen, Y.T.; Ventosa, E.; Scheu, C.; Ludwig, A.; Schuhmann, W. Discovery of a Multinary Noble Metal–Free Oxygen Reduction Catalyst. Adv. Energy Mater. 2018, 8, 1802269. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Bagger, A.; Rossmeisl, J. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Wang, A.-L.; Wan, H.-C.; Xu, H.; Tong, Y.-X.; Li, G.-R. Quinary PdNiCoCuFe Alloy Nanotube Arrays as Efficient Electrocatalysts for Methanol Oxidation. Electrochim. Acta 2014, 127, 448–453. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2016, 16, 70–81. [Google Scholar] [CrossRef]
- Löffler, T.; Ludwig, A.; Rossmeisl, J.; Schuhmann, W. What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew. Chem. Int. Ed. 2021, 60, 26894–26903. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Körmann, F. Surface segregation in Cr-Mn-Fe-Co-Ni high entropy alloys. Appl. Surf. Sci. 2020, 533, 147471. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Ye, Y.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2015, 19, 349–362. [Google Scholar] [CrossRef]
- Mackay, A.L. On complexity. Crystallogr. Rep. 2001, 46, 524–526. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Li, Y.J.; Savan, A.; Ludwig, A. Atomic scale understanding of phase stability and decomposition of a nanocrystalline CrMnFeCoNi Cantor alloy. Appl. Phys. Lett. 2021, 119, 201910. [Google Scholar] [CrossRef]
- Li, Y.; Kostka, A.; Savan, A.; Ludwig, A. Atomic-scale investigation of fast oxidation kinetics of nanocrystalline CrMnFeCoNi thin films. J. Alloy. Compd. 2018, 766, 1080–1085. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Li, T.; Yao, Y.; Huang, Z.; Xie, P.; Liu, Z.; Yang, M.; Gao, J.; Zeng, K.; Brozena, A.H.; Pastel, G.; et al. Denary oxide nanoparticles as highly stable catalysts for methane combustion. Nat. Catal. 2021, 4, 62–70. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Fang, G.; Wen, Y.; Liu, P.; Xie, G.; Liu, X.; Sun, S. Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 2019, 7, 6499–6506. [Google Scholar] [CrossRef]
- Li, S.; Tang, X.; Jia, H.; Li, H.; Xie, G.; Liu, X.; Lin, X.; Qiu, H.-J. Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 2020, 383, 164–171. [Google Scholar] [CrossRef]
- Chen, X.; Si, C.; Gao, Y.; Frenzel, J.; Sun, J.; Eggeler, G.; Zhang, Z. Multi-component nanoporous platinum–ruthenium–copper–osmium–iridium alloy with enhanced electrocatalytic activity towards methanol oxidation and oxygen reduction. J. Power Sources 2015, 273, 324–332. [Google Scholar] [CrossRef]
- Mahmood, A.; Xie, N.; Din, M.A.U.; Saleem, F.; Lin, H.; Wang, X. Shape controlled synthesis of porous tetrametallic PtAgBiCo nanoplates as highly active and methanol-tolerant electrocatalyst for oxygen reduction reaction. Chem. Sci. 2017, 8, 4292–4298. [Google Scholar] [CrossRef]
- Chen, Y.; Zhan, X.; Bueno, S.L.A.; Shafei, I.H.; Ashberry, H.M.; Chatterjee, K.; Xu, L.; Tang, Y.; Skrabalak, S.E. Synthesis of monodisperse high entropy alloy nanocatalysts from core@shell nanoparticles. Nanoscale Horizons 2021, 6, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Rugged High-Entropy Alloy Nanowires with in Situ Formed Surface Spinel Oxide As Highly Stable Electrocatalyst in Zn–Air Batteries. ACS Mater. Lett. 2020, 2, 1698–1706. [Google Scholar] [CrossRef]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Chen, Z.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Top–Down Synthesis of Noble Metal Particles on High-Entropy Oxide Supports for Electrocatalysis. Chem. Mater. 2021, 33, 1771–1780. [Google Scholar] [CrossRef]
- Marković, N.; Ross, P. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2005, 275–374. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Löffler, T.; Waag, F.; Gökce, B.; Ludwig, A.; Barcikowski, S.; Schuhmann, W. Comparing the Activity of Complex Solid Solution Electrocatalysts Using Inflection Points of Voltammetric Activity Curves as Activity Descriptors. ACS Catal. 2021, 11, 1014–1023. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; McCarthy, D.N.; Nierhoff, A.; Hernandez-Fernandez, P.; Strebel, C.; Stephens, I.; Nielsen, J.H.; Chorkendorff, I. The Effect of Size on the Oxygen Electroreduction Activity of Mass-Selected Platinum Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 4641–4643. [Google Scholar] [CrossRef] [PubMed]
- Kramm, U.I.; Herrmann-Geppert, I.; Fiechter, S.; Zehl, G.; Zizak, I.; Dorbandt, I.; Schmeißer, D.; Bogdanoff, P. Effect of iron-carbide formation on the number of active sites in Fe–N–C catalysts for the oxygen reduction reaction in acidic media. J. Mater. Chem. A 2013, 2, 2663–2670. [Google Scholar] [CrossRef]
- Batchelor, T.A.A.; Löffler, T.; Xiao, B.; Krysiak, O.A.; Strotkötter, V.; Pedersen, J.K.; Clausen, C.M.; Savan, A.; Li, Y.; Schuhmann, W.; et al. Complex-Solid-Solution Electrocatalyst Discovery by Computational Prediction and High-Throughput Experimentation. Angew. Chem. Int. Ed. 2020, 60, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2021, 2104339. [Google Scholar] [CrossRef] [PubMed]
- Schweinar, K.; Nicholls, R.L.; Rajamathi, C.R.; Zeller, P.; Amati, M.; Gregoratti, L.; Raabe, D.; Greiner, M.; Gault, B.; Kasian, O. Probing catalytic surfaces by correlative scanning photoemission electron microscopy and atom probe tomography. J. Mater. Chem. A 2019, 8, 388–400. [Google Scholar] [CrossRef]
- Li, Y.J.; Savan, A.; Kostka, A.; Stein, H.S.; Ludwig, A. Accelerated atomic-scale exploration of phase evolution in compositionally complex materials. Mater. Horizons 2017, 5, 86–92. [Google Scholar] [CrossRef]
- Marshal, A.; Pradeep, K.; Music, D.; Zaefferer, S.; De, P.; Schneider, J. Combinatorial synthesis of high entropy alloys: Introduction of a novel, single phase, body-centered-cubic FeMnCoCrAl solid solution. J. Alloy. Compd. 2016, 691, 683–689. [Google Scholar] [CrossRef]
- Casalongue, H.S.; Kaya, S.; Viswanathan, V.; Miller, D.J.; Friebel, D.; Hansen, H.A.; Nørskov, J.K.; Nilsson, A.; Ogasawara, H. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 2013, 4, 2817. [Google Scholar] [CrossRef]
- Jacobse, L.; Huang, Y.-F.; Koper, M.T.M.; Rost, M.J. Correlation of surface site formation to nanoisland growth in the electrochemical roughening of Pt(111). Nat. Mater. 2018, 17, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique Electrochemical Adsorption Properties of Pt-Skin Surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar] [CrossRef]
- Weaver, M.; Chang, S.-C.; Leung, L.-W.; Jiang, X.; Rubel, M.; Szklarczyk, M.; Zurawski, D.; Wieckowski, A. Evaluation of absolute saturation coverages of carbon monoxide on ordered low-index platinum and rhodium electrodes. J. Electroanal. Chem. 1992, 327, 247–260. [Google Scholar] [CrossRef]
- Cuesta, A.; Couto, A.; Rincón, A.; Pérez, M.C.; López-Cudero, A.; Gutiérrez, C. Potential dependence of the saturation CO coverage of Pt electrodes: The origin of the pre-peak in CO-stripping voltammograms. Part 3: Pt(poly). J. Electroanal. Chem. 2006, 586, 184–195. [Google Scholar] [CrossRef]
- Vidaković, T.; Christov, M.; Sundmacher, K. The use of CO stripping for in situ fuel cell catalyst characterization. Electrochimica Acta 2007, 52, 5606–5613. [Google Scholar] [CrossRef]
- Rudi, S.; Cui, C.; Gan, L.; Strasser, P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5, 408–418. [Google Scholar] [CrossRef]
- Green, C.L.; Kucernak, A. Determination of the platinum and ruthenium surface areas in platinum-ruthenium alloy electrocatalysts by underpotential deposition of Copper. I. Unsupported catalysts. J. Phys. Chem. B 2002, 106, 1036–1047. [Google Scholar] [CrossRef]
- Nagel, T.; Bogolowski, N.; Baltruschat, H. Towards a determination of the active surface area of polycrystalline and nanoparticle electrodes by Cu upd and CO oxidation. J. Appl. Electrochem. 2006, 36, 1297–1306. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- McCrory, C.; Jung, S.; Ferrer, I.M.; Chatman, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.M.; Risch, M. Seven steps to reliable cyclic voltammetry measurements for the determination of double layer capacitance. J. Physics: Energy 2021, 3, 3. [Google Scholar] [CrossRef]
- Čolić, V.; Tymoczko, J.; Maljusch, A.; Ganassin, A.; Schuhmann, W.; Bandarenka, A.S. Experimental Aspects in Benchmarking of the Electrocatalytic Activity. ChemElectroChem 2014, 2, 143–149. [Google Scholar] [CrossRef]
- Lasia, A. Impedance Spectroscopy Applied to the Study of Electrocatalytic Processes. Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, Netherlands, 2018; pp. 241–263. [Google Scholar]
- van der Vliet, D.; Strmcnik, D.S.; Wang, C.; Stamenkovic, V.R.; Markovic, N.M.; Koper, M.T. On the importance of correcting for the uncompensated Ohmic resistance in model experiments of the Oxygen Reduction Reaction. J. Electroanal. Chem. 2010, 647, 29–34. [Google Scholar] [CrossRef]
- Popkirov, G. A technique for series resistance measurement and ohmic drop correction under potentiostatic control. J. Electroanal. Chem. 1993, 359, 97–103. [Google Scholar] [CrossRef]
- DOE Durability Working Group. Rotating Disk-Electrode Aqueous Electrolyte Accelerated Stress Tests for PGM Electrocatalyst/Support Durability Evaluation. 2011. Available online: https://www.energy.gov/sites/default/files/2015/08/f25/fcto_dwg_pgm_electrocatalyst_support_aqueous_ast.pdf (accessed on 24 November 2021).
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.; Li, D.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374. [Google Scholar] [CrossRef]
- Du, C.; Tan, Q.; Yin, G.; Zhang, J. 5—Rotating Disk Electrode Method. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 171–198. [Google Scholar]
- Mayrhofer, K.; Strmcnik, D.; Blizanac, B.; Stamenkovic, V.; Arenz, M.; Markovic, N. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochimica Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Martens, S.; Asen, L.; Ercolano, G.; Dionigi, F.; Zalitis, C.; Hawkins, A.; Bonastre, A.M.; Seidl, L.; Knoll, A.C.; Sharman, J.; et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 2018, 392, 274–284. [Google Scholar] [CrossRef]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of Platinum: Limits for the Deployment of Electrochemical Energy Conversion? Angew. Chem. Int. Ed. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).