Poly(Lactic-co-glycolic) Acid and Phospholipids Hybrid Nanoparticles for Regeneration of Biological Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Dynamic Light Scattering
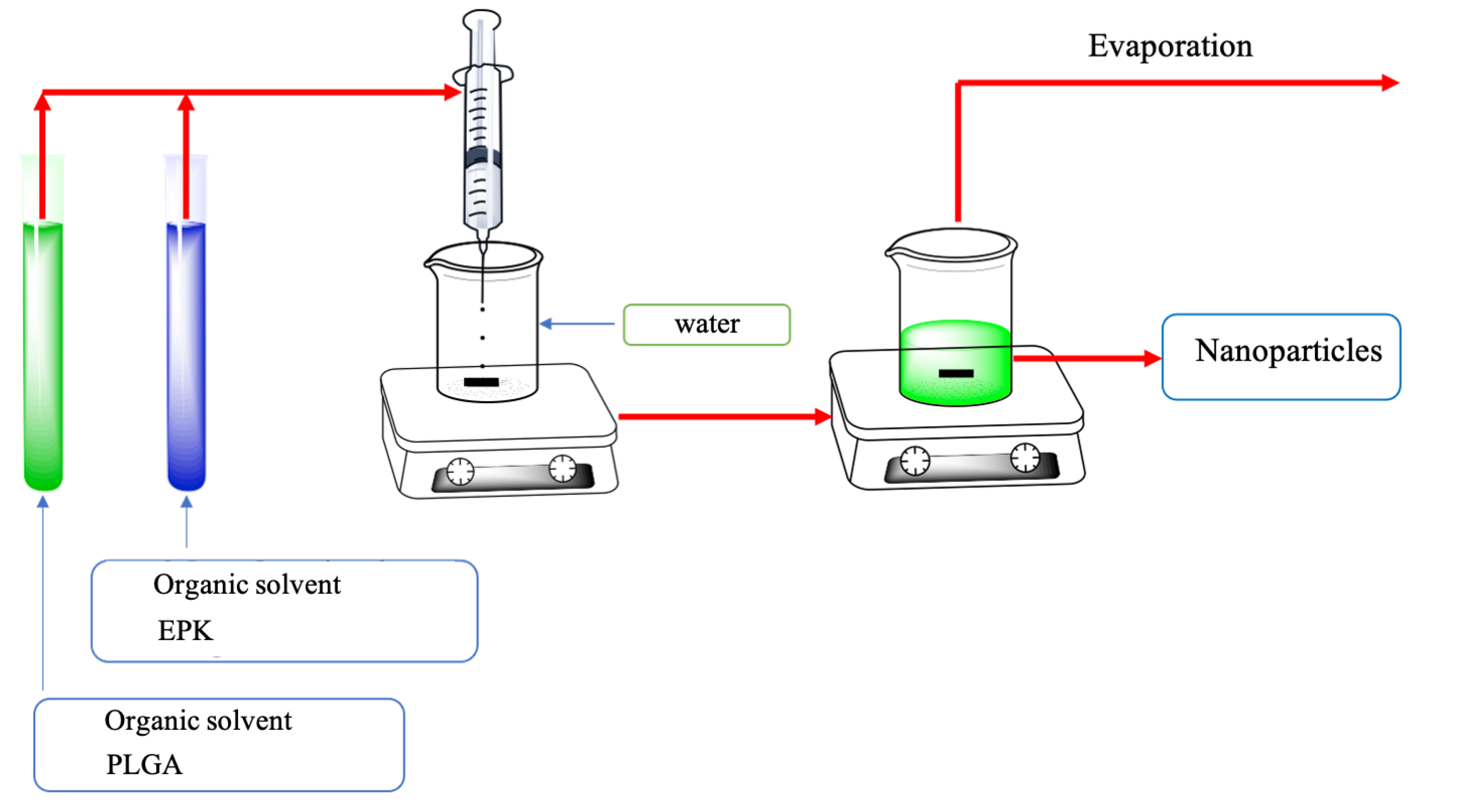
2.3. Preparation of Hybrid Nanoparticles
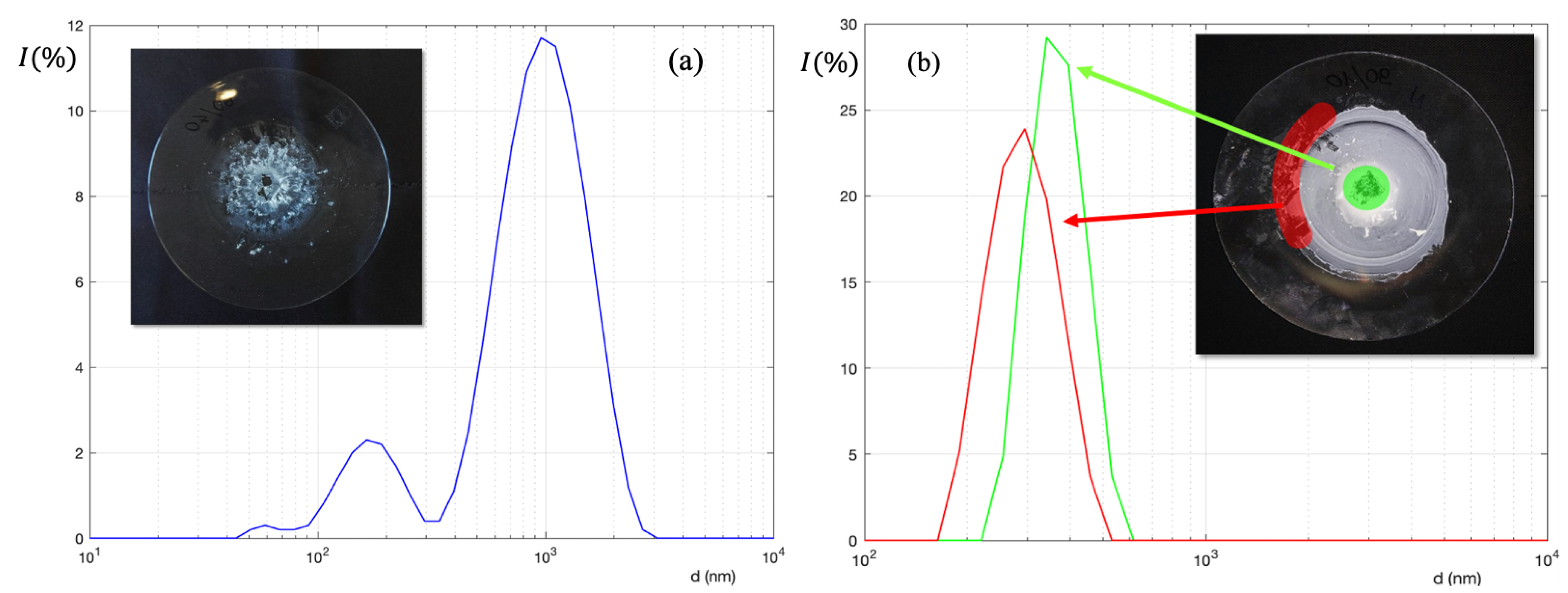
2.4. Preparation of Patches
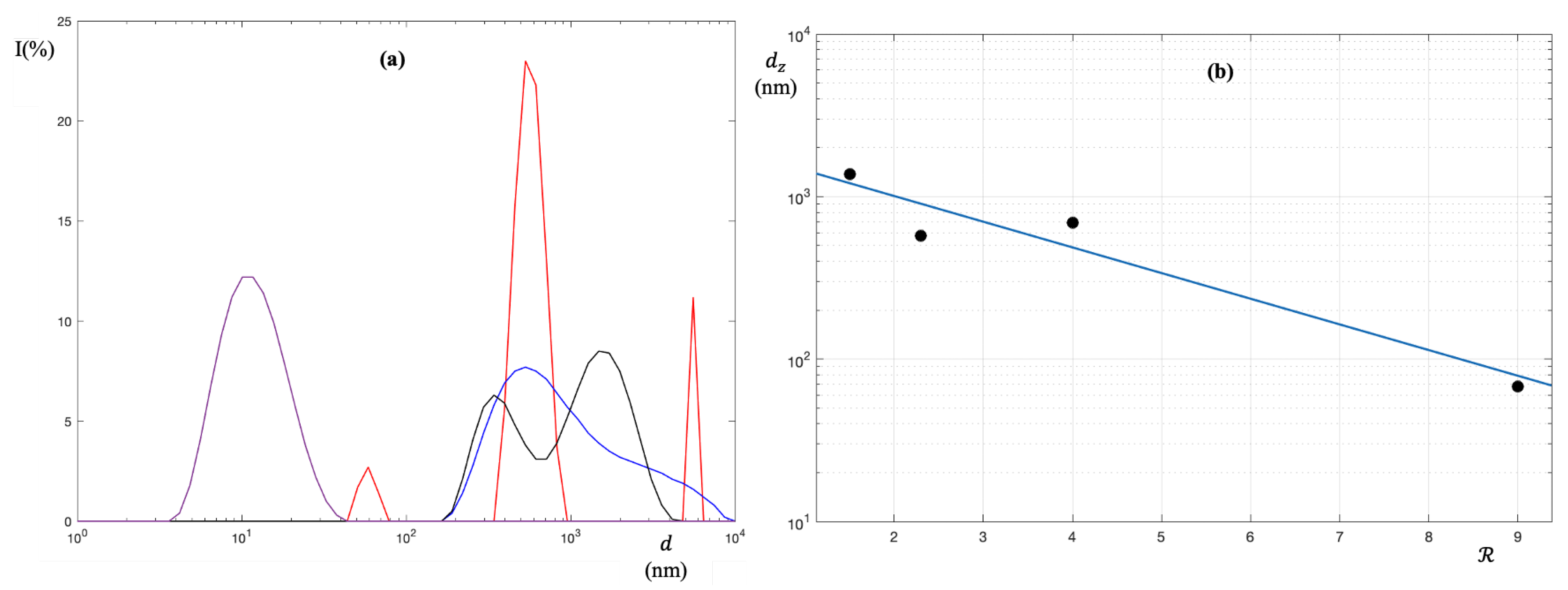
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Producing gelatin nanoparticles as delivery system for bovine serum albumin. Iran. Biomed. J. 2014, 18, 34. [Google Scholar]
- Naderi-Meshkin, H.; Andreas, K.; Matin, M.M.; Sittinger, M.; Bidkhori, H.R.; Ahmadiankia, N.; Bahrami, A.R.; Ringe, J. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol. Int. 2014, 48, 72–84. [Google Scholar] [CrossRef]
- Lopez, F.; Venditti, F.; Ambrosone, L.; Colafemmina, G.; Ceglie, A.; Palazzo, G. Gelatin microemulsion-based gels with the cationic surfactant cetyltrimethylammonium bromide: A self-diffusion and conductivity study. Langmuir 2004, 20, 9449–9452. [Google Scholar] [CrossRef]
- Ghidoni, I.; Chlapanidas, T.; Bucco, M.; Crovato, F.; Marazzi, M.; Vigo, D.; Torre, M.L.; Faustini, M. Alginate cell encapsulation: New advances in reproduction and cartilage regenerative medicine. Cytotechnology 2008, 58, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Venditti, F.; Cinelli, G.; Ceglie, A. The novel hexadecyltrimethylammonium bromide (CTAB) based organogel as reactor for ester synthesis by entrapped Candida rugosa lipase. Process Biochem. 2006, 41, 114–119. [Google Scholar] [CrossRef]
- Sailor, M.J.; Park, J.-H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef]
- Ma, D. Hybrid nanoparticles: An introduction. Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–6. [Google Scholar]
- Teekamp, N.; Duque, L.F.; Frijlink, H.W.; Hinrichs, W.L.J.; Olinga, P. Production methods and stabilization strategies for polymer-based nanoparticles and microparticles for parenteral delivery of peptides and protein. Expert Opin. Drug Deliv. 2015, 12, 1311–1331. [Google Scholar] [CrossRef]
- Lee, P.-C.; Zan, B.-S.; Chen, L.-T.; Chung, T.-W. Multifunctional PLGA-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int. J. Nanomed. 2019, 14, 1533. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Bufalo, G.; Lopez, F.; Ambrosone, L. Cooperativity between Dimerization and Binding Equilibria in the Ternary System Laponite-Indocyanine Green-Water. ChemEngineering 2021, 5, 6. [Google Scholar] [CrossRef]
- Bufalo, G.; Florio, C.; Cinelli, G.; Lopez, F.; Cuomo, F.; Ambrosone, L. Principles of minimal wrecking and maximum separation of solid waste to innovate tanning industries and reduce their environmental impact: The case of paperboard manufacture. J. Clean. Prod. 2018, 174, 324–332. [Google Scholar] [CrossRef]
- Ambrosone, L.; Cinelli, G.; Mosca, M.; Ceglie, A. Susceptibility of water-emulsified extra virgin olive oils to oxidation. J. Am. Oil Chem. Soc. 2006, 83, 165–170. [Google Scholar] [CrossRef]
- Di Biasio, A.; Ambrosone, L.; Cametti, C. Numerical simulation of dielectric spectra of aqueous suspensions of non-spheroidal differently shaped biological cells. J. Phys. D Appl. Phys. 2008, 42, 401–410. [Google Scholar] [CrossRef]
- Molino, B.; Bufalo, G.; De Vincenzo, A.; Ambrosone, L. Semiempirical model for assessing dewatering process by flocculation of dredged sludge in an artificial reservoir. Appl. Sci. 2020, 10, 3051. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Zou, P.; Stern, S.T.; Sun, D. PLGA/liposome hybrid nanoparticles for short-chain ceramide delivery. Pharm. Res. 2014, 31, 684–693. [Google Scholar] [CrossRef][Green Version]
- Ciccocioppo, R.; Cantore, A.; Chaimov, D. Regenerative medicine: The red planet for clinicians. Intern. Emerg. Med. 2019, 14, 911–921. [Google Scholar] [CrossRef]
- Passaro, F.; Testa, G.; Ambrosone, L.; Costagliola, C.; Tocchetti, C.B.; Di Nezza, F.; Russo, M.; Pirozzi, F.; Abete, P.; Russo, T. Nanotechnology-based cardiac targeting and direct cardiac reprogramming: The Betrothed. Stem Cells Int. 2017, 2017, 4940397. [Google Scholar] [CrossRef]
- Passaro, F.; Tocchetti, C.G.; Spinetti, G.; Paudice, F.; Amborsone, L.; Costagliola, C.; Cacciatore, F.; Abete, P.; Testa, G. Targeting fibrosis in the failing heart with nanoparticles. In Advanced Drug Delivery Reviews; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Guan, J.; Chen, W.; Yang, M.; Wu, E.; Qian, J.; Zhan, C. Regulation of in vivo delivery of nanomedicines by herbal medicines. In Advanced Drug Delivery Reviews; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Testa, G; Ambrosone, L; Costagliola, C. Biodegradable Polymer Medical Device. European Patent n. PCT/IB2015/059278, 2 December 2015.
- Baldelli, A.; Ou, J.; Li, W.; Amirfazli, A. Spray-On Nanocomposite Coatings: Wettability and Conductivity. Langmuir 2020, 36, 11393–11410. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Rytting, E.; Lebhardt, T.; Wang, X.; Kissel, T. Preparation of nanoparticles by solvent displacement for drug delivery: A shift in the “ouzo region” upon drug loading. Eur. J. Pharm. Sci. 2010, 41, 244–253. [Google Scholar] [CrossRef]
- Costagliola, C.; Semeraro, F.; Zeppa, L.; Bufalo, G.; Cardone, M.; Romano, M.; Ambrosone, L. Some physicochemical remarks on spontaneous emulsification of vitreal tamponades. BioMed Res. Int. 2014, 2014, 243056. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. Polymer Solutions; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Uehara, E.; Deguchi, T. Statistical and hydrodynamic properties of topological polymers for various graphs showing enhanced short-range correlation. J. Chem. Phys. 2016, 145, 164905. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Ceglie, A.; Ambrosone, L. Effect of membrane composition on lipid oxidation in liposomes. Chem. Phys. Lipids 2011, 164, 158–165. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minó, A.; Cinelli, G.; Paventi, G.; Testa, G.; Passaro, F.; Lopez, F.; Ambrosone, L. Poly(Lactic-co-glycolic) Acid and Phospholipids Hybrid Nanoparticles for Regeneration of Biological Tissue. ChemEngineering 2022, 6, 10. https://doi.org/10.3390/chemengineering6010010
Minó A, Cinelli G, Paventi G, Testa G, Passaro F, Lopez F, Ambrosone L. Poly(Lactic-co-glycolic) Acid and Phospholipids Hybrid Nanoparticles for Regeneration of Biological Tissue. ChemEngineering. 2022; 6(1):10. https://doi.org/10.3390/chemengineering6010010
Chicago/Turabian StyleMinó, Antonio, Giuseppe Cinelli, Gianluca Paventi, Gianluca Testa, Fabiana Passaro, Francesco Lopez, and Luigi Ambrosone. 2022. "Poly(Lactic-co-glycolic) Acid and Phospholipids Hybrid Nanoparticles for Regeneration of Biological Tissue" ChemEngineering 6, no. 1: 10. https://doi.org/10.3390/chemengineering6010010
APA StyleMinó, A., Cinelli, G., Paventi, G., Testa, G., Passaro, F., Lopez, F., & Ambrosone, L. (2022). Poly(Lactic-co-glycolic) Acid and Phospholipids Hybrid Nanoparticles for Regeneration of Biological Tissue. ChemEngineering, 6(1), 10. https://doi.org/10.3390/chemengineering6010010







