Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K
Abstract
:1. Introduction
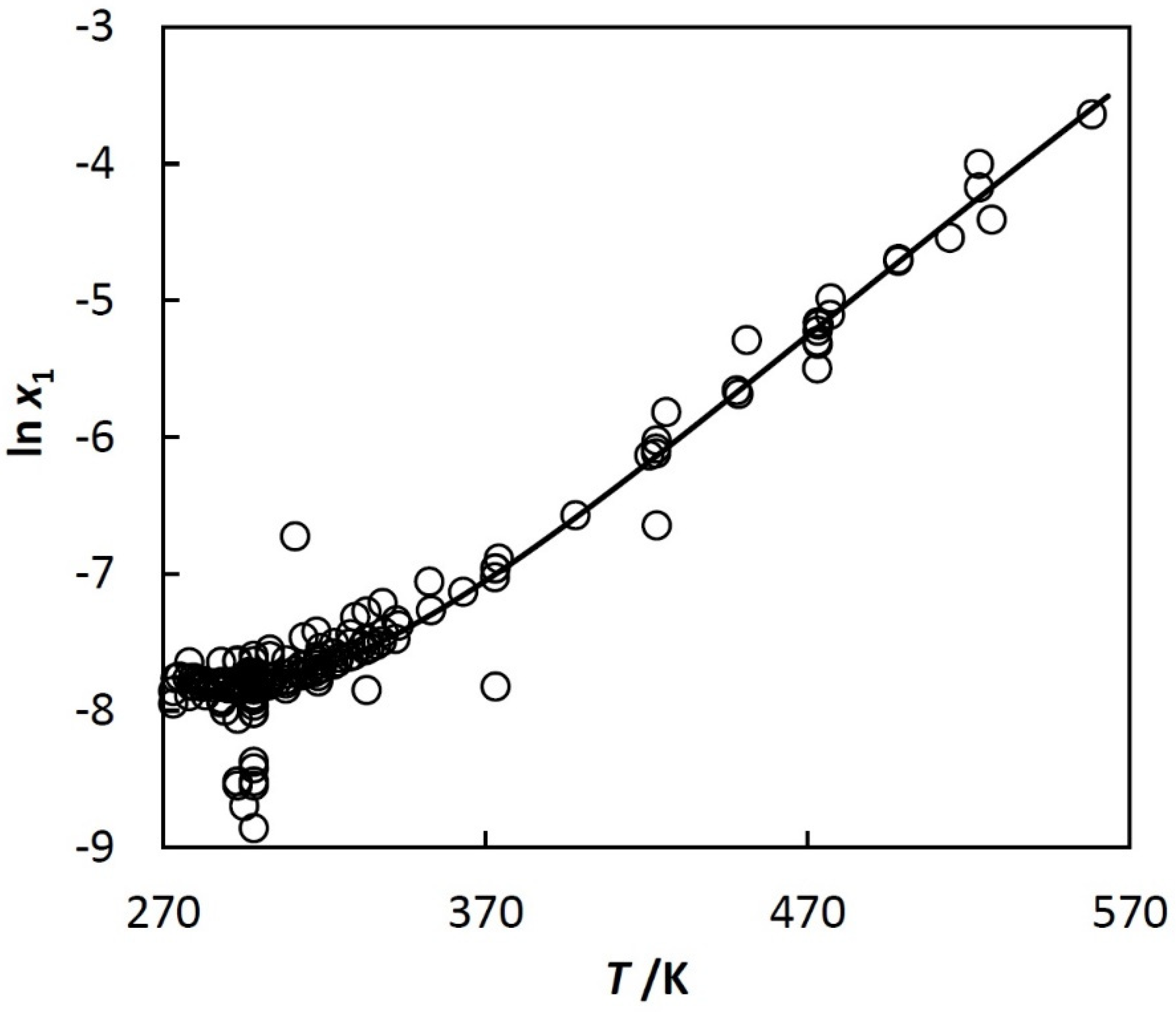
- Solubilities of hydrocarbons in water are approximated with a thermodynamically based equation described in the next section. The two coefficients of this equation are linearly dependent on the excluded volume of the hydrocarbon molecule, which enables prediction.
- Solubilities of water in hydrocarbons are calculated using liquid–liquid equilibrium calculations. The input data for these calculations are concentrations in the second liquid phase predicted by the previously mentioned smoothing equation.
2. Solubility of Hydrocarbons in Water
3. Solubility of Water in Hydrocarbons
4. Calculated Solubility Values
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Tsonopoulos, C. Thermodynamic analysis of the mutual solubilities of normal alkanes and water. Fluid Phase Equilibria 1999, 156, 21–33. [Google Scholar] [CrossRef]
- Tsonopoulos, C. Thermodynamic analysis of the mutual solubilities of normal alkanes and water. Fluid Phase Equilibria 2001, 186, 185–206. [Google Scholar] [CrossRef]
- Oracz, P.; Góral, M. Application of the Unified Functional Activity Coefficient (UNIFAC) and Analytical Solution of Groups (ASOG) for the Calculation of Mutual Solubilities in Water Systems of Alkanes, Arenes, and Alkanols. J. Chem. Eng. Data 2011, 56, 4853–4861. [Google Scholar] [CrossRef]
- Magnussen, T.; Rasmussen, P.; Fredenslund, A. UNIFAC parameter table for prediction of liquid-liquid equilibria. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 331–339. [Google Scholar] [CrossRef]
- Gupte, P.A.; Danner, R.P. Prediction of Liquid-Liquid Equilibria with UNIFAC: A Critical Evaluation. Ind. Eng. Chem. Res. 1987, 26, 2036–2042. [Google Scholar] [CrossRef]
- Hooper, H.; Michel, S.; Prausnitz, J.M. Correlation of liquid-liquid equilibria for some water-organic liquid systems in the region 20–250 °C. Ind. Eng. Chem. Res. 1988, 27, 2182–2187. [Google Scholar] [CrossRef]
- Voutsas, E.C.; Tassios, D.P. An Analysis of the UNIFAC-Type Group-Contribution Models at the Highly Dilute Region. 2. Empirical Improvements with Application to Water/Hydrocarbon Mixtures. Ind. Eng. Chem. Res. 1997, 36, 4973–4976. [Google Scholar] [CrossRef]
- Kang, J.W.; Diky, V.; Frenkel, M. New modified UNIFAC parameters using critically evaluated phase equilibrium data. Fluid Phase Equilibria 2015, 388, 128–141. [Google Scholar] [CrossRef]
- Kang, J.W.; Diky, V.; Frenkel, M. Corrigendum to “New modified UNIFAC parameters using critically evaluated phase equilibrium data” [Fluid Phase Equilib. 388 (2015) 128-141]. Fluid Phase Equilibria 2017, 440, 122–123. [Google Scholar] [CrossRef]
- Gmehling, J.; Li, J.; Schiller, M. A Modified UNIFAC Model. 2. Present Parameter Matrix and Results. Ind. Eng. Chem. Res. 1993, 32, 178–193. [Google Scholar] [CrossRef]
- Yakoumis, I.V.; Kontogeorgis, G.M.; Voutsas, E.C.; Hendriks, E.M.; Tassios, D.P. Prediction of Phase Equilibria in Binary Aqueous Systems Containing Alkanes, Cycloalkanes, and Alkenes with the Cubic-plus-Association Equation of State. Ind. Eng. Chem. Res. 1998, 37, 4175–4182. [Google Scholar] [CrossRef]
- Voutsas, E.C.; Boulougouris, G.C.; Economou, I.G.; Tassios, D.P. Water/Hydrocarbon Phase Equilibria Using the Thermodynamic Perturbation Theory. Ind. Eng. Chem. Res. 2000, 39, 797–804. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Coutinho, J.A.P.; Queimad, A.J. Mutual solubilities of hydrocarbons and water with the CPA EoS. Fluid Phase Equilibria 2007, 258, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Economou, I.G.; Tsonopoulos, C. Association models and mixing rules in equations of state for water/hydrocarbon mixtures. Chem. Eng. Sci. 1997, 52, 511–525. [Google Scholar] [CrossRef]
- Vega, L.F.; Llovell, F.; Blas, F.J. Capturing the Solubility Minima of n-Alkanes in Water by Soft-SAFT. J. Phys. Chem. B 2009, 113, 7621–7630. [Google Scholar] [CrossRef]
- Ahmed, S.; Ferrando, N.; de Hemptinne, J.-C.; Simonin, J.-P.; Bernard, O.; Baudouin, O. A New PC-SAFT Model for Pure Water, Water–Hydrocarbons, and Water–Oxygenates Systems and Subsequent Modeling of VLE, VLLE, and LLE. J. Chem. Eng. Data 2016, 61, 4178–4190. [Google Scholar] [CrossRef]
- Landra, C.; Satyro, M.A. Mutual Solubility of Water and Hydrocarbons. J. Chem. Eng. Data 2016, 61, 525–534. [Google Scholar] [CrossRef]
- Haarmann, N.; Enders, S.; Sadowski, G. Modeling binary mixtures of n-alkanes and water using PC-SAFT. Fluid Phase Equilibria 2018, 470, 203–211. [Google Scholar] [CrossRef]
- Safamirzaei, M.; Modarress, H. Modeling and predicting solubility of n-alkanes in water. Fluid Phase Equilibria Equilibria 2011, 309, 53–61. [Google Scholar] [CrossRef]
- Klamt, A. Prediction of the mutual solubilities of hydrocarbons and water with COSMO-RS. Fluid Phase Equilibria 2003, 206, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Morgado, P.; Barras, J.; Duarte, P.; Filipe, E.J.M. Solubility of water in n-alkanes: New experimental measurements and molecular dynamics simulations. Fluid Phase Equilibria 2020, 503, 112322. [Google Scholar] [CrossRef]
- Mączyński, A.; Góral, M.; Wiśniewska–Gocłowska, B.; Skrzecz, A.; Shaw, D. Mutual Solubilities of Water and Alkanes. Mon. Chem. 2003, 134, 633–653. [Google Scholar] [CrossRef]
- Maczynski, A.; Wisniewska–Goclowska, B.; Goral, M. Recommended Liquid-Liquid Equilibrium Data, Part 1: Binary C5–C11 Alkane—Water Systems. J. Phys. Chem. Ref. Data 2003, 33, 549–577. [Google Scholar] [CrossRef]
- Goral, M.; Wisniewska–Goclowska, B.; Maczynski, A. Recommended Liquid-Liquid Equilibrium Data, Part 2: Binary Unsaturated hydrocarbon—Water Systems. J. Phys. Chem. Ref. Data 2004, 33, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Goral, M.; Wisniewska–Goclowska, B.; Maczynski, A. Recommended Liquid-Liquid Equilibrium Data, Part 3: Binary Alkylbenzene—Water Systems. J. Phys. Chem. Ref. Data 2004, 33, 1159–1190. [Google Scholar] [CrossRef]
- Maczynski, A.; Shaw, D.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Kapuku, F.; Hefter, G.T.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 1. C5 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 441–476. [Google Scholar] [CrossRef] [Green Version]
- Maczynski, A.; Shaw, D.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Szafranski, A.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 2. Benzene with Water and Heavy Water. J. Phys. Chem. Ref. Data 2005, 34, 477–552. [Google Scholar] [CrossRef] [Green Version]
- Maczynski, A.; Shaw, D.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Maczynska, Z.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 3. C6H8–C6H12 Hydrocarbons with Water and Heavy Water. J. Phys. Chem. Ref. Data 2005, 34, 657–708. [Google Scholar] [CrossRef] [Green Version]
- Maczynski, A.; Shaw, D.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 4. C6H14 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 709–753. [Google Scholar] [CrossRef] [Green Version]
- Maczynski, A.; Shaw, D.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 5. C7 Hydrocarbons with Water and Heavy Water. J. Phys. Chem. Ref. Data 2005, 34, 1399–1488. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Maczynska, Z.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 6. C8H8–C8H10 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 1489–1553. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 7. C8H12–C8H18 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 2261–2298. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A. Erratum: IUPAC-NIST Solubility Data Series. 81. Hydrocarbons with Water and Seawater Revised and Updated. Part 7. C8H12–C8H18 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 2261. [Google Scholar] [CrossRef]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 8. C9 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2005, 34, 2299–2345. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 9. C10 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2006, 35, 93–151. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 10. C11 and C12 Hydrocarbons with Water and Heavy Water. J. Phys. Chem. Ref. Data 2006, 35, 153–203. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.; Maczynski, A.; Goral, M.; Wisniewska–Goclowska, B.; Skrzecz, A.; Owczarek, I.; Blazej, K.; Haulait–Pirson, M.-C.; Hefter, G.T.; Kapuku, F.; et al. IUPAC-NIST Solubility Data Series, Hydrocarbons with Water and Seawater–Revised and Updated, Part 11. C13–C36 Hydrocarbons with Water. J. Phys. Chem. Ref. Data 2006, 35, 687–784. [Google Scholar] [CrossRef] [Green Version]
- Góral, M. Cubic equation of state for calculation of phase equilibria in association systems. Fluid Phase Equilibria 1996, 118, 27–59. [Google Scholar] [CrossRef]
- Dohányosová, P.; Sarraute, S.; Dohnal, V.; Majer, V.; Gomes, M.C. Aqueous Solubility and Related Thermodynamic Functions of Nonaromatic Hydrocarbons as a Function of Molecular Structure. Ind. Eng. Chem. Res. 2004, 43, 2805–2815. [Google Scholar] [CrossRef]


| Formula | Name | CAS RN | xh | xw |
|---|---|---|---|---|
| C5H6 | 1,3-Cyclopentadiene | 542-92-7 | 1.50·10−4 1.80·10−4 [24] | 1.93·10−3 |
| C5H8 | Cyclopentene | 142-29-0 | 1.89·10−4 1.62·10−4 [24] | 1.33·10−3 |
| C5H8 | 2-Methyl-1,3-butadiene | 78-79-5 | 1.38·10−4 1.70·10−4 [24] | 1.98·10−3 |
| C5H8 | 3-Methyl-1,2-butadiene | 598-25-4 | 3.63·10−4 | 3.44·10−3 |
| C5H8 | 3-Methyl-1-butyne | 598-23-2 | 4.69·10−4 | 3.47·10−3 |
| C5H8 | 1,2-Pentadiene | 591-95-7 | 2.42·10−4 | 3.39·10−3 |
| C5H8 | cis-1,3-Pentadiene | 1574-41-0 | 1.07·10−4 | 1.95·10−3 |
| C5H8 | trans-1,3-Pentadiene | 2004-70-8 | 1.02·10−4 | 1.97·10−3 |
| C5H8 | 1,4-Pentadiene | 591-93-5 | 2.54·10−4 | 3.50·10−3 |
| C5H8 | 2,3-Pentadiene | 591-96-8 | 2.92·10−4 | 3.41·10−3 |
| C5H8 | 1-Pentyne | 627-19-0 | 3.58·10−4 4.15·10−4 [24] | 3.43·10−3 |
| C5H8 | 2-Pentyne | 627-21-4 | 2.60·10−4 | 3.40·10−3 |
| C5H10 | Cyclopentane | 287-92-3 | 4.31·10−5 4.22·10−5 [23] | 3.86·10−4 |
| C5H10 | 2-Methyl-1-butene | 563-46-2 | 7.42·10−5 | 1.55·10−3 |
| C5H10 | 2-Methyl-2-butene | 513-35-9 | 7.31·10−5 8.30·10−5 [24] | 1.50·10−3 |
| C5H10 | 3-Methyl-1-butene | 563-45-1 | 6.23·10−5 | 1.68·10−3 |
| C5H10 | 1-Pentene | 109-67-1 | 4.90·10−5 3.80·10−5 [24] | 1.63·10−3 |
| C5H10 | cis-2-Pentene | 627-20-3 | 5.38·10−5 | 1.55·10−3 |
| C5H10 | trans-2-Pentene | 646-04-8 | 5.67·10−5 | 1.55·10−3 |
| C5H12 | 2-Methylbutane | 78-78-4 | 1.33·10−5 1.24·10−5 [23] | 6.61·10−4 |
| C5H12 | Pentane | 109-66-0 | 1.12·10−5 1.19·10−5 [23] | 6.26·10−4 4.81·10−4 [23] |
| C6H6 | Benzene | 71-43-2 | 4.09·10−4 4.11·10−4 [25] | 2.94·10−3 3.09·10−3 [25] |
| C6H8 | 1,4-Cyclohexadiene | 628-41-1 | 2.19·10−4 1.57·10−4 [24] | 3.43·10−3 |
| C6H10 | Cyclohexene | 110-83-8 | 5.35·10−5 4.67·10−5 | 1.34·10−3 1.69·10−3 |
| C6H10 | 1,5-Hexadiene | 592-42-7 | 6.46·10−5 | 3.34·10−3 |
| C6H10 | 1-Hexyne | 693-02-7 | 8.58·10−5 7.89·10−5 [24] | 3.32·10−3 |
| C6H12 | Cyclohexane | 110-82-7 | 1.33·10−5 1.25·10−5 [23] | 4.00·10−4 3.75·10−4 [23] |
| C6H12 | 2,3-Dimethyl-1-butene | 563-78-0 | 2.98·10−5 | 1.51·10−3 |
| C6H12 | 2,3-Dimethyl-2-butene | 563-79-1 | 1.34·10−5 | 1.49·10−3 |
| C6H12 | 3,3-Dimethyl-1-butene | 558-37-2 | 2.75·10−5 | 1.62·10−3 |
| C6H12 | 2-Ethyl-1-butene | 760-21-4 | 2.42·10−5 | 1.48·10−3 |
| C6H12 | 1-Hexene | 592-41-6 | 1.12·10−5 1.18·10−5 [24] | 1.57·10−3 1.89·10−3 [24] |
| C6H12 | cis-2-Hexene | 7688-21-3 | 1.47·10−5 | 1.51·10−3 |
| C6H12 | trans-2-Hexene | 4050-45-7 | 9.37·10−6 | 1.56·10−3 |
| C6H12 | cis-3-Hexene | 7642-09-3 | 1.57·10−5 | 1.51·10−3 |
| C6H12 | trans-3-Hexene | 13269-52-8 | 9.51·10−6 | 1.56·10−3 |
| C6H12 | Methylcyclopentane | 96-37-7 | 1.03·10−5 0.96·10−5 [23] | 4.52·10−4 |
| C6H12 | 2-Methyl-1-pentene | 763-29-1 | 2.12·10−5 1.70·10−5 [24] | 1.51·10−3 |
| C6H12 | 2-Methyl-2-pentene | 625-27-4 | 1.87·10−5 | 1.49·10−3 |
| C6H12 | 3-Methyl-1-pentene | 760-20-3 | 2.56·10−5 | 1.53·10−3 |
| C6H12 | cis-3-Methyl-2-pentene | 922-62-3 | 1.87·10−5 | 1.49·10−3 |
| C6H12 | trans-3-Methyl-2-pentene | 616-12-6 | 1.11·10−5 | 1.53·10−3 |
| C6H12 | 4-Methyl-1-pentene | 691-37-2 | 2.13·10−5 | 1.56·10−3 |
| C6H12 | cis-4-Methyl-2-pentene | 691-38-3 | 2.00·10−5 | 1.55·10−3 |
| C6H12 | trans-4-Methyl-2-pentene | 674-76-0 | 1.18·10−5 | 1.59·10−3 |
| C6H14 | 2,2-Dimethylbutane | 75-83-2 | 4.08·10−6 3.85·10−6 [23] | 6.70·10−4 |
| C6H14 | 2,3-Dimethylbutane | 79-29-8 | 3.85·10−6 3.99·10−6 [23] | 6.25·10−4 |
| C6H14 | Hexane | 110-54-3 | 2.43·10−6 2.50·10−6 [23] | 6.09·10−4 5.10·10−4 [23] |
| C6H14 | 2-Methylpentane | 107-83-5 | 2.88·10−6 2.88·10−6 [23] | 6.41·10−4 |
| C6H14 | 3-Methylpentane | 96-14-0 | 3.48·10−6 3.74·10−6 [23] | 6.04·10−4 4.5·10−4 [23] |
| C7H8 | 1,3,5-Cycloheptatriene | 544-25-2 | 1.13·10−4 1.21·10−4 [24] | 3.37·10−3 |
| C7H8 | Methylbenzene | 108-88-3 | 1.09·10−4 1.12·10−4 [25] | 2.81·10−3 2.80·10−3 [25] |
| C7H12 | 1-Heptyne | 628-71-7 | 1.98·10−5 1.76·10−5 [24] | 3.21·10−3 |
| C7H12 | 1-Methylcyclohexene | 591-49-1 | 1.23·10−5 0.97·10−5 [24] | 1.37·10−3 |
| C7H14 | Cycloheptane | 291-64-5 | 4.30·10−6 5.50·10−6 [24] | 3.88·10−4 |
| C7H14 | 1,1-Dimethylcyclopentane | 1638-26-2 | 3.58·10−6 | 4.90·10−4 |
| C7H14 | cis-1,2-Dimethylcyclopentane | 1192-18-3 | 2.71·10−6 | 4.73·10−4 |
| C7H14 | trans-1,2-Dimethylcyclopentane | 822-50-4 | 3.25·10−6 | 4.84·10−4 |
| C7H14 | cis-1,3-Dimethylcyclopentane | 2532-58-3 | 3.38·10−6 | 4.85·10−4 |
| C7H14 | trans-1,3-Dimethylcyclopentane | 1759-58-6 | 3.25·10−6 | 4.85·10−4 |
| C7H14 | Ethylcyclopentane | 1640-89-7 | 2.50·10−6 | 4.65·10−4 |
| C7H14 | 1-Heptene | 592-76-7 | 2.43·10−6 | 1.54·10−3 1.70·10−3 [24] |
| C7H14 | 2-Heptene | 592-77-8 | 3.63·10−6 2.70·10−6 [24] | 1.51·10−3 |
| C7H14 | Methylcyclohexane | 108-87-2 | 2.90·10−6 2.90·10−6 [23] | 4.66·10−4 |
| C7H14 | 2,3,3-Trimethyl-1-butene | 594-56-9 | 3.16·10−6 | 1.62·10−3 |
| C7H16 | 3-Ethylpentane | 617-78-7 | 9.24·10−7 | 5.82·10−4 |
| C7H16 | 2,2-Dimethylpentane | 590-35-2 | 8.84·10−7 7.90·10−7 [23] | 6.60·10−4 |
| C7H16 | 2,3-Dimethylpentane | 565-59-3 | 1.05·10−6 0.94·10−6 [23] | 5.90·10−4 |
| C7H16 | 2,4-Dimethylpentane | 108-08-7 | 8.04·10−7 7.92·10−7 [23] | 6.58·10−4 |
| C7H16 | 3,3-Dimethylpentane | 562-49-2 | 1.21·10−6 1.06·10−6 [23] | 5.99·10−4 |
| C7H16 | Heptane | 142-82-5 | 5.31·10−7 5.30·10−7 [23] | 6.00·10−4 6.70·10−4 [23] |
| C7H16 | 2-Methylhexane | 591-76-4 | 6.32·10−7 4.57·10−7 [23] | 6.23·10−4 |
| C7H16 | 3-Methylhexane | 589-34-4 | 7.69·10−7 8.89·10−7 [23] | 6.02·10−4 |
| C7H16 | 2,2,3-Trimethylbutane | 464-06-2 | 1.33·10−6 | 7.51·10−4 |
| C8H10 | 1,2-Dimethylbenzene | 95-47-6 | 3.55·10−5 3.61·10−5 [25] | 2.62·10−3 2.60·10−3 [25] |
| C8H10 | 1,3-Dimethylbenzene | 108-38-3 | 2.74·10−5 2.70·10−5 [25] | 2.68·10−3 2.60·10−3 [25] |
| C8H10 | 1,4-Dimethylbenzene | 106-42-3 | 2.63·10−5 2.66·10−5 [25] | 2.68·10−3 2.70·10−3 [25] |
| C8H10 | Ethylbenzene | 100-41-4 | 3.19·10−5 3.18·10−5 [25] | 2.67·10−3 2.60·10−3 [25] |
| C8H14 | 1,7-Octadiene | 3710-30-3 | 2.92·10−6 | 3.11·10−3 |
| C8H14 | 1-Octyne | 629-05-0 | 4.36·10−6 4.40·10−6 [24] | 3.12·10−3 |
| C8H16 | Cyclooctane | 292-64-8 | 1.22·10−6 1.27·10−6 [23] 0.93·10−6 [39] | 3.97·10−4 |
| C8H16 | 1,1-Dimethylcyclohexane | 590-66-9 | 1.02·10−6 | 4.83·10−4 |
| C8H16 | cis-1,2-Dimethylcyclohexane | 2207-01-4 | 7.89·10−7 9.60·10−7 [23] 9.57·10−7 [39] | 4.69·10−4 |
| C8H16 | trans-1,2-Dimethylcyclohexane | 6876-23-9 | 9.27·10−7 7.59·10−7 [39] | 4.78·10−4 |
| C8H16 | cis-1,3-Dimethylcyclohexane | 638-04-0 | 1.00·10−6 | 4.80·10−4 |
| C8H16 | trans-1,3-Dimethylcyclohexane | 2207-03-6 | 9.01·10−7 | 4.76·10−4 |
| C8H16 | cis-1,4-Dimethylcyclohexane | 624-29-3 | 9.01·10−7 | 4.76·10−4 |
| C8H16 | trans-1,4-Dimethylcyclohexane | 2207-04-7 | 1.02·10−6 | 4.83·10−4 |
| C8H16 | Ethylcyclohexane | 1678-91-7 | 7.72·10−7 6.26·10−7 [39] | 4.63·10−4 |
| C8H16 | 1-Ethyl-1-methylcyclopentane | 16747-50-5 | 5.3410−7 | 5.27·10−4 |
| C8H16 | 1-Octene | 111-66-0 | 4.72·10−7 4.00·10−7 [24] | 1.52·10−3 |
| C8H16 | trans-2-Octene | 13389-42-9 | 8.59·10−7 | 1.47·10−3 |
| C8H16 | Propylcyclopentane | 2040-96-2 | 5.24·10−7 | 4.87·10−4 |
| C8H16 | 1,1,2-Trimethylcyclopentane | 4259-00-1 | 5.42·10−7 | 5.54·10−4 |
| C8H16 | 1,1,3-Trimethylcyclopentane | 4516-69-2 | 4.28·10−7 | 6.06·10−4 |
| C8H18 | 2,2-Dimethylhexane | 590-73-8 | 1.88·10−7 2.01·10−7 [39] | 6.49·10−4 |
| C8H18 | 2,3-Dimethylhexane | 584-94-1 | 2.21·10−7 | 5.98·10−4 |
| C8H18 | 2,4-Dimethylhexane | 589-43-5 | 2.00·10−7 | 6.32·10−4 |
| C8H18 | 3,3-Dimethylhexane | 563-16-6 | 2.47·10−7 | 6.11·10−4 |
| C8H18 | 2,5-Dimethylhexane | 592-13-2 | 1.56·10−7 1.79·10−7 [39] | 6.51·10−4 |
| C8H18 | 3-Ethylhexane | 619-99-8 | 1.94·10−7 | 5.94·10−4 |
| C8H18 | 3-Ethyl-2-methylpentane | 609-26-7 | 2.75·10−7 | 5.86·10−4 |
| C8H18 | 3-Ethyl-3-methylpentane | 1067-08-9 | 3.56·10−7 | 5.63·10−4 |
| C8H18 | 2-Methylheptane | 592-27-8 | 1.22·10−7 | 6.28·10−4 |
| C8H18 | 3-Methylheptane | 589-81-1 | 1.55·10−7 1.25·10−7 [23] | 6.07·10−4 |
| C8H18 | 4-Methylheptane | 589-53-7 | 1.54·10−7 | 6.12·10−4 |
| C8H18 | Octane | 111-65-9 | 1.03·10−7 1.04·10−7 [23] | 6.04·10−4 5.01·10−4 [23] |
| C8H18 | 2,2,3,3-Tetramethylbutane | 594-82-1 | 5.23·10−7 | 5.21·10−4 |
| C8H18 | 2,2,3-Trimethylpentane | 564-02-3 | 3.33·10−7 | 6.00·10−4 |
| C8H18 | 2,2,4-Trimethylpentane | 540-84-1 | 2.53·10−7 3.23·10−7 [23] | 6.69·10−4 5.07·10−4 [23] |
| C8H18 | 2,3,3-Trimethylpentane | 560-21-4 | 3.91·10−7 | 5.71·10−4 |
| C9H12 | 1-Ethyl-2-methylbenzene | 611-14-3 | 1.00·10−5 1.12·10−5 [25] | 2.53·10−3 |
| C9H12 | 1-Ethyl-3-methylbenzene | 620-14-4 | 7.81·10−6 | 2.57·10−3 |
| C9H12 | 1-Ethyl-4-methylbenzene | 622-96-8 | 6.63·10−6 | 2.56·10−3 |
| C9H12 | (1-Methylethyl)benzene | 98-82-8 | 1.00·10−5 1.09·10−5 [25] | 2.60·10−3 |
| C9H12 | Propylbenzene | 103-65-1 | 8.85·10−6 8.23·10−6 [25] | 2.57·10−3 |
| C9H16 | 1-Nonyne | 3452-09-3 | 0.92·10−6 1.00·10−6 [24] | 3.08·10−3 |
| C9H18 | Butylcyclopentane | 2040-95-1 | 9.86·10−8 | 5.08·10−4 |
| C9H18 | (1-Methylethyl)cyclohexane | 696-29-7 | 1.18·10−7 | 5.26·10−4 |
| C9H18 | 1-Nonene | 124-11-8 | 7.98·10−8 | 1.51·10−3 |
| C9H18 | Propylcyclohexane | 1678-92-8 | 9.03·10−8 | 5.19·10−4 |
| C9H20 | 3,3-Diethylpentane | 1067-20-5 | 1.54·10−7 | 5.22·10−4 |
| C9H20 | 2,2-Dimethylheptane | 1071-26-7 | 4.20·10−8 | 6.38·10−4 |
| C9H20 | 2,3-Dimethylheptane | 3074-71-3 | 4.08·10−8 | 6.11·10−4 |
| C9H20 | 2,4-Dimethylheptane | 2213-23-2 | 5.10·10−8 | 6.22·10−4 |
| C9H20 | 2,5-Dimethylheptane | 2216-30-0 | 4.69·10−8 | 6.17·10−4 |
| C9H20 | 2,6-Dimethylheptane | 1072-05-5 | 3.63·10−8 | 6.36·10−4 |
| C9H20 | 3,3-Dimethylheptane | 4032-86-4 | 6.66·10−8 | 5.97·10−4 |
| C9H20 | 3,4-Dimethylheptane | 922-28-1 | 5.35·10−8 | 5.96·10−4 |
| C9H20 | 3,5-Dimethylheptane | 926-82-9 | 6.14·10−8 | 6.02·10−4 |
| C9H20 | 4,4-Dimethylheptane | 1068-19-5 | 7.00·10−8 | 6.04·10−4 |
| C9H20 | 3-Ethyl-2,2-dimethylpentane | 16747-32-3 | 1.00·10−7 | 5.88·10−4 |
| C9H20 | 3-Ethyl-2,3-dimethylpentane | 16747-33-4 | 1.37·10−7 | 5.32·10−4 |
| C9H20 | 3-Ethyl-2-methylhexane | 16789-46-1 | 5.79·10−8 | 5.99·10−4 |
| C9H20 | 3-Ethyl-3-methylhexane | 3074-76-8 | 1.02·10−7 | 5.60·10−4 |
| C9H20 | 3-Ethyl-4-methylhexane | 3074-77-9 | 7.31·10−8 | 5.77·10−4 |
| C9H20 | 3-Ethyl-2,4-dimethylpentane | 1068-87-7 | 6.60·10−8 | 6.00·10−4 |
| C9H20 | 4-Ethylheptane | 2216-32-2 | 4.86·10−8 | 5.94·10−4 |
| C9H20 | 3-Ethylheptane | 15869-80-4 | 4.59·10−8 | 5.92·10−4 |
| C9H20 | 2-Methyloctane | 3221-61-2 | 2.72·10−8 | 6.18·10−4 |
| C9H20 | 4-Methyloctane | 2216-34-4 | 3.53·10−8 | 6.07·10−4 |
| C9H20 | Nonane | 111-84-2 | 1.91·10−8 1.71·10−8 [23] | 6.08·10−4 5.60·10−4 [23] |
| C9H20 | 2,2,3,3-Tetramethylpentane | 7154-79-2 | 1.49·10−7 | 5.49·10−4 |
| C9H20 | 2,2,3,4-Tetramethylpentane | 1186-53-4 | 7.70·10−8 | 6.16·10−4 |
| C9H20 | 2,2,4,4-Tetramethylpentane | 1070-87-7 | 9.23·10−8 | 6.45·10−4 |
| C9H20 | 2,3,3,4-Tetramethylpentane | 16747-38-9 | 1.11·10−7 | 5.62·10−4 |
| C9H20 | 2,2,3-Trimethylhexane | 16747-25-4 | 7.53·10−8 | 6.14·10−4 |
| C9H20 | 2,2,4-Trimethylhexane | 16747-26-5 | 7.21·10−8 | 6.34·10−4 |
| C9H20 | 2,2,5-Trimethylhexane | 3522-94-9 | 5.67·10−8 | 6.60·10−4 5.34·10−4 [23] |
| C9H20 | 2,3,3-Trimethylhexane | 16747-28-7 | 8.91·10−8 | 5.83·10−4 |
| C9H20 | 2,3,4-Trimethylhexane | 921-47-1 | 6.05·10−8 | 5.99·10−4 |
| C9H20 | 2,3,5-Trimethylhexane | 1069-53-0 | 5.58·10−8 | 6.29·10−4 |
| C9H20 | 2,4,4-Trimethylhexane | 16747-30-1 | 8.22·10−8 | 6.12·10−4 |
| C9H20 | 3,3,4-Trimethylhexane | 16747-31-2 | 1.08·10−7 | 5.62·10−4 |
| C10H14 | Butylbenzene | 104-51-8 | 2.35·10−6 2.40·10−6 [25] | 2.46·10−3 |
| C10H14 | sec-Butylbenzene | 135-98-8 | 2.84·10−6 2.36·10−6 [25] | 2.51·10−3 |
| C10H14 | tert-Butylbenzene | 98-06-6 | 2.19·10−6 | 2.53·10−3 |
| C10H14 | 1,2-Diethylbenzene | 135-01-3 | 2.19·10−6 | 2.47·10−3 |
| C10H14 | 1,3-Diethylbenzene | 141-93-5 | 2.32·10−6 | 2.48·10−3 |
| C10H14 | 1,4-Diethylbenzene | 105-05-5 | 1.75·10−6 | 2.47·10−3 |
| C10H14 | 1-Ethyl-2,3-dimethylbenzene | 933-98-2 | 1.73·10−6 | 2.40·10−3 |
| C10H14 | 1-Ethyl-2,4-dimethylbenzene | 874-41-9 | 1.97·10−6 | 2.44·10−3 |
| C10H14 | 1-Ethyl-3,5-dimethylbenzene | 934-74-7 | 2.19·10−6 | 2.47·10−3 |
| C10H14 | 2-Ethyl-1,3-dimethylbenzene | 2870-04-4 | 1.89·10−6 | 2.43·10−3 |
| C10H14 | 2-Ethyl-1,4-dimethylbenzene | 1758-88-9 | 2.03·10−6 | 2.45·10−3 |
| C10H14 | 4-Ethyl-1,2-dimethylbenzene | 934-80-5 | 1.91·10−6 | 2.43·10−3 |
| C10H14 | Isobutylbenzene | 538-93-2 | 4.78·10−6 | 2.51·10−3 |
| C10H14 | 1-Methyl-2- (1-methylethyl)benzene | 527-84-4 | 2.38·10−6 | 2.49·10−3 |
| C10H14 | 1-Methyl-3- (1-methylethyl)benzene | 535-77-3 | 2.55·10−6 | 2.51·10−3 |
| C10H14 | 1-Methyl-4- (1-methylethyl)benzene | 99-87-6 | 2.03·10−6 | 2.50·10−3 2.68·10−3 [25] |
| C10H14 | 1-Methyl-2-propylbenzene | 1074-17-5 | 2.12·10−6 | 2.46·10−3 |
| C10H14 | 1-Methyl-3-propylbenzene | 1074-43-7 | 2.28·10−6 | 2.48·10−3 |
| C10H14 | 1-Methyl-4-propylbenzene | 1074-55-1 | 2.19·10−6 | 2.47·10−3 |
| C10H14 | 1,2,3,4-Tetramethylbenzene | 488-23-3 | 1.34·10−6 | 2.34·10−3 |
| C10H14 | 1,2,3,5-Tetramethylbenzene | 527-53-7 | 1.59·10−6 | 2.38·10−3 |
| C10H14 | 1,2,4,5-Tetramethylbenzene | 95-93-2 | 2.26·10−6 | 2.40·10−3 |
| C10H16 | d-Limonene | 5989-27-5 | 7.64·10−7 | 3.07·10−3 |
| C10H20 | 1-Butylcyclohexane | 1678-93-9 | 1.53·10−8 | 5.40·10−4 |
| C10H20 | 1-Decene | 872-05-9 | 1.27·10−8 | 1.50·10−3 |
| C10H20 | Pentylcyclopentane | 3741-00-2 | 1.68·10−8 1.50·10−8 [23] | 5.29·10−4 |
| C10H22 | 2,4-Dimethyl-3- (1-methylethyl)pentane | 13475-79-1 | 1.47·10−8 | 6.23·10−4 |
| C10H22 | 2,3-Dimethyloctane | 7146-60-3 | 7.58·10−9 | 6.16·10−4 |
| C10H22 | 2,4-Dimethyloctane | 4032-94-4 | 1.01·10−8 | 6.27·10−4 |
| C10H22 | 2,5-Dimethyloctane | 15869-89-3 | 9.23·10−9 | 5.87·10−4 |
| C10H22 | 2,6-Dimethyloctane | 2051-30-1 | 8.65·10−9 | 6.20·10−4 |
| C10H22 | 3,3-Dimethyloctane | 4110-44-5 | 1.21·10−8 | 6.07·10−4 |
| C10H22 | 3,5-Dimethyloctane | 15869-93-9 | 1.18·10−8 | 6.10·10−4 |
| C10H22 | 4,5-Dimethyloctane | 15869-96-2 | 1.08·10−8 | 6.07·10−4 |
| C10H22 | 4-Ethyl-2,2-dimethylhexane | 52896-99-8 | 8.34·10−8 | 5.31·10−4 |
| C10H22 | 3-Ethyl-2,2,4-trimethylpentane | 52897-18-4 | 2.28·10−8 | 6.03·10−4 |
| C10H22 | 3-Ethyl-2,3,4-trimethylpentane | 52897-19-5 | 3.35·10−8 | 5.45·10−4 |
| C10H22 | 2-Methylnonane | 871-83-0 | 3.82·10−9 | 6.52·10−4 |
| C10H22 | 3-Methylnonane | 5911-04-6 | 6.37·10−9 | 6.08·10−4 |
| C10H22 | 4-Methylnonane | 17301-94-9 | 6.90·10−9 | 6.10·10−4 |
| C10H22 | 5-Methylnonane | 15869-85-9 | 7.00·10−9 | 6.12·10−4 |
| C10H22 | 2,2,3,3,4-Pentamethylpentane | 16747-44-7 | 3.23·10−8 | 5.63·10−4 |
| C10H22 | 2,2,3,4,4-Pentamethylpentane | 16747-45-8 | 2.18·10−8 | 5.93·10−4 |
| C10H22 | 4-Propylheptane | 3178-29-8 | 1.20·10−8 | 6.09·10−4 |
| C10H22 | 2,2,3,3-Tetramethylhexane | 13475-81-5 | 3.72·10−8 | 5.60·10−4 |
| C10H22 | 2,2,3,5-Tetramethylhexane | 52897-09-3 | 2.09·10−8 | 6.27·10−4 |
| C10H22 | 2,2,5,5-Tetramethylhexane | 1071-81-4 | 1.50·10−8 | 6.88·10−4 |
| C10H22 | 2,3,3,4-Tetramethylhexane | 52897-10-6 | 2.99·10−8 | 5.62·10−4 |
| C10H22 | 2,3,3,5-Tetramethylhexane | 52897-11-7 | 4.23·10−8 | 5.75·10−4 |
| C10H22 | 2,3,4,4-Tetramethylhexane | 52897-12-8 | 2.35·10−8 | 5.83·10−4 |
| C10H22 | 2,3,4,5-Tetramethylhexane | 52897-15-1 | 1.52·10−8 | 6.17·10−4 |
| C10H22 | 3,3,4,4-Tetramethylhexane | 5171-84-6 | 4.90·10−8 | 5.56·10−4 |
| C10H22 | 2,2,4-Trimethylheptane | 14720-74-2 | 1.47·10−8 | 6.43·10−4 |
| C10H22 | 2,2,6-Trimethylheptane | 1190-83-6 | 1.10·10−8 | 6.55·10−4 |
| C10H22 | 2,4,6-Trimethylheptane | 2613-61-8 | 1.39·10−8 | 6.44·10−4 |
| C10H22 | 2,5,5-Trimethylheptane | 1189-99-7 | 9.65·10−9 | 6.50·10−4 |
| C10H22 | 3,3,5-Trimethylheptane | 7154-80-5 | 1.73·10−8 | 6.11·10−4 |
| C10H22 | Decane | 124-18-5 | 3.33·10−9 2.50·10−9 [23] | 6.15·10−4 5.70·10−4 [23] |
| C11H16 | Pentylbenzene | 538-68-1 | 5.20·10−7 4.68·10−7 [25] | 2.38·10−3 |
| C11H22 | 1-Undecene | 821-95-4 | 1.94·10−9 | 1.50·10−3 |
| C11H24 | Undecane | 1120-21-4 | 4.79·10−10 5.07·10−10 [23] | 6.29·10−4 6.0·10−4 [23] |
| C12H18 | 1,4-Diisopropylbenzene | 100-18-5 | 1.81·10−7 | 2.40·10−3 |
| C12H18 | Hexylbenzene | 1077-16-3 | 0.98·10−7 1.01·10−7 [25] | 2.31·10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral, M.; Oracz, P. Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K. ChemEngineering 2021, 5, 89. https://doi.org/10.3390/chemengineering5040089
Góral M, Oracz P. Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K. ChemEngineering. 2021; 5(4):89. https://doi.org/10.3390/chemengineering5040089
Chicago/Turabian StyleGóral, Marian, and Paweł Oracz. 2021. "Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K" ChemEngineering 5, no. 4: 89. https://doi.org/10.3390/chemengineering5040089
APA StyleGóral, M., & Oracz, P. (2021). Predicted Mutual Solubilities in Water + C5-C12 Hydrocarbon Systems. Results at 298 K. ChemEngineering, 5(4), 89. https://doi.org/10.3390/chemengineering5040089