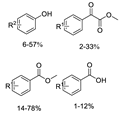
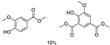
Abstract
Lignin, a complex aromatic polymer with different types of methoxylated phenylpropanoid connections, enables the sustainable supply of value-added chemicals and biofuels through its use as a feedstock. Despite the development of numerous methodologies that upgrade lignin to high-value chemicals such as drugs and organic synthesis intermediates, the variety of valuable products obtained from lignin is still very limited, mainly delivering hydrocarbons and oxygenates. Using selective oxidation and activation cleavage of lignin, we can obtain value-added aromatics, including phenols, aldehydes, ketones, and carboxylic acid. However, biorefineries will demand a broad spectrum of fine chemicals in the future, not just simple chemicals like aldehydes and ketones containing simple C = O groups. In particular, most n-containing aromatics, which have found important applications in materials science, agro-chemistry, and medicinal chemistry, such as amide, aniline, and nitrogen heterocyclic compounds, are obtained through n-containing reagents mediating the oxidation cleavage in lignin. This tutorial review provides updates on recent advances in different classes of chemicals from the catalytic oxidation system in lignin depolymerization, which also introduces those functionalized products through a conventional synthesis method. A comparison with traditional synthetic strategies reveals the feasibility of the lignin model and real lignin utilization. Promising applications of functionalized compounds in synthetic transformation, drugs, dyes, and textiles are also discussed.
1. Introduction
Lignin is a complex aromatic polymer with a three-dimensional amorphous structure consisting of a benzene ring and different types of methoxylated phenylpropanoid connections [1,2]. Due to its abundant organic carbon composition and inactive property [3], lignin has been burned to produce heat and power in biorefinery processes or treated as a side waste in the pulp and paper industry [4]. Several researchers have examined various strategies for the production of value-added chemicals, biofuels, and other platform chemicals from biorefinery of lignin. These depolymerization and upgrade strategies can be broadly classified into catalyzed depolymerization, including hydrolysis, hydrotreatment, and chemical oxidation, and other depolymerization methods (biodegradation, pyrolysis, liquid-phase reforming, and gasification), through which lignin can be transformed to aromatics to alleviate the dependency on petrochemicals [5]. Over the past few decades, several researchers have developed state-of-the-art methodologies to upgrade lignin to high-value chemicals such as drugs and organic synthesis intermediates. Among these methodologies, selective oxidation and activation cleavage of lignin to functionalized compounds seem to be highly efficient choices. Several reviews [6,7,8,9], have summarized the oxidation depolymerization of lignin in either one-step or multi-step reactions involving oxidation. In particular, the selective oxidation of a multi-step reaction provides a basis for the streamlined conversion of intermediates into high-value chemicals [8,10,11]. Among the different basic classes of reactions in organic chemistry, oxidative reactions are attractive because they often generate various rich O-containing/unsaturated functional products, and using the same substrates, we can obtain different chemicals under different oxidative conditions [12]. Carboxylic acids and aromatic aldehydes are the main oxidation products of lignin and its model compounds [13]. In particular, oxidative depolymerization of lignin is a widely used valorization technique and an efficient strategy that focuses on producing even more species of high-value-added chemicals, mainly including synthetic intermediates for drugs and cosmetics.
The relevant reviews on oxidized lignin are written from the perspective of oxidation methods and oxidation substrates (lignin and its model compounds). There has been no overview focusing on the products. For example, how many platform compounds have been obtained in recent years from lignin oxidation methods? What valuable platform compounds can be obtained by oxidative depolymerization of lignin? Herein, we summarize promising strategies for the generation of functionalized monomer compounds based on the selective oxidation and activation cleavage of lignin: aromatic amides, aniline compounds, esters, phenols, aldehydes, ketones, and carboxylic acid.
2. Nitrogen-Containing Functionalized Aromatics
Nitrogen-containing compounds are frequently used in the textile, agricultural, pharmaceutical, and chemical industries to produce amines, dyes, and pesticides. Among them, the most commonly used nitrogen-containing compounds in organic synthesis are aromatic amines and their derivatives, which are usually used as feedstocks or solvents. In addition, ammonia and azo compounds are also used as raw materials in the production of dyes and pesticides [14].
As for nitrogen heterocyclic compounds, in most cases, the synthesis method uses alcohols [15,16,17,18] as the reaction substrate to produce carbonyl compounds in situ, where they are then reacted with nitrogen-containing reagents to transform them into nitrogen heterocyclic compounds such as pyridines, pyrroles, pyrimidines, quinolines, pyrazines, and quinoxalines, as well as other heterocyclic compounds, isoxazole, and imidazole heterocycles [19]. Lignin, a complex polymer consisting of a large number of hydroxyl groups, can be regarded as having alcohol unit connections of various types. Similarly, using n-containing reagents, the subsequent organic condensation reactions cleave oxidized lignin to generate the corresponding nitrogen heterocyclic compounds.
3. Aromatic Amides
The amide bond, as one of the essential functional groups, is the main chemical bond of proteins, and it also appears in some pharmaceutical molecular textile polymers [20,21,22,23,24] Methods using carboxylic acids and amines are the most common and direct methods to generate amides. However, the simple condensation reaction must happen in harsh conditions because the constructed ammonium salts need to be heated to transform into amides [20].
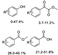
Based on the understanding of the structure of native lignin, β-O-4 (β-aryl ether) is, by far, the largest linkage in lignin [4]. In this key connection, it is important to oxidize and break the propanediol segment. In particular, the oxidation of β-O-4 alcohol to β-O-4 ketone provides the possibility of amine condensation. Then, nitrogen-containing products can be obtained through an amine-mediated reaction [25]. Chiba, Loh, and Wang et al. developed a method for the chemical conversion of β-O-4 lignin models through Cu-catalyzed aerobic oxidation and amine-mediated amide bond formation [26] As a result, intermolecular Cβ–H oxidation and Cβ–O cleavage of the β-O-4 ketone with secondary amines generate α-keto amides as the major products and small phenol products under oxidative conditions, but Cα–Cβ is cleaved to deliver aromatic amides as by-products (Scheme 1, Equation (3)) [26]. Subsequently, Liu et al. developed a novel approach for the synthesis of benzanilides via reacting β-O-4 lignin ketone models with anilines over a CuII catalyst in DMSO solution under an air atmosphere [27]. Comparing the above two systems, the later approach largely preserved the phenol products and obtained different amines because of selective C–C cleavage instead of C–O cleavage (Scheme 1, Equation (2)). Recently, Wang et al. added novel bond cleavage pathways by introducing amines/ammonia into lignin cracking. Similar to the mechanism proposed by Liu and co-workers, control experiments designed by Loh and co-workers indicated that amines act as nucleophiles attacking at the Cα or Cβ position of the oxidized β-O-4 linkage to be cleaved. As a result, primary and secondary aliphatic amines cleave the Cα-Cβ bond on lignin ketone models and form Cα-N bonds to generate aromatic amides. If ammonia is present as a nucleophile, the generation of amides and α-keto amides is dependent on the selectivity of Cα-N and Cβ-N, owing to the competition between oxygen and ammonia [28] (Scheme 1, Equations (1)–(4)).
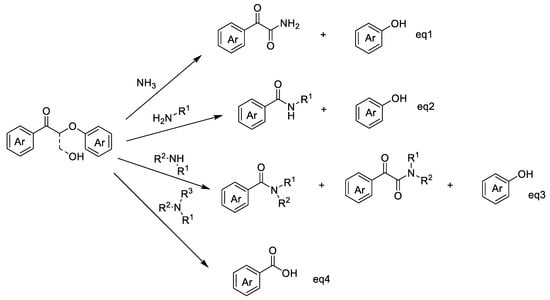
Scheme 1.
Different substituted amines transform oxidized lignin models to various types of aromatic amides.
4. Nitrogen Heterocyclic Compounds and Nitriles
The varied family of n-containing heterocyclic rings contains different species such as three-membered ring aziridines, [29,30,31] five-membered ring pyrrole, and five-membered ring pyridine (shown in Figure 1), many of which have found important applications in materials science, agrochemistry, and medicinal chemistry. Therefore, there is continuing interest in the development of convenient, efficient, and environmentally benign synthetic methods for the construction of nitrogen-containing heterocycles [32].
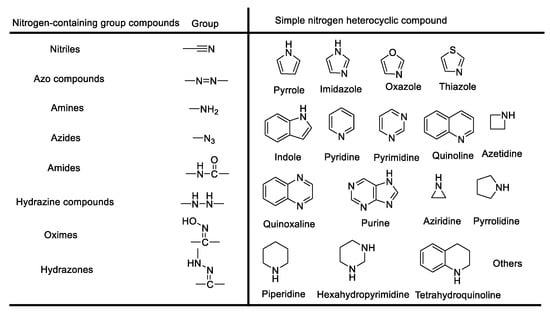
Figure 1.
General classification of nitrogen-containing functionalized compounds in the organic chemistry field.
The nitrile-group pharmaceuticals are widely used in clinical treatment due to the good biocompatibility and biological activity of nitrile-containing chemicals [33] Further, the nitriles are used in the preparation of important functional materials such as dyes and textiles [34,35] As an intermediate raw material for organic synthesis, cyanide can be used to construct amides, carboxylic acids, amines, aldehydes, and ketones [36]. Recent achievements in a large number of active nitrile-containing drug molecules mainly include the antidepressant vilazodone; entacapone, for treating Parkinson’s disease; Lodoxamide, an anti-inflammatory agent; and febuxostat, for treating gout [33,37,38,39,40].
In traditional synthesis methods, the synthesis of nitrogen heterocyclic compounds requires cyclization/oxidative aromatization with n-containing reagents and hydrocarbons [41,42,43]. As for nitriles, general methods include oxime rearrangement [44] and coupling reactions involving cyanogen reagents [45,46,47,48,49]. However, these typical methodologies for the synthesis of nitrogen heterocyclic compounds and nitriles may have only a limited range of applications because of the toxic reagents and expensive feedstock. Thinking about inexpensive and environmentally benign starting materials, lignin must be one of them.
Hydroxylamine and hydrazine are generally used as nitrogen reagents and reducing agents in classical organic synthesis. In particular, the oximes, which are formed via the condensation of carbonyl groups with hydroxylamine, are versatile intermediates for the generation of amides. Recently, Zhang and Wang’s group introduced NH2OH-mediated lignin conversion to isoxazole and nitrile [50,51] (Scheme 2 Equation (3)). Both products were obtained in one step via the same oxime intermediate, followed by condensation of β-hydroxyl ketone with hydroxylamine and then Beckmann rearrangement to construct isoxazole and nitrile, respectively. Moreover, based on the carbonyl condensation property and hydrazine as the amination and reducing agent, Heinrich et al. developed an attractive strategy through Wolff–Kishner reduction for C-O bond cleavage in lignin ketone models and further cyclization to pyrazole. Meanwhile, the above system also obtained corresponding monophenols due to the cleavage of C-O bonds (Scheme 2 Equation (2)) [52]. Zhong et al. reported a copper-catalyzed system or an efficient iodine/tert-butyl hydroperoxide (I2/TBHP) catalytic system for catalytic oxidative cyclization of lignin models with 2-amino azaarenes to synthesize imidazo heterocycles (Scheme 2 Equation (1)) [53,54].
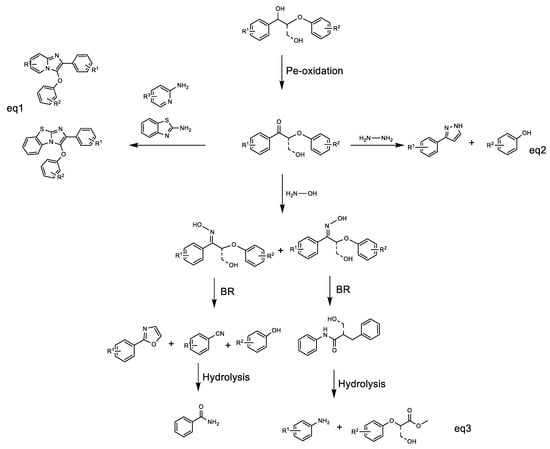
Scheme 2.
Different n-containing reagents transforming oxidized lignin models to various types of n-substituted aromatics, including nitrogen heterocyclic compounds and nitriles.
5. Aniline Compounds
The nitrogen element mainly exists in nature as nitrogen, ammonia, and bio-organic amines. Among them, most nitrogen-containing compounds exist in the form of amines. Many natural products with physiological activities such as anesthesia, excitement, and anti-inflammatory properties also contain amino functional groups, which are all alkaloid compounds. Aromatic amines are used as a synthetic raw material for dyes, and the method of producing dyes by oxidizing aromatic amines created the dye industry [55]. The various methods for aniline synthesis, including reduction of aromatic nitro compounds, amino substitution of halogenated aromatic compounds, and other decoration of aromatic hydrocarbons by NH-R groups, generally require harsh reaction conditions and toxic substrates [56,57]. Moreover, the direct modification of aromatic hydrocarbons by NH-R groups has three difficulties: the high C-H bond dissociation energy, the use of poisonous aniline catalyst, and difficulty in keeping the aniline products stable in an oxidative atmosphere [58,59,60,61,62,63,64,65]. To date, various efficient approaches have been developed to promote C–H primary aromatic hydrocarbon activation and amination, including electrochemical catalysis, [66] photoredox catalysis [67,68], and novel electrophilic amination reagents [69].
The preparation of aromatic amides and benzonitriles with pre-oxidized lignin is described above; thus, the amides and benzonitriles produced could be further hydrolyzed to aniline (Scheme 2, Equation (3)) and carboxylic acids, [70] respectively. Recently, Nicewicz et al. realized the selective cleavage of aryl ether in lignin via photocatalyzed nucleophilic aromatic substitution using amines under mild conditions. A lignin β-O-4 ketone model was used, and selective O-4 bond cleavage occurred and generated aniline, while other groups on the arenes were maintained (Scheme 3, Equation (2)) [71]. Besides this, Jiao et al. recently developed amination using NaN3 and realized a transition from alkylarenes to anilines via redox-neutral site-directed carbon–carbon amination (Scheme 3, Equation (1)) [72]. This transformation of aromatics involved the use of O2 as an environmentally benign oxidant. Subsequently, the secondary alcohol intermediates could also be transformed into the corresponding anilines without any oxidant. Therefore, lignin model compounds containing a propanediol segment were redox-neutrally depolymerized into corresponding primary arylamines. Based on the cleavage of the β-O-4 ketone model, tandem selective oxidation, oximation, and acylation reactions were used, followed by imine-participated ether cleavage via 1,4-aryl migration and a subsequent hydrolysis reaction, to yield anilines and a-hydroxy ketones as products [73] (Scheme 3, Equation (3)).
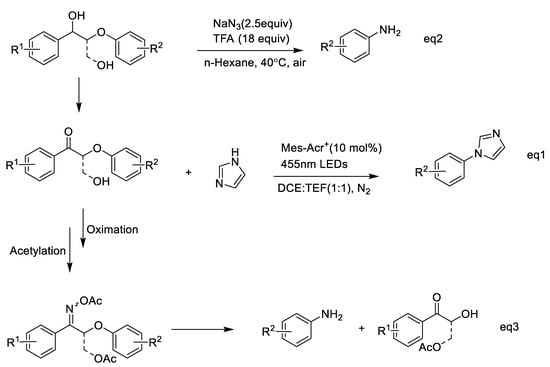
Scheme 3.
Selective oxidation and n-reagent-participating activation cleavage of lignin linkages to yield anilines.
6. Esters
The dehydrated products of acids and alcohols are esters, which have fragrance and are widely used as food additives [74] As a kind of carboxylic acid derivative, esters are an important class of chemicals and are widely used in the preparation of fine chemicals, natural products, medicines, and agrochemicals [75]. In addition to the esters traditionally obtained by the dehydration reaction of carboxylic acid and alcohol, esters can also be prepared from other carboxylic acid derivatives such as amides, acid anhydrides, and acid chlorides (Scheme 4). The esterification procedure is reversible and suffers from inherent drawbacks, such as the fact that it is hard to increase the conversion rate and isolate or separate the catalysts and/or products in time [76] Aldehydic compounds in an alcohol-containing solution are also able to provide oxide esterification effectively, so they are used as a supplement to traditional protocols [77,78,79,80,81,82,83,84]. In recent years, benzylic alcohol and methanol as substrates for esterification have attracted more attention; they have been used for direct, economical, and environmentally friendly conversion into esters in the presence of catalysts and oxidants, [85,86,87,88,89,90] in which the alkali additive is usually able to increase the yield of esters [90].
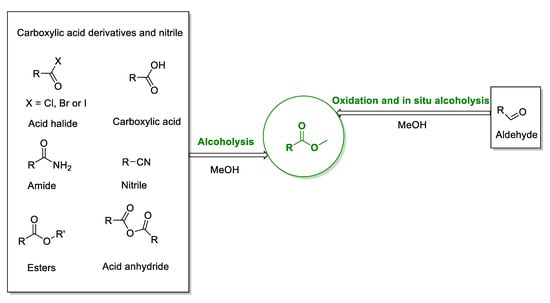
Scheme 4.
The general methods of synthesizing esters in organic synthesis.
There are many methods to obtain functional carbonyl compounds via the tandem sequence oxidation reaction and functionalization of C-C bonds in lignin. However, while some splendid achievements have been made in the oxidative depolymerization of lignin models and real lignin to acids or aldehydes, the direct transformation of lignin to esters through oxidative cleavages is still limited. Recent reports have described efficient protocols for converting lignin models or lignin to aromatic esters and very few types of short-chain non-aromatic esters using homogeneous and heterogeneous catalysts with oxidants. In addition, those methods have only provided limited product types, including functional aromatic esters and diethyl maleate.
7. Aromatic Esters
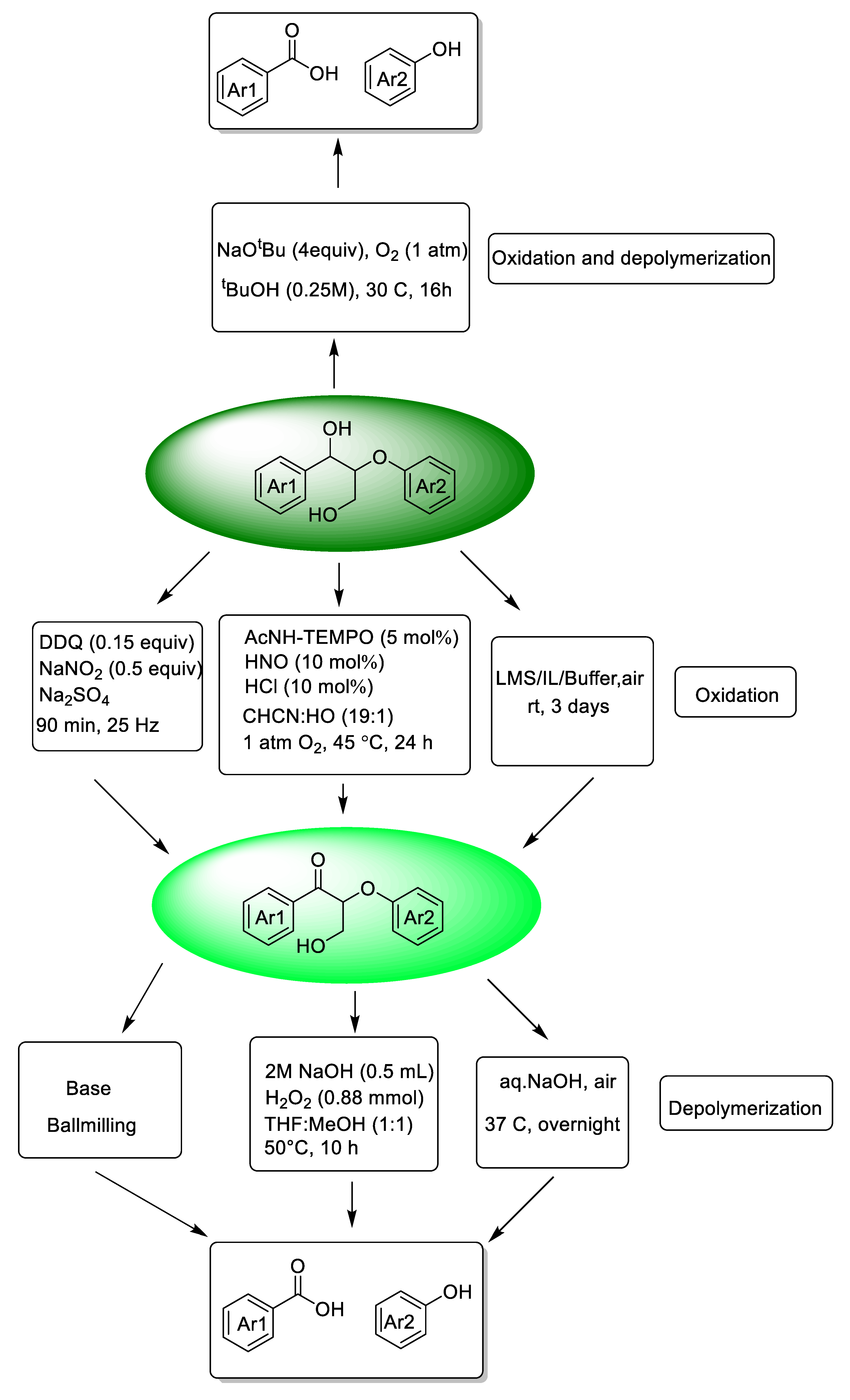
In the past few years, novel strategies for the oxidative depolymerization of lignin model compounds and real lignin transformation into aromatic esters have been developed, including the use of heterogeneous catalysts [32] or homogeneous catalysts [25] Among these, oxidative systems for lignin depolymerization strategies to obtain functional esters in high yield and with excellent selectivity may be designed by treating lignin as a secondary alcohol [91]. Ideally, researchers use secondary benzyl alcohol as a model compound in the screening reaction system and then apply the final reaction system to the conversion of lignin model compounds or real lignin. Besides this, researchers also use model compounds to verify the mechanism by designing a series of control experiments [91,92,93].
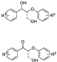
The cleavage of the β-O-4-type bonds of lignin to produce discrete molecular compounds is frequently studied as the first step in this field for the valorization of lignin. Indeed, many papers have described the catalytic transformation of β-O-4-linked dimeric compounds to final aromatic esters. For example, the catalytic transformation of 2-phenoxy-1-phenylethanol to methyl benzoate was successfully achieved using a single-atom Co catalyst at low oxygen pressure in MeOH [94]. Nitrogen-doped carbon-modified cobalt nanoparticles were also used as a catalyst in the same conversion [95]. For instance, the covalent triazine framework (CTF) has been used as a catalyst for oxidative cleavage of β-O-4 ketone models to form aromatic esters [96] A strong-base-modified covalent triazine framework (30KCTF) remarkably enhanced the cleavage of the β-O-4 linkage [92]. Inexpensive copper homogeneous catalysts also promote the aerobic oxidation cleavage and esterification of C(OH)–C bonds or -CO–C bonds [93,97] and, according to related reports, 2-phenoxy phenethyl alcohols [93] and β-O-4 ketone models [97] are all converted to aromatic esters in O2 atmosphere with an inexpensive copper salts catalyst. Moreover, Zhang et al. developed an attractive strategy based on Baeyer–Villiger (BV) oxidation for C-C bond cleavage in lignin ketone models and further alcoholysis to esters, [98] and Meier et al. also used BV oxidation after the mechanochemical treatment of lignin by porphyrin-catalyzed oxidation, with further alcoholysis to esters [99].
8. Non-Aromatic Acid Esters
In addition to aromatic esters, carbonyl esters also include non-aromatic esters such as fatty acid glycerides, essential nutrients needed by humans. Similar to the synthesis of amides, esters can also be obtained by condensation reactions of acids and alcohols or halogenated hydrocarbons. The direct oxidation of aldehyde and alcohol during in situ esterification undergoes two steps of oxidation and condensation to construct O = C-O [100,101]. The oxidation of abundant phenol units and side-chain alcohol units on lignin can form acids and a variety of functional esters in situ in an alcohol system. Compared with the formation of aromatic esters, the cleavage of lignin to produce non-aromatic esters may require more severe oxidation conditions.
At present, there are not many types of non-aromatic acids prepared by oxidative depolymerization of lignin. Diethyl maleate is another class of functionalized ester based on carboxylic acid derivatives from lignin conversion (Scheme 5). Strategies for the selective production of diethyl maleate via oxidative cleavage of lignin aromatic units have been developed, including the use of heterogeneous catalysts [13] or ionic liquids [102]. Li et al. used polyoxometalate ionic liquids to promote cleavage of a lignin aromatic unit to obtain bulk chemical diethyl maleate, [13] and according to their report, lignin aromatic units were converted to diethyl maleate in the intensive synergistic effect between the acidic depolymerization, oxidative aromatic ring cleavage, and in situ esterification [13]. Besides this, Liu’s group also designed novel hierarchical Ce-Cu/MFI nanosheets and used them for the selective oxidative cleavage of organosolv lignin. As a result, the reaction pathway underwent a two-step process in which the lignin was converted to monophenols by selective cleavage of the C-C bonds, and these monophenols were then transformed to final products of diethyl maleate and other C3-C5 esters via benzene ring-cleavage [102].
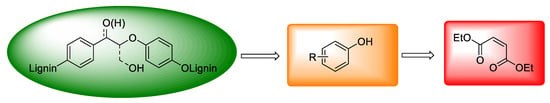
Scheme 5.
The general route from lignin oxidation (green) to DEM production (red).
9. Quinones
In the plant kingdom, many natural products with physiological activity contain phenolic hydroxyl or quinone structures. Flavonoids are one such representative present in plants. However, phenolic substances are easily oxidized to produce corresponding quinones due to the electron-donating effect of phenolic hydroxyl groups [103]. Quinones have been used as pigments and drugs in human life and industry for a long time [104] and may be the longest used chemical, even longer than any other kind of natural compound. More than 4000 years ago, humans used plant-derived quinones as medicines, laxatives, and emetics; therefore, the important position of quinones is well established.
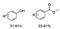
In addition to phenols, highly active aromatic compounds such as anthracene, phenanthrene, and anilines can be transformed to the corresponding quinones by chemical oxidation, even directly by molecular oxygen oxidation. Lignin is a kind of abundant polyphenolic polymer that imparts the potential to generate quinones. Recently, Parks et al. used Co (salen) catalysis to form benzoquinones and benzaldehydes through oxidation of lignin models belonging to the monomeric lignin models syringyl (S), vanillyl (G), and 4-hydroxybenzyl alcohol (H) [105]. In particular, this group attempted to combine computational and experimental approaches to investigate the mechanism of Co (salen)-catalyzed oxidation of the monomeric lignin models. Density functional theory calculations and experiments revealed that S oxidation occurs more easily to form dimethoxybenzoquinone than G and H oxidation with a pyridine-coordinated Co (salen) catalyst, but adding bulky, noncoordinating bases enhanced the oxidation of G to form methoxybenzoquinone (Scheme 6, Equation (1)) [105]. Similarly, Fu’s group also reported that a vanadium catalyst with base additive could be used in the C−C cleavage of β-O-4 lignin phenolic model compounds to generate benzoquinone products and acrolein derivatives (Scheme 6, Equation (2)) [106]. Moreover, Bolm and co-workers used a ball milling method to treat phenolic model compounds with HO−TEMPO/KBr/Oxone (TEMPO is 2,2,6,6- tetramethyl-1-piperidinyloxy), resulting in the production of the corresponding quinones and phenol derivatives (Scheme 6, Equation (3)) [107].
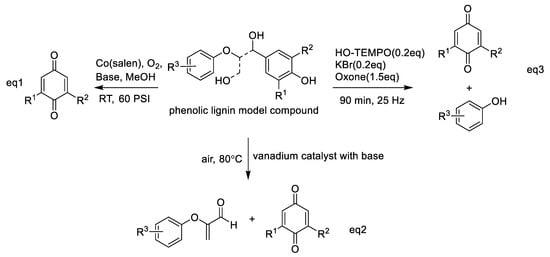
Scheme 6.
Oxidation of phenolic lignin model compounds with different catalysts to produce corresponding quinones and other compounds.
10. Carboxylic Acids
Carboxylic acids, as a fundamental raw material, have shown promise in the construction of complex small molecules [108,109]. Using transformations of carboxylic acid derivatives, we can obtain roughly four categories of products: anhydrides, esters, amides, and hydrocarbons [110].
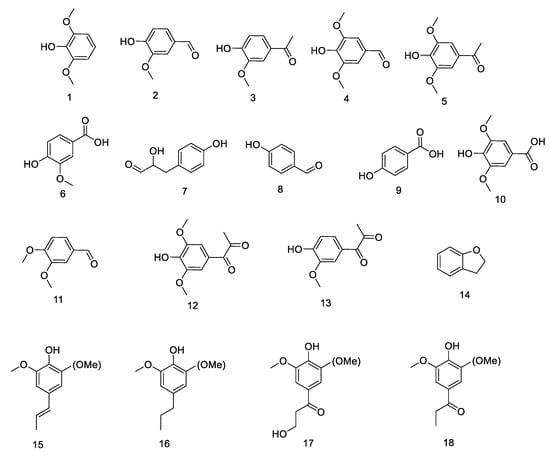
In the past few years, many types of carboxylic acid products have been obtained from the oxidative depolymerization of lignin or lignin model compounds, including aromatic acids such as vanillic acid, [111] veratric acid, [112] and benzoic acids, [113] and non-aromatic acids such as muconic acid, muconolactones,114 and C4 dicarboxylic acids (succinic, mulic, maleic, fumaric, and tartaric) [111,112,113,114,115]. Particularly, the oxidation of either phenolic or non-phenolic lignin model compounds is capable of producing aromatic acids or non-aromatic acids [116]. Among these, several acids have been obtained in high yield or selectivity from lignin-derived monomers through novel strategies of oxidative depolymerization of lignin model compounds, including a strong oxidant continuous oxidation process [111,115] a synergistic process of strong base and oxidant, [111,113,114,115,116,117,118] a catalyst/laccase oxidative process, [114,115,116,117,118,119,120,121] photocatalysis, [122] and ionic liquids [123].
11. Aromatic Acids
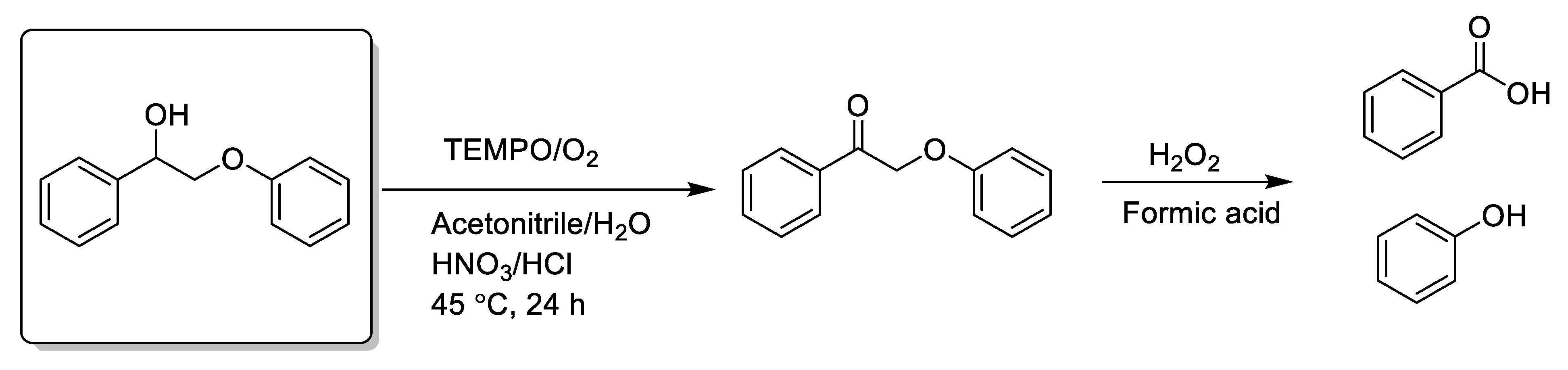
Earlier, we mentioned that esters can be obtained by BV reaction of β-O-4 ketone-structured lignin model compounds. A kind of lignin model compound, β-O-4 alcohol undergoes a two-step oxidation strategy, resulting in Cα-Cβ cleavage; then, pre-oxidation of the β-O-4 alcohol dehydrogenates the substrate to b-O-4 ketone, and an atom is then inserted into the medium ketone to form an ester. As we mentioned, BV products can generate aromatic esters by the alcoholysis step. However, Zhang et al. used formic acid instead of alcohol to hydrolyze the products after BV reaction, and corresponding aromatic acids and phenols were generated; [124] according to this report, aromatic acids were obtained via H2O2 oxidative C-C bond cleavage of a lignin model compound in a formic acid system under metal-catalyst-free conditions (Scheme 7a). Similarly, Yang, Stahl, and Su’s group developed a successful protocol that using the synergistic effect of strong base and oxidant without a transition-metal catalyst to convert lignin model compounds to high-value aromatic acids (Scheme 7b) [113,118,125,126]. One of the reasons why lignin transformation to monoaromatic chemicals and fine chemicals is difficult is that the lignin structure is highly complex and heterogeneous, depending on both the natural lignin structure and the biomass fractionation method [127,128]. The use of a homogeneous catalysis system may be a good alternative for the conversion of lignin.
Scheme 7.
Oxidation of lignin or lignin model compounds by different oxidative strategies to obtain corresponding aromatic acids and other compounds.
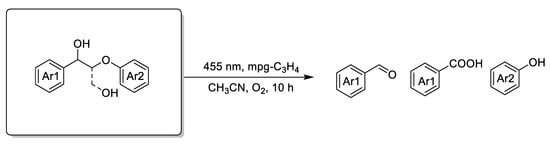
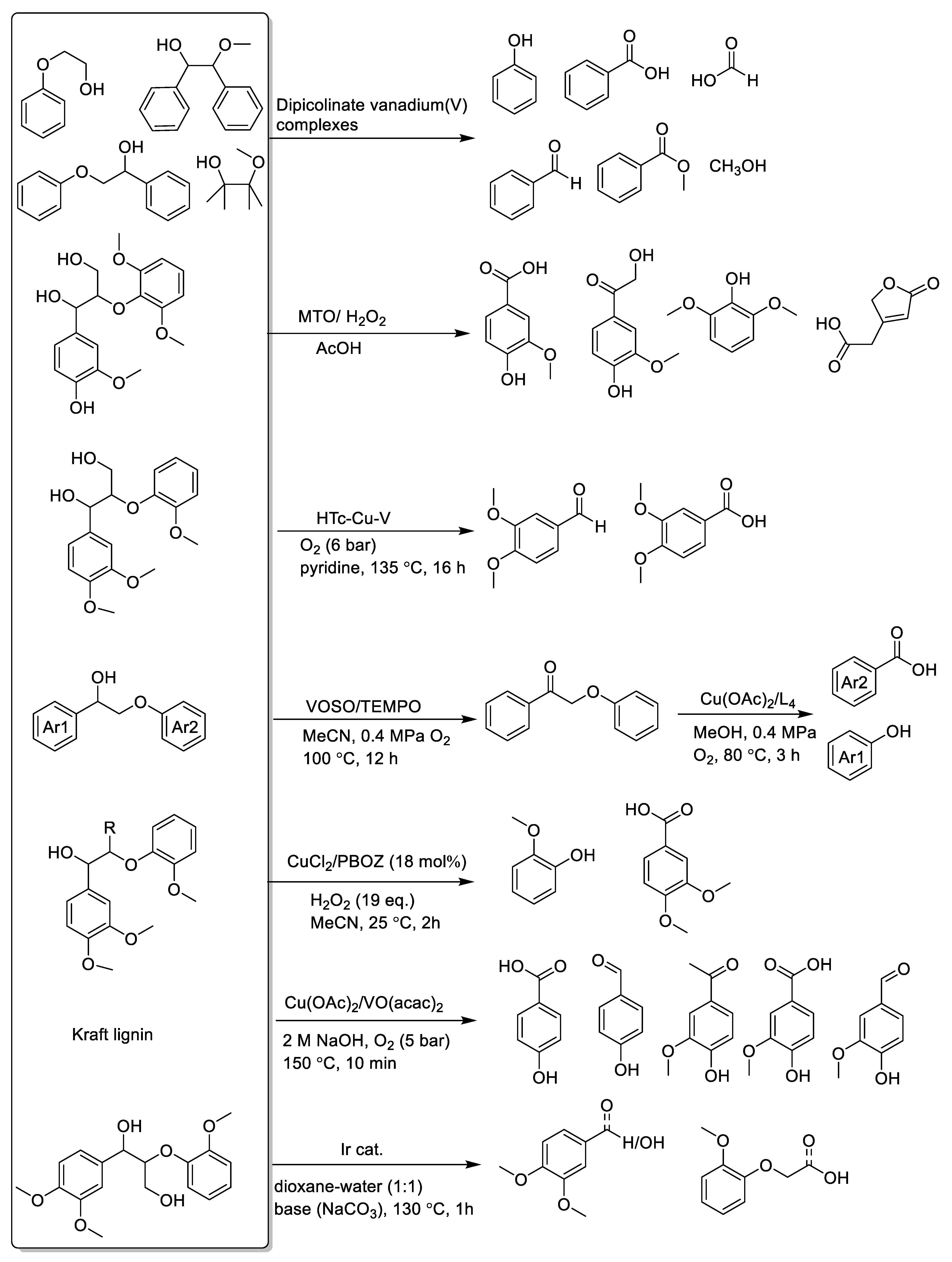
Copper, vanadium, and rhenium catalysts116,129 are used for the oxidative cleavage of lignin for the generation of acids and aldehydes. In recent years, a series of vanadium- and copper-based catalysts were prepared to act on the oxidation of lignin model compounds (Scheme 7c) [129,130,131,132,133]. Corma et al. used a dioxygen V(acac)3/Cu(NO3)2 3H2O catalyst system with high catalytic activity in the cleavage of the lignin model compound erythro-1-(3,4-dimethoxyphenyl)-2-(2-methoxyphe-noxy)-1,3-propanediol; with molecular oxygen as the oxidant, the main conversion product was veratric acid [112]. Vanadium-based complexes mainly act on the C–H bond of β-O-4 model compounds to cause cleavage of the hydroxy carbon and form an intermediate ketone or aldehyde. The result is that the C–C bond connecting the hydroxyl group is broken [134]. By contrast, copper-based catalysts can break Cα–C aryl bonds, even Cα–Cβ bonds, in lignin phenolic β-O-4 model compounds without limitation of hydroxyl carbons [97,135]. Wang et al. used a VOSO4/TEMPO [(2,2,6,6-tetramethylpiperidin-1-yl)oxyl)] catalyst to form β-O-4 ketone and followed this with cleavage of the C-C bond of β-O-4 linkages to acid and phenols by oxidation over a Cu/1,10-phenanthroline catalyst [136]. Zheng et al. achieved a high conversion rate of 83% after 2 h at room temperature when using CuCl2/polybenzoxazine catalysts to oxidize the cleavage of C-C and C-O bonds of lignin selectively to oxidize lignin β-O-4 model compounds to aromatic acids, monophenolics, and aromatic aldehydes [137,138]. Riisager prepared a homogeneous vanadium–copper catalyst for the oxidation depolymerization of Kraft lignin to high-value aromatics (vanillin, vanillic acid, and acetovanillone) [139]. Saladino et al. reported methylrhenium trioxide (MTO)/H2O2 systems for application to the selective oxidation of lignin model compounds and lignins to generate aromatic acids and other compounds [116]. Besides this, Bruijnincx et al. reported a novel method using Cp*Ir-bipyridonate as a catalyst to promote the depolymerization and isolation of lignin model compounds and real lignin without any oxidants; according to this report, the conversion undergoes selective primary alcohol dehydrogenation, retro-aldol (Cα–Cβ) bond cleavage, and in situ transformation of the aldehyde products to alcohols and carboxylic acids by similar Cannizzaro reaction [121]. The above homogeneous metal complexes, both V-based catalysts and Cu-based catalysts, were able to achieve excellent results in oxidative lignin valorization. However, it is difficult to realize organic product separation and recycling of homogeneous catalysts. Researchers have attempted to find generally highly efficient heterogeneous catalysts for lignin conversion. Wang’s group introduced a one-step oxidative method that used mesoporous and heterogeneous graphitic carbon nitrides (mpg-C3N4) to cleave the β-O-4 or β-1 linkages in lignin models (Scheme 7d). Because of the mesoporous graphitic carbon nitride photocatalyst, lignin models with β-O-4 and β-1 linkages were aerobically oxidized into aromatic aldehydes (acids) and phenolic esters even at room temperature [122]. Recently, a two-step process was developed for selective oxidation of β-O-4 lignin model compounds in which β-O-4 lignin model compounds are first converted to β-O-4 ketone models and then proceed to the next step (shown in Table 1).

Table 1.
Transformation of lignin or its model compounds to aromatic esters.
- BV oxidation strategy for lignin oxidative cleavage.
- Synergistic strategy of strong base and oxidant for lignin oxidative cleavage.
- Copper, vanadium, rhenium, or Ir homogeneous catalysis strategies for lignin oxidative cleavage.
- Heterogeneous catalysis strategy for lignin oxidative cleavage.
12. Non-Aromatic Acids
Lignin is composed of three phenylpropane units on the aromatic rings, named p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units [140]. These units are abundant in methoxy substituents on aromatic rings. The use of a strong oxidation system to break aromatic rings to produce low-carbon acids is promising.
Non-aromatic acids can be generated via oxidation of lignin monomers connected to various hydroxyl [115] or carbonyl-containing compounds [141]. Depending on the oxidants and catalyst, the formation and species of these compounds vary (shown in Scheme 8). In Rodrigues’s work, using H2O2 as an oxidant, lignin model compounds (p-hydroxybenzoic acid, vanillic acid, and syringic acid) and hardwood and softwood lignin samples were oxidized to obtain dicarboxylic acids [115]. In addition, C4 dicarboxylic acids (DCA) were also obtained from lignin oxidation with transition metal oxide (Fe, Co, Cu) modified catalysts [142,143,144]. For instance, recent reports developed methods for the generation of carboxylic acids from the oxidation of lignin by a Fenton oxidation process, with a Fenton reagent consisting of an iron catalyst [145]. However, because lignin depolymerization to dicarboxylic acids by the Fenton oxidation process suffers from poor yield and raw material utilization, Doherty et al. introduced a process based on the use of sodium percarbonate in bagasse lignin depolymerization [111]. Using Doherty’s strategy, higher DCA yields (predominantly oxalic, malonic, and succinic acids) were obtained, without residual solids. Besides this, some other low carbonic acids (lactic acid, formic acid acetic acid, acrylic acid, and malonic acid) were also obtained from oxidation isolation of lignin using vanadium-modified catalysts and oxidants [146,147]. At last, researchers have gradually deepened their understanding of the structure of lignin and promoted the greater potential of lignin. For example, using oxidative cleavage of catechols for the synthesis of muconic acid and muconolactones opens a new route to lignin upgrading [114]. Besides this, Yuan et al. developed a novel catalytic oxidation system consisting of a copper catalyst in MeOH–ChCl/O2 DES capable of acetic acid and acetovanillone synthesis via the selective oxidative cleavage of C–C bonds in lignin side chains [148]. Furthermore, the wet oxidation of lignin and phenolic lignin model compounds easily forms carboxylic acids, including formic acid, acetic acid, and unsaturated dicarboxylic acids with four carbon atoms [149].
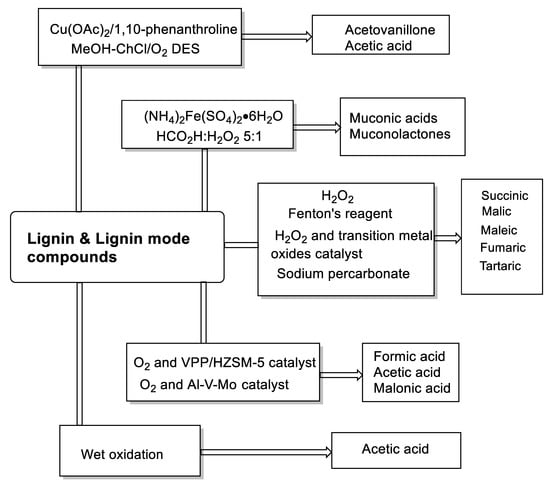
Scheme 8.
Oxidation of lignin or lignin model compounds by different oxidative strategies to obtain corresponding non-aromatic acids.
13. Phenols, Aldehydes, and Ketones
Recently, phenolic derivatives have attracted people′s attention owing to their anti-microbial, anti-cancer, anti-viral, anti-inflammatory, hypolipidemic, and hypoglycemic effects. Soybeans, flaxseed, and olives are particularly rich in phenolic compounds [150]. Traditional industrial production processes of phenols mainly include the sulfonation method and cumene method. Other substituted phenols are usually constructed by nucleophilic substitution reactions or diazonium hydrolysis.
In aldehydes and ketones, the carbonyl (C = O) carbon in the aldehyde and ketone structure is vulnerable to attack by nucleophiles such as S-containing and n-containing reagents to construct corresponding C-S and C-N bonds; therefore, aromatic ketones and benzaldehyde as chemical feedstocks are often used in the synthesis of complex natural compounds and as intermediate raw materials in pharmaceuticals and agriculture [151,152,153]. These aldehydes and ketones are generated from corresponding alcohols via organic transformation reactions [154]. Among those reactions are many classic organic name reactions, including Swern oxidation [155,156,157,158,159,160,161] and DesseMartin periodinane oxidation [162,163,164]. Therefore, those oxidation methods can also be applied to lignin oxidation and depolymerization into aldehydes and ketones. Lignin oxidation cleavage can be used to produce phenolic products [165] such as vanillin and syringaldehyde, which, connected with the carbonyl group, can also be classified as aldehydes or ketones.
In this section, we summarize the novel strategies using oxidative depolymerization of lignin or lignin model compounds to obtain lignin-derived aromatic compounds (phenols, aldehydes, and ketones). Besides this, we mentioned earlier that phenols can be obtained in several systems of oxidative depolymerization of lignin, including those systems used to produce acids, esters, or amides, so we will not repeat it here. The oxidation cleavage of different lignin model compounds in different catalytic systems produces all, one, or two of the product kinds of phenols, aldehydes, and ketones. In the past few years, researchers have developed main methods, including lignin catalytic oxidation and simultaneous depolymerization, catalytic oxidation then depolymerization, and lignin pre-oxidation then redox catalytic depolymerization, to achieve the above transformation [166].
14. Phenols and Aldehydes or Ketones
The oxidation of Kraft lignin is a potential method to generate functionalized phenols. Using catalytic oxidation with a simultaneous depolymerization process mainly produces phenolics containing a carbonyl functional group (shown in Scheme 9). Firstly, lignin can be depolymerized with a system containing transition metal catalysts and oxidants. For example, Sakdaronnarong et al. exploited microwave-assisted lignin oxidative depolymerization to produce high-value phenolic compounds such as syringol, vanillin, acetovanillone, syringaldehyde, and acetosyringone using bimetallic Cu (OH)2 and Fe2O3 catalysts in NaOH solution with 2.5% (w/w) hydrogen peroxide [167]. Qiu’s group applied hydrothermal oxidation and depolymerization of lignin via a CuO/Fe2(SO4)3/NaOH catalysis system with H2O2 as the oxidant to generate monophenolic compounds [168]. Phenolic products were obtained from lignin depolymerization via a CuSO4/H2O2 system catalyzed under microwave irradiation [169]. Besides this, Bhaskar’s group also introduced the generation of phenolics by oxidative depolymerization of prot lignin and alkali lignin in the presence of cobalt-supported TiO2, CeO2, and ZrO2 catalysts at 140 °C for 1 h [170]. Additionally, Welton et al. exploited ionic liquids under oxidant conditions to oxidize and depolymerize lignin in a one-pot process resulting in an array of phenols and functionalized aromatics, vanillin, and syringaldehyde as the main products [166]. Secondly, lignin can be depolymerized without a transition metal catalyst. For instance, Qi’s group demonstrated oxidative cleaving with molecular oxygen (O2) to depolymerize native lignin into oxygenated phenolic monomers [171]. Thirdly, lignin was depolymerized with Mo catalyst, ionic liquids promoted the aerobic oxidation of lignin, and lignin was converted to functionalized phenols containing vanillin and methyl vanillate in the presence of H3PMo12O40 catalyst in an alcohol system [172]. Natte et al. reported molybdenum pyrophosphate supported on CeO2 (MoPO/CeO2) catalyst for the oxidative depolymerization of alkali lignin to generate vanillin [173]. Lastly, for the same transformation producing aromatic aldehydes, aerobic oxidation of lignin in alkali conditions facilitated efficient production of vanillin/syringaldehyde [174,175,176].
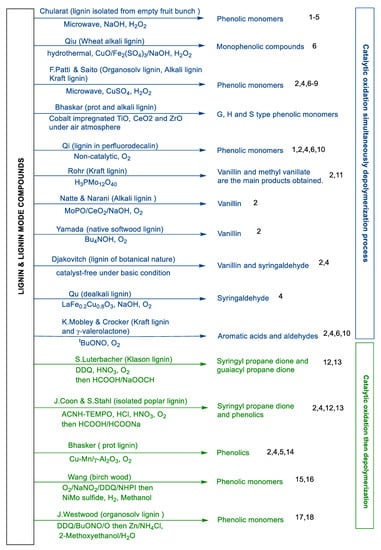
Scheme 9.
Distribution and composition of main products after catalytic oxidation simultaneous to/followed by depolymerization of kinds of lignins.
Catalytic oxidation followed by depolymerization of lignin also mainly produces phenolics containing a carbonyl functional group [177]. Luterbacher et al. used 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as an oxidant/catalyst to oxidize the a-OH of lignin into a ketone; then, the oxidized lignin was depolymerized using a formic acid/sodium formate system to a single product reaching 80% yield (syringyl propane dione) and 10–13% guaiacyl propane dione [178]. Similar to Luterbacher’s work, Stahl and Bhaskar et.al. used a catalytic aerobic oxidation process, followed by formic-acid-induced [179] or water/ethanol-water co-solvent [180] hydrolytic depolymerization resulting in monomeric phenolics containing carbonyl groups. Besides this, Wang et al. used catalytic oxidation with the O2/NaNO2/DDQ/NHPI system followed by a hydrogenation depolymerization process with NiMo sulfide catalyst to produce phenol monomers in 32% yield from birch powder [181]. Westwood et al. also put an organosolv lignin through a two-step depolymerization process similar to the above to generate phenolic monomers [182].
15. Phenols and Ketones
In recent years, the two-step conversion strategy of lignin via pre-oxidation followed by redox catalytic depolymerization has been popular in the valorization of lignin, with the ability to obtain corresponding phenols and ketones as shown in Scheme 10. According to recent reports, the redox step involves photoredox [117,119,141,143,183] and biomimetic redox [70,135].
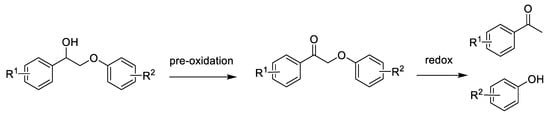
Scheme 10.
Oxidation of β-O-4 lignin model compounds by pre-oxidation followed by redox strategies to obtain corresponding phenols and ketones.
Prof. J. Zhang elaborated on the conversion of lignin model compounds via photoredox catalysis in detail [133]. For biomimetic redox, using nucleophilic thiolates and reductive thiols as redox mediators entails reductive cleavage of keto aryl ether bonds by an SN2 mechanism with the thiol redox mediator glutathione to form phenol and acetophenone products [70,135].
The hydrogenolysis of oxidized lignin using a catalyst with a hydrogenation function can also achieve a similar conversion to redox catalysis. For instance, Wang et al. introduced a nickel catalyst for the depolymerization of oxidized lignin to phenols and aromatic ketones. This group also developed a method of photocatalytic oxidation–hydrogenolysis of lignin β-O-4 models in one pot to offer ketones and phenols. According to Wang’s report, the Pd/ZnIn2S4 catalyst is used in aerobic oxidation, and TiO2- NaOAc/Ethanol was used in the hydrogenolysis system [137]. Similarly, He et al. obtained corresponding aromatic ketones and phenols via visible-light photocatalytic oxidation and in situ carbonic-acid-facilitated hydrogenolysis [140].
16. Conclusions
In this review, we summarized recent advances in the conversion of lignin via selective oxidation to functionalized lignin monomers, including aromatic amides, aniline compounds, esters, phenols, aldehydes, ketones, carboxylic acids, and quinones, since lignin-derived functional chemicals generated via oxidation are of higher value than lignin used for other traditional applications, such as for heat and fuels. The use of different catalytic oxidation systems and types of oxidants in the solvent can achieve diverse lignin products.
For oxidative depolymerization to multiple monomers, lignin needs to undergo a series of tandem chemical reactions, including the cracking of C–O bonds, C–C bonds, or other linkages within the lignin. The catalytic oxidation systems of lignin and types of chemicals used in the solvent have been comprehensively overviewed. Through unremitting exploration and attempts by scientific researchers, more types of products have also been obtained from the oxidation depolymerization of lignin. In addition to acids and aldehydes, other high value-added chemicals have been obtained, such as maleic acid esters, aromatic acid esters, and aromatic amines. Furthermore, O2 and H2O2 are the most popular oxidants for lignin.
Catalytic oxidation systems of lignin and the types of products obtained after the oxidation process were introduced herein in detail, including summaries of which reaction systems produce a certain chemical and how to achieve the conversion of a certain chemical. This has further certain reference significance for emphasizing the value of lignin in the preparation of chemicals and expanding the application range of lignin.
Despite the many depolymerization strategies that have been employed for lignin valorization, not many species of functional chemicals have been obtained. Lignin is the only renewable aromatic chemical source in nature, so it is often used as a feedstock for derived chemicals. The selective oxidation processes in lignin valorization are still challenging because they often exhibit poor selectivity and generate several types of products. Isolating a pure product is difficult when obtaining several chemicals from lignin. It is suggested that selective oxidation strategies for lignin transformation be further improved to obtain pure fine chemicals. Lastly, new functional chemicals from lignin should be explored to exploit natural lignin to its greatest potential.
Author Contributions
J.L. and L.M. supervised and designed the research. X.-Z.W. wrote the original manuscript. J.L. reviewed and corrected the manuscript. All authors discussed the results and assisted during manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the National Key R&D Program of China (2018YFB1501500), National Natural Science Foundation of China (51976225).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Additional Information
Correspondence and requests for materials should be addressed to J.G.L. or L.L.M.
References
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular Self-Assembled Chaos: Polyphenolic Lignin’s Barrier to Cost-Effective Lignocellulosic Biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.A.; Hommes, G.; Schäffer, A.; Corvini, P.F.-X. Multi-catalysis reactions: New prospects and challenges of biotechnology to valorize lignin. Appl. Microbiol. Biotechnol. 2012, 95, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2018, 302, 50–60. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafei, N.; Nezafat, Z.; Bidgoli, N.S.S. Mol. Catal. 2020, 489, 110942.
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin–Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Chatel, G.; Rogers, R.D. Review: Oxidation of Lignin Using Ionic Liquids—An Innovative Strategy to Produce Renewable Chemicals. ACS Sustain. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- Burns, N.Z.; Baran, P.S.; Hoffmann, R.W. Redox Economy in Organic Synthesis. Angew. Chem. Int. Ed. 2009, 48, 2854–2867. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, Z.; Lu, Z.; Pei, H.; Wang, H. Activation of lignin by selective oxidation: An emerging strategy for boosting lignin depolymerization to aromatics. Bioresour. Technol. 2019, 291, 121885. [Google Scholar] [CrossRef]
- Wang, D.; Weinstein, A.B.; White, P.B.; Stahl, S.S. Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev. 2018, 118, 2636–2679. [Google Scholar] [CrossRef]
- Cai, Z.; Long, J.; Li, Y.; Ye, L.; Yin, B.; France, L.J.; Dong, J.; Zheng, L.; He, H.; Liu, S.; et al. Selective Production of Diethyl Maleate via Oxidative Cleavage of Lignin Aromatic Unit. Chem 2019, 5, 2365–2377. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef] [PubMed]
- Zell, T.; Milstein, D. Hydrogenation and Dehydrogenation Iron Pincer Catalysts Capable of Metal–Ligand Cooperation by Aromatization/Dearomatization. Acc. Chem. Res. 2015, 48, 1979–1994. [Google Scholar] [CrossRef]
- Obora, Y. Recent Advances in α-Alkylation Reactions using Alcohols with Hydrogen Borrowing Methodologies. ACS Catal. 2014, 4, 3972–3981. [Google Scholar] [CrossRef]
- Nandakumar, A.; Midya, S.P.; Landge, V.; Balaraman, E. Transition-Metal-Catalyzed Hydrogen-Transfer Annulations: Access to Heterocyclic Scaffolds. Angew. Chem. Int. Ed. 2015, 54, 11022–11034. [Google Scholar] [CrossRef]
- Mastalir, M.; Glatz, M.; Pittenauer, E.; Allmaier, G.; Kirchner, K. Rhenium-Catalyzed Dehydrogenative Coupling of Alcohols and Amines to Afford Nitrogen-Containing Aromatics and More. Org. Lett. 2019, 21, 1116–1120. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Suppo, J.-S.; Campagne, J.-M. Nonclassical Routes for Amide Bond Formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nat. Cell Biol. 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Roughley, S.D.; Jordan, A. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic Amino Acid Building Blocks for Nonribosomal Peptide and Hybrid Polyketide Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124. [Google Scholar] [CrossRef]
- Song, W.; Dong, K.; Li, M. Visible Light-Induced Amide Bond Formation. Org. Lett. 2019, 22, 371–375. [Google Scholar] [CrossRef]
- Li, H.; Bunrit, A.; Li, N.; Wang, F. Heteroatom-participated lignin cleavage to functionalized aromatics. Chem. Soc. Rev. 2020, 49, 3748–3763. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chiba, S.; Loh, T.-P. Chemical conversion of β-O-4 lignin linkage models through Cu-catalyzed aerobic amide bond formation. Chem. Commun. 2013, 49, 11439. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Wu, C.; Liu, Z.; Chen, Y.; Yua, B.; Liu, Z. Copper-catalyzed synthesis of benzanilides from lignin model substrates 2-phenoxyacetophenones under an air atmosphere. New J. Chem. 2018, 42, 1223. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Liu, H.; Luo, N.; Zhang, C.; Wang, F. Amine-Mediated Bond Cleavage in Oxidized Lignin Models. ChemSusChem 2020, 13, 4660–4665. [Google Scholar] [CrossRef] [PubMed]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, S.; D’hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Van Speybroeck, V.; De Kimpe, N.; Ha, H.-J. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643. [Google Scholar] [CrossRef]
- Florio, S.; Luisi, R. Aziridinyl Anions: Generation, Reactivity, and Use in Modern Synthetic Chemistry. Chem. Rev. 2010, 110, 5128–5157. [Google Scholar] [CrossRef]
- Zhou, L.; Hossain, M.L.; Xiao, T. Synthesis ofN-Containing Heterocyclic Compounds Using Visible-light Photoredox Catalysis. Chem. Rec. 2016, 16, 319–334. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef]
- Miller, J.S.; Manson, J.L. Designer Magnets Containing Cyanides and Nitriles. Accounts Chem. Res. 2001, 34, 563–570. [Google Scholar] [CrossRef]
- Nandi, S.; Chakraborty, D.; Vaidhyanathan, R. A permanently porous single molecule H-bonded organic framework for selective CO2 capture. Chem. Commun. 2016, 52, 7249–7252. [Google Scholar] [CrossRef] [PubMed]
- Wasley, J.W.F. Comprehensive Organic Transformations. A Guide to Functional Group Preparations By Richard A. Larock. Wiley-VCH Publishers, New York. J. Med. Chem. 2000, 43, 2488. [Google Scholar] [CrossRef]
- Khan, A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin. Investig. Drugs 2009, 18, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Johnson, H.G.; Sheridan, A.Q. The characterization of lodoxamide, a very active inhibitor of mediator release, in animal and human models of asthma. Inflamm. Res. 1986, 18, 301–305. [Google Scholar] [CrossRef]
- Pascual, E.; Sivera, F.; Yasothan, U.; Kirkpatrick, P. Febuxostat. Nat. Rev. Drug Discov. 2009, 8, 191–192. [Google Scholar] [CrossRef]
- Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Visible-Light-Induced Oxidation/[3+2] Cycloaddition/Oxidative Aromatization Sequence: A Photocatalytic Strategy To Construct Pyrrolo[2,1-a]isoquinolines. Angew. Chem. Int. Ed. 2011, 50, 7171–7175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Das, A.; Bui, L.; Zhou, H.; Curran, D.P.; Rueping, M. Oxygen Switch in Visible-Light Photoredox Catalysis: Radical Additions and Cyclizations and Unexpected C–C-Bond Cleavage Reactions. J. Am. Chem. Soc. 2013, 135, 1823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, X.; Thomoson, C.S.; Dolbier, W.R. Photoredox-Catalyzed Intramolecular Aminodifluoromethylation of Unactivated Alkenes. Org. Lett. 2015, 17, 3528–3531. [Google Scholar] [CrossRef]
- Hyodo, K.; Togashi, K.; Oishi, N.; Hasegawa, G.; Uchida, K. Brønsted Acid Catalyzed Nitrile Synthesis from Aldehydes Using Oximes via Transoximation at Ambient Temperature. Org. Lett. 2017, 19, 3005–3008. [Google Scholar] [CrossRef]
- Pradal, A.; Evano, G. A vinylic Rosenmund–von Braun reaction: Practical synthesis of acrylonitriles. Chem. Commun. 2014, 50, 11907–11910. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Shechter, H. Preparation of Nitriles from Halides and Sodium Cyanide. An Advantageous Nucleophilic Displacement in Dimethyl Sulfoxide1a. J. Org. Chem. 1960, 25, 877–879. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, A.; Chen, H.; Liu, Y. General and Mild Nickel-Catalyzed Cyanation of Aryl/Heteroaryl Chlorides with Zn(CN)2: Key Roles of DMAP. Org. Lett. 2017, 19, 2118–2121. [Google Scholar] [CrossRef]
- Sundermeier, M.; Zapf, A.; Mutyala, S.; Baumann, W.; Sans, J.; Weiss, S.; Beller, M. Progress in the Palladium-Catalyzed Cyanation of Aryl Chlorides. Chem.-A Eur. J. 2003, 9, 1828–1836. [Google Scholar] [CrossRef]
- Anbarasan, P.; Schareina, T.; Beller, M. Recent developments and perspectives in palladium-catalyzed cyanation of aryl halides: Synthesis of benzonitriles. Chem. Soc. Rev. 2011, 40, 5049–5067. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; He, J.; Zhang, Y. Transformation of lignin model compounds to N-substituted aromatics via Beckmann rearrangement. Green Chem. 2018, 20, 3318–3326. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Liu, H.; Luo, N.; Lu, J.; Zhang, C.; Wang, F. NH2OH–Mediated Lignin Conversion to Isoxazole and Nitrile. ACS Sustain. Chem. Eng. 2018, 6, 3748–3753. [Google Scholar] [CrossRef]
- Hofmann, L.E.; Hofmann, D.; Prusko, L.; Altmann, L.-M.; Heinrich, M.R. Sequential Cleavage of Lignin Systems by Nitrogen Monoxide and Hydrazine. Adv. Synth. Catal. 2020, 362, 1485–1489. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Li, T.; Wang, S.; Zhong, G. Copper-Catalyzed Oxidative Cyclization of 2-Amino-azaarenes with Lignin Models: Synthesis of 3-Phenoxy Imidazo Heterocycles. J. Org. Chem. 2017, 82, 5222–5229. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Zhong, G.; Chen, Y.; Wang, S.; Rong, C.; Liu, T.; Sun, H.; Wang, Y. A Metal-Free Synthesis of 3-Phenoxyimidazo Heterocycles by Catalytic Oxidative Cyclization of 2-Amino-azaarenes with Lignin Models. Synthesis 2018, 50, 3169–3176. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. Spectroelectrochemistry of aromatic amine oxidation: An insight into the indo dye formation. Res. Chem. Intermed. 2005, 31, 103–112. [Google Scholar] [CrossRef]
- Newhouse, T.; Baran, P.S.; Hoffmann, R.W. The economies of synthesis. Chem. Soc. Rev. 2009, 38, 3010–3021. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon−Heteroatom Bond-Forming Reductive Eliminations of Amines, Ethers, and Sulfides. Accounts Chem. Res. 1998, 31, 852–860. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon–heteroatom bond formation catalysed by organometallic complexes. Nat. Cell Biol. 2008, 455, 314–322. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Dialkylbiaryl phosphines in Pd-catalyzed amination: A user’s guide. Chem. Sci. 2011, 2, 27–50. [Google Scholar]
- Klinkenberg, J.L.; Hartwig, J.F. Catalytic Organometallic Reactions of Ammonia. Angew. Chem. Int. Ed. 2011, 50, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, Z.; Kwon, D.-H.; Coombs, J.; Jones, S.; Behnke, N.; Ess, D.H.; Kürti, L. Rapid heteroatom transfer to arylmetals utilizing multifunctional reagent scaffolds. Nat. Chem. 2016, 9, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, N.; Shimojo, K.; Hirano, K.; Komagawa, S.; Yoshida, K.; Wang, C.; Miyamoto, K.; Saito, T.; Takita, R.; Uchiyama, M. Direct Hydroxylation and Amination of Arenes via Deprotonative Cupration. J. Am. Chem. Soc. 2016, 138, 9166–9171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, Z.; Behnke, N.; Gao, H.; Kürti, L. Non-Deprotonative Primary and Secondary Amination of (Hetero)Arylmetals. J. Am. Chem. Soc. 2017, 139, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, T.; Shimizu, A.; Yoshida, J.-I. ChemInform Abstract: Electrochemical C-H Amination: Synthesis of Aromatic Primary Amines via N-Arylpyridinium Ions. Cheminform 2013, 44, 5000–5003. [Google Scholar] [CrossRef]
- Romero, N.A.; Margrey, K.A.; Tay, N.E.; Nicewicz, D.A. Site-selective arene C-H amination via photoredox catalysis. Science 2015, 349, 1326–1330. [Google Scholar] [CrossRef]
- Zheng, Y.-W.; Chen, B.; Ye, P.; Feng, K.; Wang, W.; Meng, Q.-Y.; Wu, L.-Z.; Tung, C.-H. Photocatalytic Hydrogen-Evolution Cross-Couplings: Benzene C–H Amination and Hydroxylation. J. Am. Chem. Soc. 2016, 138, 10080–10083. [Google Scholar] [CrossRef]
- Paudyal, M.P.; Adebesin, A.M.; Burt, S.R.; Ess, D.H.; Ma, Z.; Kürti, L.; Falck, J.R. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science 2016, 353, 1144–1147. [Google Scholar] [CrossRef]
- Klinger, G.E.; Zhou, Y.; Foote, J.A.; Wester, A.M.; Cui, Y.; Alherech, M.; Stahl, S.S.; Jackson, J.E.; Hegg, E.L. Nucleophilic Thiols Reductively Cleave Ether Linkages in Lignin Model Polymers and Lignin. ChemSusChem 2020, 13, 4394–4399. [Google Scholar] [CrossRef]
- Tay, N.E.S.; Nicewicz, D.A. Cation Radical Accelerated Nucleophilic Aromatic Substitution via Organic Photoredox Catalysis. J. Am. Chem. Soc. 2017, 139, 16100–16104. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Huang, X.; Luo, X.; Zhang, C.; Wei, J.; Pan, J.; Liang, Y.; Zhu, Y.; Qin, Q.; et al. From alkylarenes to anilines via site-directed carbon–carbon amination. Nat. Chem. 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Li, H.; Bunrit, A.; Lu, J.; Gao, Z.; Luo, N.; Liu, H.; Wang, F. Photocatalytic Cleavage of Aryl Ether in Modified Lignin to Non-phenolic Aromatics. ACS Catal. 2019, 9, 8843–8851. [Google Scholar] [CrossRef]
- Sumby, K.; Grbin, P.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Otera, J. Esterification: Methods, Reactions, and Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449. [Google Scholar] [CrossRef]
- Abiko, A.; Roberts, J.C.; Takemasa, T.; Masamune, S. ChemInform Abstract: KMnO4 Revisited: Oxidation of Aldehydes to Carboxylic Acids in the tert-Butyl Alcohol-Aqueous NaH2PO4 System. Tetrahedron Lett. 1986, 27, 4537. [Google Scholar] [CrossRef]
- Garegg, P.J.; Olsson, L.; Oscarson, S. Synthesis of Methyl (Ethyl 2-O-acyl-3,4-di-O-benzyl-1-thio-.beta.-D-glucopyranosid)uronates and Evaluation of Their Use as Reactive .beta.-Selective Glucuronic Acid Donors. J. Org. Chem. 1995, 60, 2200–2204. [Google Scholar] [CrossRef]
- Travis, B.R.; Sivakumar, M.; Hollist, A.G.O.; Borhan, B. Facile Oxidation of Aldehydes to Acids and Esters with Oxone. Org. Lett. 2003, 5, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, R.; Barkakaty, B.; Talukdar, B.; Patel, B.K. Peroxovanadium-Catalyzed Oxidative Esterification of Aldehydes†. J. Org. Chem. 2003, 68, 2944–2947. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Rajabi, J. An improved protocol for aerobic oxidation of acetals to esters catalyzed by N-hydroxy phthalimide (NHPI) and lipophilic Co(II) complexes. J. Mol. Catal. A Chem. 2005, 226, 165–169. [Google Scholar] [CrossRef]
- Gopinath, R.; Patel, B. A Catalytic Oxidative Esterification of Aldehydes Using V2O5−H2O2. Org. Lett. 2000, 2, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Darcel, C. Iron-Catalyzed One-Pot Oxidative Esterification of Aldehydes. Eur. J. Org. Chem. 2009, 2009, 1144–1147. [Google Scholar] [CrossRef]
- Malik, P.; Chakraborty, D. Bi2O3-catalyzed oxidation of aldehydes with t-BuOOH. Tetrahedron Lett. 2010, 51, 3521. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Meng, L.; Deng, Y.; Li, Y.; Lei, A. Palladium-Catalyzed Aerobic Oxidative Direct Esterification of Alcohols. Angew. Chem. 2011, 123, 5250–5254. [Google Scholar] [CrossRef]
- Gowrisankar, S.; Neumann, H.; Beller, M. General and Selective Palladium-Catalyzed Oxidative Esterification of Alcohols. Angew. Chem. 2011, 123, 5245–5249. [Google Scholar] [CrossRef]
- Oliveira, R.D.L.; Kiyohara, P.K.; Rossi, L. Clean preparation of methyl esters in one-step oxidative esterification of primary alcohols catalyzed by supported gold nanoparticles. Green Chem. 2009, 11, 1366–1370. [Google Scholar] [CrossRef]
- Zweifel, T.; Naubron, J.-V.; Grützmacher, H. Catalyzed Dehydrogenative Coupling of Primary Alcohols with Water, Methanol, or Amines. Angew. Chem. 2009, 121, 567–571. [Google Scholar] [CrossRef]
- Yamamoto, N.; Obora, Y.; Ishii, Y. Iridium-Catalyzed Oxidative Methyl Esterification of Primary Alcohols and Diols with Methanol. J. Org. Chem. 2011, 76, 2937–2941. [Google Scholar] [CrossRef]
- Arita, S.; Koike, T.; Kayaki, Y.; Ikariya, T. Aerobic Oxidative Kinetic Resolution of Racemic Secondary Alcohols with Chiral Bifunctional Amido Complexes. Angew. Chem. 2008, 120, 2481–2483. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Shang, S.; Li, G.; Lv, Y.; Gao, S.; Dai, W. Cobalt Nanoparticles-Catalyzed Widely Applicable Successive C−C Bond Cleavage in Alcohols to Access Esters. Angew. Chem. Int. Ed. 2020, 59, 19268–19274. [Google Scholar] [CrossRef]
- Zhu, G.; Shi, S.; Zhao, L.; Liu, M.; Gao, J.; Xu, J. Catalytic Activation of Carbon–Hydrogen Bonds in Lignin Linkages over Strong-Base-Modified Covalent Triazine Frameworks for Lignin Oxidative Cleavage. ACS Catal. 2020, 10, 7526–7534. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Yan, J.; Liu, S.; Liu, H.; Liu, Z.; Wang, W.; He, Z.; Han, B. Aerobic Oxidative Cleavage and Esterification of C(OH)–C Bonds. Chem 2020, 6, 3288–3296. [Google Scholar] [CrossRef]
- Liu, S.; Bai, L.; van Muyden, A.P.; Huang, Z.; Cui, X.; Fei, Z.; Li, X.; Hu, X.; Dyson, P.J. Oxidative cleavage of β-O-4 bonds in lignin model compounds with a single-atom Co catalyst. Green Chem. 2019, 21, 1974–1981. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Li, G.; Shang, S.; Lv, Y.; Niu, J.; Gao, S. Nitrogen-Doped Carbon-Modified Cobalt-Nanoparticle-Catalyzed Oxidative Cleavage of Lignin β-O-4 Model Compounds under Mild Conditions. ACS Sustain. Chem. Eng. 2018, 6, 14188–14196. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, S.; Liu, M.; Zhu, G.; Wang, M.; Du, W.; Gao, J.; Xu, J. Covalent triazine framework catalytic oxidative cleavage of lignin models and organosolv lignin. Green Chem. 2018, 20, 1270–1279. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.H.; Lu, J.M.; Li, H.J.; Zhang, X.C.; Liu, H.F.; Luo, N.C.; Wang, F. Acid promoted C–C bond oxidative cleavage of β-O-4 and β-1 lignin models to esters over a copper catalyst. Green Chem. 2017, 19, 702. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; He, J.; Zhang, Y. Highly effective C–C bond cleavage of lignin model compounds. Green Chem. 2017, 19, 3135–3141. [Google Scholar] [CrossRef]
- Yao, S.G.; Mobley, J.K.; Ralph, J.; Crocker, M.; Parkin, S.; Selegue, J.P.; Meier, M.S. Mechanochemical Treatment Facilitates Two-Step Oxidative Depolymerization of Kraft Lignin. ACS Sustain. Chem. Eng. 2018, 6, 5990–5998. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, J.; Liu, C.; Lei, A. Direct oxidative esterification of alcohols. Dalton Trans. 2014, 43, 13460–13470. [Google Scholar] [CrossRef]
- Ekoue-Kovi, K.; Wolf, C. One-Pot Oxidative Esterification and Amidation of Aldehydes. Chem.-Eur. J. 2008, 14, 6302–6315. [Google Scholar] [CrossRef]
- Li, L.; Kong, J.; Zhang, H.; Liu, S.; Zeng, Q.; Zhang, Y.; Ma, H.; He, H.; Long, J.; Li, X. Selective aerobic oxidative cleavage of lignin Csingle bondC bonds over novel hierarchical Ce-Cu/MFI nanosheets. Appl. Catal. B Environ. 2020, 279, 119343. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Trouillas, P.; Martin, N.; Dizhbite, T.; Krasilnikova, J.; Telysheva, G. Elucidation of antioxidant properties of wood bark derived saturated diarylheptanoids: A comprehensive (DFT-supported) understanding. Phytochemistry 2014, 103, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Monks, T.J.; Jones, D.C. The Metabolism and Toxicity of Quinones, Quinonimines, Quinone Methides, and Quinone-Thioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Alam, S.; Nziko, V.D.P.N.; Johnston, R.C.; Ivanov, A.S.; Mou, Z.; Turpin, D.B.; Rudie, A.W.; Elder, T.J.; Bozell, J.J.; et al. Co(salen)-Catalyzed Oxidation of Lignin Models to Form Benzoquinones and Benzaldehydes: A Computational and Experimental Study. ACS Sustain. Chem. Eng. 2020, 8, 7225–7234. [Google Scholar] [CrossRef]
- Hanson, S.K.; Wu, R.; Silks, L.A.P. C-C or C-O Bond Cleavage in a Phenolic Lignin Model Compound: Selectivity Depends on Vanadium Catalyst. Angew. Chem. Int. Ed. 2012, 51, 3410–3413. [Google Scholar] [CrossRef]
- Dabral, S.; Wotruba, H.; Hernández, J.G.; Bolm, C. Mechanochemical Oxidation and Cleavage of Lignin β-O-4 Model Compounds and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 3242–3254. [Google Scholar] [CrossRef]
- Goossen, L.J.; Gooßen, L.J.; Deng, G.; Levy, L.M. Synthesis of Biaryls via Catalytic Decarboxylative Coupling. Science 2006, 313, 662–664. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Gooßen, K.; Rodriguez, N.; Blanchot, M.; Linder, C.; Zimmermann, B. New catalytic transformations of carboxylic acids. Pure Appl. Chem. 2008, 80, 1725–1733. [Google Scholar] [CrossRef]
- Nagayama, K.; Shimizu, I.; Yamamoto, A. Direct Hydrogenation of Carboxylic Acids to Corresponding Aldehydes Catalyzed by Palladium Complexes. Bull. Chem. Soc. Jpn. 2001, 74, 1803–1815. [Google Scholar] [CrossRef]
- Cronin, D.J.; Zhang, X.; Bartley, J.; Doherty, W.O.S. Lignin Depolymerization to Dicarboxylic Acids with Sodium Percarbonate. ACS Sustain. Chem. Eng. 2017, 5, 6253–6260. [Google Scholar] [CrossRef]
- Mottweiler, J.; Puche, M.; Räuber, C.; Schmidt, T.; Concepción, P.; Corma, A.; Bolm, C. Copper- and Vanadium-Catalyzed Oxidative Cleavage of Lignin using Dioxygen. ChemSusChem 2015, 8, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Yang, J.W. Transition-metal-free conversion of lignin model compounds to high-value aromatics: Scope and chemoselectivity. Green Chem. 2018, 20, 3761–3771. [Google Scholar] [CrossRef]
- Coupé, F.; Petitjean, L.; Anastas, P.T.; Caijo, F.; Escande, V.; Darcel, C. Sustainable oxidative cleavage of catechols for the synthesis of muconic acid and muconolactones including lignin upgrading. Green Chem. 2020, 22, 6204–6211. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Barreiro, M.F.; Rodrigues, A.E. Effect of Methoxy Substituents on Wet Peroxide Oxidation of Lignin and Lignin Model Compounds: Understanding the Pathway to C4 Dicarboxylic Acids. Ind. Eng. Chem. Res. 2021, 60, 3543–3553. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorganic Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef]
- Magallanes, G.; Kärkäs, M.D.; Bosque, I.; Lee, S.; Maldonado, S.; Stephenson, C.R.J. Selective C–O Bond Cleavage of Lignin Systems and Polymers Enabled by Sequential Palladium-Catalyzed Aerobic Oxidation and Visible-Light Photoredox Catalysis. ACS Catal. 2019, 9, 2252–2260. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, L.; Xu, W.; Dushkin, A.V.; Su, W. Mechanochemical cleavage of lignin models and lignin via oxidation and a subsequent base-catalyzed strategy. Green Chem. 2020, 22, 3489–3494. [Google Scholar] [CrossRef]
- Bosque, I.; Magallanes, G.; Rigoulet, M.; Kärkäs, M.D.; Stephenson, C.R.J. Redox Catalysis Facilitates Lignin Depolymerization. ACS Central Sci. 2017, 3, 621–628. [Google Scholar] [CrossRef]
- Kang, J.; Irmak, S.; Wilkins, M. Conversion of lignin into renewable carboxylic acid compounds by advanced oxidation processes. Renew. Energy 2019, 135, 951–962. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Teunissen, L.W.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Iridium-catalysed primary alcohol oxidation and hydrogen shuttling for the depolymerisation of lignin. Green Chem. 2018, 20, 3214–3221. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Lu, J.; Zeng, S.; Wang, M.; Luo, N.; Xu, S.; Wang, F. Photocatalytic Cleavage of C–C Bond in Lignin Models under Visible Light on Mesoporous Graphitic Carbon Nitride through π–π Stacking Interaction. ACS Catal. 2018, 8, 4761–4771. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; De Gregorio, G.F.; Labidi, J.; Welton, T. Willow Lignin Oxidation and Depolymerization under Low Cost Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 5277–5288. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y. Metal Catalyst-Free Oxidative C−C Bond Cleavage of a Lignin Model Compound by H2O2 in Formic acid. ChemSusChem 2020, 13, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, L.; Wallraf, A.-M.; Räuber, C.; Grande, P.M.; Anders, N.; Gertler, C.; Werner, B.; Klankermayer, J.; Leitner, W.; et al. Depolymerization of Laccase-Oxidized Lignin in Aqueous Alkaline Solution at 37 °C. ACS Sustain. Chem. Eng. 2019, 7, 11150–11156. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective Metal-Free Aerobic Alcohol Oxidation in Lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.; Thorn, D.L. Aerobic Oxidation of Lignin Models Using a Base Metal Vanadium Catalyst. Inorg. Chem. 2010, 49, 5611–5618. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.W.; Bauer, S.; Sorek, H.; Sreekumar, S.; Wang, K.; Toste, F.D. Studies on the Vanadium-Catalyzed Nonoxidative Depolymerization of Miscanthus giganteus-Derived Lignin. ACS Catal. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Yan, L.; Yu, H.-Z.; Zhang, Q.; Fu, Y. Mechanism of Vanadium-Catalyzed Selective C–O and C–C Cleavage of Lignin Model Compound. ACS Catal. 2016, 6, 4399–4410. [Google Scholar] [CrossRef]
- Zhang, J. Conversion of Lignin Models by Photoredox Catalysis. ChemSusChem 2018, 11, 3071–3080. [Google Scholar] [CrossRef]
- Amadio, E.; Di Lorenzo, R.; Zonta, C.; Licini, G. Vanadium catalyzed aerobic carbon–carbon cleavage. Coord. Chem. Rev. 2015, 301, 147–162. [Google Scholar] [CrossRef]
- Klinger, G.E.; Zhou, Y.; Hao, P.; Robbins, J.; Aquilina, J.M.; Jackson, J.E.; Hegg, E.L. Biomimetic Reductive Cleavage of Keto Aryl Ether Bonds by Small-Molecule Thiols. ChemSusChem 2019, 12, 4775–4779. [Google Scholar] [CrossRef]
- Wang, M.; Lu, J.; Zhang, X.; Li, L.; Li, H.; Luo, N.; Wang, F. Two-Step, Catalytic C–C Bond Oxidative Cleavage Process Converts Lignin Models and Extracts to Aromatic Acids. ACS Catal. 2016, 6, 6086–6090. [Google Scholar] [CrossRef]
- Luo, N.; Wang, M.; Li, H.; Zhang, J.; Liu, H.; Wang, F. Photocatalytic Oxidation–Hydrogenolysis of Lignin β-O-4 Models via a Dual Light Wavelength Switching Strategy. ACS Catal. 2016, 6, 7716–7721. [Google Scholar] [CrossRef]
- Ren, X.; Wang, P.; Han, X.; Zhang, G.; Gu, J.; Ding, C.; Zheng, X.; Cao, F. Depolymerization of Lignin to Aromatics by Selectively Oxidizing Cleavage of C–C and C–O Bonds Using CuCl2/Polybenzoxazine Catalysts at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 6548–6556. [Google Scholar] [CrossRef]
- Walch, F.; Abdelaziz, O.Y.; Meier, S.; Bjelić, S.; Hulteberg, C.P.; Riisager, A. Oxidative depolymerization of Kraft lignin to high-value aromatics using a homogeneous vanadium–copper catalyst. Catal. Sci. Technol. 2021, 11, 1843–1853. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; He, X.; Li, H.-R.; He, L.-N. Photocatalytic Oxidation and Subsequent Hydrogenolysis of Lignin β-O-4 Models to Aromatics Promoted by In Situ Carbonic Acid. ACS Sustain. Chem. Eng. 2018, 6, 15032–15039. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Bosque, I.; Matsuura, B.S.; Stephenson, C. Photocatalytic Oxidation of Lignin Model Systems by Merging Visible-Light Photoredox and Palladium Catalysis. Org. Lett. 2016, 18, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Bourbiaux, D.; Pu, J.; Rataboul, F.; Djakovitch, L.; Geantet, C.; Laurenti, D. Reductive or oxidative catalytic lignin depolymerization: An overview of recent advances. Catal. Today 2021, 373, 24–37. [Google Scholar] [CrossRef]
- Das, A.; König, B. Transition metal- and photoredox-catalyzed valorisation of lignin subunits. Green Chem. 2018, 20, 4844–4852. [Google Scholar] [CrossRef]
- Aguilar, C.A.V.; Barreiro, M.F.; Rodrigues, A.E. Catalytic wet peroxide oxidation of vanillic acid as a lignin model compound towards the renewable production of dicarboxylic acids. Chem. Eng. Res. Des. 2020, 159, 115–124. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, S.; Ekanayake, U.M.; Fang, Z.; Cullen, P.J.; Bazaka, K.; Ostrikov, K. Power-to-chemicals: Low-temperature plasma for lignin depolymerisation in ethanol. Bioresour. Technol. 2020, 318, 123917. [Google Scholar] [CrossRef]
- Lotfi, S.; Bahrpaima, K.; Boffito, D.C.; Patience, G.S. Gas–Solid Oxidation of Unwashed Lignin to Carboxylic Acids. Energy Fuels 2020, 34, 9683–9696. [Google Scholar] [CrossRef]
- Lotfi, S.; Boffito, D.C.; Patience, G.S. Gas-Phase Partial Oxidation of Lignin to Carboxylic Acids over Vanadium Pyrophosphate and Aluminum-Vanadium-Molybdenum. ChemSusChem 2015, 8, 3424–3432. [Google Scholar] [CrossRef]
- Yu, Q.; Song, Z.; Chen, X.; Fan, J.; Clark, J.H.; Wang, Z.; Sun, Y.; Yuan, Z. A methanol–choline chloride based deep eutectic solvent enhances the catalytic oxidation of lignin into acetovanillone and acetic acid. Green Chem. 2020, 22, 6415. [Google Scholar] [CrossRef]
- Suzuki, H.; Cao, J.; Jin, F.; Kishita, A.; Enomoto, H.; Moriya, T. Wet oxidation of lignin model compounds and acetic acid production. J. Mater. Sci. 2006, 41, 1591–1597. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, S.; Sankar, R.V.; Gunanathan, C. Ruthenium-Catalyzed α-Alkylation of Ketones Using Secondary Alcohols to β-Disubstituted Ketones. Org. Lett. 2020, 22, 7879–7884. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.-J.; Chauvin, R. Synthesis of diarylketones through carbonylative coupling. Chem. Soc. Rev. 1995, 24, 89–95. [Google Scholar] [CrossRef]
- McClelland, K.P.; Weiss, E.A. Selective Photocatalytic Oxidation of Benzyl Alcohol to Benzaldehyde or C–C Coupled Products by Visible-Light-Absorbing Quantum Dots. ACS Appl. Energy Mater. 2018, 2, 92–96. [Google Scholar] [CrossRef]
- Lima, M.J.; Silva, A.M.; Silva, C.G.; Faria, J.L. A microfluidic reactor application for the continuous-flow photocatalytic selective synthesis of aromatic aldehydes. Appl. Catal. A Gen. 2020, 608, 117844. [Google Scholar] [CrossRef]
- Mancuso, A.J.; Huang, S.-L.; Swern, D. Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide “activated” by oxalyl chloride. J. Org. Chem. 1978, 43, 2480–2482. [Google Scholar] [CrossRef]
- Omura, K.; Swern, D. Oxidation of alcohols by “activated” dimethyl sulfoxide. a preparative, steric and mechanistic study. Tetrahedron 1978, 34, 1651–1660. [Google Scholar] [CrossRef]
- Mancuso, A.J.; Swern, D. Activated Dimethyl Sulfoxide: Useful Reagents for Synthesis. Synthesis 1981, 165–183. [Google Scholar] [CrossRef]
- Tidwell, T.T. Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update. Synthesis 1990, 857–870. [Google Scholar] [CrossRef]
- Nishide, K.; Patra, P.K.; Matoba, M.; Shanmugasundaram, K.; Node, M. A practical improvement of odorless Corey–Kim and Swern oxidations. Green Chem. 2004, 6, 142–146. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Miyata, H.; Ataka, K.; Mae, K.; Yoshida, J.-I. Room-Temperature Swern Oxidations by Using a Microscale Flow System. Angew. Chem. 2005, 117, 2465–2468. [Google Scholar] [CrossRef]
- Pichlmair, S.; Marques, M.; Green, M.P.; Martin, H.J.; Mulzer, J. A Novel Approach toward the Synthesis of Kendomycin: Selective Synthesis of aC-Aryl Glycoside as a Single Atropisomer. Org. Lett. 2003, 5, 4657–4659. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- de Lera, A.R.; Okamura, W.H. The stereospecific formation of an exocyclic alkene by a consecutive radical cyclization-elimination. Tetrahedron Lett. 1987, 28, 2941. [Google Scholar]
- Gao, Y.; Liu, J.; Wang, L.; Xiao, M.; Du, Y. Total Syntheses of Cochliomycin B and Zeaenol. Eur. J. Org. Chem. 2014, 10, 2092–2098. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Kim, Y.; Lee, J.-H. Chemicals from lignin: Recent depolymerization techniques and upgrading extended pathways. Curr. Opin. Green Sustain. Chem. 2018, 14, 33–39. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Prado, R.; Vriamont, C.; Erdocia, X.; Labidi, J.; Hallett, J.P.; Welton, T. Oxidative Depolymerization of Lignin Using a Novel Polyoxometalate-Protic Ionic Liquid System. ACS Sustain. Chem. Eng. 2016, 4, 6031–6036. [Google Scholar] [CrossRef]
- Panyadee, R.; Posoknistakul, P.; Jonglertjunya, W.; Kim-Lohsoontorn, P.; Laosiripojana, N.; Matsagar, B.M.; Wu, K.C.-W.; Sakdaronnarong, C. Sequential Fractionation of Palm Empty Fruit Bunch and Microwave-Assisted Depolymerization of Lignin for Producing Monophenolic Compounds. ACS Sustain. Chem. Eng. 2018, 6, 16896–16906. [Google Scholar] [CrossRef]
- Ouyang, X.; Ruan, T.; Qiu, X. Effect of solvent on hydrothermal oxidation depolymerization of lignin for the production of monophenolic compounds. Fuel Process. Technol. 2016, 144, 181–185. [Google Scholar] [CrossRef]
- Dai, J.; Styles, G.N.; Patti, A.F.; Saito, K. CuSO4/H2O2-Catalyzed Lignin Depolymerization under the Irradiation of Microwaves. ACS Omega 2018, 3, 10433–10441. [Google Scholar] [CrossRef]
- Kumara, A.; Biswasa, B.; Bhaskar, T. Effect of cobalt on titania, ceria and zirconia oxide supported catalysts on the oxidative depolymerization of prot and alkali lignin. Bioresour. Technol. 2020, 299, 122589. [Google Scholar] [CrossRef]
- Hafezisefat, P.; Lindstrom, J.K.; Brown, R.C.; Qi, L. Non-catalytic oxidative depolymerization of lignin in perfluorodecalin to produce phenolic monomers. Green Chem. 2020, 22, 6567–6578. [Google Scholar] [CrossRef]
- Voitl, T.; von Rohr, P.R. Oxidation of Lignin Using Aqueous Polyoxometalates in the Presence of Alcohols. ChemSusChem 2008, 1, 763–769. [Google Scholar] [CrossRef]
- Rawat, S.; Gupta, P.; Singh, B.; Bhaskar, T.; Natte, K.; Narani, A. Molybdenum-catalyzed oxidative depolymerization of alkali lignin: Selective production of Vanillin. Appl. Catal. A Gen. 2020, 598, 117567. [Google Scholar] [CrossRef]
- Hosoya, T.; Yamamoto, K.; Miyafuji, H.; Yamada, T. Selective production of bio-based aromatics by aerobic oxidation of native soft wood lignin in tetrabutylammonium hydroxide. RSC Adv. 2020, 10, 19199–19210. [Google Scholar] [CrossRef]
- Almada, C.C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Oxidative depolymerization of lignins for producing aromatics: Variation of botanical origin and extraction methods. Biomass-Convers. Biorefinery 2020, 1–14. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhu, J.-P.; Zhang, Z.-J.; Qu, Y.-S. Preparation of Syringaldehyde from Lignin by Catalytic Oxidation of Perovskite-Type Oxides. ACS Omega 2020, 5, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Motagamwala, A.H.; Karlen, S.D.; Dumesic, J.A.; Ralph, J.; Mobley, J.K.; Crocker, M. A comparative study of secondary depolymerization methods on oxidized lignins. Green Chem. 2019, 21, 3940–3947. [Google Scholar] [CrossRef]
- Lan, W.; de Bueren, J.B.; Luterbacher, J.S. Highly Selective Oxidation and Depolymerization of α,γ-Diol-Protected Lignin. Angew. Chem. Int. Ed. 2019, 58, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rahimi, A.; Ulbrich, A.; Alherech, M.; Motagamwala, A.H.; Bhalla, A.; Sousa, L.D.C.; Balan, V.; Dumesic, J.A.; Hegg, E.L.; et al. Lignin Conversion to Low-Molecular-Weight Aromatics via an Aerobic Oxidation-Hydrolysis Sequence: Comparison of Different Lignin Sources. ACS Sustain. Chem. Eng. 2018, 6, 3367–3374. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, B.; Saini, K.; Kumar, A.; Kumar, J.; Krishna, B.B.; Bhaskar, T. Copper and manganese bimetallic catalysts for oxidation of prot lignin: Effects of metal oxide on product yield. Biomass-Convers. Biorefinery 2021, 1–14. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Lu, J.; Zhang, X.; MacArthur, K.; Heggen, M.; Wang, F. Promoting Lignin Depolymerization and Restraining the Condensation via an Oxidation−Hydrogenation Strategy. ACS Catal. 2017, 7, 3419–3429. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C. A Photochemical Strategy for Lignin Degradation at Room Temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).