Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. sPSB Sulfonation and Characterization
2.3. Free Fatty Acid (FFA) Esterification
2.3.1. Catalyst Liquid Take-Up
2.3.2. Analytical Methods
- N.A.: acidity number (mg KOH/g of the sample)
- V: volume of solution used in the titration (mL)
- V0: volume of solution used in the titration of blank (ethanol) (mL)
- C: titrant solution concentration (mol/L)
- MW: titrant molecular weight (g/mol)
- W: sample mass (g)
- NA0: initial mixture acidity number
- NAt: mixture acidity number at time t.
2.3.3. Viscosity Test
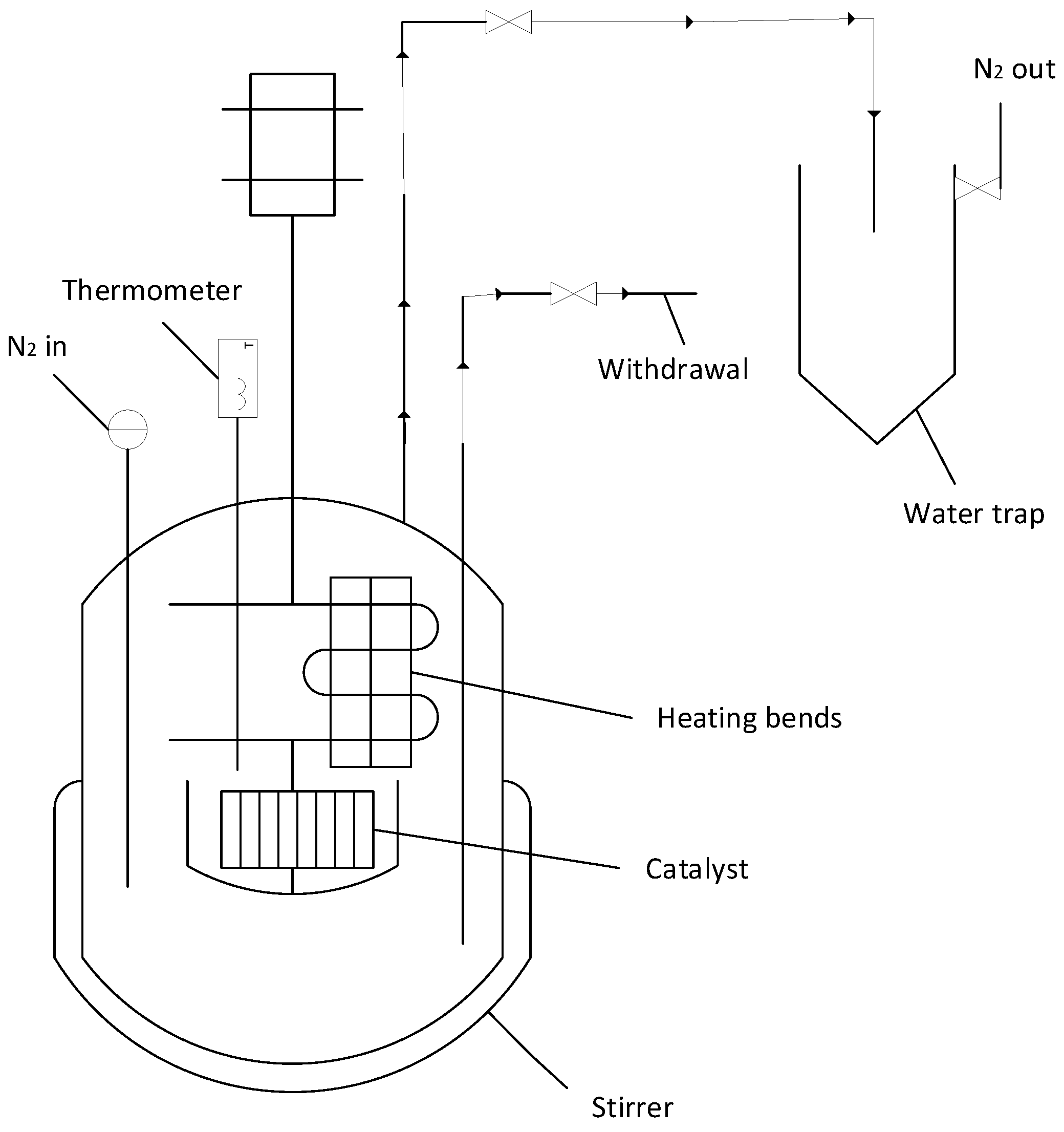
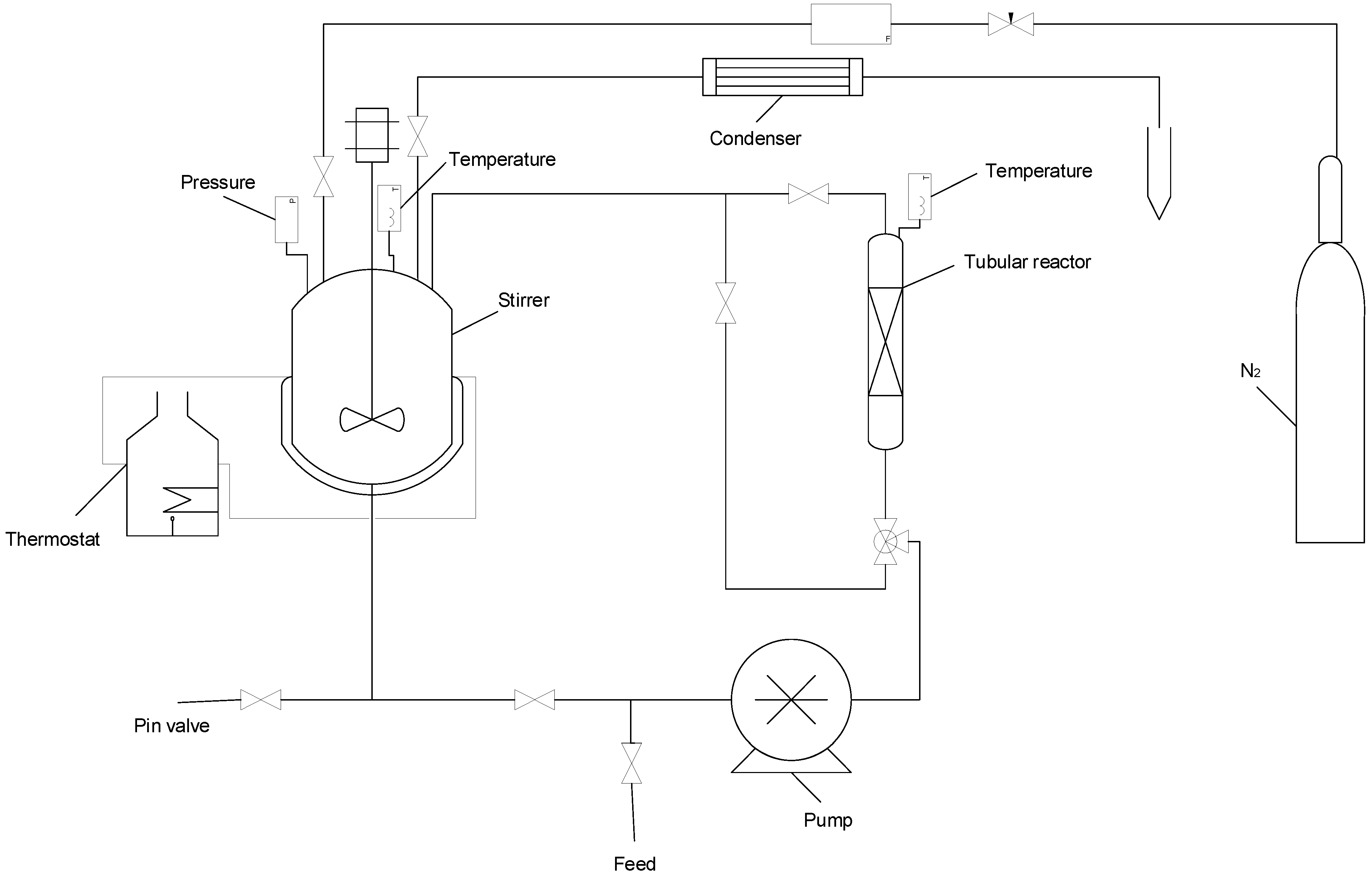
2.3.4. Experimental Tests
3. Results
3.1. Catalyst Take-Up Results
3.2. Blank Tests
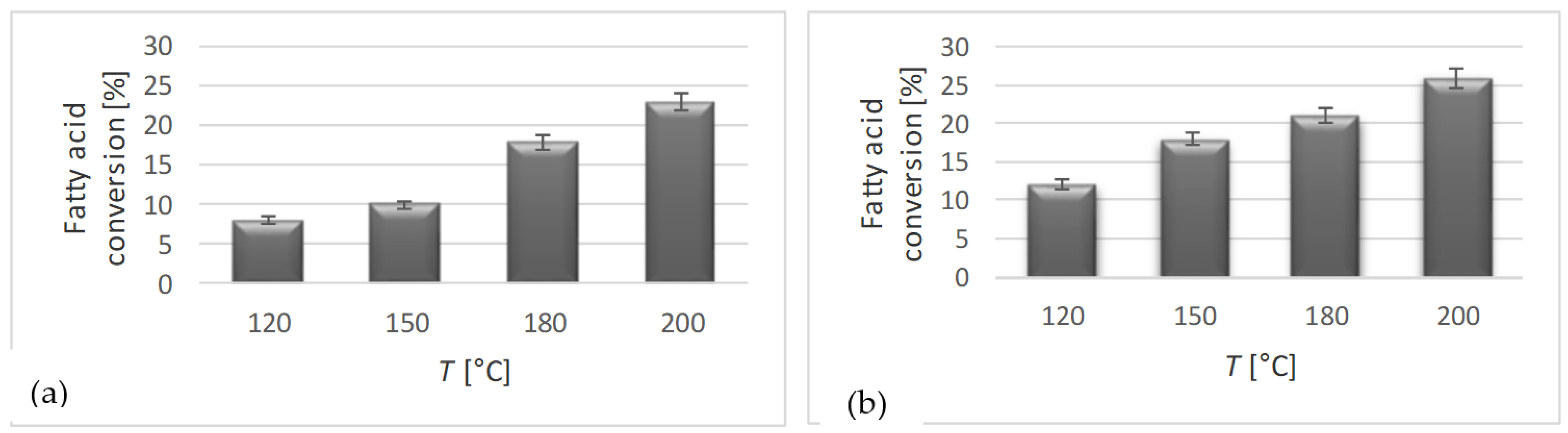
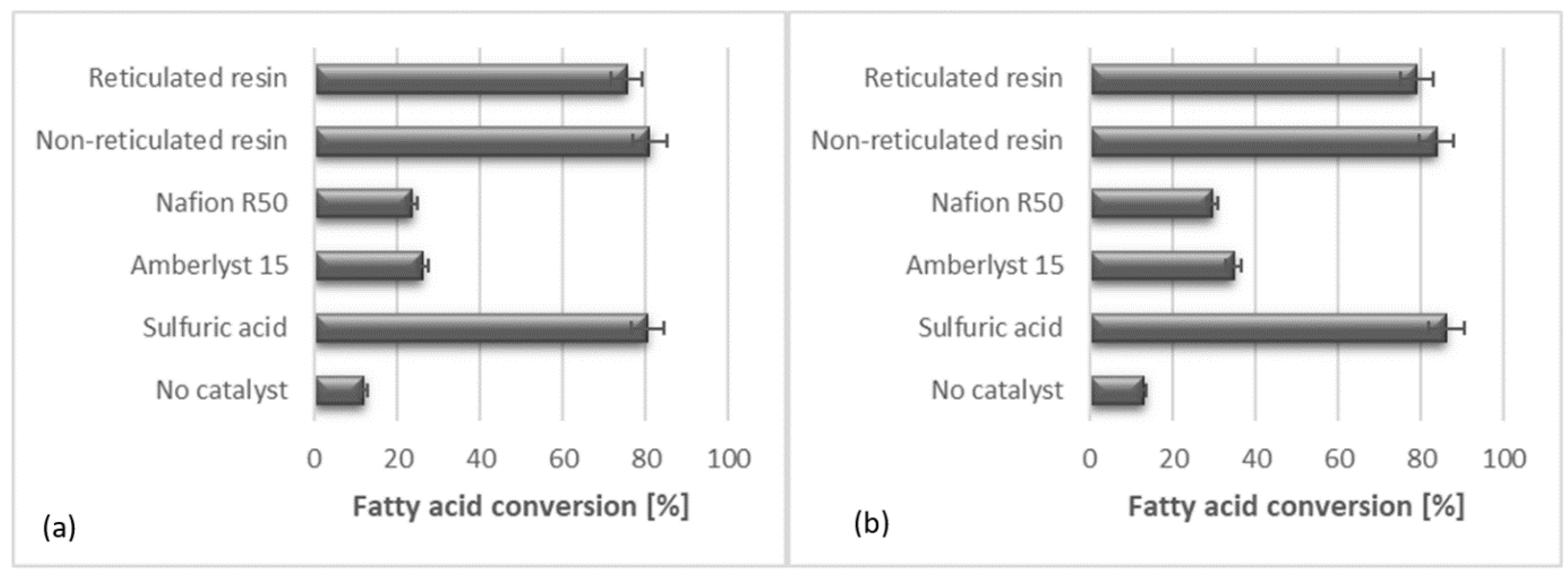
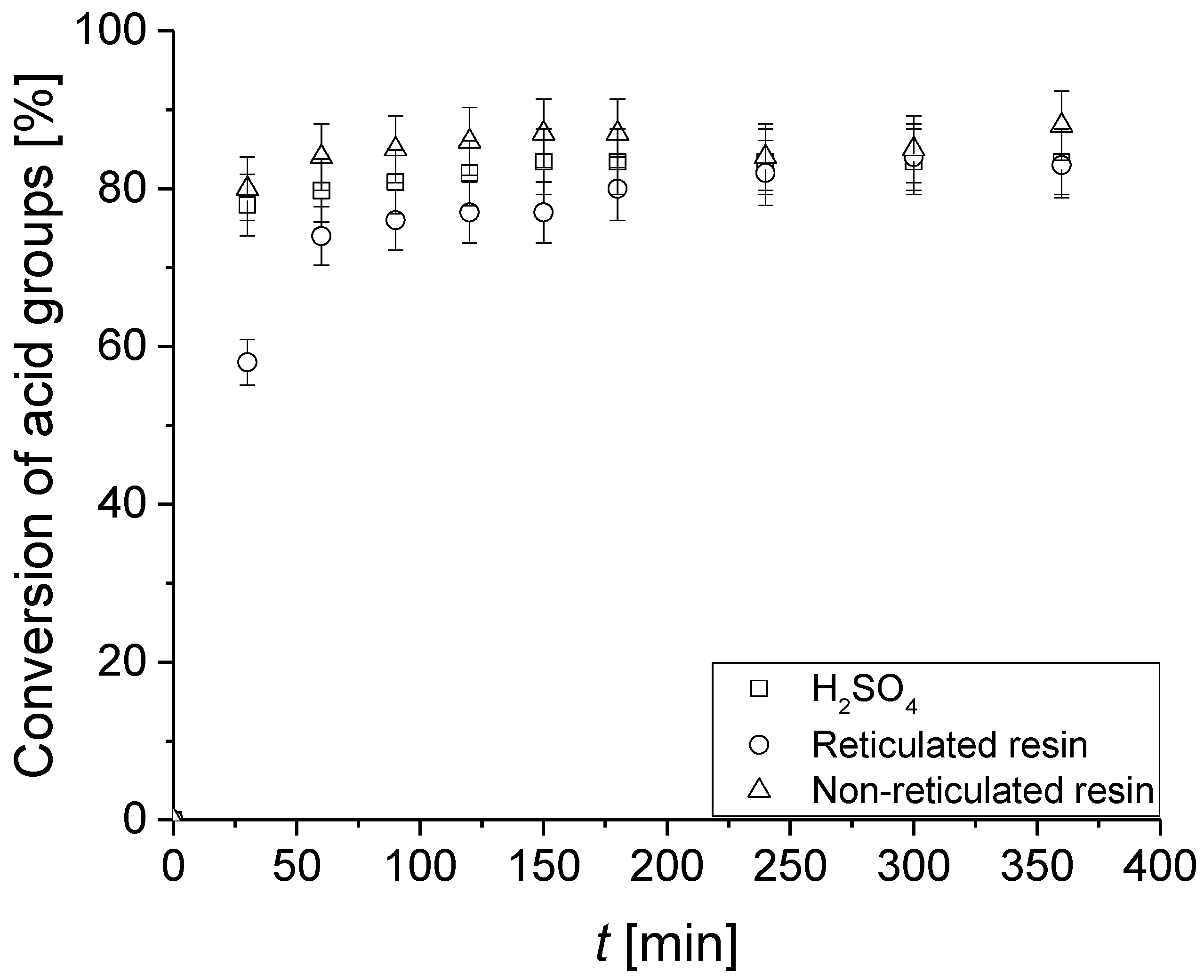
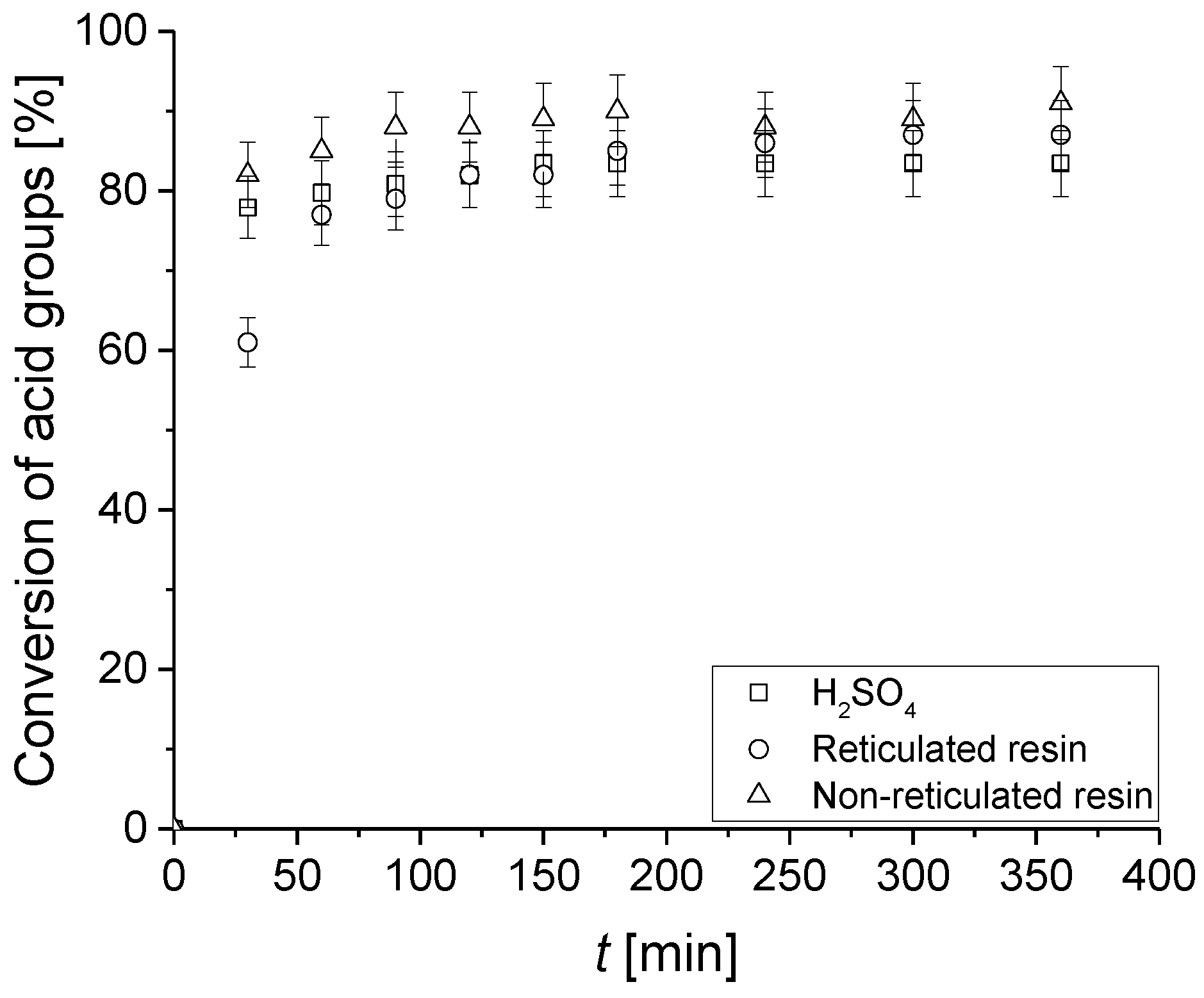
3.3. Catalytic Tests
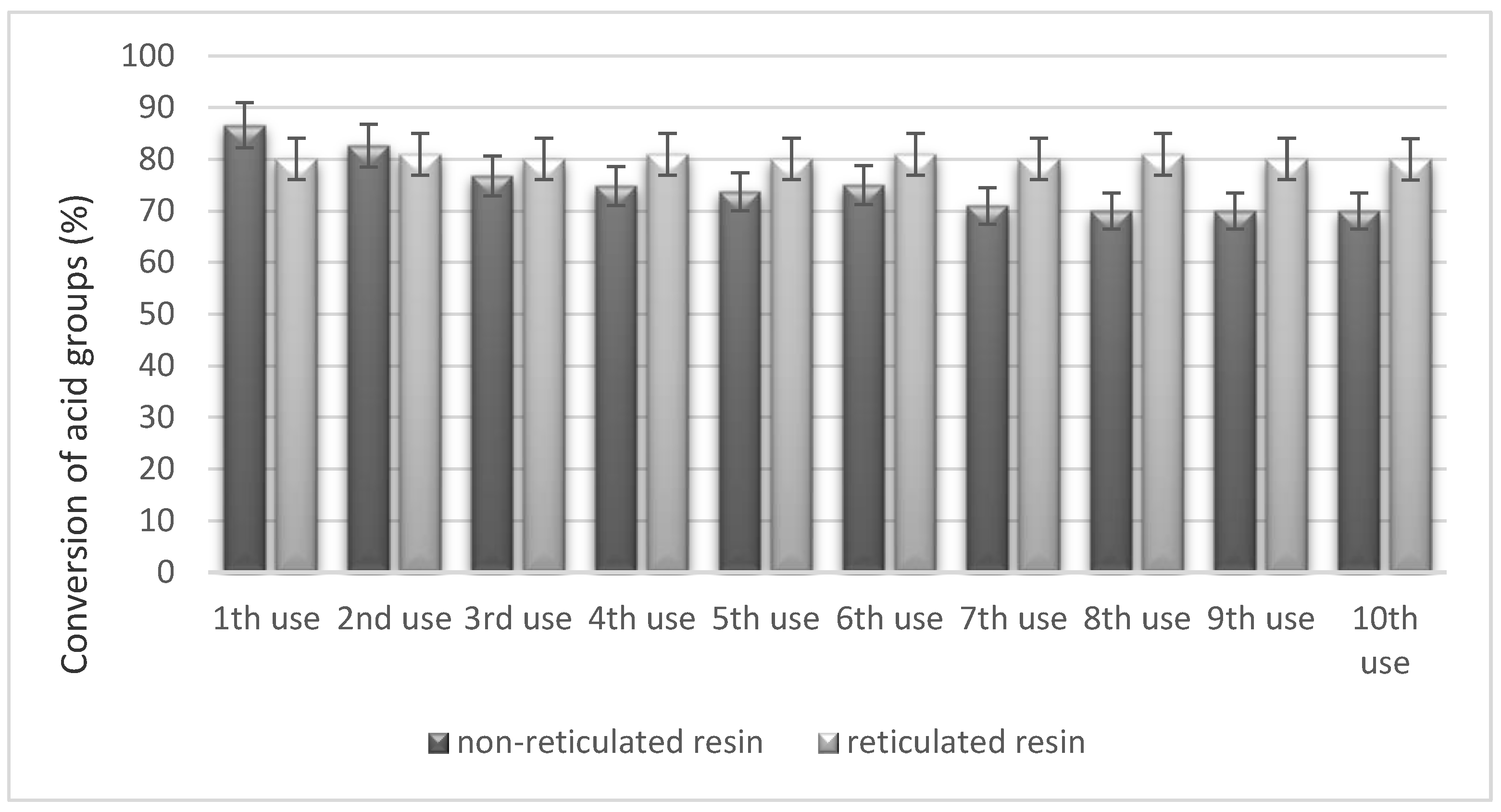
3.4. Reusability Test of the Catalysts in the PBR Loop Reactor
3.5. Viscosity Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in auto motive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Biolubricant: Science and Technology; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- McNutt, J.; He, Q. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil—Biodegradable and potential base stoke for industrial lubricants. Ind. Crop. Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Dalbey, W.E.; Biles, R.W. Respiratory Toxicology of Mineral Oils in Laboratory Animals. Appl. Occup. Environ. Hyg. 2003, 18, 921–929. [Google Scholar] [CrossRef]
- Urbanus, J.H.; Lobo, R.C.; Riley, A.J. European Hazard Classification Advice for Crude Oil—Derived Lubricant Base Oils Compared with the Proposed Mineral Oil Mist TLV®. Appl. Occup. Environ. Hyg. 2003, 18, 815–817. [Google Scholar] [CrossRef]
- Dalbey, W.E.; McKee, R.H.; Goyak, K.O.; Biles, R.W.; Murray, J.; White, R. Acute, Subchronic, and Developmental Toxicological Properties of Lubricating Oil Base Stocks. Int. J. Toxicol. 2014, 33, 110–135. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Luther, R.; Mang, T. Lubricant base fluids based on renewable raw materials Their catalytic manufacture and modification. Appl. Catal. A Gen. 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Yazan, D.M.; Mandras, G.; Garau, G. Environmental and economic sustainability of integrated production in bio-refineries: The thistle case in Sardinia. Renew. Energy 2017, 102, 349–360. [Google Scholar] [CrossRef]
- Turco, R.; Pischetola, C.; Di Serio, M.; Vitiello, R.; Tesser, R.; Santacesaria, E. Selective Epoxidation of Soybean Oil in the Presence of H-Y Zeolite. Ind. Eng. Chem. Res. 2017, 56, 7930–7936. [Google Scholar] [CrossRef]
- Turco, R.; Tesser, R.; Vitiello, R.; Russo, V.; Andini, S.; Di Serio, M. Synthesis of biolubricant basestocks from epoxidized soybean oil. Catalysts 2017, 7, 309. [Google Scholar] [CrossRef]
- Vitiello, R.; Tesser, R.; Russo, V.; Turco, R.; Andini, S.; Di Serio, M. Loop reactor modeling for lubricants synthesis. Chem. Eng. J. 2017, 329, 295–304. [Google Scholar] [CrossRef]
- Vitiello, R.; Li, C.; Russo, V.; Tesser, R.; Turco, R.; Di Serio, M. Catalysis for esterification reactions: A key step in the biodiesel production from waste oils. Rend. Fis. Acc. Lincei 2017, 28, 117–123. [Google Scholar] [CrossRef]
- Maquirriain, M.A.; Querini, C.A.; Pisarello, M.L. Glycerine esterification with free fatty acids: Homogeneous catalysis. Chem. Eng. Res. Des. 2021. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw Materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Sharma, B.K.; Stipanovic, A.J. Development of a new oxidation stability test method for lubricating oils using high-pressure differential scanning calorimetry. Thermochim. Acta 2003, 402, 1–18. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Asadaukas, S. Lubricant base stocks from vegetable oils. Ind. Crop. Prod. 2000, 11, 277–282. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Fernando, S.; Hanna, M.; Adhikari, S. Lubricity Characteristics of Selected Vegetable Oils, Animal Fats, and their Derivatives. Appl. Eng. Agric. 2007, 23, 5–11. [Google Scholar] [CrossRef]
- Soni, S.; Agarwal, M. Lubricants from renewable energy sources—A review. Green Chem. Lett. Rev. 2014, 7, 359–382. [Google Scholar] [CrossRef]
- Syahrullail, S.; Zubil, B.M.; Azwadi, C.S.N.; Ridzuan, M.J.M. Experimental evaluation of palm oil as lubricant in cold forward extrusion process. Int. J. Mech. Sci. 2011, 53, 549–555. [Google Scholar] [CrossRef]
- Regueira, T.; Lugo, L.; Fandino, O.; Lopez, E.R.; Fernandez, J. Compressibilities and viscosities of reference and vegetable oils for their use as hydraulic fluids and lubricants. Green Chem. 2011, 13, 1293–1302. [Google Scholar] [CrossRef]
- Siniawski, M.T.; Saniei, N.; Stoyanov, P. Influence of humidity on the tribological performance of unmodified soybean and sunflower oils. Lubr. Sci. 2011, 23, 301–311. [Google Scholar] [CrossRef]
- Cermak, S.C.; Biresaw, G.; Isbell, T.A.; Evangelista, R.L.; Vaughn, S.F.; Murray, R. New crop oils—Properties as potential lubricants. Ind. Crop. Prod. 2013, 44, 232–239. [Google Scholar] [CrossRef]
- Benessere, V.; Cucciolito, M.E.; Poggetto, G.D.; Di Serio, M.; Granados, M.L.; Ruffo, F.; Vitagliano, A.; Vitiello, R. Strategies for immobilizing homogeneous zinc catalysts in biodiesel production. Catal. Commun. 2014, 56, 81–85. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salimon, J. The Effects of Various Acid Catalyst on the Esterification of Jatropha Curcas Oil based Trimethylolpropane Ester as Biolubricant Base Stock. E-J. Chem. 2011, 8, 33–40. [Google Scholar] [CrossRef]
- Oh, J.; Yang, S.; Kim, C.; Choi, I.; Kim, J.H.; Lee, H. Synthesis of biolubricants using sulfated zirconia catalysts. Appl. Catal. A Gen. 2013, 455, 164–171. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Kleinova, A.; Fodran, P.; Brncalova, L.; Cvengros, J. Substituted esters of stearic acid as potential lubricants. Biomass Bioenergy 2008, 32, 366–371. [Google Scholar] [CrossRef]
- Aronne, A.; Di Serio, M.; Vitiello, R.; Clayden, N.J.; Minieri, L.; Imparato, C.; Piccolo, A.; Pernice, P.; Carniti, P.; Gervasini, A. An Environmentally Friendly Nb-P-Si Solid Catalyst for Acid-Demanding Reactions. J. Phys. Chem. C 2017, 121, 17378–17389. [Google Scholar] [CrossRef]
- Russo, V.; Taddeo, F.; Cogliano, T.; Vitiello, R.; Esposito, R.; Tesser, R.; Salmi, T.; Di Serio, M. Investigation of the intrinsic reaction kinetics and the mass transfer phenomena of nonanoic acid esterification with 2-ethylhexanol promoted by sulfuric acid or Amberlite IR120. Chem. Eng. J. 2021, 408, 127236. [Google Scholar] [CrossRef]
- Russo, V.; Rossano, C.; Salucci, E.; Tesser, R.; Salmi, T.; Di Serio, M. Intraparticle diffusion model to determine the intrinsic kinetics of ethyl levulinate synthesis promoted by Amberlyst 15. Chem. Eng. Sci. 2020, 228, 115974. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.E.; Holmen, A.; Keurentjes, J.T.F. Comparison of commercial solid acid catalysts for the esterification of acetic acid with butanol. Appl. Catal. A Gen. 2006, 297, 182–188. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Wang, M.K. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 48, 6162–6172. [Google Scholar] [CrossRef]
- Kimura, M.; Nakato, T.; Okuhara, T. Water-tolerant solid acid catalysis of Cs2.5H0.5PW12O40 for hydrolysis of esters in the presence of excess water. Appl. Catal. A Gen. 1997, 165, 227–240. [Google Scholar] [CrossRef]
- Turco, R.; Vitiello, R.; Tesser, R.; Vergara, A.; Andini, S.; Di Serio, M. Niobium Based Catalysts for Methyl Oleate Epoxidation Reaction. Top. Catal. 2017, 60, 1054–1061. [Google Scholar] [CrossRef][Green Version]
- Turco, R.; Di Serio, M. Sustainable synthesis of epoxidized Cynara C. Seed oil. Catalysts 2020, 10, 721. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospect of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Am. Soc. Agric. Eng. 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Suwannakarn, K. Biodiesel Production from High Free Fatty Acid Content Feedstocks; Clemson University, Tiger Prints: Clemson, SC, USA, 2008; p. 207. [Google Scholar]
- Vitiello, R.; Buonerba, A.; Tesser, R.; Di Serio, M.; Grassi, A.; Santacesaria, E. Use of waste materials for biodiesel production. DGMK Tagungsbericht 2012, 3, 49–54. [Google Scholar]
- Buonerba, A.; Cuomo, C.; Ortega Sanchez, S.; Canton, P.; Grassi, A. Gold Nanoparticles Incarcerated in Nanoporous Syndiotactic Polystyrene Matrices as New and Efficient Catalysts for Alcohol Oxidations. Chem. Eur. J. 2012, 18, 709–715. [Google Scholar] [CrossRef]
- Bodamer, G.W.; Kunin, R. Behavior of Ion Exchange Resins in Solvents Other Than Water—Swelling and Exchange Characteristics. Ind. Eng. Chem. 1953, 45, 2577–2580. [Google Scholar] [CrossRef]
- ASTM D974-14e2. Standard Test Method for Acid and Base Number by Color-Indicator Titration; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Santacesaria, E.; Di Serio, M.; Tesser, R.; Casale, L.; Verde, D.; Turco, R.; Bertola, A. Use of a corrugated plates heat exchanger reactor for obtaining biodiesel with very high productivity. Energy Fuels 2009, 23, 5206–5212. [Google Scholar] [CrossRef]
- Russo, V.; Protasova, L.; Turco, R.; De Croon, M.H.J.M.; Hessel, V.; Santacesaria, E. Hydrogen peroxide decomposition on manganese oxide supported catalyst: From batch reactor to continuous microreactor. Ind. Eng. Chem. Res. 2013, 52, 7668–7676. [Google Scholar] [CrossRef]
| Run | Alcohol | T [°C] |
|---|---|---|
| 1 | 1,3-propanediol | 120 |
| 2 | 1,3-propanediol | 150 |
| 3 | 1,3-propanediol | 180 |
| 4 | 1,3-propanediol | 200 |
| 5 | Glycerol | 120 |
| 6 | Glycerol | 150 |
| 7 | Glycerol | 180 |
| 8 | Glycerol | 200 |
| Resin | Acidity [meqH+/g] | T max [°C] |
|---|---|---|
| Amberlyst 15 | 4.5 | 120 |
| Nafion R50 | 0.8 | 190 |
| Sulfonic non-reticulated resin | 5.4 | 200 |
| Sulfonic reticulated resin | 4.3 | 200 |
| Run | Alcohol | Acid | Catalyst |
|---|---|---|---|
| 9 | 1,3-propanediol | Oleic acid | Amberlyst 15 |
| 10 | 1,3-propanediol | Oleic acid | Nafion R50 |
| 11 | 1,3-propanediol | Oleic acid | Sulfonic reticulated resin |
| 12 | 1,3-propanediol | Oleic acid | Sulfonic non-reticulated resin |
| 13 | 1,3-propanediol | Oleic acid | H2SO4 |
| 14 | Glycerol | Oleic acid | Amberlyst 15 |
| 15 | Glycerol | Oleic acid | Nafion R50 |
| 16 | Glycerol | Oleic acid | Sulfonic reticulated resin |
| 17 | Glycerol | Oleic acid | Sulfonic non-reticulated resin |
| 18 | Glycerol | Oleic acid | H2SO4 |
| Run | Alcohol | Catalyst | Time [h] |
|---|---|---|---|
| 19 | 1,3-propanediol | H2SO4 | 3 |
| 20 | 1,3-propanediol | Non-reticulated resin | 3 |
| 21 | 1,3-propanediol | Reticulated resin | 3 |
| 22 | 1,3-propanediol | H2SO4 | 6 |
| 23 | 1,3-propanediol | Non-reticulated resin | 6 |
| 24 | 1,3-propanediol | Reticulated resin | 6 |
| Run | Alcohol | Catalyst | Time [h] |
|---|---|---|---|
| 25 | Glycerol | H2SO4 | 3 |
| 26 | Glycerol | Non-reticulated resin | 3 |
| 27 | Glycerol | Reticulated resin | 3 |
| 28 | Glycerol | Non-reticulated resin | 6 |
| 29 | Glycerol | Non-reticulated resin | 6 |
| 30 | Glycerol | H2SO4 | 6 |
| Product | Viscosity at 40 °C [mPa·s] | Viscosity at 100 °C [mPa·s] |
|---|---|---|
| Synthetic base lubricant | 25.1 | 7.2 |
| 80 N lubricant base oil | 12.0 | 3.2 |
| 150 N lubricant base oil | 29.6 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitiello, R.; Taddeo, F.; Russo, V.; Turco, R.; Buonerba, A.; Grassi, A.; Di Serio, M.; Tesser, R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering 2021, 5, 46. https://doi.org/10.3390/chemengineering5030046
Vitiello R, Taddeo F, Russo V, Turco R, Buonerba A, Grassi A, Di Serio M, Tesser R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering. 2021; 5(3):46. https://doi.org/10.3390/chemengineering5030046
Chicago/Turabian StyleVitiello, Rosa, Francesco Taddeo, Vincenzo Russo, Rosa Turco, Antonio Buonerba, Alfonso Grassi, Martino Di Serio, and Riccardo Tesser. 2021. "Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts" ChemEngineering 5, no. 3: 46. https://doi.org/10.3390/chemengineering5030046
APA StyleVitiello, R., Taddeo, F., Russo, V., Turco, R., Buonerba, A., Grassi, A., Di Serio, M., & Tesser, R. (2021). Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering, 5(3), 46. https://doi.org/10.3390/chemengineering5030046













