Alkali-Added Catalysts Based on LaAlO3 Perovskite for the Oxidative Coupling of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Reaction
3. Results and Discussion
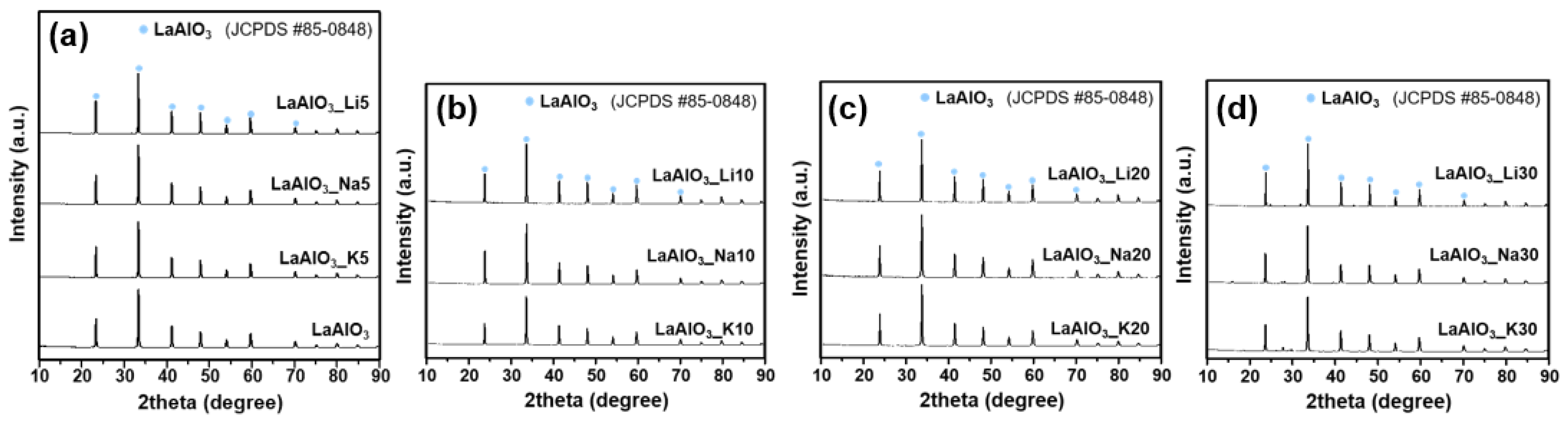
3.1. Formation of LaAlO3_XY (X = Li, Na, K, Y = mol %) Catalysts
3.2. Catalyst Performance of LaAlO3_XY (X = Li, Na, K, Y = mol %) Catalysts in the OCM
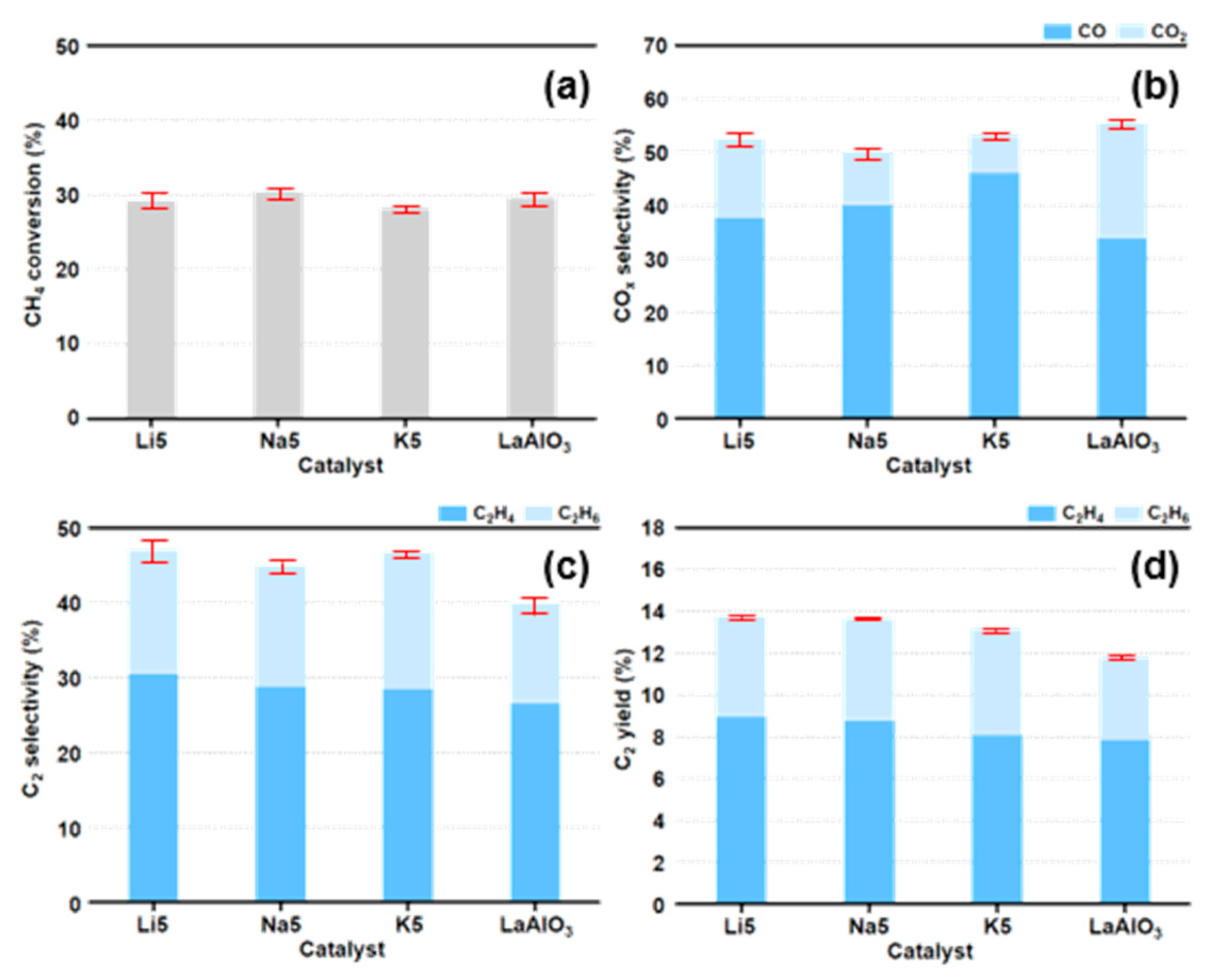
3.3. Catalyst Performance of LaAlO3_X5 (X = Li, Na, K) Catalysts in the OCM
3.4. Physical Properties and Chemical Composition of LaAlO3_X5 (X = Li, Na, K) Catalysts
3.5. Investigation of the Active Site of LaAlO3_X5 (X = Li, Na, K) and LaAlO3 Catalysts on the OCM
3.5.1. The Active Site of LaAlO3_X5 (X = Li, Na, K) and LaAlO3 Catalysts for C2 Selectivity and COx Selectivity
3.5.2. The Active Site of LaAlO3_X5 (X = Li, Na, K) and LaAlO3 for COx Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 10, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.L.; Igenegbai, V.O.; Linic, S. A viewpoint on direct methane conversion to ethane and ethylene using oxidative coupling on solid catalysts. ACS Catal. 2016, 6, 4340–4346. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, Q.; Wu, Z.; Ma, D. Methane activation: The past and future. Energy Environ. Sci. 2014, 7, 2580–2591. [Google Scholar] [CrossRef]
- Lee, J.; Yasin, M.; Park, S.; Chang, I.S.; Ha, K.S.; Lee, E.Y.; Lee, J.; Kim, C. Gas-liquid mass transfer coefficient of methane in bubble column reactor. Korean J. Chem. Eng. 2015, 32, 1060–1063. [Google Scholar] [CrossRef]
- Sudheer, P.D.V.N.; David, Y.; Chae, C.G.; Kim, Y.J.; Baylon, M.G.; Baritugo, K.A.; Kim, T.W.; Kim, M.S.; Na, J.G.; Park, S.J. Advances in the biological treatment of coal for synthetic natural gas and chemicals. Korean J. Chem. Eng. 2016, 33, 2788–2801. [Google Scholar] [CrossRef]
- Wood, D.A.; Nwaoha, C.; Towler, B.F. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012, 9, 196–208. [Google Scholar] [CrossRef]
- Nwaoha, C.; Wood, D.A. A review of the utilization and monetization of Nigeria’s natural gas resources: Current realities. J. Nat. Gas Sci. Eng. 2014, 18, 412–432. [Google Scholar] [CrossRef]
- Araujo, G.C.D.; Lima, S.; Rangel, M.D.C.; Parola, V.L.; Pena, M.A.; Fierro, J.L.G. Characterization of precursors and reactivity of LaNi1-xCoxO3 for the partial oxidation of methane. Catal. Today 2005, 107–108, 906–912. [Google Scholar] [CrossRef]
- Valderrama, G.; Boldwasser, M.R.; Navarro, C.U.D.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Matìnez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–180, 785–791. [Google Scholar] [CrossRef]
- Perenìguez, R.; González-DelaCruz, V.M.; Holgado, J.P.; Caballero, A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts. Appl. Catal. B 2010, 93, 346–353. [Google Scholar] [CrossRef]
- Enger, B.C.; Lodeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Nipan, G.D.; Artukh, V.A.; Yusupov, V.S.; Loktev, A.S.; Spesivtsev, N.A.; Dedov, A.G.; Moiseev, I.I. Effect of pressure on the phase composition of Li(Na)/W/Mn/SiO2 composites and their catalytic activity for oxidative coupling of methane. Inorg. Mater. 2014, 50, 912–916. [Google Scholar] [CrossRef]
- Colmenares, M.G.; Simon, U.; Yildiz, M.; Arndt, S.; Schomaecker, R.; Thormas, A.; Rosowski, F.; Gurlo, A.; Goerke, O. Oxidative coupling of methane on the Na2WO4-MnxOy catalyst: COK-12 as an inexpensive alternative to SBA-15. Catal. Commun. 2016, 85, 75–78. [Google Scholar] [CrossRef]
- Wang, H.; Schmack, R.; Paul, B.; Albrecht, M.; Sokolov, S.; Rummler, S.; Kondratenko, E.V.; Kraehnert, R. Porous silicon carbide as a support for Mn/Na/W/SiC catalyst in the oxidative coupling of methane. Appl. Catal. A 2017, 537, 33–39. [Google Scholar] [CrossRef]
- Jeon, W.; Lee, J.Y.; Lee, M.; Choi, J.W.; Ha, J.M.; Suh, D.J.; Kim, I.W. Oxidative coupling of methane to C2 hydrocarbons on theMg-Ti mixed oxide-supported catalysts at the lower reaction temperature: Role of surface oxygen atoms. Appl. Catal. A 2013, 464–465, 68–77. [Google Scholar] [CrossRef]
- Kwon, D.; Yang, I.; Sim, Y.; Ha, J.M.; Jung, J.C. A K2NiF4-type La2Li0.5Al0.5O4 catalyst for the oxidative coupling of methane (OCM). Catal. Commu. 2019, 128, 105702. [Google Scholar] [CrossRef]
- Sim, Y.; Kwon, D.; An, S.; Ha, J.M.; Oh, T.S.; Jung, J.C. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane. Mol. Catal. 2020, 489, 110925. [Google Scholar] [CrossRef]
- Nipan, G.D. Melt-assisted phase transformations of A/W/Mn/SiO2 (A=Li, Na, K, Rb, Cs) composite catalysts. Inorg. Mater. 2017, 53, 553–559. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Moiseev, I.I.; Aboukais, A.; Lamonier, J.F.; Filimonov, I.N. Oxidative coupling of methane catalyzed unexpected synergistic effect of the oxide mixtures. Appl. Cataly. A 2003, 245, 209–220. [Google Scholar] [CrossRef]
- Driscoll, D.J.; Martir, W.; Wang, J.X.; Lunsford, J.H. Formation of gas-phase methyl radicals over magnesium oxide. J. Am. Chem. Soc. 1985, 107, 58–63. [Google Scholar] [CrossRef]
- Arndt, S.; Laugel, G.; Levchenko, S.; Horn, R.; Baerns, M.; Scheffler, M. A Critical assessment of Li/MgO-based catalysts for the oxidative coupling of methane. Catal. Rev. 2011, 53, 424–514. [Google Scholar] [CrossRef]
- Ghose, R.; Hwang, H.T.; Varma, A. Oxidative coupling of methane using catalysts synthesized by solution combustion method: Catalyst optimization and kinetic studies. Appl. Catal. A 2014, 472, 39–46. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, D.; Gao, R.; Wang, Z.; Yang, J. Scale up and stability test for oxidative coupling of methane over Na2WO4-Mn/SiO2 catalyst in a 200 mL fixed-bed reactor. J. Nat. Gas Chem. 2008, 17, 59–63. [Google Scholar] [CrossRef]
- Gholipour, Z.; Malekzadeh, A.; Hatami, R.; Mortazavi, Y.; Khodadadi, A. Oxidative coupling of methane over (Na2WO4+Mn or Ce)/SiO2 catalysts: In situ measurement of electrical conductivity. J. Nat. Gas Chem. 2010, 19, 35–42. [Google Scholar] [CrossRef]
- Rollilns, B.; Grosjean, N.; Poulston, S. The impact of the presence of various relevant gases during aging of MnNa2WO4/SiO2 catalyst on its performance for the oxidative coupling of methane. Appl. Catal. A 2020, 598, 117606. [Google Scholar] [CrossRef]
- Kidamorn, O.; Tiyatha, W.; Chukeaw, T.; Niamnuy, C.; Chareonpanich, M.; Sohn, H.; Seubsai, A. Synthesis of value-added chemicals via oxidative coupling of methanes over Na2WO4-TiO2-MnOx/SiO2 catalysts with alkali or alkali earth oxide additives. ACS Omega 2020, 5, 13612–13620. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomacker, R. Mn-Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A 2012, 425–426, 53–61. [Google Scholar] [CrossRef]
- Nibbelke, R.H.; Scheerova, J.; Decroon, M.H.J.M.; Maring, G.B. The oxidative coupling of methane over MgO-based catalysts: A steady-state isotope transient kinetic analysis. J. Catal. 1995, 156, 106–119. [Google Scholar] [CrossRef]
- Luo, L.; You, R.; Liu, Y.; Yang, J.; Zhu, Y.; Wen, W.; Pan, Y.; Qi, F.; Huang, W. Gas-phase reaction network of Li/MgO-catalyzed oxidative coupling of methane and oxidative dehydrogenation of ethane. ACS Catal. 2019, 9, 2514–2520. [Google Scholar] [CrossRef]
- Qian, K.; You, R.; Guan, Y.; Wen, W.; Tian, Y.; Pan, Y.; Huang, W. Single-site catalysis of Li-MgO catalysts for oxidative coupling of methane reaction. ACS Catal. 2020, 10, 15142–15148. [Google Scholar] [CrossRef]
- Tang, L.G.; Yamaguchi, D.; Wong, L.; Burke, N.; Chiang, K. The promoting effect of ceria on Li/MgO catalysts for the oxidative coupling of methane. Catal. Today 2011, 178, 172–180. [Google Scholar] [CrossRef]
- Gu, S.; Oh, H.S.; Choi, J.W.; Suh, D.J.; Jae, J.; Choi, J.; Ha, J.M. Effects of metal or metal oxide additives on oxidative coupling of methane using Na2WO4/SiO2 catalysts: Reducibility of metal additives to manipulate the catalytic activity. Appl. Catal. A 2018, 562, 114–119. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rane, V.H.; Pandit, M.Y. Comparison of alkali metal promoted MgO catalysts for their surface acidity/basicity and catalytic activity/selectivity in the oxidative coupling of methane. J. Chem. Tech. Biotechnol. 1997, 68, 177–186. [Google Scholar] [CrossRef]
- Elkins, T.W.; Roberts, S.J.; Hagelin-Weaver, H.E. Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling of methane. Appl. Catal. A 2016, 528, 175–190. [Google Scholar] [CrossRef]
- Miro, E.; Santamaria, J.; Wolf, E.E. Oxidative coupling of methane on alkali metal-promoted nickel titanate: I. Catalyst characterization and transient studies. J. Catal. 1990, 124, 451–464. [Google Scholar] [CrossRef]
- Rane, V.H.; Chaudhari, S.T.; Choudhary, V.R. Influence of alkali metal doping on surface properties and catalytic activity/selectivity of CaO catalysts in oxidative coupling of methane. J. Nat. Gas Chem. 2008, 17, 313–320. [Google Scholar] [CrossRef]
- Ito, T.; Wang, J.; Lin, C.H.; Lunsford, J.H. Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst. J. Am. Chem. Soc. 1985, 107, 5062–5068. [Google Scholar] [CrossRef]
- Kim, I.; Lee, G.; Na, H.B.; Ha, J.M.; Jung, J.C. Selective oxygen species for the oxidative coupling of methane. Mol. Catal. 2017, 435, 13–23. [Google Scholar] [CrossRef]
- Sim, Y.; Kwon, D.; Ha, J.M.; Jung, J.C. Preparation of LaAlO3 perovskite catalysts by simple solid-state method for oxidative coupling of methane. Catal. Today 2020, 352, 134–139. [Google Scholar] [CrossRef]
- Berger, T.; Schuh, J.; Sterrer, M.; Diwald, O.; KnÖzinger, E. Lithium ion induced surface reactivity changes on MgO nanoparticles. J. Catal. 2007, 247, 61–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Wang, X.; Sun, J.; Si, R.; Zhou, H. Collective and individual impacts of the cascade doping of alkali cations in perovskite single crystals. J. Mater. Chem. C 2020, 43, 15350–15360. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Baltrusaitis, J.; Wachs, I.E. Oxidative coupling of methane (OCM) by SiO2-supported tungsten oxide catalysts promoted with Mn and Na. ACS Catal. 2019, 9, 5912–5928. [Google Scholar] [CrossRef]
- Zhang, H.B.; Lin, G.D.; Wan, H.L.; Liu, Y.D.; Weng, W.Z.; Cai, J.X.; Shen, Y.F.; Tsai, K.R. Active-oxygen species on non-reducible rare-earth-oxide-based catalysts in oxidative coupling of methane. Catal. Lett. 2001, 73, 141–147. [Google Scholar] [CrossRef]
- Lunsford, J.H. The catalytic oxidative coupling of methane. Angew. Chem. Int. Ed. Engl. 1995, 34, 970–980. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3- tubular membrane reactors. Catal. Today 2005, 104, 160–167. [Google Scholar] [CrossRef]
- He, Y.; Yang, B.; Cheng, G. On the oxidative coupling of methane with carbon dioxide over CeO2/ZnO nanocatalysts. Catal. Today 2004, 98, 595–600. [Google Scholar] [CrossRef]
- Lee, M.R.; Park, M.J.; Jeon, W.; Choi, J.W.; Suh, Y.W.; Suh, D.J. A kinetic model for the oxidative coupling of methane over Na2WO4/Mn/SiO2. Fuel Process. Technol. 2012, 96, 175–182. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, S.; Choi, J.W.; Suh, D.J.; Song, K.H.; Ha, J.M. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O2, surface lattice oxygen, and CO2 on the C2 selectivity. RSC Adv. 2020, 10, 35889. [Google Scholar] [CrossRef]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]




| Catalyst | SBET (m2/g) a |
|---|---|
| LaAlO3_Li5 | 1.4 |
| LaAlO3_Na5 | 2.9 |
| LaAlO3_K5 | 3.1 |
| LaAlO3 | 3.5 |
| Catalyst | La | Al | Li | Na | K |
|---|---|---|---|---|---|
| mol % | |||||
| LaAlO3_Li5 | 53.3 | 46.7 | - | - | - |
| LaAlO3_Na5 | 52.6 | 46.3 | - | 1.1 | - |
| LaAlO3_K5 | 52.8 | 46.5 | - | - | 0.7 |
| Catalyst | Relative Amount (%) | Olat(e)/Olat(n) | ||
|---|---|---|---|---|
| Oads (533.0 eV) | Olat(e) (531.0 eV) | Olat(n) (528.8 eV) | ||
| LaAlO3_Li5 | 3.6 | 38.6 | 57.9 | 0.67 |
| LaAlO3_Na5 | 3.7 | 38.5 | 57.8 | 0.67 |
| LaAlO3_K5 | 3.5 | 37.7 | 58.8 | 0.64 |
| LaAlO3 | 3.7 | 34.6 | 61.7 | 0.56 |
| Catalyst | ||||
|---|---|---|---|---|
| LaAlO3_Li5 | LaAlO3_Na5 | LaAlO3_K5 | LaAlO3 | |
| Peak (°C) | 565.1 | 585.9 | 609.4 | 548.5 |
| Peak area (a.u.) a | 1563.3 | 1842.2 | 2817.9 | 1466.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Cho, J.; Kwon, D.; Jung, J.C. Alkali-Added Catalysts Based on LaAlO3 Perovskite for the Oxidative Coupling of Methane. ChemEngineering 2021, 5, 14. https://doi.org/10.3390/chemengineering5010014
An S, Cho J, Kwon D, Jung JC. Alkali-Added Catalysts Based on LaAlO3 Perovskite for the Oxidative Coupling of Methane. ChemEngineering. 2021; 5(1):14. https://doi.org/10.3390/chemengineering5010014
Chicago/Turabian StyleAn, Suna, JeongHyun Cho, Dahye Kwon, and Ji Chul Jung. 2021. "Alkali-Added Catalysts Based on LaAlO3 Perovskite for the Oxidative Coupling of Methane" ChemEngineering 5, no. 1: 14. https://doi.org/10.3390/chemengineering5010014
APA StyleAn, S., Cho, J., Kwon, D., & Jung, J. C. (2021). Alkali-Added Catalysts Based on LaAlO3 Perovskite for the Oxidative Coupling of Methane. ChemEngineering, 5(1), 14. https://doi.org/10.3390/chemengineering5010014






