Insights from Mathematical Modelling into Process Control of Oxygen Transfer in Batch Stirred Tank Bioreactors for Reducing Energy Requirement
Abstract
1. Introduction
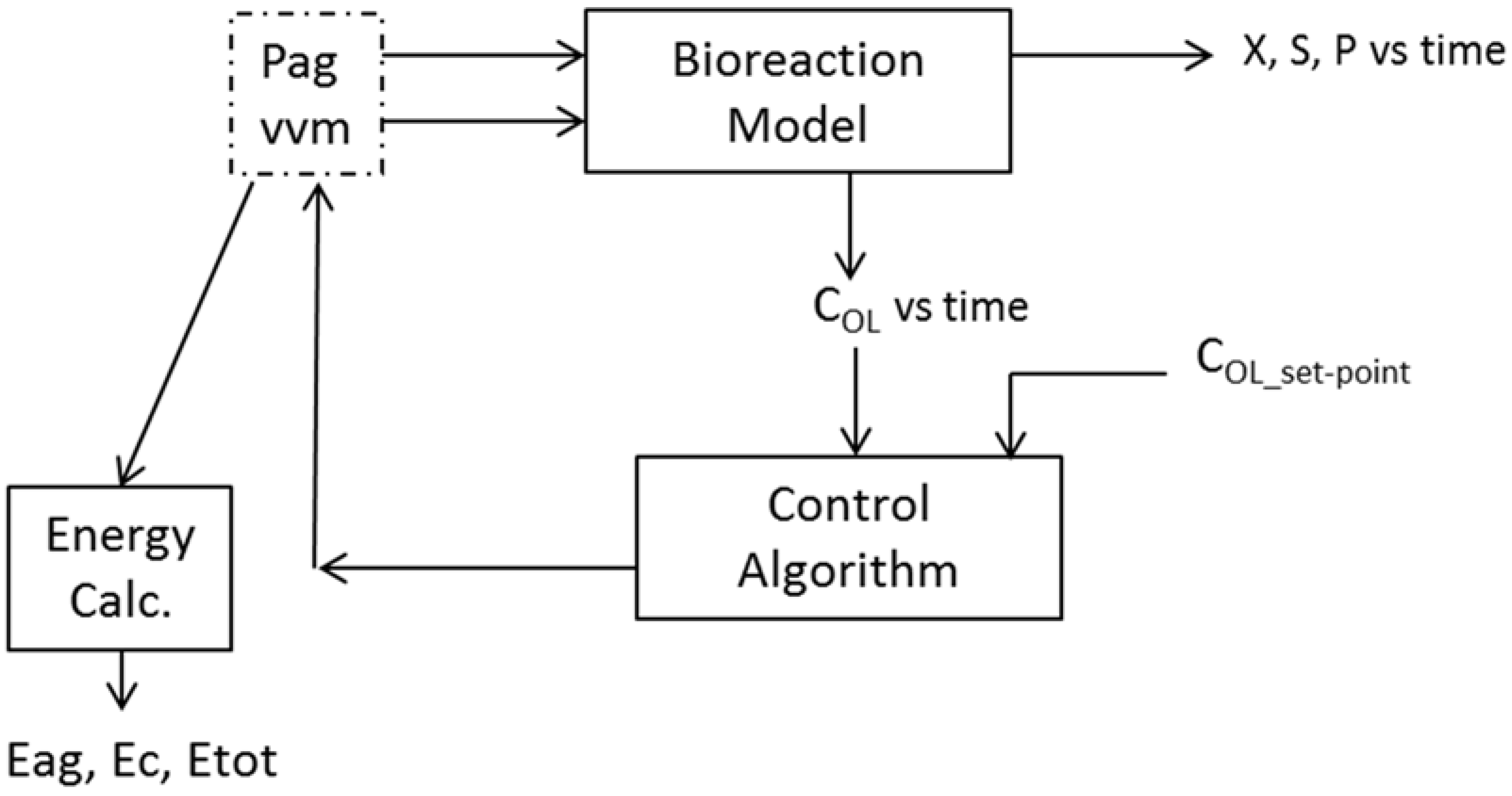
2. Mathematical Modelling and Aeration System Control Strategies
2.1. Mathematical Modelling
2.2. Aeration System Control Strategies
3. Results and Discussion
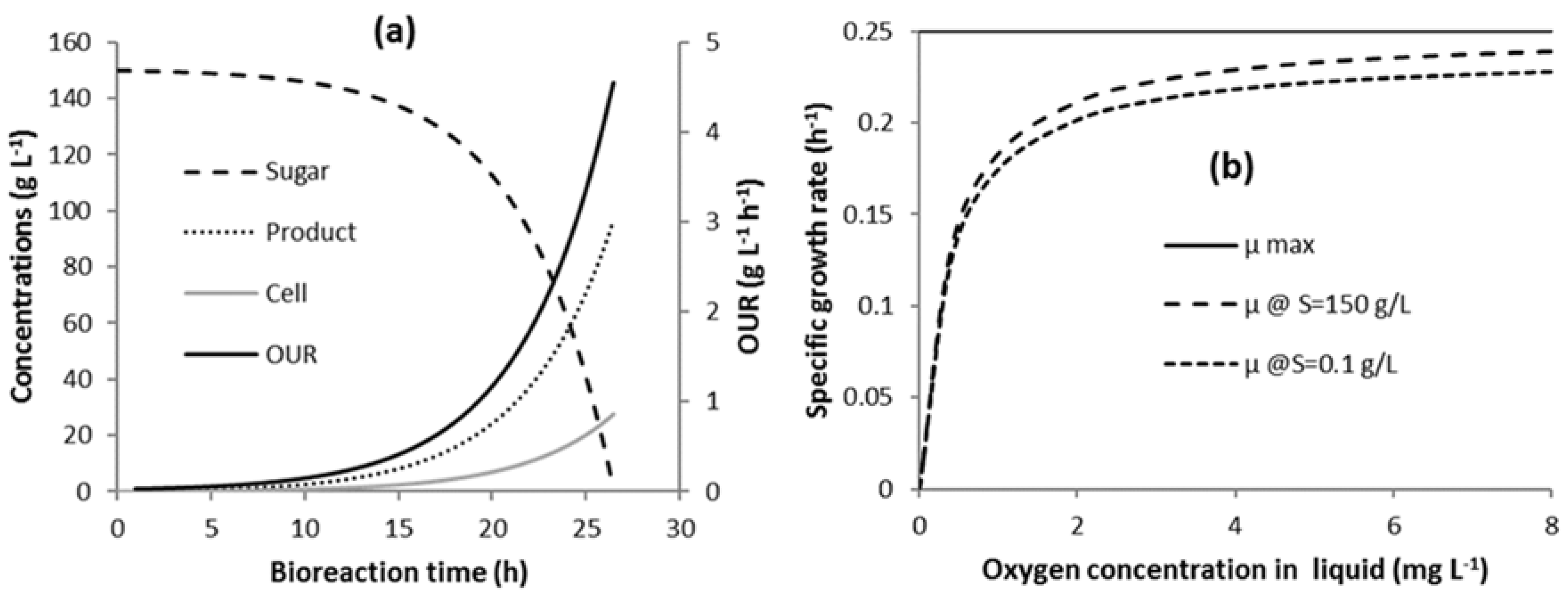
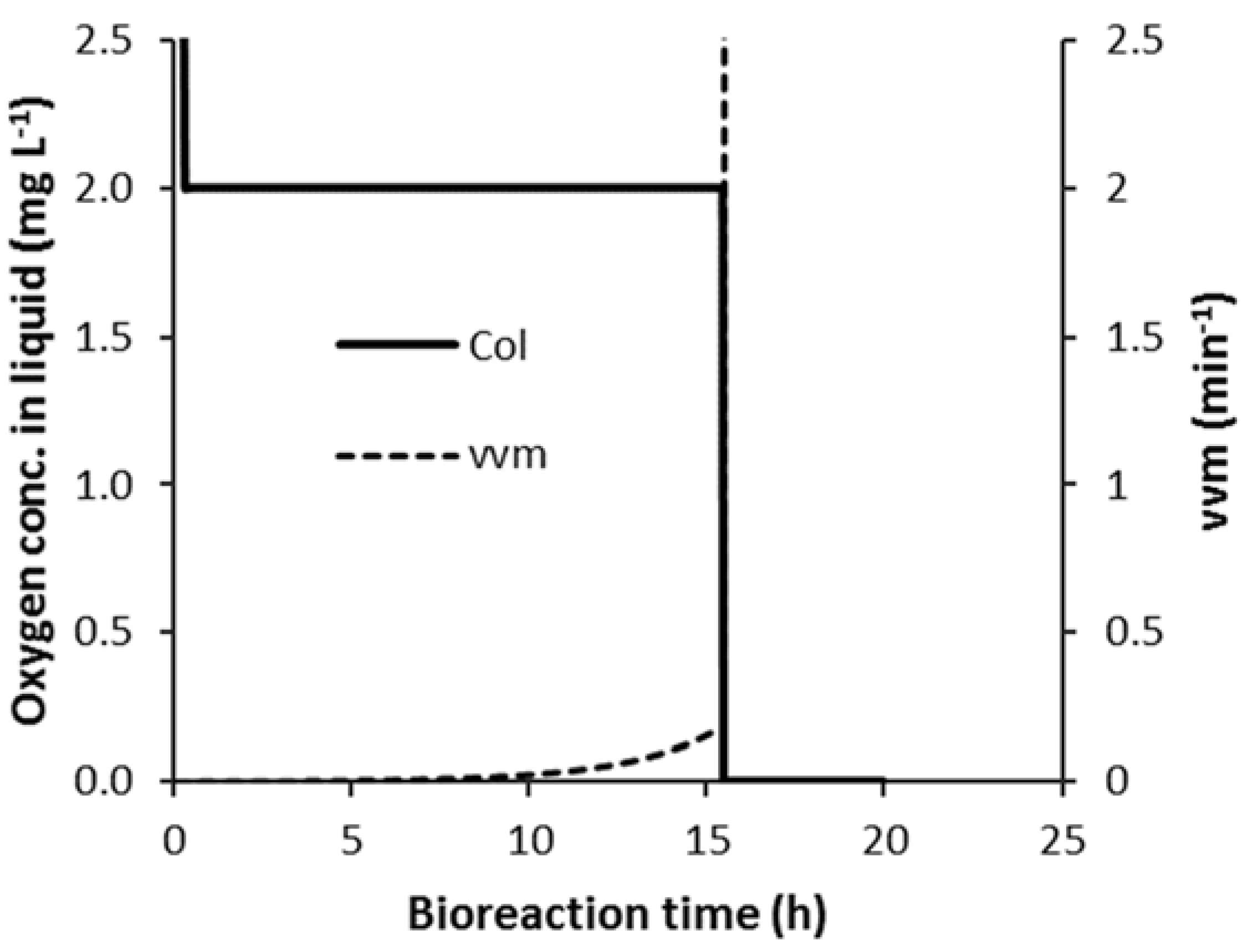
3.1. Bioreaction Progression
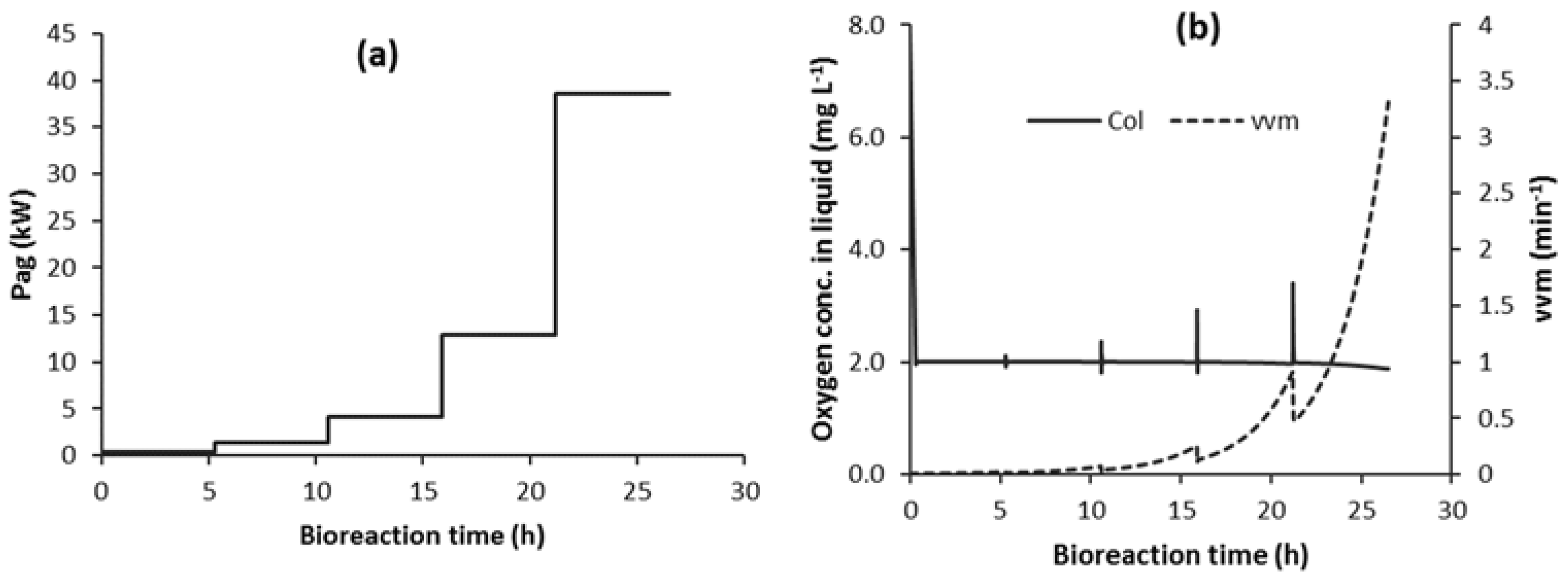
3.2. Control of vvm in Strategy C1
- (1)
- Set a low Kp value (1 or 10 for example) and set Ki = Kd = 0.
- (2)
- While Ki = Kd = 0, increase Kp value until COL sustained oscillations appear. The Kp value where oscillations appear is called Kp_lim.
- (3)
- Measure oscillation period T0.
- (4)
- Tune PID parameters using Equations (2) to (4).
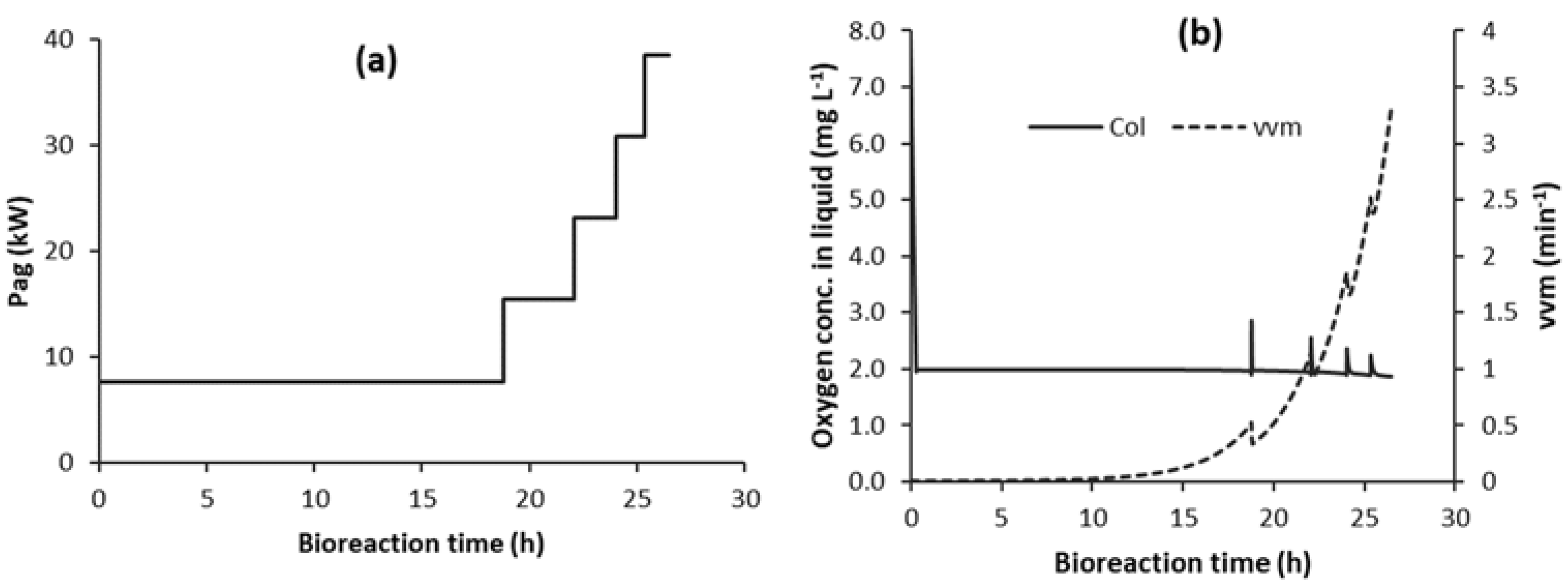
3.3. Energy Reduction and Minimisation
3.3.1. Energy Minimisation Using Strategy Cmin
3.3.2. Energy Reduction Using Strategy C1-N
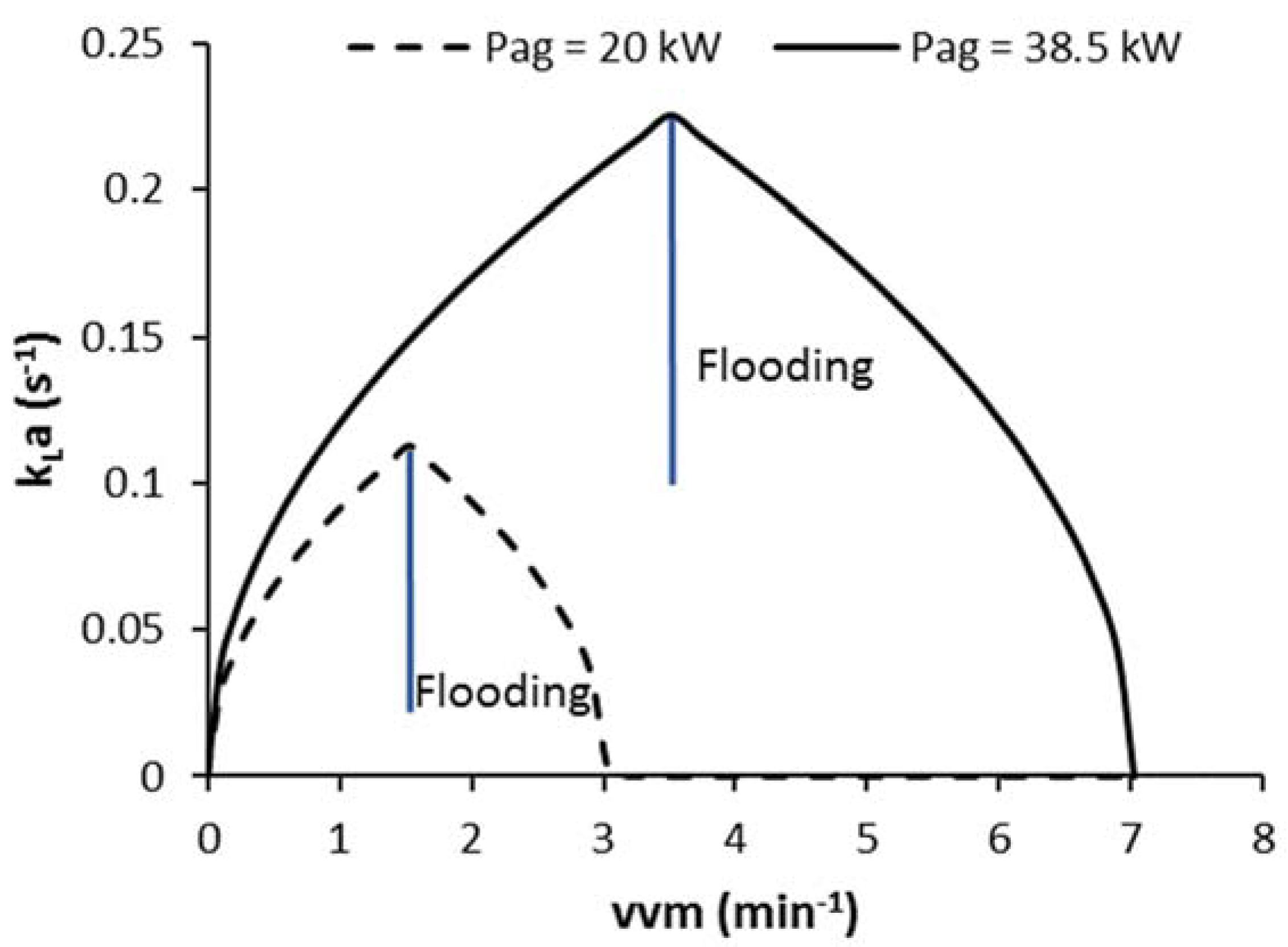
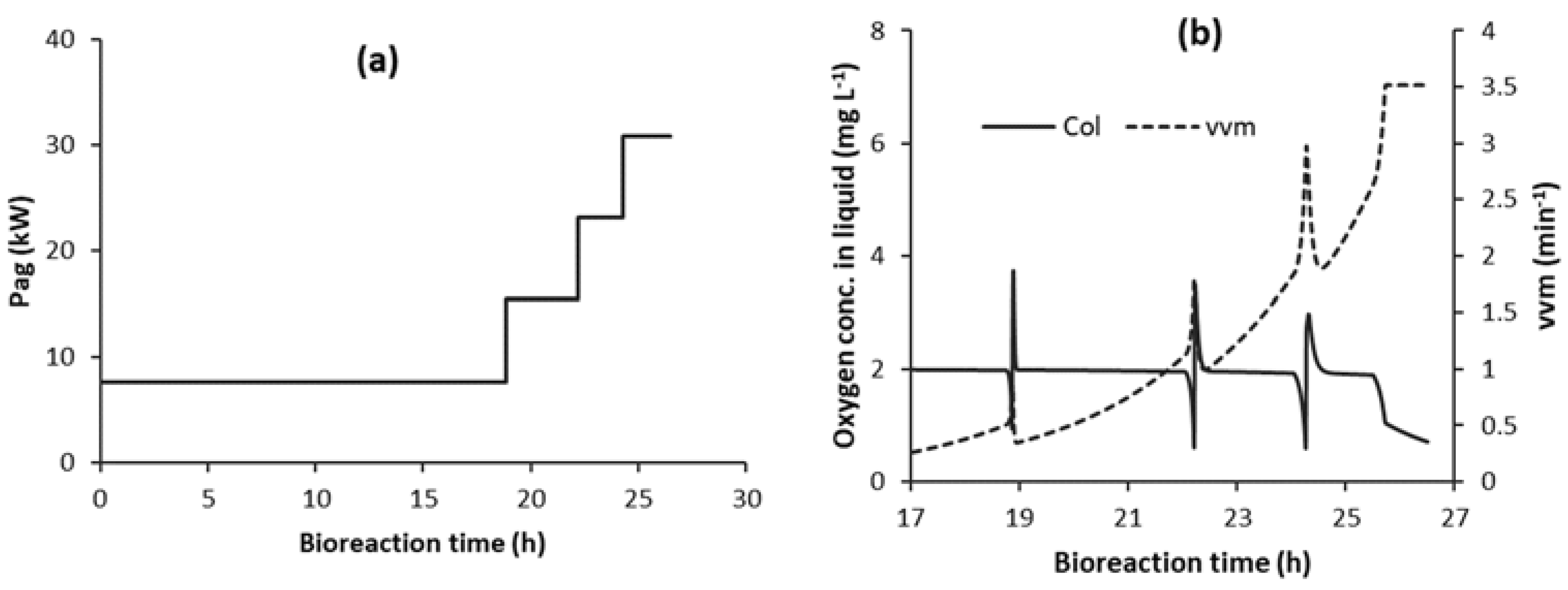
3.4. Implementation of Strategy C1-N with Detection of Impeller Flooding using Oxygen Sensor Data (C1F-N)
3.4.1. Detection of Impeller Flooding Using Oxygen Sensor Data and Implementation of Strategy C1F-N
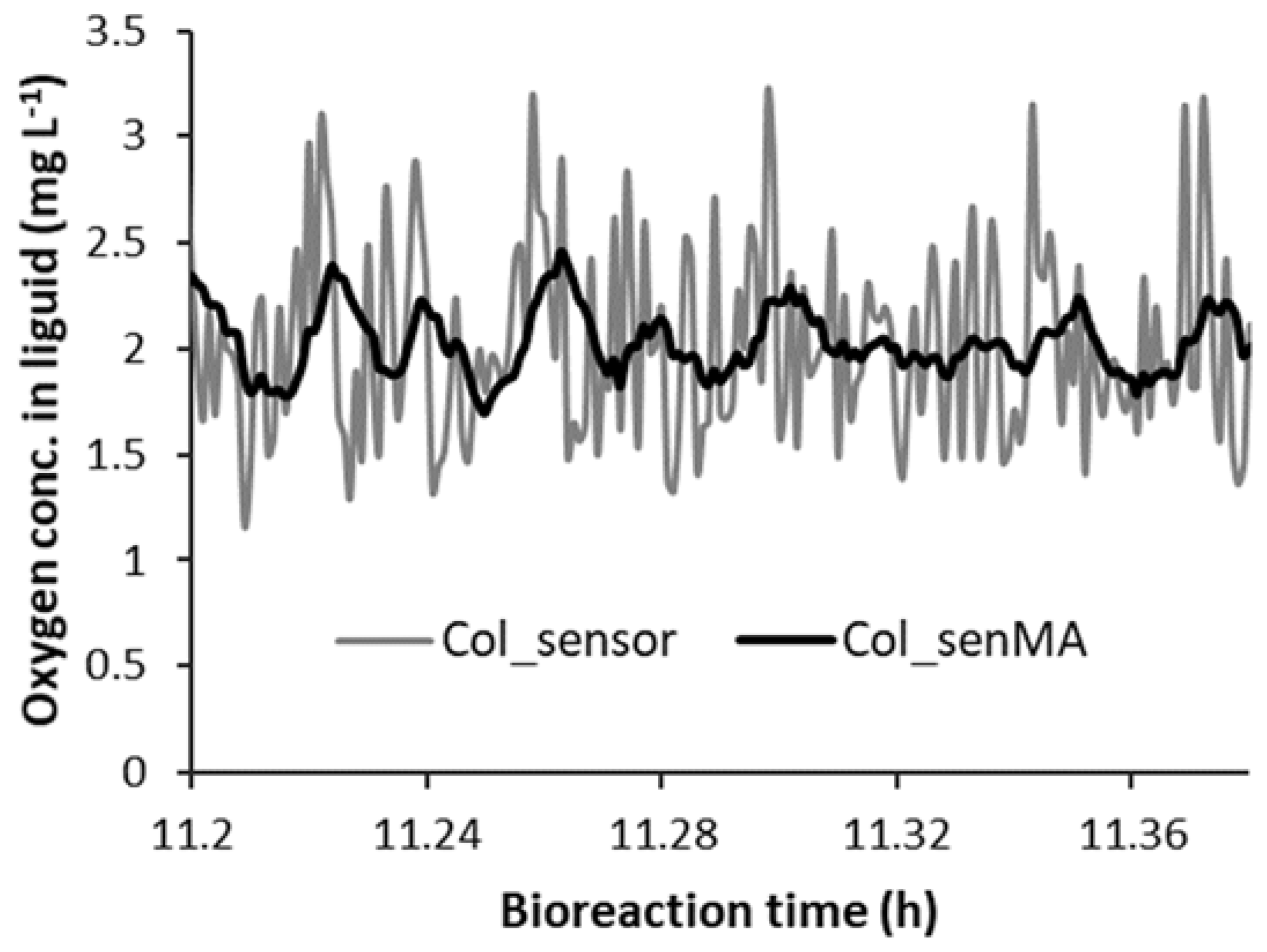
3.4.2. Dealing with Random Fluctuations in the Oxygen Sensor Measurements and Unwanted Pag Step Increases
3.4.3. Impact of Operating at Lower Values of COL_FLD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Symbols
| COL | oxygen concentration in the bioreaction liquid (mg L−1) |
| COL_FLD | oxygen concentration at which Pag step increase occurs (mg L−1) |
| COL_sensor | simulated oxygen sensor measurement (mg L−1) |
| OL_senMA | moving average of COL_sensor values (mg L−1) |
| COL_sp | oxygen concentration controller set-point (mg L−1) |
| Eag | agitator electrical energy (GJ) |
| Ec | compressor electrical energy (GJ) |
| Etot | sum of agitator and compressor electrical energy (GJ) |
| e(t) | difference between O2 concentration set-point and simulated value (mg L−1) |
| FG | inlet air volumetric flowrate (m3 h−1) |
| kLa | volumetric oxygen mass transfer coefficient (h−1) |
| KdKiKp | PID controller constants |
| N | number of time increments |
| OUR | oxygen uptake rate (g L−1 h−1) |
| OTR | oxygen transfer rate (g L−1 h−1) |
| P | product concentration (g L−1) |
| Pag | agitator mechanical power input (kW) |
| S | sugar concentration (g L−1) |
| t | time (hours) |
| T0 | oscillation period PID controller (h) |
| VL | bioreactor working volume (m−3) |
| vvm | volume of air per minute per unit bioreactor working volume (min−1) |
| air superficial velocity (m h−1) | |
| air superficial velocity at onset of flooding (m h−1) | |
| X | cell concentration (g L−1) |
| Y | maximum random fluctuation amplitude in oxygen concentration (mg L−1) |
| µ | specific growth rate (h−1) |
| µmax | maximum specific growth rate (h−1) |
| τiτd | PID controller parameters (h) |
References
- García-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.; Orejas, J. Oxygen transfer in a stirred reactor in laboratory scale. Lat. Am. Appl. Res. 2001, 31, 433–439. [Google Scholar]
- Demirtas, M.U.; Kolhatkar, A.; Kilbane, J.J. Effect of aeration and agitation on growth rate of Thermus thermophilus in batch mode. J. Biosci. Bioeng. 2003, 95, 113–117. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, S.-J.; Chen, T.-L. The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem. Eng. J. 2006, 32, 239–243. [Google Scholar] [CrossRef]
- Emily, L.; Nandong, J.; Samyudia, Y. Experimental Investigation on the Impact of Aeration Rate and Stirrer Speed on Micro-Aerobic Batch Fermentation. J. Appl. Sci. 2009, 9, 3126–3130. [Google Scholar] [CrossRef][Green Version]
- Radchenkova, N.; Vassilev, S.; Martinov, M.; Kuncheva, M.; Panchev, I.; Vlaev, S.; Kambourova, M. Optimization of the aeration and agitation speed of Aeribacillus palidus 418 exopolysaccharide production and the emulsifying properties of the product. Process. Biochem. 2014, 49, 576–582. [Google Scholar] [CrossRef]
- Tervasmäki, P.; Latva-Kokko, M.; Taskila, S.; Tanskanen, J. Effect of oxygen transfer on yeast growth—Growth kinetic and reactor model to estimate scale-up effects in bioreactors. Food Bioprod. Process. 2018, 111, 129–140. [Google Scholar] [CrossRef]
- Felder, M.; Simmons, A.; Shambaugh, R.; Sikavitsas, V. Effects of Flow Rate on Mesenchymal Stem Cell Oxygen Consumption Rates in 3D Bone-Tissue-Engineered Constructs Cultured in Perfusion Bioreactor Systems. Fluids 2020, 5, 30. [Google Scholar] [CrossRef]
- Alves, S.S.; Vasconcelos, J.M.T. Optimisation of agitation and aeration in fermenters. Bioprocess Eng. 1996, 14, 119–123. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; McMillan, J.D. Aeration costs in stirred-tank and bubble column bioreactors. Biochem. Eng. J. 2017, 127, 161–166. [Google Scholar] [CrossRef]
- Benz, G.T. Impeller selection for agitated aerobic fermenters. Chem. Eng. Prog. 2004, 100, 18S–20S. [Google Scholar]
- Buffo, M.; Corrêa, L.; Esperança, M.; Cruz, A.J.G.; Farinas, C.; Badino, A.C. Influence of dual-impeller type and configuration on oxygen transfer, power consumption, and shear rate in a stirred tank bioreactor. Biochem. Eng. J. 2016, 114, 130–139. [Google Scholar] [CrossRef]
- Schaepe, S.; Kuprijanov, A.; Sieblist, C.; Jenzsch, M.; Simutis, R.; Lübbert, A. kLa of stirred tank bioreactors revisited. J. Biotechnol. 2013, 168, 576–583. [Google Scholar] [CrossRef]
- Gakingo, G.; Clarke, K.; Louw, T. A numerical investigation of the hydrodynamics and mass transfer in a three-phase gas-liquid-liquid stirred tank reactor. Biochem. Eng. J. 2020, 157, 107522. [Google Scholar] [CrossRef]
- Rahimi, M.J.; Sitaraman, H.; Humbird, D.; Stickel, J.J. Computational fluid dynamics study of full-scale aerobic bioreactors: Evaluation of gas–liquid mass transfer, oxygen uptake, and dynamic oxygen distribution. Chem. Eng. Res. Des. 2018, 139, 283–295. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Neto, J.M.; Filho, R. Hydrodynamics and mass transfer in bubble column, conventional airlift, stirred airlift and stirred tank bioreactors, using viscous fluid: A comparative study. Biochem. Eng. J. 2017, 118, 70–81. [Google Scholar] [CrossRef]
- Nienow, A.W. Mass transfer and mixing across the scales in animal cell culture. In Animal Cell Culture; Al-Rubeai, M., Ed.; Volume 9, Cell Engineering; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Benz, G.T. Optimize power consumption in aerobic fermenters. Chem. Eng. Prog. 2003, 99, 32–35. [Google Scholar]
- Benz, G.T. Cut agitator power costs. Chem. Eng. Prog. 2012, 108, 40–43. [Google Scholar]
- Zamouche, R.; Bencheikh-Lehocine, M.; Meniai, A.-H. Oxygen transfer and energy savings in a pilot-scale batch reactor for domestic wastewater treatment. Desalination 2007, 206, 414–423. [Google Scholar] [CrossRef]
- Hu, D.; Luo, K.; Ma, H.; Min, H.; Zhao, Y.; Cui, Y.; Wang, S.; Ning, N.; Zhang, L.; Liu, W. A sustainability anti-infective pharmaceutical wastewater treatment technology: Multi-stage vertical variable diameter membrane bioreactor with DO online controlling. Bioresour. Technol. 2020, 311, 123507. [Google Scholar] [CrossRef] [PubMed]
- Chitra, M.; Natarajan, P.; Abraham, A. Dissolved Oxygen Control of Batch Bioreactor using Model Reference Adaptive Control scheme. IFAC-PapersOnLine 2018, 51, 13–18. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jegatheesan, V.; Shi, G. Dissolved Oxygen Control in Activated Sludge Process Using a Neural Network-Based Adaptive PID Algorithm. Appl. Sci. 2018, 8, 261. [Google Scholar] [CrossRef]
- Simutis, R.; Lübbert, A. Bioreactor control improves bioprocess performance. Biotechnol. J. 2015, 10, 1115–1130. [Google Scholar] [CrossRef]
- Jonelis, K.; Brazauskas, K.; Levisauskas, D. A system for dissolved oxygen control in industrial aeration tank. Inf. Technol. Control 2012, 41, 46–52. [Google Scholar] [CrossRef][Green Version]
- Oliveira, R.; Simutis, R.; De Azevedo, S.F. Design of a stable adaptive controller for driving aerobic fermentation processes near maximum oxygen transfer capacity. J. Process. Control 2004, 14, 617–626. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Emde, F.; Regestein, L.; Büchs, J. Computational minimization of the specific energy demand of large-scale aerobic fermentation processes based on small-scale data. Chem. Eng. Sci. 2016, 153, 270–283. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Gloanec, F.; Michel, E.; Blondy, J.; Lauzeral, A. Application of Mathematical Modelling to Reducing and Minimising Energy Requirement for Oxygen Transfer in Batch Stirred Tank Bioreactors. ChemEngineering 2019, 3, 14. [Google Scholar] [CrossRef]
- Buffo, M.M.; Esperança, M.N.; Béttega, R.; Farinas, C.S.; Badino, A.C. Oxygen Transfer and Fragmentation of Aspergillus niger Pellets in Stirred Tank and Concentric-Duct Airlift Bioreactors. Ind. Biotechnol. 2020, 16, 67–74. [Google Scholar] [CrossRef]
- Seborg, D.E.; Edgar, T.F.; Mellichamp, D.A.; Doyle, F.J., III. Process Control and Dynamics; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bakker, A.; Smith, J.M.; Meyers, K.J. How to disperse gases in liquids. Chem. Eng. 1994, 12, 98–104. [Google Scholar]

| Control Strategy | Agitator Eag | Compressor Ec | Total Etot | Energy Savings (%) |
|---|---|---|---|---|
| C1 | 4104 | 459 | 4563 | - |
| Cmin | 746 | 706 | 1452 | 68.2 |
| C1-2 | 2174 | 463 | 2637 | 42.2 |
| C1-5 | 1221 | 529 | 1750 | 61.6 |
| C1-10 | 965 | 590 | 1555 | 65.9 |
| Control Strategy | Agitator Eag | Compressor Ec | Total Etot | Energy Savings (%) |
|---|---|---|---|---|
| C1 | 4104 | 459 | 4563 | - |
| Cmin | 746 | 706 | 1452 | 68.2 |
| Strategy C1F-N | ||||
| C1F-2 | 2303 | 520 | 2823 | 38.1 |
| C1F-5 | 1305 | 596 | 1901 | 58.3 |
| C1F-10 | 1001 | 635 | 1637 | 64.1 |
| COL_FLD (mg L−1) | Y = 0.1 | Y = 0.3 | Y = 0.5 |
|---|---|---|---|
| 0.8 | No | No | Yes |
| 1.2 | No | Yes | Yes |
| 1.5 | No | Yes | Yes |
| 1.7 | Yes | Yes | Yes |
| 1.9 | Yes | Yes | Yes |
| Colour signifies that constraint in Equation (10) was met. | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, J.J.; Gloanec, F.; Michel, E. Insights from Mathematical Modelling into Process Control of Oxygen Transfer in Batch Stirred Tank Bioreactors for Reducing Energy Requirement. ChemEngineering 2020, 4, 34. https://doi.org/10.3390/chemengineering4020034
Fitzpatrick JJ, Gloanec F, Michel E. Insights from Mathematical Modelling into Process Control of Oxygen Transfer in Batch Stirred Tank Bioreactors for Reducing Energy Requirement. ChemEngineering. 2020; 4(2):34. https://doi.org/10.3390/chemengineering4020034
Chicago/Turabian StyleFitzpatrick, John J., Franck Gloanec, and Elisa Michel. 2020. "Insights from Mathematical Modelling into Process Control of Oxygen Transfer in Batch Stirred Tank Bioreactors for Reducing Energy Requirement" ChemEngineering 4, no. 2: 34. https://doi.org/10.3390/chemengineering4020034
APA StyleFitzpatrick, J. J., Gloanec, F., & Michel, E. (2020). Insights from Mathematical Modelling into Process Control of Oxygen Transfer in Batch Stirred Tank Bioreactors for Reducing Energy Requirement. ChemEngineering, 4(2), 34. https://doi.org/10.3390/chemengineering4020034




