Obtaining Mn-Co Alloys in AISI 430 Steel from Lithium-Ion Battery Recycling: Application in SOFC Interconnectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Recycled Electrolyte Solutions: Hydrometallurgical Route
2.3. Electrochemical Measurements
3. Results
3.1. Electrolyte Solutions
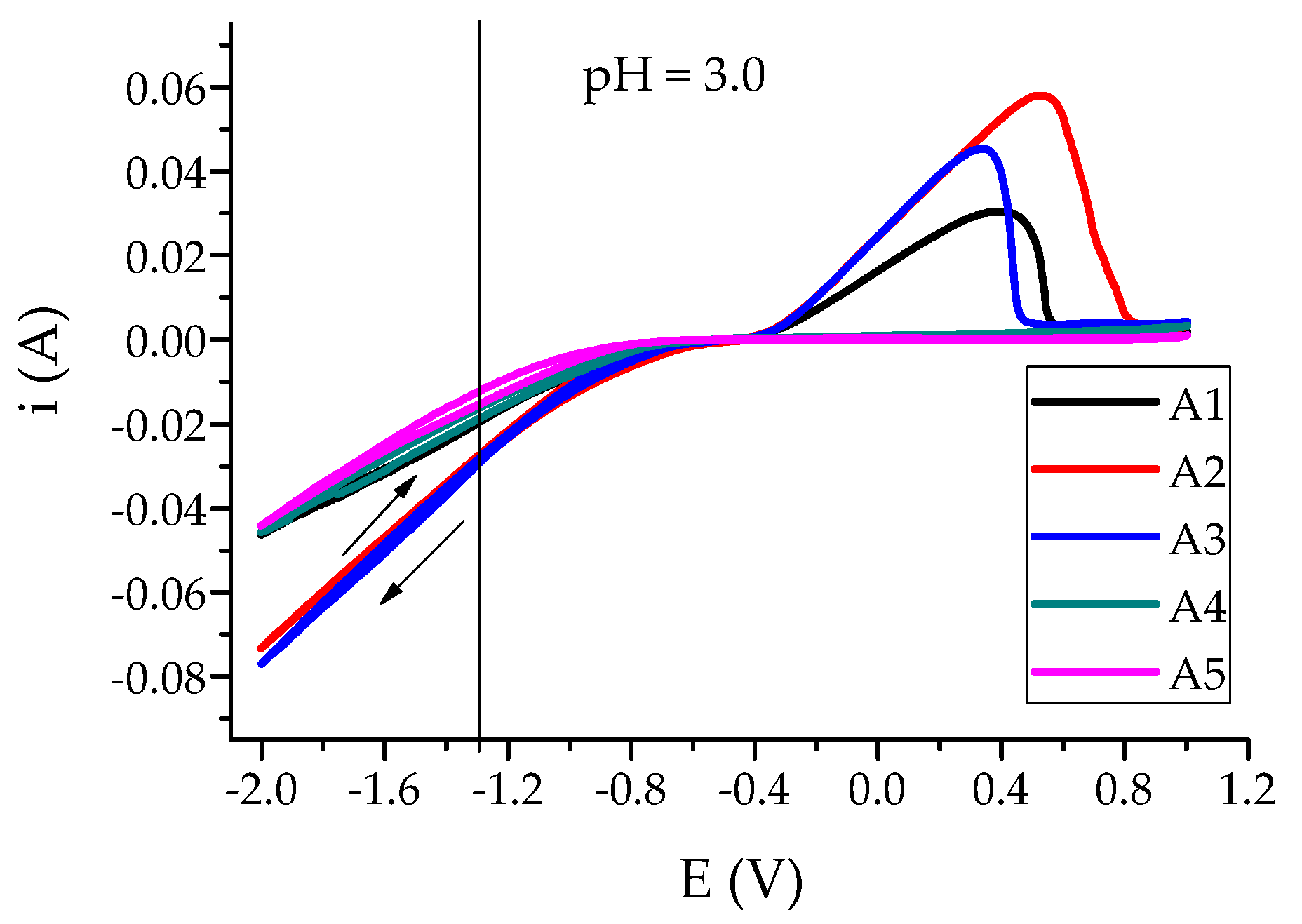
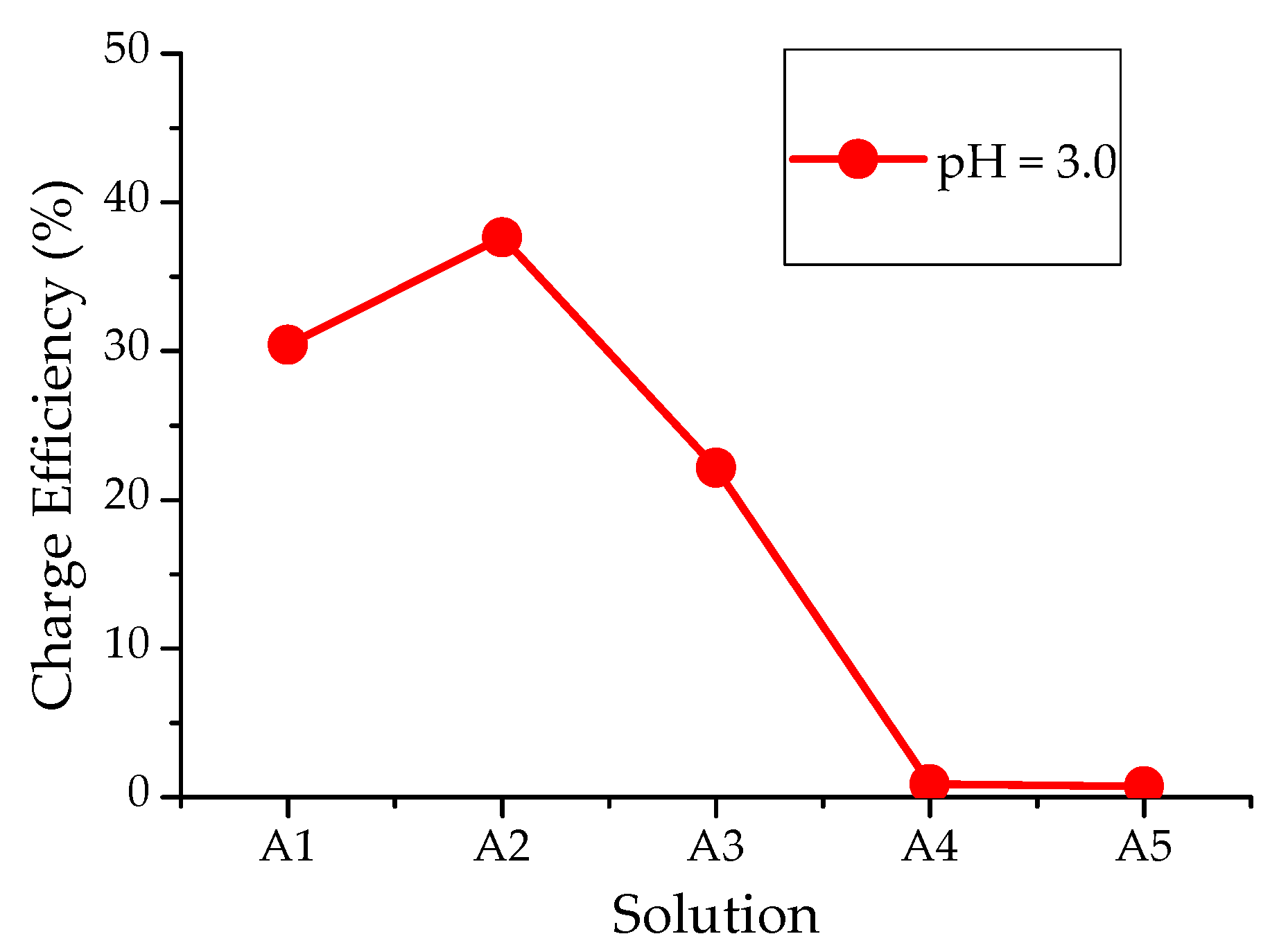
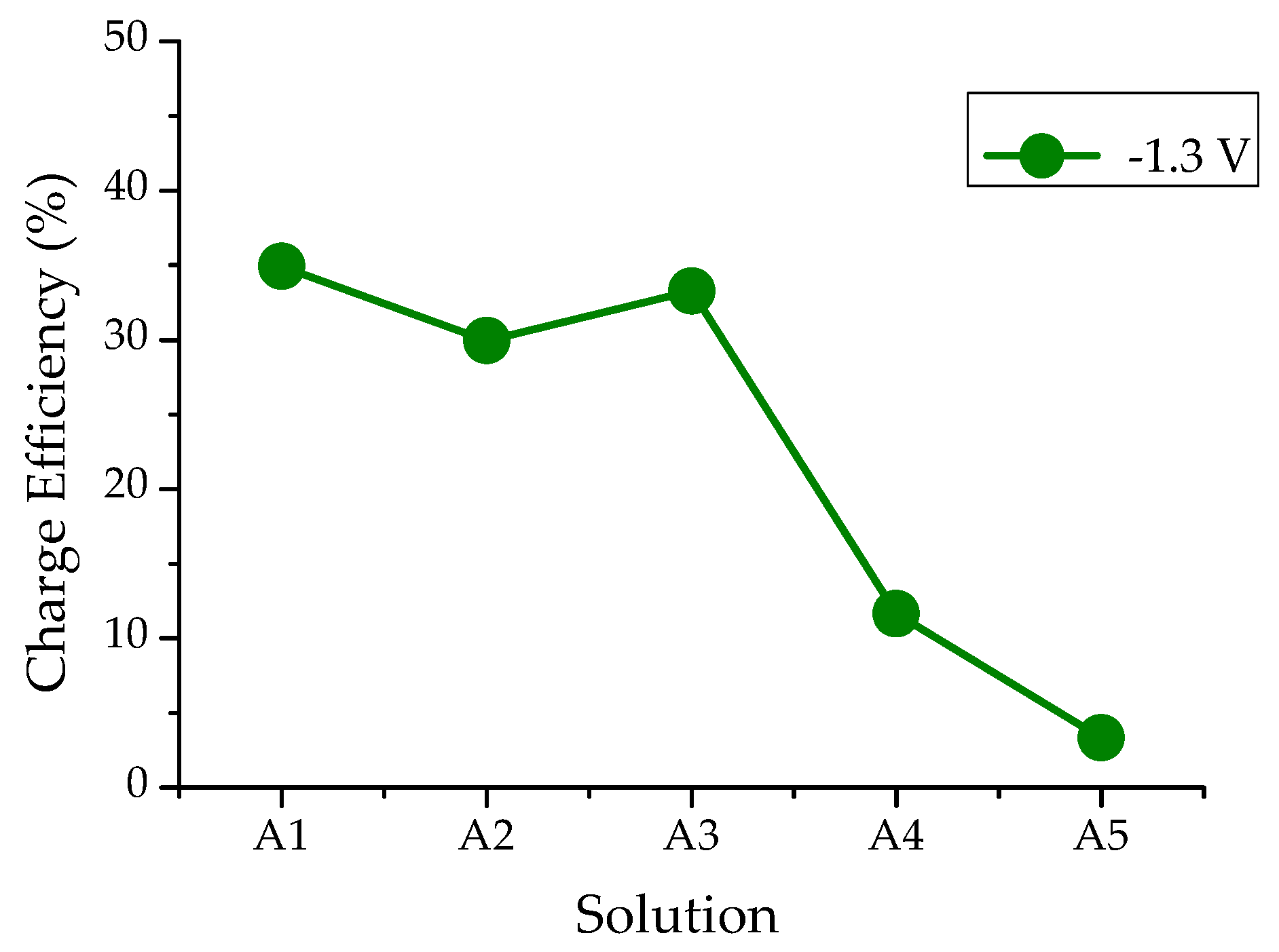
3.2. The Efficiency of Electrochemical Processes
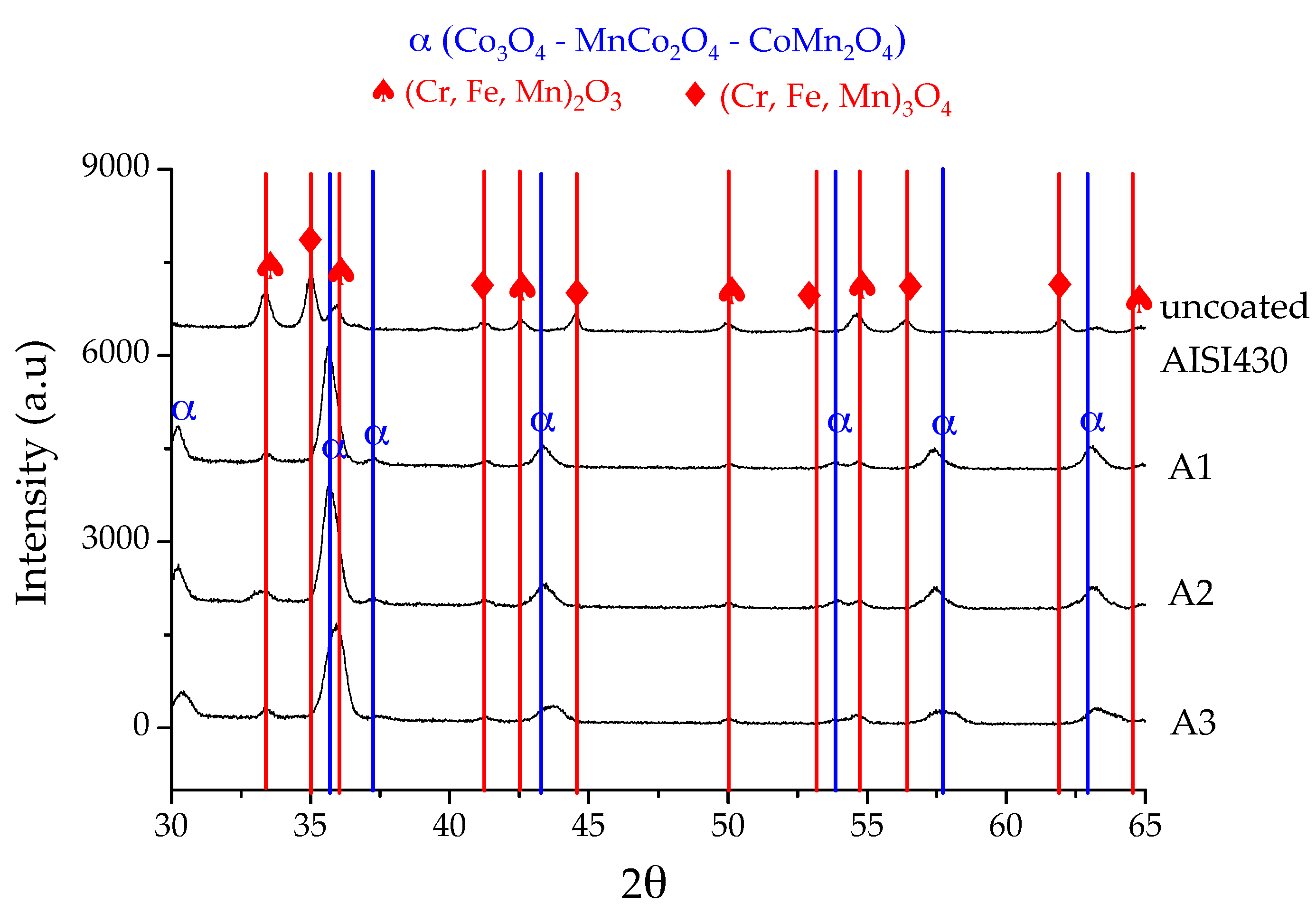
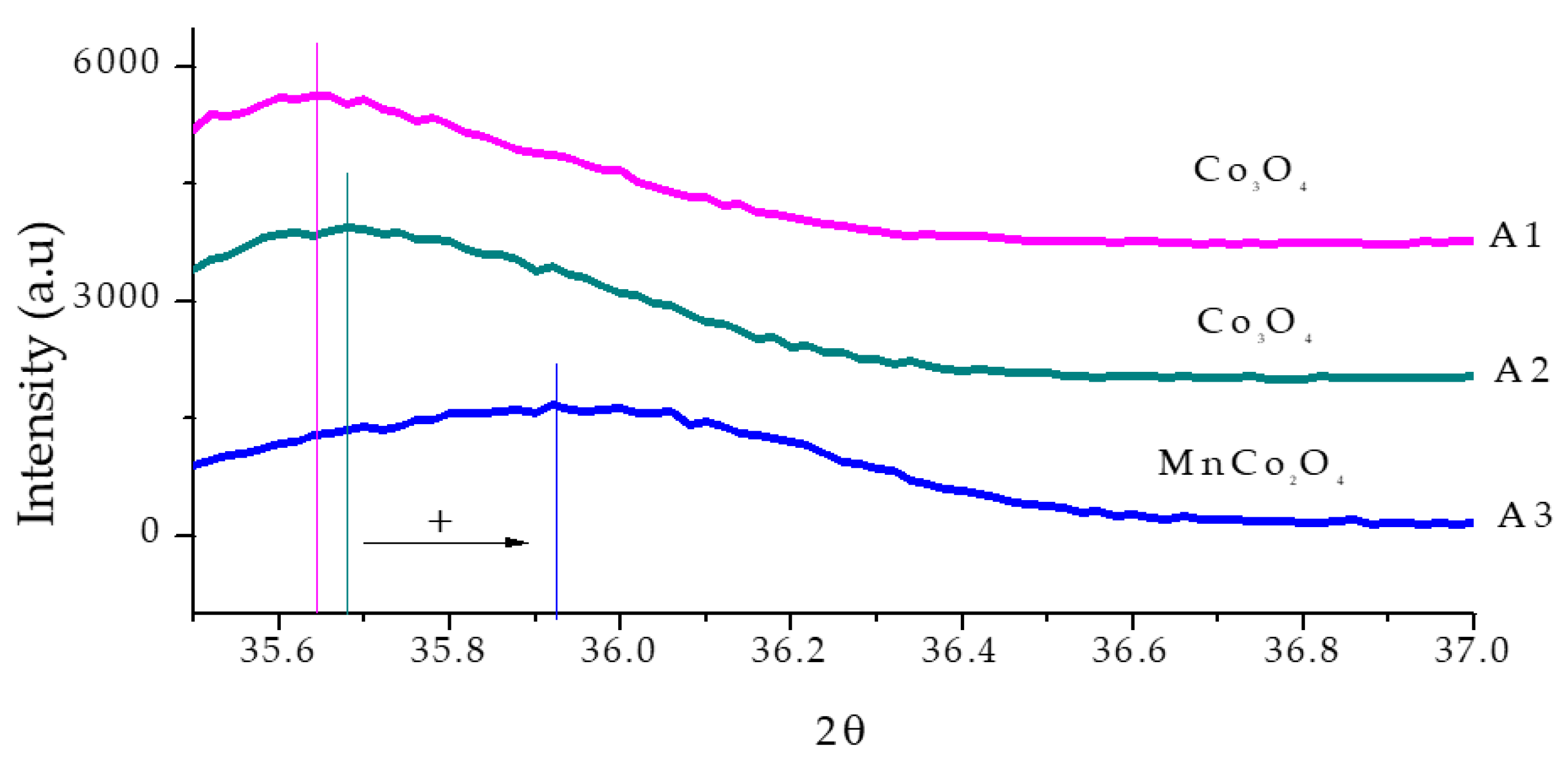
3.3. X-Ray Diffraction
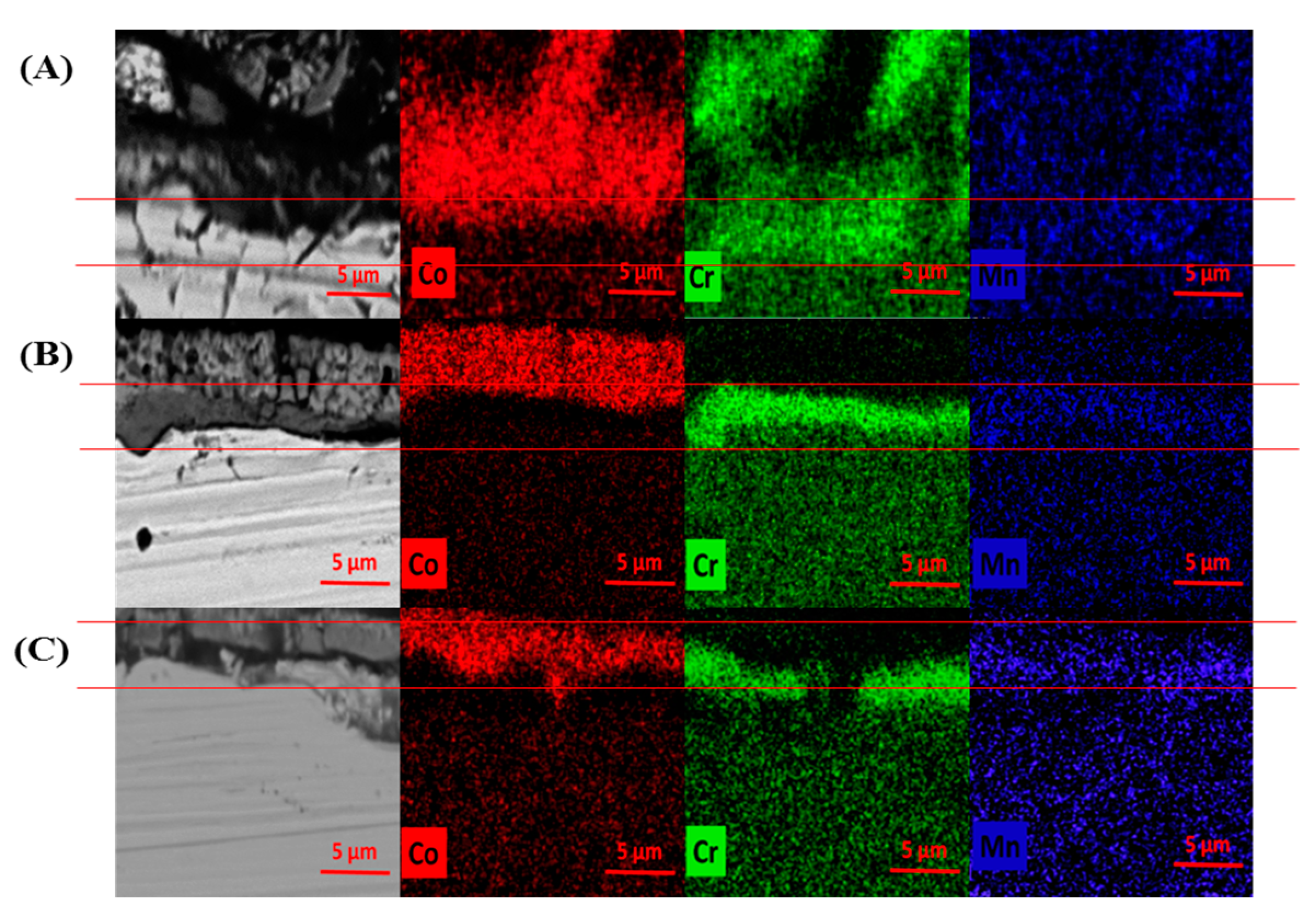
3.4. SEM and EDS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmud, L.S.; Muchtar, A.; Somalu, M.R. Challenges in fabricating planar solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2017, 72, 105–116. [Google Scholar] [CrossRef]
- Cooper, S.J.; Brandon, N.P. An Introduction to Solid Oxide Fuel Cell Materials, Technology and Applications. In Solid Oxide Fuel Cell Lifetime and Reliability: Critical Challenges in Fuel Cells; Brandon, N.P., Boldrin, P., Ruiz-Trejo, E., Eds.; Elsevier Ltd.: London, UK, 2017; pp. 1–18. ISBN 9780128097243. [Google Scholar]
- Kendall, K. History. In High-Temperature Solid Oxide Fuel Cells for the 21st Century; Kendall, K., Kendall, M., Eds.; Elsevier Ltd.: Birmingham, UK, 2016; pp. 25–50. ISBN 9780124104532. [Google Scholar]
- Janardhanan, V.M.; Deutschmann, O. Modeling of solid-oxide fuel cells. Z. Phys. Chem. 2007, 221, 443–479. [Google Scholar] [CrossRef]
- Ni, M. Modeling of solid oxide fuel cells. Sci. Bull. 2016, 61, 1311–1312. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Thallam Thattai, A.; Fan, L.; Lindeboom, R.E.F.; Spanjers, H.; Aravind, P.V. Solid Oxide Fuel Cells fuelled with biogas: Potential and constraints. Renew. Energy 2019, 134, 194–214. [Google Scholar] [CrossRef]
- Bednarz, M.; Molin, S.; Bobruk, M.; Stygar, M.; Długoń, E.; Sitarz, M.; Brylewski, T. High-temperature oxidation of the Crofer 22 H ferritic steel with Mn1.45Co1.45Fe0.1O4 and Mn1.5Co1.5O4 spinel coatings under thermal cycling conditions and its properties. Mater. Chem. Phys. 2019, 225, 227–238. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Molin, S.; Zhang, Y.; Chen, M.; Han, M. High temperature oxidation behavior of SUS430 SOFC interconnects with Mn-Co spinel coating in air. J. Alloys Compd. 2019, 787, 1327–1335. [Google Scholar] [CrossRef]
- Li, J.; Xiong, C.; Li, J.; Yan, D.; Pu, J.; Chi, B.; Jian, L. Investigation of MnCu0.5Co1.5O4 spinel coated SUS430 interconnect alloy for preventing chromium vaporization in intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 16752–16759. [Google Scholar] [CrossRef]
- Niewolak, L.; Tietz, F.; Quadakkers, W.J. Interconnects; Kendall, K., Kendall, M., Eds.; Elsevier: Jülich, Germany, 2016; ISBN 9780124104532. [Google Scholar]
- Yang, Z.; Weil, K.S.; Paxton, D.M.; Stevenson, J.W. Selection and Evaluation of Heat-Resistant Alloys for SOFC Interconnect Applications. J. Electrochem. Soc. 2003, 150, A1188–A1201. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- VDM ® Crofer 22 APU; Material Datasheet no. 4046; VDM Metals GmbH: Werdohl, Germany, 2010; p. 10.
- Jo, K.H.; Kim, J.H.; Kim, K.M.; Lee, I.; Kim, S. Development of a new cost effective Fe–Cr ferritic stainless steel for SOFC interconnect. Int. J. Hydrogen Energy 2015, 40, 9523–9529. [Google Scholar] [CrossRef]
- Hsu, C.; Yeh, A.; Shong, W.; Liu, C. Development of advanced metallic alloys for solid oxide fuel cell interconnector application. J. Alloys Compd. 2016, 656, 903–911. [Google Scholar] [CrossRef]
- Demeneva, N.V.; Kononenko, O.V.; Matveev, D.V.; Kharton, V.V.; Bredikhin, S.I. Composition-gradient protective coatings for solid oxide fuel cell interconnectors. Mater. Lett. 2019, 240, 201–204. [Google Scholar] [CrossRef]
- Bianco, M.; Linder, M.; Larring, Y.; Greco, F.; Van Herle, J. Lifetime issues for solid oxide fuel cell interconnects. In Solid Oxide Fuel Cell Lifetime and Reliability: Critical Challenges in Fuel Cells; Brandon, N.P., Boldrin, P., Ruiz-Trejo, E., Eds.; Elsevier Ltd.: London, UK, 2017; pp. 121–144. ISBN 9780128097243. [Google Scholar]
- Yeh, A.; Chen, Y.; Liu, C.; Shong, W. Development of an advanced bond coat for solid oxide fuel cell interconnector applications. J. Power Sources 2015, 296, 426–432. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, R.; Nikiforov, A.Y.; Gopalan, S.; Pal, U.B.; Basu, S.N. CuMn1.8O4 protective coatings on metallic interconnects for prevention of Cr-poisoning in solid oxide fuel cells. J. Power Sources 2018, 378, 125–133. [Google Scholar] [CrossRef]
- Stefan, E.; Neagu, D.; Blennow Tullmar, P.; Persson, Å.H.; Sudireddy, B.R.; Miller, D.; Chen, M.; Irvine, J. Spinel-based coatings for metal supported solid oxide fuel cells. Mater. Res. Bull. 2017, 89, 232–244. [Google Scholar] [CrossRef]
- Bopp, C.; Santhanam, K. Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge. ChemEngineering 2019, 3, 80. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrogen Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Hosseini, N.; Abbasi, M.H.; Karimzadeh, F.; Choi, G.M. Development of Cu1.3Mn1.7O4 spinel coating on ferritic stainless steel for solid oxide fuel cell interconnects. J. Power Sources 2014, 273, 1073–1083. [Google Scholar] [CrossRef]
- Grünwald, N.; Sohn, Y.J.; Yin, X.; Menzler, N.H.; Guillon, O.; Vaßen, R. Microstructure and phase evolution of atmospheric plasma sprayed Mn-Co-Fe oxide protection layers for solid oxide fuel cells. J. Eur. Ceram. Soc. 2019, 39, 449–460. [Google Scholar] [CrossRef]
- Molin, S.; Sabato, A.G.; Bindi, M.; Leone, P.; Cempura, G.; Salvo, M.; Cabanas Polo, S.; Boccaccini, A.R.; Smeacetto, F. Microstructural and electrical characterization of Mn-Co spinel protective coatings for solid oxide cell interconnects. J. Eur. Ceram. Soc. 2017, 37, 4781–4791. [Google Scholar] [CrossRef]
- Molin, S.; Jasinski, P.; Mikkelsen, L.; Zhang, W.; Chen, M.; Hendriksen, P.V. Low temperature processed MnCo2O4 and MnCo1.8Fe0.2O4 as effective protective coatings for solid oxide fuel cell interconnects at 750 °C. J. Power Sources 2016, 336, 408–418. [Google Scholar] [CrossRef]
- Wu, J.; Gemmen, R.S.; Manivannan, A.; Liu, X. Investigation of Mn/Co coated T441 alloy as SOFC interconnect by on-cell tests. Int. J. Hydrogen Energy 2011, 36, 4525–4529. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M.; Karaagac, O.; Haciismailoglu, M. Electrodeposited Ni-Co films from electrolytes with different Co contents. Appl. Surf. Sci. 2012, 258, 4005–4010. [Google Scholar] [CrossRef]
- Karpuz, A.; Kockar, H.; Alper, M. Properties of electrodeposited Co–Mn films: Influence of deposition parameters. Appl. Surf. Sci. 2015, 358, 605–611. [Google Scholar] [CrossRef]
- Chen, G.; Xin, X.; Luo, T.; Liu, L.; Zhou, Y.; Yuan, C.; Lin, C.; Zhan, Z.; Wang, S. Mn1.4Co1.4Cu0.2O4 spinel protective coating on ferritic stainless steels for solid oxide fuel cell interconnect applications. J. Power Sources 2015, 278, 230–234. [Google Scholar] [CrossRef]
- Puranen, J.; Laakso, J.; Honkanen, M.; Heinonen, S.; Kylmälahti, M.; Lugowski, S.; Coyle, T.W.; Kesler, O.; Vuoristo, P. High temperature oxidation tests for the high velocity solution precursor flame sprayed manganese–cobalt oxide spinel protective coatings on SOFC interconnector steel. Int. J. Hydrogen Energy 2015, 40, 6216–6227. [Google Scholar] [CrossRef]
- Sobha Jayakrishnan, D. Electrodeposition: The versatile technique for nanomaterials. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Woodhead Publishing Limited: Cambrigde, UK, 2012; pp. 86–125. ISBN 9781845699499. [Google Scholar]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Olukanni, D.; Aipoh, A.; Kalabo, I. Recycling and Reuse Technology: Waste to Wealth Initiative in a Private Tertiary Institution, Nigeria. Recycling 2018, 3, 44. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.E.; Olasehinde, F.E.; Adebayo, O.A.; Ajayi, O.J.; Ghosh, M.K.; Basu, S. Extraction of Copper and Zinc from Waste Printed Circuit Boards. Recycling 2019, 4, 36. [Google Scholar] [CrossRef]
- Tran, A.T.; Tomlin, J.; Lam, P.H.; Stinger, B.L.; Miller, A.D.; Walczyk, D.J.; Cruz, O.; Vaden, T.D.; Yu, L. Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering 2019, 3, 81. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Garcia, E.; Taroco, H. Water Electrolysis Anode Based on 430 Stainless Steel Coated with Cobalt Recycled from Li-Ion Batteries. Recycling 2018, 3, 42. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Celante, V.G.; Pietre, M.K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits. J. Power Sources 2010, 195, 3309–3315. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Garcia, E.M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Garcia, E.M.; Santos, J.S.; Pereira, E.C.; Freitas, M.B.J.G. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique. J. Power Sources 2008, 185, 549–553. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering valuable metals from LiNi x Co y Mn 1-x-y O 2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Apelt, S.; Zhang, Y.; Zhu, J.H.; Leyens, C. Electrodeposition of Co–Mn3O4 composite coatings. Surf. Coatings Technol. 2015, 280, 208–215. [Google Scholar] [CrossRef]
- Pinto, R.; Carmezim, M.J.; Montemor, M.F. Electrodeposition and isothermal aging of Co and Mn layers on stainless steel for interconnectors: Initial stages of spinel phase formation. J. Power Sources 2014, 255, 251–259. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Johnson, C.; Liu, X. DC electrodeposition of Mn–Co alloys on stainless steels for SOFC interconnect application. J. Power Sources 2008, 177, 376–385. [Google Scholar] [CrossRef]
- Yar-Mukhamedova, G.; Ved, M.; Sakhnenko, N.; Nenastina, T. Electrodeposition and properties of binary and ternary cobalt alloys with molybdenum and tungsten. Appl. Surf. Sci. 2018, 445, 298–307. [Google Scholar] [CrossRef]
- Lv, Y.; Geng, S.; Shi, Z. Effect of pH of the galvanic bath on electrodeposition of Cu-Mn3O4 composite coatings. Mater. Chem. Phys. 2017, 189, 176–182. [Google Scholar] [CrossRef]
- Talic, B.; Molin, S.; Wiik, K.; Hendriksen, P.V.; Lein, H.L. Comparison of iron and copper doped manganese cobalt spinel oxides as protective coatings for solid oxide fuel cell interconnects. J. Power Sources 2017, 372, 145–156. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Yun, L.L.; Wei, T.; Li, C.X.; Qi, Z.; Yang, G.J.; Li, C.J.; Liu, M. Aerosol sprayed Mn 1.5 Co 1.5 O 4 protective coatings for metallic interconnect of solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 20305–20313. [Google Scholar] [CrossRef]



| Element | Average Price (2018) U$/t |
|---|---|
| Cobalt | 80.490 |
| Manganese | 2.060 |
| Aluminum | 1.885 |
| Nickel | 8.931 |
| Iron | 57,86 |
| Fe (%m) | C (%m) | Mn (%m) | Si (%m) | Cr (%m) | Ni (%m) | Mo (%m) | Nb (%m) | Ti (%m) | N (ppm) | |
|---|---|---|---|---|---|---|---|---|---|---|
| AISI430 | Bal. | 0.047 | 0.23 | 0.32 | 16.22 | 0.27 | 0.02 | 0.012 | 0.003 | 518 |
| Solution | Co (mol/L) | Mn (mol/L) |
|---|---|---|
| S1 | 0.17 | 0 |
| S2 | 0 | 0.19 |
| A1 | 0.04 | ~0 |
| A2 | 0.03 | 0.01 |
| A3 | 0.02 | 0.02 |
| A4 | 0.01 | 0.03 |
| A5 | ~0 | 0.04 |
| mol/L | Mn | Co | Fe | Ni |
|---|---|---|---|---|
| Battery 1 | 0.1699 | * | * | 0.0001 |
| Battery 2 | 0.0005 | 0.1868 | * | 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, S.L.A.; Garcia, E.M.; Tarôco, H.A.; Matencio, T. Obtaining Mn-Co Alloys in AISI 430 Steel from Lithium-Ion Battery Recycling: Application in SOFC Interconnectors. ChemEngineering 2020, 4, 10. https://doi.org/10.3390/chemengineering4010010
Gonçalves SLA, Garcia EM, Tarôco HA, Matencio T. Obtaining Mn-Co Alloys in AISI 430 Steel from Lithium-Ion Battery Recycling: Application in SOFC Interconnectors. ChemEngineering. 2020; 4(1):10. https://doi.org/10.3390/chemengineering4010010
Chicago/Turabian StyleGonçalves, Sicele L. A., Eric M. Garcia, Hosane A. Tarôco, and Tulio Matencio. 2020. "Obtaining Mn-Co Alloys in AISI 430 Steel from Lithium-Ion Battery Recycling: Application in SOFC Interconnectors" ChemEngineering 4, no. 1: 10. https://doi.org/10.3390/chemengineering4010010
APA StyleGonçalves, S. L. A., Garcia, E. M., Tarôco, H. A., & Matencio, T. (2020). Obtaining Mn-Co Alloys in AISI 430 Steel from Lithium-Ion Battery Recycling: Application in SOFC Interconnectors. ChemEngineering, 4(1), 10. https://doi.org/10.3390/chemengineering4010010





