Abstract
Considering works published in the literature for more than a decade (period from January 2008 till June 2019), this paper provides an overview of recent applications of the so-called “solar furnaces”, their reactors, process chambers and related devices, aiming specifically at the processing of (solid) materials. Based on the author’s own experience, some prospects on future trends are also presented. The aim of this work is to demonstrate the tremendous potentialities of the usage of solar heat for materials processing, but also to reveal the necessity of further developing solar-driven high-temperature technologies (which are required to displace the use of electricity or natural gas). In particular, it is essential to improve the temperature homogeneity conditions inside reaction chambers for materials processing using solar heat. Moreover, new innovative modular systems, practical and flexible, for capture, concentration, control and conduction of concentrated solar radiation are suggested. Solar thermal technologies for the production of electricity, as well as solar thermochemical processes for production of gases or liquids, are outside the scope of this review.
1. Introduction
Interaction between solar light/radiation and matter is studied and applied at least since the Classic Antiquity. During the siege of Syracuse (circa 213–212 BC), Archimedes may have used mirrors acting collectively as a parabolic reflector to concentrate the sun’s rays in order to burn the Roman ships attacking the city. Nowadays, a single Fresnel lens, available on the market, for example, with dimensions 1400 × 1050 mm, fabricated from acrylic glass (PMMA polymer) and mounted in a structure equipped with an automatic sun-tracking system, is capable of concentrating the radiation on a small focal spot of approximately 8 to 10 mm diameter, and then temperatures of circa 1500 °C, or even higher, are easily attainable depending on the characteristics of the material that is being irradiated.
Either for residential, commercial, or industrial applications, solar heating and cooling technologies are available to provide hot water, space heating and cooling, pool heating, etc., but, on the contrary, the so-called high-concentration or high-flux solar applications, which pertain to the attainment of higher temperatures, are much less used. In fact, the current state-of-the-art on the use of concentrated solar energy applied to materials science and metallurgy is far from being so widely known. The main reason for it being less widespread is because high-concentration solar furnaces or other high-flux devices are relatively more difficult to build in order to operate under steady conditions. Although available in several labs distributed throughout the world, until now all high-flux solar furnaces have been designed and constructed individually, i.e., on a one-by-one basis because there are several possible optical configurations [1] which must take into account the geographical location [2] and the maximum power to be achieved.
Until now, the so-called “solar furnaces” are mainly point-focusing solar concentration facilities located throughout the world at universities, research institutes, and companies, operating at concentrations relevant to solar thermochemistry, materials processing and thermal treatments. The design of solar furnaces is mainly based on the concepts of geometrical optics, or ray optics, that describe light propagation uniquely in terms of rays [1].
The origin of current solar furnaces dates back to the beginning of the 20th century, when the Portuguese Catholic priest Manuel António Gomes created and patented “an apparatus for making industrial use of the heat of the sun and obtaining high temperatures such as are required in metallurgical and chemical researches” [3,4]. He was a very tall man, and for this reason was nicknamed ‘‘Father Himalaya”. In both his British [3] and United States [4] patents, Manuel “Himalaya” described his invention as comprising a parabolic reflecting-surface arranged to cause solar rays to converge upon a confined focus placed in the centre of a furnace, crucible, or other receiver. Two of the figures included in the Manuel Himalaya’s British patent application are depicted in Figure 1. For the 1904 World’s Fair of St. Louis (Missouri, MO, USA), he built a huge device he called the Pyreliophorus, which was apparently similar to a large convex lens that can concentrate the sun’s rays onto a small area, but in which thousands of mirrors over a surface of 80 m2 concentrated solar energy up to a temperature of around 3500 °C, enough to melt many different types of materials, including metals and rocks. The Pyreliophorus installation was one of the main attractions at the Saint Louis world’s fair, where it was awarded with two gold medals and one silver medal [5,6].
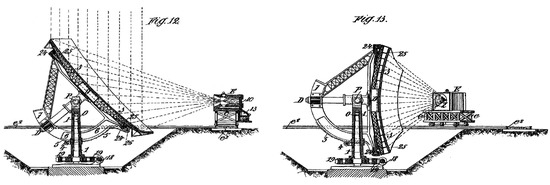
Figure 1.
Two figures of the Manuel A. Gomes Himalaya’s British patent application, dated 1901 [3].
Manuel Himalaya’s British [3] and United States [4] accepted patents (“for making industrial use of solar heat, particularly in the metallurgical and chemical arts which necessitate the use of temperatures higher than those of ordinary furnaces, including the electrical furnace”) date back to 1901 and 1905, respectively, and during those years he resided in France. More than 40 years later, and by an initiative of the French chemist Félix Trombe and his team, a so-called solar furnace was built (circa 1950) at the town of Mont-Louis, a commune in the French Pyrénées-Orientales. Then, based on the 50-kW prototype built in Mont-Louis and using the results obtained there, a much more powerful solar furnace of 1000 kW was designed to be placed (not very far from Mont-Louis) at Odeillo (Pyrénées-Orientales and Cerdagne near the Spanish border in the south of France). Work on the construction of the Odeillo solar furnace lasted from 1962 to 1968 and was commissioned in 1970 [7]. Nowadays, this 1 MW solar furnace continues serving as an installation for studying materials at very high temperatures.
Instead of providing a state-of-the-art review of the development of Concentrated Solar Power (CSP) technologies over the last decade [8] or analysing the so-called “solar thermochemical processes” [9], this review intends to highlight the most recent achievements, but also shortcomings of the existing solar furnaces to be used for materials processing. Based on the author’s own experience, some guidelines, proposed solutions, and prospects for future research work will be also presented.
2. Other Recent Overviews
A review conducted by Bushra and Hartmann [10] has shown that most articles published on reflective two-stage solar concentrators deal with applications for power generation using solar cells, and thermo-electric generators. In a work published in 2017, Levêque et al. [11] reviewed the designs and characteristics of high-flux optical systems (HFOSs), as well as challenges and opportunities in the area of HFOSs for solar thermochemical applications. This review [11] provides an exhaustive list of point-focusing on-sun HFOSs, showing the characteristics and peak fluxes of existing solar furnaces worldwide (at universities, research institutes, and companies) which use real sunlight, but also of indoor solar simulators. The discrepancy between the spectral power distributions of the solar simulators compared to real solar radiation is normally considered a reasonable compromise, in order to allow for easily controlled and repeatable experiments. When the radiation is provided by xenon arc bulbs, some significant divergence occurs in the infra-red region due to the Xe emission lines, as discussed by Petrasch et al. [12] (2007) and Alxneit and Schmit [13] (2012).
More recently (2018), the state-of-the-art technology on the use of concentrated solar energy applied to materials science and metallurgy has been comprehensively reviewed by Fernández-González et al. [14], and it was concluded that solar energy offers a great potential in applications of high temperature. As opposed to conventional processes used in metallic and non-metallic materials processing (in which the higher the temperature, the higher the energy consumption), concentrated solar energy has costs that are not dependent on the temperature, and in this way, could be competitive with other high-energy technologies (laser, plasma, etc.). The most known drawback of solar energy is the impossibility of working 24 h per day because of the sun availability, but the work of Fernández-González et al. [14] has pointed out that concentrated solar energy could find application in the recovery of wastes, as well as in the processing of a short series of products (as for instance in obtaining of hard refractory ceramics), high purity materials (as for instance the production of lime for the chemical and pharmaceutical industries) or in materials recently discovered (as for instance fullerenes and carbon nanotubes). However, it is worthwhile to note remarks made by the authors [14]: “nowadays, the interest of solar energy is mainly focused on the field of energy, both thermal and electric, except for several research projects where the possible applications of solar energy in materials science are explored. The problem observed in most of these researches connected with materials science is the lack of continuity (not in all of them but is the general keynote), meaning that a certain field is explored and then abandoned, without an attempt of scaling up to pilot plant or industrial scale (searching for a commercial application). The reason for abandoning the topic is perhaps the lack of interest by the industrial companies (generally solar processes are competitive in quality, time and temperatures with traditional methods in a laboratory scale but they are not industrially proved, and apart from that, these traditional methods are well known and currently installed in competitive plants) or the lack of promising results (but this question was not observed, in general). In fact, few projects were scaled from laboratory to pilot scale, and none of them are commercially used on an industrial scale”.
Two other review works, by Alonso et al. [15] and by Ho [16], published in 2017 and 2016, respectively, are also worth being mentioned. Alonso et al. [15] reviewed the rotary kiln technology and focused on the employment of these devices for thermal and thermochemical processes conducted by concentrating solar energy. Among the solar devices, a novel rotary kiln prototype for thermochemical processes was presented and compared with a static solar reactor. In fact, rotary movement favours the radiation heat transfer; then, some practical conclusions on the design and operation of solar rotary kilns are remarked upon, and an analysis of their current limitations is presented.
The review work authored by Ho [16] provides an analysis of high-temperature particle receivers for concentrating solar power. It reveals the concern of the technical-scientific community to find out more efficient ways to generate high temperatures and to control them adequately. Although most studies are focused in the production of electricity by concentrating solar power in a receiver located at the top of a central tower (i.e., concentrating solar power tower technologies), the use of advanced receivers, of fluidized bed type or of the so-called “particle receivers”, may contribute to the evolution of the technologies for other important industrial sectors that need the attainment of high temperature. The falling particle receivers use solid particles that are heated directly as they fall through a beam of concentrated sunlight, with particle temperatures capable of reaching 1000 °C and higher. There are several alternative designs for the particle receivers, which include: free-falling, obstructed flow, centrifugal, flow in tubes with or without fluidization, multi-pass recirculation, north- or south-facing, and face-down configurations [16].
Furthermore, in 2018, a bibliographic analysis was carried out by Rosa & Rosa [17] on the database named Web of Science™ Core Collection, and the searches were done year by year, starting in 2008, i.e., with PY = Year Published = 2008, and ending with PY = 2017. This bibliographic analysis [17] considering the period from January 2008 till December 2017, aimed to provide a vision of recent applications of the so-called solar furnaces, reactors or process chambers and corresponding accessories, used for physical, chemical or metallurgical processes requiring high (>400 °C) and very high (>1500 °C) temperatures. Research works/publications dedicated to the production of electricity by solar-thermal or photovoltaic technologies, as well as other research topics like those dealing with desalinization technologies, and works aiming for the development of systems for thermal storage, namely those that make use of molten salts or phase change materials (PCMs), were not included in the metrics of the bibliographic analysis [17]. The results of the analysis have indicated the main topics under investigation, the institutions and countries involved, as well as the scientific journals where the works have been published. It could be also concluded that the usage of concentrated solar radiation to obtain high (>400 °C) and very high (>1500 °C) temperatures, needed for physical, chemical or metallurgical processes, is presently characterized by a low percentage of publications in comparison to the other research topics in which solar energy is also used.
3. Examples of Innovative Topics of Research
3.1. Process Chambers for the Thermal Dissociation of ZnO
Design of the solar receiver, reactor or process chamber, is critically important to the utilization of solar power to drive a high temperature thermal or thermochemical process. In a review article published in 2017 by Koepf et al. [18], relevant solar reactor development projects were summarized and compared.
In fact, concerning the development of process chambers for solar thermal applications, the research studies and projects carried out by groups based in Switzerland should be highlighted, particularly those dealing with the thermal dissociation of ZnO. The objective is to perform a redox cycle in two-steps: (1) the endothermal dissociation of ZnO to Zn and O2 above 2000 K using concentrated solar energy, and (2) the subsequent oxidation of Zn with H2O/CO2 to produce H2/CO, [19]. In fact, the production of Zn from ZnO in a high temperature solar decomposition process offers a thermodynamically efficient path for converting solar energy into storable and transportable fuels. In the process, the oxide is decomposed into its elements at a temperature above 2000 K. Zn, recovered after quenching the product gases, can be used directly as a fuel in a fuel cell or battery. In summary, it is suggested that when hydrogen is needed to supply a market, Zn should be used to split water to hydrogen in an exothermic reaction; the by-product of either the power generation or the water-splitting reaction is ZnO which would be recycled to the solar process [20,21].
3.2. Solar Heat for Glass Production/Melting
The abundance of silicate raw materials on the moon, together with the extremely high cost of transporting materials from earth, has certainly motivated NASA (as early as in 1979) to consider the use of concentrated solar radiation for in-situ glass production from lunar based materials [22]. The raw material of lunar origin is lunar regolith, from which plagioclase concentrate, high silica content slag, and calcium oxide are obtained. More recently (2014), the study of the feasibility of using concentrated solar radiation to provide process heat for glass production/melting was revisited by Ahrnad et al. [23], in this case using a high-flux solar simulator. The research included static, as well as semi-continuous melting experiments. Initial proof-of-concept static melting experiments involved melting a powdered, ternary soda-lime-silica glass forming batch which demonstrated that rapid and full conversion of the crystalline raw materials into an X-ray amorphous vitreous state was possible with rapid gas removal, resulting in a completely transparent glass. Further experiments to investigate the feasibility of generating a semi-continuous flow of molten glass using the concentrated radiation were conducted, and a series of semi-continuous glass melting experiments were completed to iteratively optimise pellet feed rate, radiant power intensity and crucible geometry. After the small-scale demonstration, the authors have concluded that, due to the non-linearity of productivity and efficiency on scale-up, significant further experimental work on a larger scale is required before the commercial feasibility of solar powered glass melting can be evaluated to any reasonable degree of certainty [23].
A porous glass body is called a frit. Frits are the main component of nearly all ceramic glazes, and they are also present in many compositions of different materials where a glassy phase is needed as binder. The production of frits is conducted in continuous melting furnaces and common temperatures in furnaces ranging between 1350 and 1550 °C. Once the raw materials batch is melted, it is cooled on water at high cooling rate; thus, the melt solidifies as small pieces of glass. This melting process implies a significant energy consumption and low efficiency and productivity of the process, but recently Romero et al. [24] have demonstrated, at a laboratory scale, the feasibility of applying real concentrated solar radiation to achieve the energy needed for performing, in only one step, the preparation of glass frits of different typology from the raw materials. The process includes decarbonation, melting and homogenization of molten fluids. Five glass compositions were formulated with the aim of preparing different types of commercial frits. The raw materials used to prepare the glasses were silica sand, with low contents of iron oxide and reagent grade oxide such as Al2O3, B2O3, ZnO, PbO and ZrO2. To complete the glasses composition, alkali and alkaline earth elements were introduced as reagent grade carbonates [24].
3.3. Solar Heat for Lime and Portland Cement Clinker Production
The primary natural CO2 sources include ocean release, animal and plant respiration, organic matter decomposition, forest fires and volcanic eruptions, while the primary anthropogenic CO2 sources include fossil fuel burning, cement production, and farmland ploughing. Concrete is used more than any other manmade material in the world, and is the second most consumed substance behind water [25]. Just to remember, concrete is composed of cement, fine aggregates (sand) and coarse aggregates mixed with water which hardens with time. Portland cement is the commonly used type of cement for production of concrete. The global production of Portland cement was estimated to be roughly 4.05 Gt in 2017 and 4.10 Gt in 2018 [26]. The manufacturing of Portland cement, concrete’s key ingredient, contributes significantly to the global carbon dioxide emissions. Most CO2 emissions from cement manufacture originate from de-carbonization of limestone and burning fossil fuels [27] and are responsible for about 5% of the global anthropogenic CO2 emissions and 7% of industrial fuels use, respectively [28,29]. Total emissions from the cement manufacturing sector can, however, contribute as much as 8% of the total global anthropogenic CO2 emissions [30,31], of which 50% is derived from the calcination process and 40% from burning of fuel [32,33]. Emission of CO, NOX and SO2 from the cement industry contributes also to greenhouse and acid rain effects [34]. So, alternative fuel sources [35,36] and novel clinker formulations with less limestone [37,38] ought to be implemented. Also, by allowing concrete strength development to occur with a higher concrete design age, potentially less cement is necessary to meet the concrete strength requirements [39].
To reduce CO2 emissions and energy requirements, several authors [40,41,42] have considered that the endothermic calcination of limestone (CaCO3) to produce lime (CaO) may be conveniently carried out with solar heat. An interesting approach consisted of using a directly irradiated fluidized bed reactor to produce lime suitable for Portland cement formulation [42].
Recent work by Oliveira et al. [43] has demonstrated the viability of producing grey Portland cement clinker upon direct exposure of the raw materials under concentrated solar radiation. White clinker, in turn, could not be produced by direct irradiation in the used test setup conditions because of its low absorptance of solar energy. In the frame of this research work, a conceptual design of a reactor for producing Portland cement using concentrated solar energy was presented, as it is depicted in Figure 2. A cylindrical solar reactor with an internal conical shape is envisaged to achieve such a goal. Concentrated heat would be supplied so that the material fed on the back side, by a screw-type device, would enter the reaction chamber, turning in a similar fashion as that used in conventional rotary kilns, kept inside for suitable heating treatment, and then exiting in the discharge end where either water and/or air jets would quench the clinker. The gases of the clinkerization process ought to be collected to recover the heat and post-treat the CO2 formed. Owing to its inherent reflectance, solar white clinker requires higher process temperatures in excess of 1600 °C, and thence the necessity of using either a solar furnace with higher output power, or the indirect heating approach [43].
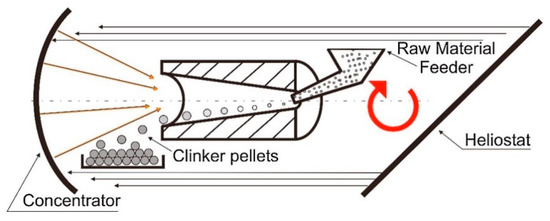
Figure 2.
Scheme of conceptual design for solar clinker production [43].
3.4. Solar Heat for Treatment of Waste Materials
Solar heat is also being used for treatment of solid waste materials, and numerous solar-driven solid waste treatments and conversion methods have been developed. A review work published in 2016 by Ugwuishiwu et al. [44] summarizes the main applications, e.g., solar incineration of Municipal Solid Waste; solar solid waste pyrolysis (pyrolysis is the thermal degradation of carbon-based materials through the use of an indirect, external source of heat, typically at temperatures of 450 °C to 750 °C, in the absence or almost complete absence of free oxygen); solar solid waste gasification (gasification is a partial oxidation process which produces a composite gas, syngas, comprised primarily of hydrogen and carbon monoxide). Depending of the type of residues, which may include several types of inedible crop residues, the gasifier operating temperature may range from 760 °C to 1540 °C [45,46,47].
3.5. Opportunities for Solar Heat in the Minerals Processing and Metallurgical Industries
The application of concentrated solar thermal heat in the minerals processing field can also lower energy costs and reduce greenhouse gas emissions. Several types of ores or mine residues have been already treated at a pilot scale using solar thermal heat for the experiments, and here are some examples:
- Successful results were obtained from solar thermal treatment of mercury mine wastes at temperatures higher than 400 °C in order to lower the Hg content [48];
- Thermal decomposition and preheating of manganese ores with solar thermal energy was conceptually proven at temperature measurements up to circa 1000 °C [49];
- The potential for alumina to be calcined with concentrated solar thermal heat has been assessed with a packed bed of boehmite (an aluminium oxyhydroxide) in a crucible positioned at the focal plane of a Fresnel concentrator by Padilla et al. [50]. The solar installation provided a power density of 260 W·cm−2 which allowed reaching temperatures higher than 1000 °C at few minutes of exposure. They reported 75% conversion after 10 min of exposure to solar radiation and the complete dehydration of boehmite, Al(OH)3, and its conversion to alumina, Al2O3, was attained after 90 min of solar radiation exposure under the static conditions described in the work [50]. Based on the fact that modern plants typically process alumina within flash calciners using particles of ~100 μm in diameter transported in a gas suspension through the reactor with residence times on the order of a few seconds, Davis et al. [51] have used a solar vortex transport reactor to processes powders of similar size to a flash calciner. They reported chemical conversion from aluminium hydroxide, Al(OH)3, to aluminium oxide or gibbsite, Al2O3, of up to 95.8% at nominal reactor temperatures over the range 890–1280 °C, and nominal residence times of approximately 3 s [51]. It is therefore proven that it is technically possible to calcine alumina without combustion and its concomitant CO2 emissions, at least during those periods when the solar resource is available. And, it is worth to remember that alumina is an intermediate product in the production of aluminium, but it is also a product in its own right. The industrial gibbsite calcination process shares similarities with the calcination of limestone, which takes place at a slightly lower range of temperature [40,41,42].
- Studies sponsored by CSIRO (Australia’s national science research agency) have identified the potential to use solar in high-temperature processing of ores such as bauxite, copper and iron ore [52]. Solar thermal energy works best at temperatures between 800 °C and 1600 °C [53] which can be achieved with existing technology. It should be noted that traditionally the conversion of heat to electricity generally operates below 600 °C.
- As an example of a high-temperature solar process allowing direct thermal route from the ore to metal, there is a recent publication [54] reporting the feasibility of using concentrated solar energy to the reduction of copper (II) oxide to metallic copper, in hydrogen atmosphere. Using a 1.5 kW thermal power vertical axis parabolic concentrator, the successful experiments were carried out using a stream of gaseous mixture 5/95 v/v H2/N2 for the reduction of CuO in H2.
- Carrying out the solar experiments also in a stream of gas, which in this case was uncracked ammonia NH3 gas (NH3 gas with suppressed extent of dissociation by flowing), other researches [55] have shown that higher nitrides of Mo (δ-MoN) and Fe (ε-Fe2N) can be successfully synthesized. For that they have developed an experimental setup consisting of a linear reaction tube made of silica glass and sample holder made of refractory steel in order to carry out nitriding experiments for powder specimens of Mo and Fe in uncracked NH3 gas at specified linear flow rate under irradiation of concentrated solar beam.
3.6. Integration of Solar Heat in the Regenerative Calcium Cycle
Integration between solar thermal technology and the “calcium looping” technology is a current topic of research undertaken by several research groups. Calcium looping (CaL), also named “the regenerative calcium cycle”, is a carbon capture technology in the form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). CaL is composed by two main steps: the forward, endothermic step is called calcination, while the backward, exothermic step is carbonation. (1) In the calcination step: solid calcium carbonate (e.g., limestone) is fed into a calciner, where it is heated to 850–950 °C to cause it to thermally decompose into gaseous carbon dioxide and solid calcium oxide (CaO) and the almost-pure stream of CO2 is then removed and purified so that it is suitable for storage or use (it can be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock). (2) In the carbonation or reverse calcination step: the solid CaO is removed from the calciner and fed into the carbonator; it is cooled to approximately 650 °C and is brought into contact with a flue gas containing a low to medium concentration of CO2; the CaO and CO2 react to form CaCO3, thus reducing the CO2 concentration in the flue gas to a level suitable for emission to the atmosphere. Calcium looping may present interesting potential for integration with the cement industry, especially if limestone calcination can be conveniently carried out using direct solar heat and the auto-thermal re-carbonation can be exploited for thermochemical energy storage applications or post-combustion CO2 capture and utilisation [56,57,58,59].
4. Some Important Characteristics of Solar Furnaces
4.1. Main Advantages of Direct Application of Concentrated Solar Radiation
Besides being free and clean, there are two other major advantageous characteristics of direct application of solar radiation on a target (material or structure): (1) the possibility to achieve rapid heating and rapid thermal cycling, and (2) the use of a wide-spectrum radiation.
4.1.1. Rapid Heating and Rapid Thermal Cycling
The possibility to achieve rapid heating and rapid thermal cycling through the intelligible use of concentrated solar radiation is undoubtedly one of the featuring advantages of solar furnaces. The creation of rapid cycles of temperature variation at the surface of a test-piece is not easy to achieve using the traditional heating-systems, whatever they are: fuel or gas combustion furnaces, electric resistance furnaces, induction furnaces, or even microwave furnaces. However, one way to generate rapid increasing of temperature is by direct exposure of the test-piece to concentrated solar radiation. Exposure to the solar radiation can be done almost instantaneously. Some examples of the use of concentrated solar radiation for rapid heating and thermal cycling are available in the literature [60,61,62,63,64].
An example of the heating-cooling cycles obtained by exposure of white alumina discs to concentrated solar radiation is shown in Figure 3. A close view of variation of temperature versus time for two thermal cycles in the period between 12:39:40 and 12:41:10 of Figure 3a is shown in Figure 3b. In solar furnaces it is a tradition to register the temperatures versus the local time (hour of the day). The solar radiation reaching the heliostat(s) depends on the hour of the day (as well as on weather conditions) and normally the best insolation conditions are used to attain high-temperature in the experiments. In the example shown in Figure 3, the heating-cooling cycles were generated by using an automatic system composed by a plate with reciprocating motion, i.e., with a repetitive back-and-forth linear motion. This type of horizontal shutter is placed close to the focal zone in order to interfere with solar radiation flux before it irradiates the discs. The cooling of the discs can be accelerated by blowing them with compressed air.
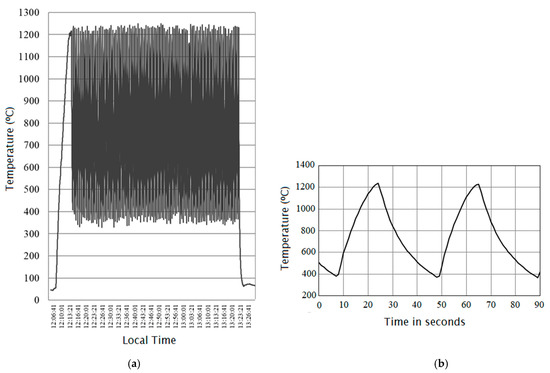
Figure 3.
(a) Example showing 100 heating-cooling obtained with a solar furnace, between 12:13 and 13:23 (local time); (b) Detail of the plot Temperature versus Time showing cycles in the period between 12:39:40 and 12:41:10 [63].
The thermal cycling shown in Figure 3, with Tmax ≈ 1200 °C and Tmin ≈ 400 °C, was conducted on a group of six circular discs (each of them with 25 mm diameter and 2 mm thickness) made of commercial high-purity dense monolithic Alumina (RAPAL® 100) [63]. The specimens’ layout placed at the focal area of a high-flux solar furnace is depicted in Figure 4 in order to explain the positioning of the discs and the location of the thermocouples that were used to measure the temperature at various locations in the vicinity of the alumina discs. Figure 4a shows six discs of RAPAL® 100 duly positioned on a zirconia felt and ready to be irradiated by concentrated sunlight. In this particular experiment, twelve type K thermocouples were used to measure the temperature at various locations in the vicinity of the discs, Figure 4b shows the locations of the thermocouples’ joints, after removing the discs, the zirconia felt, and the alumina thermocouple protection sheaths. The temperature indicated in the graphs of Figure 3 is the temperature measured by the thermocouple located at the centre i.e., the thermocouple with reference no.1 in Figure 4b. During the irradiation, the distribution of temperature was also evaluated using an infra-red camera and its software. Figure 4c depicts an image obtained using an infra-red camera, and it should be remarked that, for achieving a more homogeneous distribution of temperature at the surface of the discs, a device (named “radiation homogenizer”) composed of vertical mirrors was placed close to the focal zone [63].
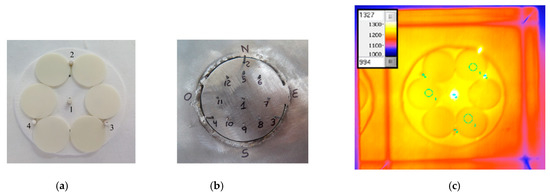
Figure 4.
(a) Discs of alumina (RAPAL® 100) ready to be irradiated; (b) Locations of the thermocouples’ joints; (c) Image obtained by an IR CAM software [63].
4.1.2. A Natural Wide-Spectrum Radiation
Contrary to most forms of radiation from various man-made sources (i.e., artificial sources of radiation, like gamma rays emitted by the radioisotopes, X-rays, microwaves, and Hertzian electromagnetic waves in the radar and radio range) solar radiation comprises a very wide spectrum of electromagnetic waves ranging from ultraviolet radiation (wavelengths between 10 nm and 400 nm) till infrared radiation (wavelengths between 700 nm and 1 mm) and including the visible part of the range. It is behind the scope of the present review to analyse and discuss the works dealing with beneficial and hazardous effects of solar radiation on humans, as well as on other living organisms (multicellular animals, plants, and fungi; or unicellular microorganisms such as bacteria). But, being a “natural” radiation, solar radiation is essential for the study of many phenomena occurring on materials and structures. Theoretically, materials can reflect, transmit, absorb and emit visible, infra-red and ultraviolet radiation coming from the Sun. Most materials do all of this in varying degrees, but some are much more prone to degradation or loss of their initial physical properties due to exposure to solar radiation for long periods of time. The durability of a material may be affected by solar irradiation mainly due to thermal effects and the specific effects caused by the wavelengths of the ultraviolet radiation. Materials more susceptible to degradation due to long-term exposure to solar radiation are polymers and polymer-based composites. One of the biggest problems caused by solar radiation is the photochemical degradation of most organic materials, which in turn causes the elasticity and plasticity of certain rubber compounds and plastic materials to be affected and can, in exceptional cases, make optical glass opaque [65]. Chemical changes induced in polymers by relatively low energy radiation such as UV or visible light are complex phenomena which may proceed via molecular or free-radical mechanisms. Nowadays, artificial weathering devices and artificial light sources are used to measure the resistance of materials to weather degradation. These type of tests are usually named laboratory-accelerated tests because their aim is to provide data in a shorter period of time than outdoor testing. Light sources for the accelerated weathering tests include Xenon long-arc lamps, fluorescent UV lamps, and carbon arc lamps [66].
4.2. Main Difficulties in the Use of Concentrated Solar Radiation
In counterpart to the advantages, there are difficulties which are inherent to the manipulation of concentrated solar radiation. Those difficulties mainly derive from the following facts:
- The solar radiation depends on the atmospheric conditions (especially if clouds appear), on the solar time and on the latitude of the site.
- After concentration, the solar radiation is essentially unidirectional. Then, the targeted objects are usually irradiated/heated in a single direction, which is not the case for most of industrial furnaces, typically dealing with temperatures higher than 400 °C, like gas furnaces, electric furnaces, micro-wave furnaces, or even optical furnaces that use a radiant energy different from the solar radiation. Note that artificial radiation sources may consist of incandescent lamps, graphite heaters, arc lamps, super high-pressure xenon gas-discharge tubes, and plasma radiators.
- Additionally, the flux of solar radiation that reaches the target is theoretically non-homogeneous. In fact, a circle illuminated with a higher concentration in the middle (see Figure 5) is theoretically obtained when the paraboloid reflection model [1] is applied to the traditional solar concentrators utilizing a point focusing solar concentrating panel assembly.
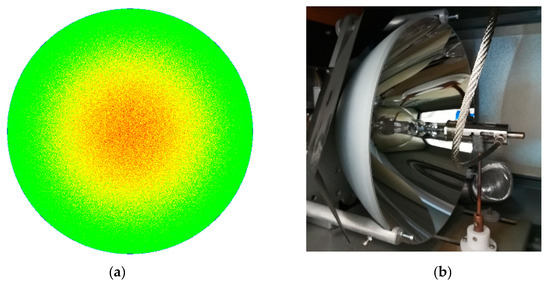 Figure 5. (a) Theoretical distribution of the irradiance (energy flux) reaching the focal plane (perpendicular to the beam) of a solar furnace, applying the paraboloid reflection model [1]; (b) Photo of a xenon-arc lamp with a paraboloid reflector.
Figure 5. (a) Theoretical distribution of the irradiance (energy flux) reaching the focal plane (perpendicular to the beam) of a solar furnace, applying the paraboloid reflection model [1]; (b) Photo of a xenon-arc lamp with a paraboloid reflector.
The profile of the flux distribution is also dependent of any geometric distortion of the concentrating system. As an example, Figure 6 shows the measured flux distribution of the irradiance (energy flux) in a plane (perpendicular to the beam and close to the focal zone), when the solar radiation is first concentrated by a parabolic concave surface. Instead of a Gaussian-type distribution, it is also suggested that the radiation flux profile produced by a parabolic concave surface in a high flux solar furnace can be approximated by a Cauchy–Lorentz distribution [67]. In conclusion, upon the traditional direct concentrated solar radiation exposure, it is rather difficult to ensure a homogenous temperature distribution throughout the exposed samples.
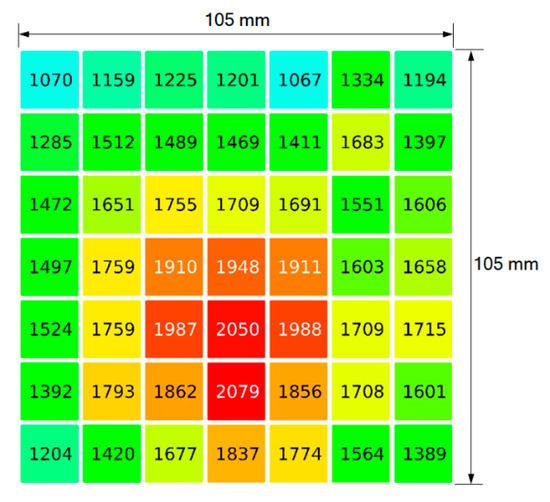
Figure 6.
Experimental (discrete) measurements of irradiance (energy flux), in kW/m2, conducted with a Gardon-type radiometer across a plane perpendicular to the radiation direction of a 60 kW (nominal power) solar furnace.
The measurements indicated in Figure 6 were made with a circular-foil heat flux transducer (Gardon-type radiometer Vatell Thermogage 1000-4; sensitivity = 2 mV per W/cm2). The radiometer was mounted in a moving xyz table and measurements were made in a 2D array with 7 × 7 = 49 positions, separated by 15 mm in both x, y directions, covering a sampling region of 105 mm × 105 mm. The values of energy flux, measured in kW/m2, are reported in Figure 6, using also a colour scheme, going from blue (cold) to red (hot).
A much more homogeneous flux distribution is needed for most of the thermal treatments used for (solid) materials processing, particularly at the industrial scale. Therefore—to overcome this difficulty, inherent to the manipulation of concentrated solar energy—light or radiation homogenizers are nowadays being developed [67,68]. Ideally a uniform incident profile would lead to the homogeneous heating of the sample if the sample is also homogeneous.
5. On-Going Activities and Prospects on Future Trends for Solar Processing of Materials
Two examples of research activities carried out at the University of Lisbon are outlined: (1) development of indirect heating techniques to improve the temperature homogeneity conditions inside reaction chambers for materials processing in solar furnaces; and (2) design of new modular systems, practical and flexible, for capture, concentration, control and conduction of solar radiation.
5.1. Novel Approach Based on Heating by Indirect Irradiation
For the majority of operations for materials processing at the industrial scale (e.g., firing of ceramics, heat treatment of metallic parts, furnaces for glass melting or for glass fritting, calcination furnaces, etc.) it is indispensable that temperature control and temperature homogeneity conditions are guaranteed. Pursuing these objectives, innovative works by Li et al. [69] and Oliveira et al. [70] have provided some guidelines for improving the design of new indirect heating setups. Figure 7 depicts a setup used for indirect heating. Different setup configurations were investigated for improving temperature uniformity and high-temperature experiments using graphite receiver discs were carried out up to 1400 °C. Graphite discs were used in both vacuum and flowing argon atmospheres [70]. Other materials were also used as receivers, namely AISI 310 stainless steel, and molybdenum disilicide (MoSi2): an intermetallic compound with very attractive properties for use as a high-temperature heating element. MoSi2 is very refractory with a melting point of 2030 °C, has excellent resistance to oxidation and a moderate density (6.24 g/cm3) [69].

Figure 7.
Photos showing a setup used for indirect heating: (a) before and (b) after being positioned inside a reaction chamber ready to be irradiated [17,69].
Recent work by Pereira et al. [1] has shown that theoretically there exists the possibility to attain homogenous distribution of concentrated solar flux by means of double reflexion using two paraboloid surfaces. As schematically presented in Figure 8, the parallel rays of solar light are reflected by the “concentrator” (concave paraboloid); then, before being concentrated to the focal point F, if these rays are reflected by a smaller paraboloid (with the same focal point of the “concentrator”)—which can be convex such as paraboloid reflector A or concave such as paraboloid reflector B—our calculations show that the reflected rays are simultaneously highly concentrated and equally distributed over the illuminated region (with a very small hole in the middle due to the shadow produced by the second paraboloid, probably too small to be detectable). Consequently, new devices, e.g., [71], can be designed to homogenise the flux of radiation near the sample (in order to equilibrate the temperatures).
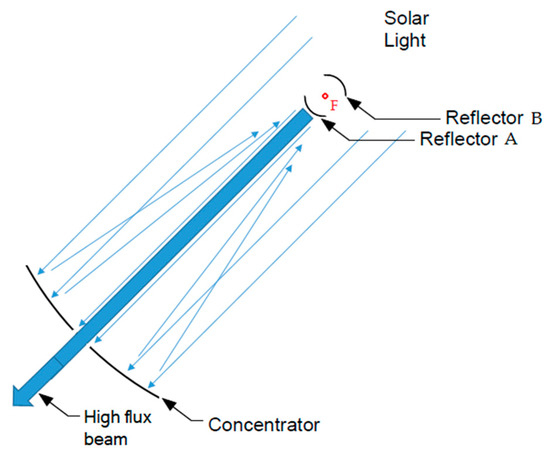
Figure 8.
Device receiving solar light, which is concentrated in a paraboloid concentrator, and then is reflected in a second paraboloid (reflector A or reflector B) to produce a high flux beam that is equally distributed over all the illuminated area.
5.2. Modular Systems for Capture, Concentration, Control and Conduction of Solar Radiation
Figure 9 depicts a computer-aided view of a modular-type system that was conceived to capture, concentrate, control and conduct solar radiation. The presented system is composed of two Fresnel lenses which, side by side, are mounted on a structure. Each Fresnel lens has, on its focus, a receiver device so that the concentrated solar radiation can be captured and then conducted by an optical waveguide made of low-loss optical fibers. Since in this case there are two Fresnel lenses, there will be two fiber-optical cables which conduct the concentrated solar radiation till the place or places where the radiation is needed.
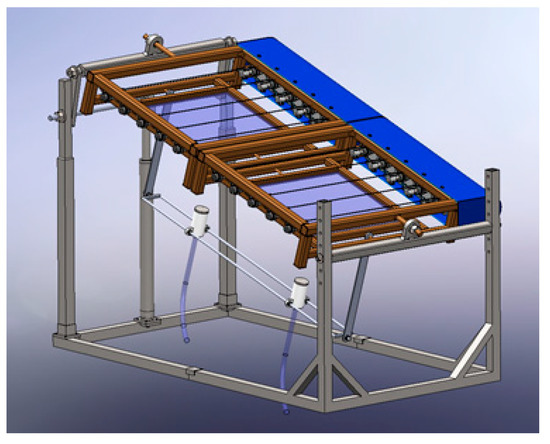
Figure 9.
Computer-aided view of a prototype (with 2 Fresnel lenses and 2 fiber-optical cables) to capture, concentrate, control and conduct solar radiation [17].
These systems can be autonomous, stationary or mobile, and easily transported and deployed on the site where they can collect the direct solar radiation. They can be applied to a variety of processes. The main advantages of such types of systems derived from the fact that fiber optical cables are flexible and, if adequately manufactured, can provide highly concentrated solar radiation to places far from the site where the solar light was collected. Additionally, as the fiber optical cables are flexible, they can be used to illuminate the target not only from one single direction, but by using several cables it is possible to illuminate the target with radiation that is coming from different directions, thus circumventing one of the major problems with traditional high-flux concentrators: the unidirectionality of the radiation pattern.
The structure of the prototype depicted in Figure 9 is designed to follow the Sun during the day and to keep the receiver device as the focus of the Fresnel lens. For control/screening of the radiation that will be transmitted by each of the fiber optical cables, on the top of each Fresnel lens there is a “shutter” or “attenuator” (similar to a venetian blind) made of very light and stiff slats. The slats are rotated by an electrical motor that can be powered by a photovoltaic source. The control of the radiation is made by the opening or closing movement of the slats. Techniques of online/robotic adaptive control are being used [72,73,74] thus allowing dealing with the presence of fast perturbations on sunlight induced by clouds. The ongoing activities are also grounded on the work done by other researchers to demonstrate both capabilities and benefits of the usage of advanced optical fibers [75,76,77,78,79,80], which allow for the transferring and directing of the concentrated solar radiation to the place of solar energy utilization.
Receiver devices to be inserted at the entrance/inlet of the fiber optical cable are now being developed to satisfy the acceptance angle of the optical cables (see Figure 10). The current research results are very promising demonstrating that fiber optical cables (bundles) can be specially designed to transmit concentrated sunlight.
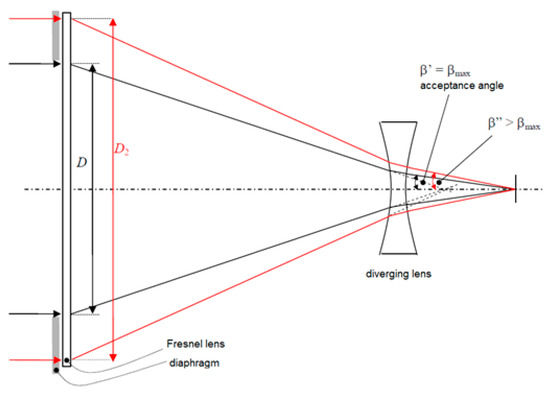
Figure 10.
Example of a concentration system (Fresnel lens) and a diverging lens to satisfy the acceptance angle of a possible conducting optical cable (not shown in the figure) [81].
6. Conclusions
This review was intentionally focussed on solar-driven high-temperature technologies for (solid) materials processing. Solar thermal technologies for the production of electricity, as well as many so-called “solar thermochemical processes” for production of gases or liquids are outside the scope of this review. Nevertheless, despite some current shortcomings for attainment of homogeneous distribution of temperature in the processed materials (due mainly to the unidirectionality of the radiation pattern and the non-homogeneous distribution of the highly concentrated radiant flux), the examples analysed in this work reveal the tremendous potentialities of the usage of solar heat for materials processing.
There is a need for innovative systems for capture, concentration, control and conduction of concentrated solar radiation. Using optical waveguide transmission lines made of low loss optical fibers, it is possible to direct the concentrated solar radiation to the place of utilization of the high-flux solar energy. Therefore, it is foreseen that most of the new systems will take advantage of the capabilities and benefits of the usage of (advanced) optical fibers, which allow for the transferring of solar radiation to locations difficult to get to.
Funding
This research was partially funded by European Union through the project titled “Integrating National Research Agendas on Solar Heat for Industrial Processes” (INSHIP: www.inship.eu) and by the Fundação para a Ciência e a Tecnologia (FCT), Portugal, through IDMEC—Instituto de Engenharia Mecânica (Pólo IST), under LAETA—Associated Laboratory for Energy, Transports and Aeronautics (project grant UID/EMS/50022/2019).
Conflicts of Interest
The author declares no conflict of interest.
References
- Pereira, J.C.G.; Fernandes, J.C.; Rosa, L.G. Mathematical Models for Simulation and Optimization of High-Flux Solar Furnaces. Math. Comput. Appl. 2019, 24, 65. [Google Scholar] [CrossRef]
- Monreal, A.; Riveros-Rosas, D.; Sanchez, M. Analysis of the influence of the site in the final energy cost of solar furnaces for its use in industrial applications. Sol. Energy 2015, 118, 286–294. [Google Scholar] [CrossRef]
- Himalaya, M.A.G. Improved Apparatus for Making Industrial Use of the Heat of the Sun and Obtaining High Temperatures. Patent GB190116181, 26 October 1901. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=19011026&DB=EPODOC&locale=en_EP&CC=GB&NR=190116181A&KC=A&ND=2 (accessed on 7 July 2019).
- Himalaya, M.A.G. Solar Apparatus for Producing High Temperatures. U.S. Patent 797,891, 22 August 1905. Available online: https://patents.google.com/patent/US797891 (accessed on 7 July 2019).
- Rodrigues, J. A Conspiração Solar do Padre Himalaya – Esboço Biográfico Dum Português pioneiro da Ecologia (The Solar Conspiration of Father Himalaya—Biographic Outline of a Portuguese Pioneer in Ecology); Árvore—Cooperativa de Actividades Artísticas: Porto, Portugal, 1999; ISBN 972-9089-44-2. [Google Scholar]
- Tinoco, A. Portugal na Exposição Universal de 1904: O Padre Himalaia e o Pirelióforo. Cadernos Sociomuseologia 2012, 42, 113–127. [Google Scholar]
- Trombe, F.; Le Phat Vinh, A. Thousand kW solar furnace, built by the National Center of Scientific Research, in Odeillo (France). Sol. Energy 1973, 15, 57–61. [Google Scholar] [CrossRef]
- Răboacă, M.S.; Badea, G.; Enache, A.; Filote, C.; Răsoi, G.; Rata, M.; Lavric, A.; Felseghi, R. Concentrating Solar Power Technologies. Energies 2019, 12, 1048. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energ. Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Bushra, N.; Hartmann, T. A review of state-of-the-art reflective two-stage solar concentrators: Technology categorization and research trends. Renew. Sustain. Energ. Rev. 2019, 114, 109307. [Google Scholar] [CrossRef]
- Levêque, G.; Bader, R.; Lipinski, W.; Haussener, S. High-flux optical systems for solar thermochemistry. Sol. Energy 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Petrasch, J.; Coray, P.; Meier, A.; Brack, M.; Haberling, P.; Wuillemin, D.; Steinfeld, A. A novel 50kW 11,000 suns high-flux solar simulator based on an array of Xenon arc lamps. J. Sol. Energy Eng. 2007, 129, 405–411. [Google Scholar] [CrossRef]
- Alxneit, I.; Schmit, H. Spectral characterization of PSI’s high-flux solar simulator. J. Sol. Energy Eng. 2012, 134. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Mochón-Csataños, J.; Sancho-Gorostiaga, J.; Verdeja, L.F. Concentrated solar energy applications in materials science and metallurgy. Sol. Energy 2018, 170, 520–540. [Google Scholar] [CrossRef]
- Alonso, E.; Gallo, A.; Roldan, M.I.; Perez-Rabago, C.A.; Fuentealba, E. Use of rotary kilns for solar thermal applications: Review of developed studies and analysis of their potential. Sol. Energy 2017, 144, 90–104. [Google Scholar] [CrossRef]
- Ho, C.K. A review of high-temperature particle receivers for concentrating solar power. Appl. Therm. Eng. 2016, 109, 958–969. [Google Scholar] [CrossRef]
- Rosa, L.G.; Rosa, N.F. Aplicação direta da radiação solar concentrada em fornos e câmaras de processo: Desenvolvimentos recentes e tendências futuras. In Proceedings of the XVI Congreso Ibérico y XII Congreso Iberoamericano de Energía Solar, Madrid, Spain, 20–24 June 2018; Available online: https://www.researchgate.net/publication/325881511_Aplicacao_direta_da_radiacao_solar_concentrada_em_fornos_e_camaras_de_processo_Desenvolvimentos_recentes_e_tendencias_futuras (accessed on 20 September 2019).
- Koepf, E.; Alxneit, I.; Wieckert, C.; Meier, A. A review of high temperature solar driven reactor technology: 25 years of experience in research and development at the Paul Scherrer Institute. Appl. Energy 2017, 188, 620–651. [Google Scholar] [CrossRef]
- Villasmil, W.; Cooper, T.; Koepf, E.; Meier, A.; Steinfeld, A. Coupled concentrating optics, heat transfer, and thermochemical modeling of a 100-kW(th) high-temperature solar reactor for the thermal dissociation of ZnO. J. Sol. Energy Eng. 2017, 139, 021015. [Google Scholar] [CrossRef]
- Palumbo, R.; Lede, J.; Boutin, O.; Ricart, E.E.; Steinfeld, A.; Möller, S.; Weidenkaff, A.; Fletcher, E.; Bielicki, J. The production of Zn from ZnO in a high temperature solar decomposition quench process ‒ I. The scientific framework for the process. Chem. Eng. Sci. 1998, 53, 2503–2517. [Google Scholar] [CrossRef]
- Haueter, P.; Möller, S.; Palumbo, R.; Steinfeld, A. The production of zinc by thermal dissociation of zinc oxide—Solar chemical reactor design. Sol. Energy 1999, 67, 161–167. [Google Scholar] [CrossRef]
- Ho, D.; Sobon, E.L. Extraterrestrial fiberglass production using solar energy. In Space Resources and Space Settlements (NASA SP-428); Billingham, J., Gilbreath, W., Eds.; National Aeronautics Space Administratio: Washington, DC, USA, 1979; pp. 225–232. Available online: https://space.nss.org/settlement/nasa/spaceres/V-2.html (accessed on 20 September 2019).
- Ahrnad, S.Q.S.; Hand, R.J.; Wieckert, C. Use of concentrated radiation for solar powered glass melting experiments. Sol. Energy 2014, 109, 174–182. [Google Scholar] [CrossRef]
- Romero, M.; Robla, J.I.; Padilla, I.; García-Hierro, J.; López-Delgado, A. Eco-efficient melting of glass frits by concentrated solar energy. Sol. Energy 2018, 174, 321–327. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Lam, R.H.W.; Lau, D. Chemical technologies for modern concrete production. Procedia Eng. 2017, 172, 1270–1277. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Cement. In Mineral commodity summaries 2019; U.S. Geological Survey: Reston, VA, USA, 2019. [Google Scholar] [CrossRef]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Mikulčić, H.; Klimeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Meier, A.; Gremaud, N.; Steinfeld, A. Economic evaluation of the industrial solar production of lime. Energy Convers. Manag. 2005, 46, 905–926. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, S.; Shao, S.; Chen, Y.; Liu, S.; Zhang, S. Aspen Plus-based simulation of a cement calciner and optimization analysis of air pollutants emission. Clean. Technol. Environ. Policy 2011, 13, 459–468. [Google Scholar] [CrossRef]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Ibris, N.; Lupiáñez, C.; Díez, L.I.; Romeo, L.M.; Montagnaro, F. Use of oxyfuel combustion ash for the production of blended cements: A synergetic solution toward reduction of CO2 emissions. Fuel Process. Technol. 2017, 156, 211–220. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Koutník, P. Preparation of β-belite using liquid alkali silicates. Mater. Construcc. 2017, 67. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11. [Google Scholar] [CrossRef]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D. Solar chemical reactor technology for industrial production of lime. Sol. Energy 2006, 80, 1355–1362. [Google Scholar] [CrossRef]
- Abanades, S.; André, L. Design and demonstration of a high temperature solar-heated rotary tube reactor for continuous particles calcination. Appl. Energy 2018, 212, 1310–1320. [Google Scholar] [CrossRef]
- Tregambi, C.; Solimene, R.; Montagnaro, F.; Salatino, P.; Marroccoli, M.; Ibris, N.; Telesca, A. Solar-driven production of lime for ordinary Portland cement formulation. Sol. Energy 2018, 173, 759–768. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Fernandes, J.C.; Galindo, J.; Rodríguez, J.; Cañadas, I.; Vermelhudo, V.; Nunes, A.; Rosa, L.G. Portland cement clinker production using concentrated solar energy—A proof-of-concept approach. Sol. Energy 2019, 183, 677–688. [Google Scholar] [CrossRef]
- Ugwuishiwu, B.O.; Owoh, I.P.; Udom, I.J. Solar energy application in waste treatment—A review. Nigerian J. Technol. 2016, 35, 432–440. [Google Scholar] [CrossRef]
- Mueller, F.; Patel, H.; Blumenthal, D.; Pozivil, P.; Das, P.; Wieckert, C.; Maiti, P.; Maiti, S.; Steinfeld, A. Co-production of syngas and potassium-based fertilizer by solar-driven thermochemical conversion of crop residues. Fuel Process. Technol. 2018, 171, 89–99. [Google Scholar] [CrossRef]
- Piatkowski, N.; Wieckert, C.; Weimer, A.W.; Steinfeld, A. Solar-driven gasification of carbonaceous feedstock—A review. Energy Environ. Sci. 2011, 4, 73–82. [Google Scholar] [CrossRef]
- Moustakas, K.; Loizidou, M. Solid waste management through the application of thermal methods. In Waste Management; Kumar, E.S., Ed.; IntechOpen: Rijeka, Croatia, 2010; ISBN 978-953-7619-84-8. Available online: http://www.intechopen.com/books/waste-management/solid-waste-management-through-the-application-of-thermal-methods (accessed on 20 September 2019). [CrossRef]
- Navarro, A.; Cañadas, I.; Rodríguez, J. Thermal treatment of mercury mine wastes using a rotary solar kiln. Minerals 2014, 4, 37–51. [Google Scholar] [CrossRef]
- Hockaday, S.A.C.; Reynolds, Q.G.; Dinter, F.; Harms, F. Solar thermal treatment of manganese ores. AIP Conf. Proc. 2018, 2033. [Google Scholar] [CrossRef]
- Padilla, I.; López-Delgado, A.; López-Andrés, S.; Álvarez, M.; Galindo, R.; Vázquez-Vaamonde, A.J. The application of thermal solar energy to high temperature processes: Case study of the synthesis of alumina from boehmite. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Müller, F.; Saw, W.L.; Steinfeld, A.; Nathan, G.J. Solar-driven alumina calcination for CO2 mitigation and improved product quality. Green Chem. 2017, 19, 2992–3005. [Google Scholar] [CrossRef]
- Eglinton, T.; Hinkley, J.; Beath, A.; Dell’Amico, M. Potential applications of concentrated solar thermal technologies in the australian minerals processing and extractive metallurgical industry. JOM 2013, 65, 1710–1720. [Google Scholar] [CrossRef]
- Ekman, B.M.; Brooks, G.; Rhamdhani, M.A. A review: Solar thermal reactors for materials production. In Energy Technology 2014: Carbon Dioxide Management and Other Technologies; Wang, C., de Bakker, J., Belt, C.A., Jha, A., Neelameggham, N.R., Pati, N.S., Prentice, L.H., Trnell, G., Brinkman, K.S., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 1–14. [Google Scholar] [CrossRef]
- Arenas, M.A.; Padilla, I.; Robla, J.A.; Vázquez, A.J.; López-Delgado, A. The use of concentrated solar energy for the reduction of CuO in H2. Sol. Energy 2019, 180, 640–647. [Google Scholar] [CrossRef]
- Shohoji, N.; Oliveira, F.A.C.; Galindo, J.; Fernandes, J.C.; Rodríguez, J.; Cañadas, I.; Rosa, L.G. Influence of Linear Flow Velocity of Uncracked Ammonia (NH3) Gas on Formation of Higher Nitrides, 𝛅-MoN and 𝛆-Fe2N, under Concentrated Solar Irradiation in the SF40 Solar Furnace at PSA. Internat. J. Mater. Chem. 2019, 9, 1–12. [Google Scholar] [CrossRef][Green Version]
- Alovisio, A.; Chacartegui, R.; Ortiz, C.; Valverde, J.M.; Verda, V. Optimizing the CSP-calcium looping integration for thermochemical energy storage. Energy Convers. Manag. 2017, 136, 85–98. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Tregambi, C.; Salatino, P.; Solimene, R.; Montagnaro, F. An experimental characterization of Calcium Looping integrated with concentrated solar power. Chem. Eng. J. 2018, 331, 794–802. [Google Scholar] [CrossRef]
- Zhai, R.; Li, C.; Qi, J.; Yang, Y. Thermodynamic analysis of CO2 capture by calcium looping process driven by coal and concentrated solar power. Energy Convers. Manag. 2016, 117, 251–263. [Google Scholar] [CrossRef]
- Douale, P.; Serror, S.; Duval, R.M.P.; Serra, J.J.; Felder, E. Thermal shocks on an electrolytic chromium coating in a solar furnace. J. Phys. IV Fr. 1999, 9, 429–434. [Google Scholar] [CrossRef]
- Kováčik, J.; Emmer, S.; Rodriguez, J.; Cañadas, I. Solar furnace: Thermal shock behaviour of TiB2 coating on steel. In Proceedings of the METAL 2014, Brno, Czech Republic, 21–23 May 2014; pp. 863–868. Available online: https://www.academia.edu/24388464/Solar_Furnace_Thermal_Shock_Behaviour_of_TIB2_Coating_on_Steel (accessed on 20 September 2019).
- Sallaberry, F.; García de Jalón, A.; Zaversky, F.; Vázquez, A.J.; López-Delgado, A.; Tamayo, A.; Mazo, M.A. Towards standard testing materials for high temperature solar receivers. Energy Procedia 2015, 69, 532–542. [Google Scholar] [CrossRef][Green Version]
- Rosa, L.G.; Rodríguez, J.; Pereira, J.; Fernandes, J.C. Thermal cycling behaviour of dense monolithic alumina. In Proceedings of the Second International Conference on Materials Chemistry and Environmental Protection—MEEP 2018, Sanya, China, 23–25 November 2018; pp. 173–179, ISBN 978-989-758-360-5. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Fernandes, J.C.; Galindo, J.; Rodríguez, J.; Canãdas, I.; Rosa, L.G. Thermal resistance of solar volumetric absorbers made of mullite, brown alumina and ceria foams under concentrated solar radiation. Sol. Energy Mater. Sol. Cells 2019, 194, 121–129. [Google Scholar] [CrossRef]
- MacCallum, J.R. Photodegradation. In Comprehensive Polymer Science; Allen, G., Bevington, J.C., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 1989; Volume 6, pp. 529–537. [Google Scholar] [CrossRef]
- McKeen, L.W. Introduction to the weathering of plastics. In The Effect of UV Light and Weather on Plastics and Elastomers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–41. [Google Scholar] [CrossRef]
- Gomez-Garcia, F.; Santiago, S.; Luque, S.; Romero, M.; Gonzalez-Aguilar, J. A new laboratory-scale experimental facility for detailed aerothermal characterizations of volumetric absorbers. AIP Conf. Proc. 2016, 1734, 030018. [Google Scholar] [CrossRef]
- Luque, S.; Bai, F.; González-Aguilar, J.; Wang, Z.; Romero, M. A parametric experimental study of aerothermal performance and efficiency in monolithic volumetric absorbers. AIP Conf. Proc. 2017, 1850, 030034. [Google Scholar] [CrossRef]
- Li, B.; Oliveira, F.A.C.; Rodríguez, J.; Fernandes, J.C.; Rosa, L.G. Numerical and experimental study on improving temperature uniformity of solar furnaces for materials processing. Sol. Energy 2015, 115, 95–108. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Fernandes, J.C.; Rodríguez, J.; Cañadas, I.; Galindo, J.; Rosa, L.G. Temperature uniformity improvement in a solar furnace by indirect heating. Sol. Energy 2016, 140, 141–150. [Google Scholar] [CrossRef]
- Martins, C.A.B. Aparelho Concentrador e Estabilizador de Raios Solares e Sistema de Transmissão de um Feixe de Raios Solares Concentrados e Estabilizados que o Contém. Portuguese Patent Pending Request No. PT20160109071, 4 January 2016. Available online: https://pt.espacenet.com/searchResults?CY=pt&DB=EPODOC&F=0&FIRST=1&LG=pt&PN=PT109071&Submit=PESQUISAR&bookmarkedResults=true&locale=pt_pt&sf=n (accessed on 22 July 2019).
- Costa, B.A.; Lemos, J.M.; Rosa, L.G. Temperature control of a solar furnace for material testing. Int. J. Syst. Sci. 2011, 42, 1253–1264. [Google Scholar] [CrossRef]
- Costa, B.A.; Lemos, J.M. Predictive adaptive temperature control in a solar furnace for material stress tests. In Proceedings of the 2012 IEEE Multi-conference on Systems and Control, Dubrovnik, Croatia, 3–5 October 2012; pp. 1340–1345. [Google Scholar] [CrossRef]
- Costa, B.A.; Lemos, J.M.; Guillot, E. Solar furnace temperature control with active cooling. Sol. Energy 2018, 159, 66–77. [Google Scholar] [CrossRef]
- Nakamura, T. Optical waveguide system for solar power applications in space. In Nonimaging Optics: Efficient Design for Illumination and Solar Concentration VI; International Society for Optics and Photonics: San Francisco, CA, USA, 2009; Volume 7423, p. 74230C. [Google Scholar] [CrossRef]
- Nakamura, T.; Smith, B.K. Solar thermal system for lunar ISRU applications: Development and field operation at Mauna Kea, HI. In Proceedings of the SPIE 8124, Nonimaging Optics: Efficient Design for Illumination and Solar Concentration VIII; 81240B (2011), San Diego, CA, USA, 21–25 August 2011. [Google Scholar] [CrossRef]
- Nakamura, T.; Smith, B.K.; Irvin, B.R. Optical waveguide solar power system for material processing in space. J. Aerosp. Eng. 2015, 28, 04014051. [Google Scholar] [CrossRef]
- Henshall, P.; Palmer, P. Concentrator pointing control concept for fiber optic augmented solar thermal propulsion systems. J. Spacecr. Rockets 2016, 53, 230–234. [Google Scholar] [CrossRef]
- Alwahabi, Z.T.; Kueh, K.C.; Nathan, G.J.; Cannon, S. Novel solid-state solar thermal simulator supplying 30,000 suns by a fibre optical probe. Opt. Express 2016, 24, A1444–A1453. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Fan, G.H.; Zhang, Y.Q.; Ji, X.F. A fresnel concentrator with fiber-optic bundle based space solar power satellite design. Acta Astronaut. 2018, 153, 122–129. [Google Scholar] [CrossRef]
- De Almeida, G. Proposta: Estrutura de Suporte do Concentrador e Dispositivos de Fixação e Regulação dos Seus Elementos Ópticos; Ed. do Autor: Lisboa, Portugal, 2019. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).